Abstract
Objective
A growing body of evidence supports an antiobesity effect of dairy products; however, the mechanisms remain unclear. The objective of this study was to explore possible intestinal mechanisms by which dairy delivers an antiobesity effect. The human intestinal cell line, NCI-H716, was used to test the hypothesis that branched-chain amino acids and dairy proteins regulate satiety hormone secretion and modulate genes involved in fatty acid and cholesterol metabolism.
Methods
In dose–response (0.5%, 1.0%, 2.0%, and 3.0%) studies, the effect of leucine, isoleucine, valine, skim milk, casein, and whey on glucagon-like peptide-1 release and the expression of selected genes were tested.
Results
Leucine, isoleucine, skim milk, and casein stimulated glucagon-like peptide-1 release (P < 0.05). Isoleucine and whey downregulated the expression of intestinal-type fatty acid binding protein (i-FABP), fatty acid transport protein 4 (FATP4), Niemann-Pick C-1–like-1 protein (NPC1L1), acetyl-coenzyme A carboxylase (ACC), fatty acid synthase (FAS), sterol regulatory element-binding protein-2 (SREBP-2), and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR; P < 0.05). Leucine and valine downregulated the expression of NPC1L1, ACC, FAS, SREBP-2, and HMGCR (P < 0.05). Casein downregulated the expression of i-FABP, FATP4, ACC, FAS, SREBP-2, and HMGCR (P < 0.05). Skim milk downregulated the expression of ACC, FAS, and SREBP-2, but not i-FABP, FATP4, and NPC1L1.
Conclusion
This work suggests that the antiobesity effect of dairy may be mediated, at least in part, by integration of events that promote glucagon-like peptide-1 secretion and inhibit expression of genes involved in intestinal fatty acid and cholesterol absorption and synthesis.
Keywords: Milk protein, Branched-chain amino acids, Lipid metabolism, Gene expression, Fatty acid transport
Introduction
Epidemiologic studies have supported the hypothesis that a dairy-rich diet is associated with lower fat accumulation, although prospective and intervention studies are not unanimous [1–6]. Our understanding of the mechanisms responsible for this association remains incomplete. The calcitropic hormones–intracellular calcium hypothesis was proposed to explain the antiobesity effect of calcium [7]. However, data from the Coronary Risk Development in Young Adults study [8], clinical trials [6,9], and animal studies [10,11] have shown greater effects of dairy products than of mineral calcium in attenuating adiposity. The data suggest that additional bioactive components of dairy products may be responsible for this augmentation.
The major protein fractions of dairy, casein and whey, have been reported to exert differential effects on gastrointestinal hormone secretion and appetite [12]. As regulators of protein metabolism, branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) have also been reported to improve glucose and cholesterol metabolism [13,14] and control appetite [15]. A study by Bowen et al. [16] suggested that the protein component of dairy appears to be more important for weight loss than calcium in over-weight adults. Therefore, likely candidates for these additional effects of dairy are casein and whey and its high concentrations of BCAAs. However, less information is available on the mechanisms by which dairy proteins and BCAAs may deliver a benefit in terms of preventing weight gain and promoting loss of body fat.
We previously demonstrated in vivo and in vitro that dietary fiber and meat hydrolysate increase the secretion of glucagon-like peptide-1 (GLP-1), a key satiety hormone, and modulate proglucagon gene expression [17–22]. Distinct from alterations in satiety, additional mechanisms may also play a role in body weight regulation by dairy and BCAAs. Three key transcription factors, designated sterol regulatory element binding proteins (SREBPs) 1a, 1c, and 2, regulate the transcription of genes involved in fatty acid and cholesterol synthesis including acetyl-coenzyme A carboxylase (ACC), fatty acid synthase (FAS), and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) [23]. In addition, intestinal-type fatty acid binding protein (i-FABP) and fatty acid transport protein-4 (FATP4) are thought to be important factors for long-chain fatty acid uptake in enterocytes [24,25], whereas Niemann-Pick C-1–like-1 protein (NPC1L1) is thought to be the principal intestinal cholesterol transporter [26,27].
In contrast to the extensive evidence for the role of diet in regulating fatty acid synthesis associated with the liver and the involvement of the SREBPs, much less is known regarding fatty acid metabolism in the intestine and particularly in response to dairy intake. Given that the intestine is the first tissue involved in mediating the effects of dairy in the whole body, our objective was to explore the molecular mechanisms by which it delivers its antiobesity benefits. We examined the direct effects of BCAAs and dairy proteins on intestinal cells in terms of GLP-1 secretion and the expression of genes involved in intestinal fatty acid and cholesterol metabolism.
Methods and materials
Chemicals
L-leucine, L-isoleucine, L-valine, casein, and whey were purchased from MP Biomedicals, LLC (Solon, OH, USA). Skim milk powder was purchased from EMD Chemicals Inc. (Darmstadt, Germany).
GLP-1 release study
The methods used for the intestinal cell culture have been published [18]. Briefly, human NCI-H716 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). For proliferation, the cells were grown in suspension in RPMI-1640 supplemented with 10% fetal bovine serum, 2 mmol/L of L-glutamine, 100 IU/mL of penicillin, and 100 mg/L of streptomycin. Two days before the experiment, 1 × 106 cells were seeded into 12-well culture plates coated with Matrigel (Becton Dickinson, Bedford, MA, USA). On the day of the experiment, supernatant was replaced by Krebs-Ringer bicarbonate buffer containing 0.2% bovine serum albumin (w/v) with or without BCAA or dairy protein. Four different concentrations (0.5%, 1.0%, 2.0%, and 3.0%, wt/vol) were selected based on our previous work [17,18] and others [28] to determine the dose–response effect of isoleucine, valine, skim milk, casein, and whey. For leucine, however, 0.5%, 1.0%, 1.5%, and 2.0% (wt/vol) were chosen in this study because leucine is poorly soluble in a 3.0% or higher solution. The concentrations selected were not shown to have any harmful effects on cell viability. Cells were incubated for 2 h. Supernatant was collected with the addition of 50 mg/L of phenylmethylsulfonyl fluoride and 34 mg/L of diprotin A. Cells were scraped and sonicated in a homogenization buffer for GLP-1 measurement. Biologically active GLP-1 (7–36) amide was measured as described by the supplier of the GLP-1 (active) radioimmunoassay kit (Linco Research, Billerica, MA, USA).
mRNA expression study
NCI-H716 cell culture
For the experiments, a total of 2 × 106 cells were seeded in six-well culture plates coated with Matrigel and grown in Dulbecco’s Minimal Eagle’s Medium high glucose, 10% fetal bovine serum, 2 mmol/L of L-glutamine, 100 IU/mL of penicillin, and 100 mg/L of streptomycin. After 24 h, the same medium without fetal bovine serum but with the addition of 0.2% bovine serum albumin and the test agents were added. After an additional 18 h, cells were washed with Hank’s balanced salt solution and stored at −80°C until RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was extracted using the RNeasy Mini Kit as described by the manufacturer (Qiagen Sciences, Mississauga, ON, Canada). Total RNA (1 μg) was reverse-transcribed using Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) with oligo-d(T)12–18 as primer. After reverse transcription, the same batch of diluted cDNA was subjected to real-time polymerase chain reaction (PCR) to amplify target genes.
Primer design
The β-actin gene was selected to normalize the expression of the target genes. The primers were designed to span two exon boundaries using Primer Express software (Beacon Designer, PRIMIER Biosoft International, Palo Alto, CA, USA), thus restricting PCR amplifications to cDNA templates only. The primers were synthesized by the UC-DNA Laboratory (University of Calgary, Calgary, AB, Canada) and are listed in Table 1. Four concentrations of primers (0.5, 1.0, 1.5, and 2.0 nmol/L) were evaluated, and formation of primer dimers was assessed by melting curve analysis. Thus, only those concentrations of primers that showed dimer-free reactions were used for the final analysis.
Table 1.
Primer sequences for real-time polymerase chain reaction
| Gene | Forward and reverse primers (5′→3′) | Amplicon size (bp) | Accession no. |
|---|---|---|---|
| β-actin | TGTCCACCTTCCAGCAGATGT GCATTTGCGGTGGACGAT |
77 | NM_001101 |
| ACC | GGCCAGATTCAAGCCATGTT TCTAGCCACTCCGCCAGATC |
101 | U19822 |
| FAS | GGTCTTGAGAGATGGCTTGC CAGGTTGACAGCAGCCAAGT |
75 | NM_004104 |
| FATP4 | CTGCCTGAGCTGCACAAAAC GTAGATAGAACAGCGGGTCTTCACA |
102 | AF055899 |
| HMGCR | CATGATTCACAACAGGTCGAAGA CCCATGTTCCAGTTCAGAACTG |
101 | NM_000859 |
| i-FABP | CCTAGAGGCTGACTCAACTGAAATC CATGAGCTGCAAGCTTCCTTT |
128 | NM_000134 |
| NPC1L1 | TTGAGGTCTTCCCCTACACGAT AGGCAGAGGCTGAGCATGAA |
91 | NM_013389 |
| SREBP-2 | CTGCACATCACAGGGAAGCTT GGGATCTTCTCCTCTGCACATT |
101 | NM_004599 |
ACC, acetyl-coenzyme A carboxylase; FAS, fatty acid synthase; FATP4, fatty acid transport protein-4; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; i-FABP, intestinal-type fatty acid binding protein; NPC1L1, Niemann-Pick C-1–like-1 protein; SREBP-2, sterol regulatory element-binding protein-2.
Real-time PCR
The same number of samples for each cDNA was analyzed to prevent bias in the results. Each sample was analyzed in duplicate in a total reaction volume of 25 μL consisting of 5 μL of dilute cDNA (1:10) and 20 μL of master mix. The master mix contained 2.5 μL of 10 × PCR buffer, 1.5 μL of 50 mmol/L of MgCl2, 0.05 μL of 100 mmol/L of dNTPs, 2.5 μL of sybergreen (1:5000), 1.0 μL of fluorescein (1:10 000), 0.2 μL of Taq DNA polymerase (Invitrogen) and the required amount of forward and reverse primers. Reactions were run on an iCycler thermal cycler (Bio-Rad, Hercules, CA, USA) using the following cycling conditions: 95°C for 1:30 min and 40 cycles at 95°C for 25 s, 60°C for 20 s, and 72°C for 20 s. For each experiment, a non-template reaction was included as a negative control. The β-actin primers were included in the reaction as internal controls for all genes. The specificity of the PCR reactions was confirmed by melting curves analysis of the products and by size verification of the amplicons in a conventional agarose gel.
The threshold cycle values (Ct) were determined at the same fluorescence threshold line for each gene. The fold change in target gene relative to the β-actin was determined using the 2−ΔΔCt method [29].
Statistical methods
Results are presented as the average of a minimum of three independent experiments. Data are expressed as mean ± SEM. Analyses were carried out with SPSS 15 for Windows (SPSS Inc., Chicago, IL, USA). To determine treatment effects and compare differences among group means, data were analyzed by one-way analysis of variance followed by post hoc Duncan’s multiple range test. Statistical significance was accepted at P < 0.05.
Results
Dose–response effects of BCAAs and dairy proteins on GLP-1 secretion
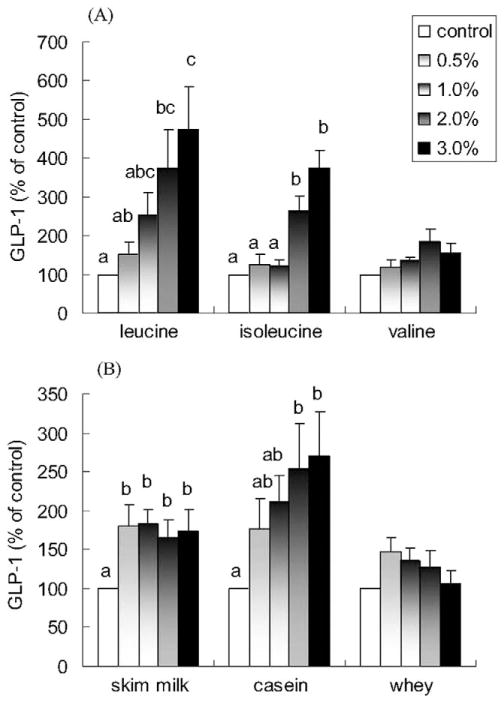
The BCAAs, leucine and isoleucine, induced a concentration-dependent increase in GLP-1 release. The increase in GLP-1 release with 3% leucine and isoleucine was approximately 474% and 264% above control, respectively (P < 0.05; Fig. 1A). In comparison, valine only induced a minimal increase in GLP-1 release (P = 0.073 for the 2% concentration versus control).
Fig. 1.
Effects of branched-chain amino acids, skim milk, casein, and whey on GLP-1 release in NCI-H716 cells. Cells (1 × 106) were incubated for 2 h with increasing concentrations of (A) leucine, isoleucine, valine and (B) skim milk, casein, and whey. Secretion into the medium was normalized to the total cellular content and expressed as a percentage of the control value. Due to lower solubility, leucine concentrations were 1.5% and 2.0% instead of 2.0% and 3.0%. Values are means ± SEMs of four independent experiments. Treatments with different letters are significantly different (P < 0.05). GLP-1, glucagon-like peptide-1.
Skim milk and casein also stimulated GLP-1 release (P < 0.05; Fig. 1B). A dose-dependent effect was observed with added casein but not with added skim milk, which appeared to already have a threshold at 0.5%. GLP-1 release was increased from 176% to 270% when casein was increased from 0.5% to 3.0% (P < 0.05; Fig. 1B). Although 0.5% whey increased GLP-1 release by 148%, the differences failed to reach statistical significance (P > 0.1).
Dose–response effects of BCAA on intestinal mRNA expression of genes for fatty acid and cholesterol metabolism
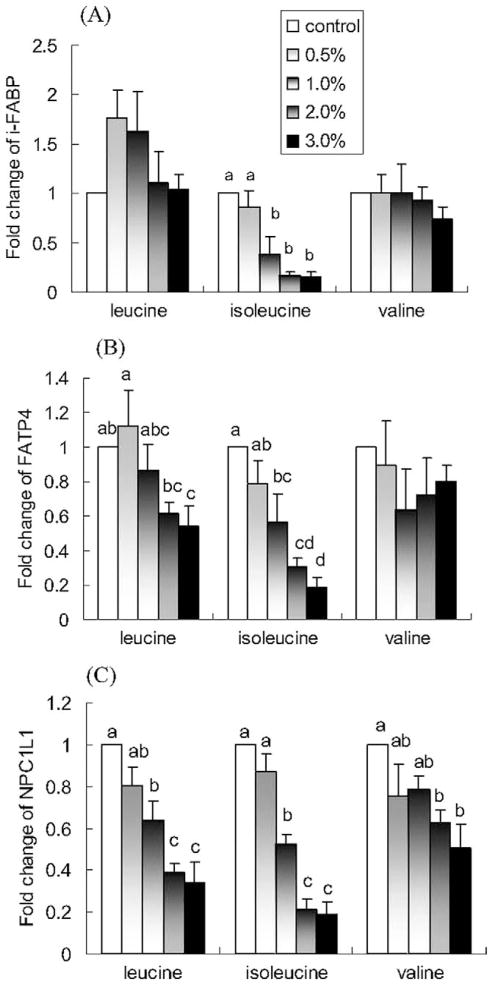
Isoleucine induced a concentration-dependent decrease in the expression of i-FABP, FATP4, and NPC1L1 (P < 0.05; Fig. 2). The 2% isoleucine treatment reduced i-FABP expression by 84%, FABP-4 expression by 70%, and NPC1L1 expression by 79% compared with control. Leucine also induced a concentration-dependent decrease in the expression of FATP4 and NPC1L1 (P < 0.05), but not i-FABP (P > 0.05). Valine induced a concentration-dependent decrease in the expression of NPC1L1 (P < 0.05), but not i-FABP and FATP4 (P > 0.05).
Fig. 2.
Effects of leucine, isoleucine, and valine on in vitro mRNA expression of (A) i-FABP, (B) FATP4, and (C) NPC1L1. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Polymerase chain reaction products for genes of interest were normalized to β-actin mRNA as a control. Due to lower solubility, leucine concentrations are 1.5% and 2.0% instead of 2.0% and 3.0%. Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). FATP4, fatty acid transport protein-4; i-FAB, intestinal-type fatty acid binding protein; NPC1L1, Niemann-Pick C-1–like-1 protein.
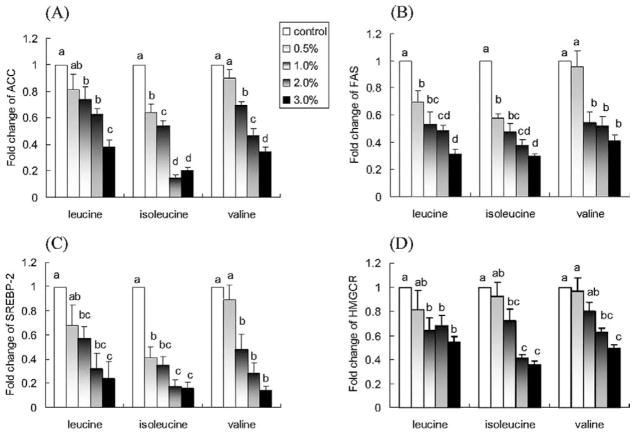
Leucine, isoleucine, and valine downregulated the expression of ACC and FAS (P < 0.05; Fig. 3A,B). They also induced a concentration-dependent decrease in the expression of SREBP-2 and HMGCR (P < 0.05; Fig. 3C,D).
Fig. 3.
Effect of leucine, isoleucine, and valine on in vitro mRNA expression of (A) ACC, (B) FAS, (C) SREBP-2, and (D) HMGCR. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Due to lower solubility, leucine concentrations are 1.5% and 2.0% instead of 2.0% and 3.0%. Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). ACC, acetyl-coenzyme A carboxylase; FAS, fatty acid synthase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; SREBP-2, sterol regulatory element binding protein-2.
Dose–response effects of dairy proteins on mRNA expression of genes involved in fatty acid and cholesterol metabolism
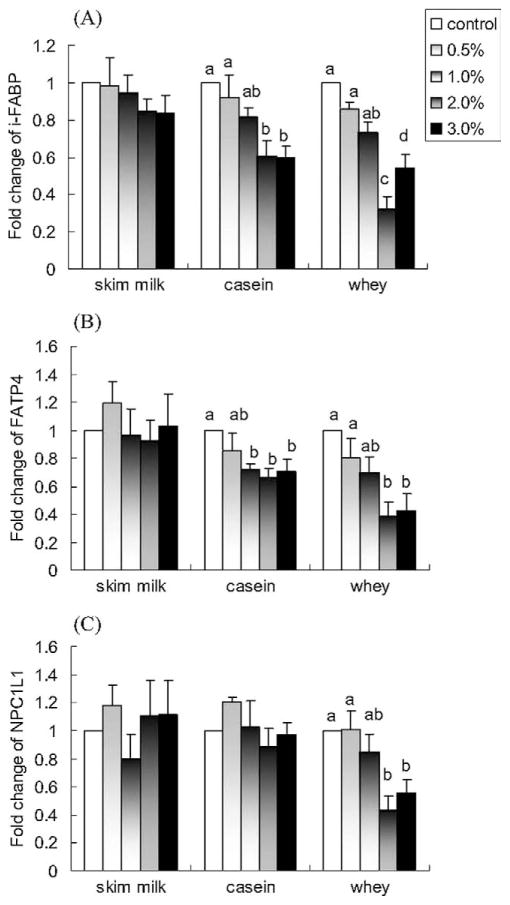
Casein and whey induced a concentration-dependent decrease in the expression of i-FABP and FATP4 (P < 0.05; Fig. 4A,B). Interestingly, there were no significant effects of skim milk on the expression of i-FABP and FATP4 (P > 0.05; Fig. 4A,B). Whey also induced a concentration-dependent decrease in the expression of NPC1L1 (P < 0.05; Fig. 4C), whereas skim milk and casein did not affect the expression of NPC1L1 (P > 0.05; Fig. 4C). The results also demonstrated that 2.0% whey appears to be the most effective dose for the modulation of expression of these three genes, with downregulations of 68% on i-FABP, 61% on FATP, and 57% on NPC1L1, respectively.
Fig. 4.
Effects of skim milk, casein, and whey on in vitro mRNA expression of (A) i-FABP, (B) FATP4, and (C) NPC1L1. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). FATP4, fatty acid transport protein-4; i-FABP, intestinal-type fatty acid binding protein; NPC1L1, Niemann-Pick C-1–like-1 protein.
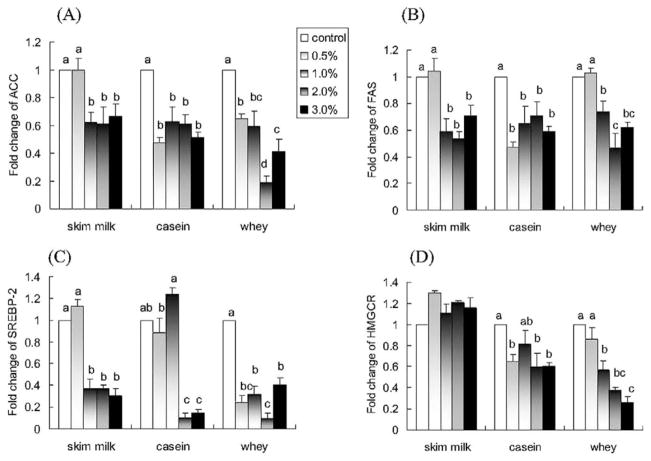
As shown in Figures 5A and 5B, skim milk and casein downregulated the expression of ACC and FAS (P < 0.05), with a plateau reached at 1.0% skim milk and casein corresponding to ~40% inhibition of ACC and FAS expressions. Whey also induced a concentration-dependent decrease in the expression of ACC and FAS (P < 0.05). Whey at 2.0% appeared to be the most effective dose, with down-regulations of 81% for ACC and 54% for FAS, respectively.
Fig. 5.
Effects of skim milk, casein, and whey on in vitro mRNA expression of (A) ACC, (B) FAS, (C) SREBP-2, and (D) HMGCR. NCI-H716 cells (2 × 106) were incubated for 42 h with Dulbecco’s Minimal Eagle’s Medium plus 0.2% bovine serum albumin with or without one of the treatments (w/v). Values are means ± SEMs of four experiments. Treatments with different letters are significantly different (P < 0.05). ACC, acetyl-coenzyme A carboxylase; FAS, fatty acid synthase; HMGCR, 3-hydroxy-3-methylglutaryl-cenzyme A reductase; SREBP-2, sterol regulatory element binding protein-2.
Skim milk, casein, and whey also induced a concentration-dependent decrease in the expression of SREBP-2 (P < 0.05; Fig. 5C) with 2% concentrations causing decreases of 63% with skim milk, 90% with casein, and 91% with whey compared with control. Casein and whey, but not skim milk, induced a concentration-dependent decrease in the expression of HMGCR (P < 0.05; Fig. 5D).
Discussion
Several novel findings arise from this work to support an intestinal mechanism as part of the antiobesity effects of dairy products. This study demonstrates that the individual BCAAs, leucine and isoleucine, and the dairy proteins, skim milk and casein, potently stimulate GLP-1 release in vitro. In addition to augmentation of the release of this satiety and glucoregulatory hormone, we also demonstrated that isoleu-cine and whey downregulated the expression of several genes involved in intestinal fatty acid transport (i-FABP, FATP4), cholesterol absorption (NPC1L1), lipogenesis (ACC, FAS), and cholesterol metabolism (SREBP-2, HMGCR). Leucine, valine, skim milk, and casein were associated with a downregulation of the expression of ACC, FAS, and SREBP-2. These data suggest that dairy proteins and their high concentrations of BCAAs may contribute to dairy’s antiobesity effect by integration of intestinal events that promote GLP-1 secretion and inhibit expression of genes involved in fatty acid and cholesterol absorption and synthesis. In addition to its high protein quality and calcium content, the potential benefits of dairy products for weight management may reflect its ability to modulate the transport and metabolism of fatty acids and cholesterol in the intestine.
The casein content of milk represents about 80% of milk protein. The digestion and absorption of casein and whey differ in that casein, unlike whey, coagulates in the stomach due to its precipitation by gastric acid [30,31]. This coagulation results in a slower gastric emptying time for casein and a smaller postprandial increase in plasma amino acids compared with the non-coagulating whey. It is well known that several key satiety hormones, including cholecystokinin, peptide YY, GLP-1, and glucose-dependent insulino-tropic polypeptide, are released from the gastrointestinal tract in response to nutrients [32]. It might be predicted that whey, a “fast protein,” would be more satiating than casein, a “slow protein.” Conflicting results, however, have been reported. A human study by Hall et al. [12] demonstrated that postabsorptive increases in plasma amino acids together with cholecystokinin and GLP-1 act as potential mediators of the increased satiety response to the “fast protein” whey. However, a study in human subjects conducted by Calbet and Holst [33] indicated that similar gastric emptying rates and GLP-1 and peptide YY plasma responses were elicited by the four solutions (casein whole protein, whey whole protein, casein hydrolysate, and whey peptide hydrolysate). The solutions utilized by Calbet and Holst [33] were matched for volume, nitrogen content, energy density, osmolality, pH, and temperature and this may explain the differences between various reports.
It is interesting to note that protein and amino acids are not all equally effective in stimulating GLP-1 release. We previously demonstrated that meat hydrolysate is a potent GLP-1 secretagogue and that a mixture of essential amino acids but not non-essential amino acids trigger GLP-1 release [17]. Diepvens et al. [34] also showed that ingestion of milk protein results in significantly higher plasma GLP-1 levels in humans than a vegetable protein, pea protein hydrolysate. Cordier-Bussat et al. [28] demonstrated that meat and albumin egg hydrolysates but not bovine serum albumin and a mixture of amino acids stimulate GLP-1 secretion in a murine intestinal cell line. Our results show a direct effect of skim milk and casein but not whey on stimulating GLP-1 release in the human intestinal NCI-H716 cell line by 1.6-and 2.5-fold, respectively. These findings suggest that promoting secretion of satiety hormones from the gastrointestinal tract with direct contact with dairy proteins is one mechanism by which dairy may exert its antiobesity effects. Surprisingly and contrary to our hypothesis, the increase in GLP-1 release in response to whey treatments failed to reach statistical significance. It is not inherently obvious why we would have observed the null results of GLP-1 in response to whey. From our previous studies demonstrating that soluble dietary fiber, which slows gastrointestinal transit, promotes GLP-1 release [19,21], it may be argued that a slower emptying rate and prolonged intestinal absorption of casein may stimulate GLP-1 release from the L cells of the distal small intestine and colon. It is interesting to note the recent observation by Diepvens et al. [34] that GLP-1 concentrations after milk protein and whey protein ingestion in humans were 145.3 ± 194.9 and 96.7 ± 150.2 pmol/L, respectively. Although these differences were not significant, likely due to the large variation seen in the groups, the direct exposure of intestinal cells to skim milk versus whey as performed in our study may accentuate this difference. The results from in vitro and in vivo studies may demonstrate a difference in the direct effects of nutrients on en-teroendocrine cells and the effects of dairy protein when other compensatory mechanisms are present during in vivo digestion and absorption. The differences noted between in vitro studies that examine direct effects of nutrients on intestinal cells in an isolated system and in vivo studies could be attributed to the reduced exposure of distal intestinal cells to the major macronutrients. Further studies will be needed to fully differentiate the role of whey and casein versus skim milk in the regulation of satiety hormone secretion and food intake.
In addition to whole protein, the amino acid composition of dairy protein may also play an important role in reducing fat accumulation. BCAAs have attracted the greatest attention given the relatively high proportion of BCAAs (~21%) found in dairy [35]. The role of BCAAs as substrates for protein synthesis is well documented. Recent studies have shown that BCAAs improve glucose and cholesterol metabolism in mice [14] and decrease insulin resistance in adipocytes by the insulin-signaling pathway [36]. Our present results demonstrate for the first time that leucine and isoleucine induce a concentration-dependent increase in GLP-1 release. There was an impressive 4.7- and 2.6-fold increase of GLP-1 release in response to 2% leucine and isoleucine, respectively. These findings support our recent work showing that a solution of essential amino acids (which includes the BCAAs) stimulates GLP-1 release from the NCI-H716 cell line but a solution of non-essential amino acids does not [17].
A recent study by Cota et al. [15] showed that central administration of leucine decreased food intake and body weight through the mammalian target of rapamycin (mTOR) pathway, an evolutionarily conserved mechanism for nutritional control of cellular growth and function. Numerous other studies have confirmed the role of leucine in activating the mTOR signaling pathway in certain cells including the intestine [37–39]. In the presence of leucine or other recently identified amino acids such as arginine, p70 S6 kinase and eIF-4E binding protein 1 are activated by mTOR. Low-dose central administration of leucine reduces food intake in conjunction with increased levels of S6K1 and S6 phosphorylation in the arcuate nucleus [15]. Because rapamycin attenuates the anorexic effect of leucine administration, the reduction in food intake seen with leucine has been ascribed to activation of the mTOR pathway [15].
Although the liver is clearly a major site for lipid metabolism in the body, the intestine is also an important contributor and both tissues play a role in the overall lipid balance in the body [40]. Given the rising incidence of obesity and insulin resistance, treatments that target lipid metabolism in the intestine and inhibit intestinal fat absorption are increasingly important. Although fatty acids are hydrophobic and are capable of rapidly diffusing through the lipid bilayer when present in high concentrations [41], a large body of evidence supports the presence of a protein-mediated carrier system that operates at low substrate concentrations [24,42]. I-FABP and FATP4 are thought to be key factors for long-chain fatty acid uptake in enterocytes [24,25], whereas NPC1L1 is thought to play a critical role in intestinal cholesterol absorption [26,27]. Our present results demonstrate that isoleucine and whey downregulated the expression of i-FABP, FATP4, and NPC1L1, with a plateau at 2.0% concentration corresponding to about 65% inhibition of i-FABP, 60% of FABP, and 70% of NPC1L1. Leucine and valine downregulated the expression of NPC1L1, whereas casein downregulated the expression of i-FABP and FATP4. These findings suggest that BCAAs and dairy proteins may decrease fatty acid and cholesterol absorption by modulating mRNA transcription of lipid-carrier proteins. The results also suggest that BCAAs may primarily inhibit cholesterol absorption, whereas dairy proteins appear to exert a larger effect on genes involved in inhibiting fatty acid absorption.
Studies accumulated during the past decade have revealed that fatty acid and cholesterol biosynthetic processes are controlled by SREBPs. SREBPs transcriptionally activate a cascade of enzymes required for endogenous fatty acid, cholesterol, triacylglycerol, and phospholipid synthesis [23]. SREBP-2 primarily activates genes involved in cholesterol synthesis (e.g., HMGCR, the rate-limiting enzyme in cholesterol synthesis). SREBP-1a and -1c have greater effects on genes involved in fatty acid synthesis (e.g., ACC and FAS). Our present study showed that all BCAAs, skim milk, casein, and whey markedly downregulated ACC, FAS, SREBP-2, and HMGCR, except HMGCR with skim milk. Although this is the first work to demonstrate an effect of dairy proteins and BCAAs on these genes, we previously demonstrated that the soluble dietary fiber, β-glucan, inhibits the uptake of long-chain fatty acids in rat intestinal explants and downregulates FAS, ACC, SREBP-1a and -1c mRNA levels and i-FABP and FATP4 mRNA [43]. The present findings suggest that BCAAs and dairy proteins may also decrease fatty acid and cholesterol synthesis by modulating mRNA transcription of these enzymes. Although our in vitro system allows us to examine the direct effect of dairy proteins and BCAAs on genes involved in intestinal cholesterol and fatty acid metabolism, it will be important to assess these changes in vivo when multiple integrated systems are at play.
Our results showed that although skim milk, casein, and whey have a similar effective trend on mRNA expression of ACC, FAS, and SREBP-2, skim milk had no significant effect on i-FABP and FATP4, whereas whey and casein did. Although the reason for this is not clear, several studies have evaluated the effects of intact protein versus peptide hydrolysates. Calbet and Holst [33] showed that although solutions of casein, whey, casein hydrolysate, and whey hydrolysate emptied at similar rates from the stomach, the peptide hydrolysates stimulated 50% more gastric acid secretion than intact proteins. Although similar levels of GLP-1 and peptide YY were observed across the four solutions, glucose-dependent insulinotropic polypeptide was greater with the hydrolysates. More recently, Diepvens et al. [34] observed modest indications that a pea protein hydrolysate induced greater satiety compared with milk protein or a combination of whey protein and pea protein hydrolysate. Differences such as those described in the previous studies are often ascribed to the rate of appearance and concentration of amino acids achieved in the bloodstream. As a fast protein, whey is expected to elicit a more rapid appearance of amino acids in the bloodstream, whereas casein with its ability to form clots in the stomach is absorbed more slowly and therefore is characterized by a delayed appearance of amino acids in the blood. Calbet and Holst [33] would suggest, however, that when all variables such as energy density, pH, osmolality, volume, and nitrogen content are matched, relatively similar rates of appearance of amino acids in blood for plant and animal intact proteins and hydrolysates are observed. Directly applicable to our findings is the observation that skim milk empties from the intestine more slowly (25 min) than whey, casein, and their hydrolysates (18–21 min) [44]. Although the majority of work in this area has addressed satiety hormone secretion, the specific properties of skim milk in contrast to whey and casein that govern its effects on gastric emptying, rate of appearance of amino acids in the blood, and delivery of nutrients along the intestinal tract may also provide some insight into the differences we observed in fatty acid transporter expression among skim milk, casein, and whey.
The concentrations of BCAAs used in this study were higher than their physiologic levels in blood in vivo. However, the objective of this study was to determine BCAA–enterocyte interactions in a direct manner, thereby reflecting luminal concentrations of amino acids. Adibi and Mercer [45] assessed the concentrations of amino acids in the intestinal lumen, mucosa, and plasma after a protein-rich meal in humans. The concentrations of total amino acids in jejunal contents 3 h after a test meal were 29.29 μmol/mL for free amino acids and 117.97 μmol/mL for peptide amino acids [45]. In the more distal segment of the intestine, the ileal contents contained concentrations of total amino acids after a meal that were 19.74 μmol/mL for free amino acids and 65.29 μmol/mL for peptide amino acids. Individually, leucine was approximately 1 and 4 μmol/L for free and peptide amino acids. Therefore, our 0.5–3% doses ranging from approximately 38 to 229 μmol/mL of total amino acid exposure would represent a range from physiologic to high physiologic to supraphysiologic. This sort of range is meaningful to examine not only for understanding physiologic responses to meals but also to determine any potential for therapeutic doses. Furthermore, no toxic effects on cells in vitro have been reported for concentrations up to 3% in previous studies [17,18,28].
Conclusion
The present study demonstrates that BCAAs and dairy proteins stimulate GLP-1 release and downregulate the expression of genes involved in fatty acid and cholesterol absorption and synthesis in vitro. These findings provide an intestinal mechanism whereby dairy may deliver a benefit for the prevention and treatment of overweight and obesity.
Acknowledgments
The authors thank Kristine Lee for her excellent technical support.
This work was supported by the Dairy Farmers of Canada, Natural Sciences and Engineering Research Council of Canada, and Canadian Institutes of Health Research.
References
- 1.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. 2005;82:523–30. doi: 10.1093/ajcn.82.3.523. [DOI] [PubMed] [Google Scholar]
- 2.Brooks BM, Rajeshwari R, Nicklas TA, Yang SJ, Berenson GS. Association of calcium intake, dairy product consumption with overweight status in young adults (1995–1996): the Bogalusa Heart Study. J Am Coll Nutr. 2006;25:523–32. doi: 10.1080/07315724.2006.10719568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon LB, Pellizzon MA, Jawad AF, Tershakovec AM. Calcium and dairy intake and measures of obesity in hyper- and normocholesterolemic children. Obes Res. 2005;13:1727–38. doi: 10.1038/oby.2005.211. [DOI] [PubMed] [Google Scholar]
- 4.Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intakes in relation to long-term weight gain in US men. Am J Clin Nutr. 2006;83:559–66. doi: 10.1093/ajcn.83.3.559. [DOI] [PubMed] [Google Scholar]
- 5.Snijder MB, van der Heijden AA, van Dam RM, Stehouwer CD, Hiddink GJ, Nijpels G, et al. Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? The Hoorn Study Am J Clin Nutr. 2007;85:989–95. doi: 10.1093/ajcn/85.4.989. [DOI] [PubMed] [Google Scholar]
- 6.Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res. 2005;13:1218–25. doi: 10.1038/oby.2005.144. [DOI] [PubMed] [Google Scholar]
- 7.Xue B, Greenberg AG, Kraemer FB, Zemel MB. Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes. FASEB J. 2001;15:2527–9. doi: 10.1096/fj.01-0278fje. [DOI] [PubMed] [Google Scholar]
- 8.Pereira MA, Jacobs DR, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity and the insulin resistance syndrome in young adults. The CARDIA study. JAMA. 2002;287:2081–9. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 9.Zemel MB, Richards J, Mathis S, Mildstead A, Gebhardt L, Silva E. Dairy augmentation of total and central weight loss in obese subjects. Int J Obes Relat Metab Disord. 2005;29:391–7. doi: 10.1038/sj.ijo.0802880. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-dihydroxyvitamin D3 inhibits uncoupling protein 2 expression in human adipocytes. FASEB J. 2002;16:1808–10. doi: 10.1096/fj.02-0255fje. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Zemel MB. Calcium and dairy products inhibit weight and fat regain during ad libitum consumption following energy restriction in Ap2-agouti transgenic mice. J Nutr. 2004;134:3054–60. doi: 10.1093/jn/134.11.3054. [DOI] [PubMed] [Google Scholar]
- 12.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 13.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–94. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu Y-H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multi-mechanisms. Diabetes. 2007;56:1647–54. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 15.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 16.Bowen J, Noakes M, Clifton PM. Effect of calcium and dairy foods in high protein, energy-restricted diets on weight loss and metabolic parameters in overweight adults. Int J Obes Relat Metab Disord. 2005;29:957–65. doi: 10.1038/sj.ijo.0802895. [DOI] [PubMed] [Google Scholar]
- 17.Reimer RA. Meat hydrolysate and essential amino acid-induced glucagon like peptide-1 secretion in the human enteroendocrine NCI-H716 cell line is regulated by extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases. J Endocrinol. 2006;190:1–13. doi: 10.1677/joe.1.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reimer RA, Darimont C, Nicolas-Metral V, Gremlich S, Rüegg UT, Macé K. A human cellular model for studying the regulation of glucagon-like peptide 1 secretion. Endocrinology. 2001;142:4522–8. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- 19.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–56. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- 20.Reimer RA, Russell JC. Glucose tolerance, lipids and GLP-1 secretion in JCR:LA-cp rats fed a high protein fiber diet. Obesity. 2008;16:40–6. doi: 10.1038/oby.2007.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimer RA, Thomson ABR, Rajotte R, Basu TK, Ooraikul B, McBurney MI. A physiological level of rhubarb fiber increases proglucagon gene expression and modulates intestinal glucose uptake in rats. J Nutr. 1997;127:1923–8. doi: 10.1093/jn/127.10.1923. [DOI] [PubMed] [Google Scholar]
- 22.Reimer RA, Thomson ABR, Rajotte R, Basu TK, Ooraikul B, McBurney MI. Proglucagon messenger ribonucleic acid and intestinal glucose uptake are modulated by fermentable fiber and food intake in diabetic rats. Nutr Res. 2000;20:851–64. [Google Scholar]
- 23.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839–48. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Besnard P, Niot I, Poirier H, Clement L, Bernard A. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Mol Cell Biochem. 2002;239:139–47. [PubMed] [Google Scholar]
- 25.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 26.Altmann W, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 27.Davis R, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole body cholesterol homeostasis. J Biol Chem. 2004;279:33586–92. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 28.Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, et al. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide-1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–45. doi: 10.2337/diabetes.47.7.1038. [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 30.Billeaud C, Guillet J, Sandler B. Gastric emptying in infants with or without gastro-oesophageal reflux according to the type of milk. Eur J Clin Nutr. 1990;44:577–83. [PubMed] [Google Scholar]
- 31.Miller MJ, Witherly SA, Clark DA. Casein: a milk protein with diverse biologic consequences. Proc Soc Exp Biol Med. 1990;195:143–59. doi: 10.3181/00379727-195-43129. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Phil Trans R Soc Lond B Biol Sci. 2006;361:1187–209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr. 2004;43:127–39. doi: 10.1007/s00394-004-0448-4. [DOI] [PubMed] [Google Scholar]
- 34.Diepvens K, Haberer D, Westerterp-Plantenga MS. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes Relat Metab Disord. 2008;32:510–8. doi: 10.1038/sj.ijo.0803758. [DOI] [PubMed] [Google Scholar]
- 35.Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:261S–7. doi: 10.1093/jn/133.1.261S. [DOI] [PubMed] [Google Scholar]
- 36.Hinault C, Van Obberghen E, Mothe-Satney I. Role of amino acids in insulin signaling in adipocytes and their potential to decrease insulin resistance of adipose tissue. J Nutr Biochem. 2006;17:374–8. doi: 10.1016/j.jnutbio.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakago T, Takaoka M, et al. Arginine and leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–43. [PubMed] [Google Scholar]
- 38.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–31. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 39.Nakajo T, Yamtsuji T, Ban H, Shigematsu S, Haisa M, Motoki T, et al. Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem Biophys Res Commun. 2005;326:174–80. doi: 10.1016/j.bbrc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Alvaro A, Sola R, Rosales R, Ribalta J, Anguera A, Masana L, Vallve JC. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life. 2008 Jul 18; doi: 10.1002/iub.110. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Kamp F, Hamilton JA. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc Natl Acad Sci U S A. 1992;89:11367–70. doi: 10.1073/pnas.89.23.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stremmel W. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid-binding protein. J Clin Invest. 1988;82:2001–10. doi: 10.1172/JCI113820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drozdowski LA, Reimer RA, Temelli F, Bell RC, Vasanthan T, Thomson ABR. β-Glucan extracts inhibit the in vitro intestinal uptake of lipids and downregulate I-FABP4 genes involved in fatty acid synthesis and cholesterol metabolism in rats. J Nutr Biochem. 2008 doi: 10.1016/j.jnutbio.2009.04.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahe S, Huneau J-F, Marteau P, Thuiller F, Tome D. Gastroileal nitrogen and electrolyte movements after bovine milk ingestion in humans. Am J Clin Nutr. 1992;56:410–6. doi: 10.1093/ajcn/56.2.410. [DOI] [PubMed] [Google Scholar]
- 45.Adibi SA, Mercer DW. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 1973;52:1586–94. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]