Abstract
Objective
To characterize the gastrointestinal tract at the onset and in well-established obesity.
Methods and Procedures
Lean (+/?) and obese (cp/cp) male JCR:LA-cp rats lacking a functional leptin receptor were killed at 3.5 weeks and 9 months of age and plasma concentrations of satiety hormones determined. The small intestine, colon, and stomach were measured, weighed, and mRNA levels of satiety genes quantified.
Results
At the onset of obesity, obese rats had greater intestine, colon, and liver mass when adjusted for body weight compared to lean rats. Conversely, adult rats with established obesity had lower intestine and colon mass and length after adjustment for body weight. Early changes in gene expression included decreased ghrelin mRNA levels in stomach and increased peptide YY (PYY) mRNA levels in duodenum of young obese rats. After massive accumulation of adipose tissue had occurred, adult obese rats had increased proglucagon and ghrelin mRNA expression in the proximal intestine. In the distal small intestine, obese rats had lower proglucagon, ghrelin, and PYY mRNA levels. Finally, at the onset and in well-established obesity, obese rats had higher plasma insulin, amylin, glucagon like peptide-1 (GLP-1), and PYY, a finding, with the exception of insulin, unique to this model. Plasma total ghrelin levels were significantly lower at the onset of obesity and established obesity compared to the lean rats.
Discussion
Several defects are manifested in the obese gut early on in the disease before the accumulation of large excesses of body fat and represent potential targets for early intervention in obesity.
INTRODUCTION
Research specifically aimed at preventing and treating obesity is accumulating; however, our understanding of the complex pathophysiology of obesity remains limited. Although obesity has many contributing factors, altered dietary intake is clearly a pivotal factor, and, therefore, understanding the role of changes in the structure and function of the intestine, the first interface between diet and the body in the lean and obese state, is critical. This knowledge is fundamentally important in identifying specific defects in the gut and ultimately new obesity therapies in the future. Moreover, a clear understanding of the physiology of the preobese gut is a crucial first step in the development of early interventions for obesity. This is especially important given the current failure rate of most traditional weight loss remedies (1) and the increasing need for preventive measures.
The gut is an active enteroendocrine organ which mediates appetite and nutrient absorption through its physical structures and the secretion of an array of hormones (2). To date, the physical characteristics of the obese gut remain undetermined and may differ before the onset of obesity and in well-established obesity. Of the gut hormones, glucagon like peptide-1 (GLP-1), peptide YY (PYY), and ghrelin have been identified as key peptides in appetite regulation and are altered in the obese state (3).
Proglucagon is synthesized by L-cells found in an increasing gradient from the small intestine to colon, as well as in pancreatic islets and neuronal cell bodies in the brain stem (4). GLP-1 (7–36) amide (GLP-1), the major bioactive form, is produced through posttranslational conversion of proglucagon by prohormone convertase 1/3 (5). Upon secretion, GLP-1 (7–36) amide is rapidly degraded into GLP-1 (9–36) by dipeptidyl peptidase IV (6). GLP-1 secretion is stimulated by mixed meals or individual nutrients, including glucose, fatty acids, essential amino acids, and dietary fiber (7–10). In addition to its well-known insulinotropic actions, GLP-1 also affects appetite in part via the “ileal brake” mechanism, which regulates the flow of nutrients from the stomach to the small intestine (11). Reductions in energy intake with GLP-1 (7–36) amide infusion are similar for obese (9%) and normal weight (13%) subjects (12). Reduced GLP-1 may contribute to the development or maintenance of obesity. While some authors describe reduced circulating GLP-1 in obesity, others have failed to reproduce these findings (13,14). Given these discrepancies, we suggest that characterization of GLP-1 concentrations in the preobese and obese state is essential in determining whether defects are a consequence of obesity, or whether they precede its development and are, therefore, amenable to preventive strategies.
PYY is colocalized with GLP-1 in the intestinal L-cells along an increasing gradient from stomach to colon (15). PYY is secreted in response to a variety of nutrients, including glucose, lipids, amino acids, and short chain fatty acids (16–18). Once secreted, PYY1–36 is converted to the active form by dipeptidyl peptidase IV, which cleaves the N-terminal residues to form PYY3–36 (19). In healthy humans, PYY3–36 accounts for 36.9% of total circulating plasma PYY in the fasted state and 54.2% postprandially (20). PYY3–36 acts on the hypothalamic arcuate nucleus to reduce appetite and energy intake by inhibiting the release of neuropeptide Y, a potent orexigenic hormone (21). Within the gut PYY3–36 promotes satiety by contributing to the “ileal break” effect (22). PYY3–36 has been proposed as a possible therapeutic target for obesity; however, there is dispute over its physiological role in regulating food intake (23). While attenuated PYY levels have been reported in obese subjects (24,25), reports of reduced energy intake with PYY3–36 administration have not been as consistent (26–28). It has been suggested that lower concentrations of PYY could promote the development of obesity; however, at present, PYY levels at the onset of obesity remain undetermined and this hypothesis untested.
Ghrelin is the only peripheral orexigenic peptide identified to date (29). Several different forms of the peptide exist due to alternative splicing of proghrelin, resulting in the active n-octanoyl-modified form (acylated ghrelin) and the major circulating form, the non-acylated or desacylated form (desacyl ghrelin) (29). The active form plays an important role in increasing gastric emptying, stimulating food intake, and increasing adipogenesis and weight gain (29). Conversely, recent research suggests that des-acyl ghrelin is not inactive but may exert effects that are antagonistic to the active form of the peptide in terms of food intake (30).
The primary site of ghrelin synthesis is the mucosal epithelium of the stomach; however, it is also produced in lower amounts by endocrine cells along the entire intestinal tract (31). Total and active ghrelin concentrations are highest at fasting and reduced by feeding with a high-carbohydrate diet producing the largest change from baseline and a high-protein diet a longer period of attenuation (32). Patients with anorexia nervosa and patients recently experiencing weight loss have higher total plasma ghrelin, while obese subjects tend to have lower total and active ghrelin than normal weight subjects (33–35). Furthermore, fasting ghrelin in obese subjects has been negatively correlated with percent body fat, as well as fasting insulin and leptin concentrations (33). Although reports of decreased ghrelin concentrations in obesity appear consistent, the timing of these changes is unknown and ghrelin levels at the onset of obesity have yet to be tested.
The gut is a major contributor to the array of anorexigenic and orexigenic peptides influencing overall energy homeostasis. Defining its role in the pathophysiology of obesity is critical. Our objective was to differentiate obesity with respect to the physical and endocrine characteristics of the gut at the onset and in well-established obesity. Identification of early defects in the obese gut will ultimately allow novel, targeted therapies to be designed for prevention of obesity.
METHODS AND PROCEDURES
Two sets of JCR:LA-cp rats were received from the colony of Spencer Proctor (University of Alberta, Edmonton, Canada) and maintained on a non-purified diet (5P14 Mod Rod Eq, Richmond, IN). The first set of lean (+/?) (n = 7) and obese (cp/cp) (n = 7) rats, 3.5 weeks of age, were used to characterize obesity at its onset. For the experiment characterizing the physiology of well-established obesity, a second set consisting of lean (+/?) (n = 7) and obese (cp/cp) (n = 8) rats 9 months of age were used. In both experiments a cardiac bleed was done to obtain plasma for quantification of satiety hormones, and the gastrointestinal tract was dissected for analysis of physical characteristics and gene expression.
Animal model
Lean and obese male JCR:LA-cp rats were chosen because they display the autosomal recessive cp gene originally isolated by Koletsky (36). Due to the lack of a functional leptin receptor, they are severely obese and exhibit marked hyperphagia, hypertriglyceridemia, hyperinsulinemia, and hyperleptinemia. At killing, young rats were not fasted in an attempt to minimize potential stress caused by a prolonged fast in rats that had only been weaned from the dam 4 days prior. The 9-month-old rats, however, were fasted to ensure a consistent metabolic status between the groups. On the day of killing, the rats were anesthetized with halothane, a cardiac blood sample taken, and the rats killed by cervical dislocation. The intestine and major desired organs were removed and weighed and the length of intestinal segments determined under tension from a 5 g weight. Samples from the proximal end of all intestinal segments (duodenum, jejunum, ileum, and colon) were immediately snap frozen in liquid nitrogen and stored at −80 °C. All procedures were approved by the University of Calgary Animal Care Committee and conformed to procedures set forth for the Care and Use of Laboratory Animals.
RNA isolation and real-time-PCR
Total RNA was extracted from the small intestine segments, colon, and stomach using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed with an input of 1 μg of total RNA using the 1st strand cDNA synthesis kit for real-time-PCR (Invitrogen, Carlsbad, CA) with oligo d(T)15 as a primer. The resultant cDNA was amplified using primers synthesized by University of Calgary Core DNA Services (Calgary, Alberta, Canada). Primers used for amplification of cDNAs of interest were : 5′-ACCGCCCTGAGATTACTTTTCTG-3′ (forward) and 5′-AGTTCTCTTTCCAGGTTCACCAC-3′ (reverse) for proglucagon; 5′-AGAGGCGCCAGCTAACAAGTAA-3′ (forward) and 5′-GCAGGAGAGTGCTGGGAGTT-3′ (reverse) for ghrelin; 5′-AGCGGTATGGGAAAAGAGAAGTC-3′ (forward) and 5′-ACCACTGGTCCACACCTTCTG-3′ (reverse) for PYY; 5′-TATCGGCAATGAGCGGTTCC-3′ (forward) and 5′-AGCACTG TGTTGGLATAGAGG-3′ (reverse) for actin. The PCR was heated for 1 min 30 s then 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 20 s in a DNA iCycler apparatus (BIO-RAD, Hercules, CA). A melt curve showed the melting point of the PCR product of interest. Actin primers were included as an internal control. The 2−ΔΔCT method was utilized for the data analysis, where threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold (37). The ΔCT is the difference in threshold cycles for the gene of interest and actin, and the ΔΔCT is the difference between the ΔCT for obese rats and the ΔCT for lean rats. Relative expression levels are presented as fold-changes to the lean group, for which levels were set to 1.
Radioimmunoassays and biochemistry
Approximately 8 ml of blood was collected from each rat into a chilled tube containing 1 mg/ml of EDTA, aprotinin (500 kallikrein inhibitor units/ml; Sigma Chemical), and diprotin-A (0.1 mmol/l). The blood was centrifuged at 1,600 × g for 15 min at 4 °C and aliquots stored at −80 °C. Analysis of active GLP-1, amylin, insulin, and glucagon was carried out using the Rat Endocrine LINCOplex kit by Linco Research (St. Charles, MO). Samples of 10 μl of plasma were analyzed using antibody-immobilized beads specific for the hormones glucagon, active GLP-1, insulin, and total amylin. Quantification of the hormones was performed using the Luminex100 (Luminex, Austin, TX). Plasma total ghrelin levels were quantified using a commercial RIA kit (Linco Research), which has 100% specificity for rat ghrelin and utilizes an antibody that does not require the presence of the octanoyl group on Serine 3 and, therefore, recognizes both the acylated and desacylated form of ghrelin. A rat Total PYY ELISA kit (Diagnostic Systems Laboratories) was used to quantify plasma PYY1–36 and PYY3–36.
Statistical analysis
Data and figures are presented as means ± s.e.m. Statistical comparisons between the lean and obese animals within each age group were done using one-way ANOVA using STATA software (STATA, College Station, TX). Values were considered significant when P ≤ 0.05.
RESULTS
Body weight of the obese rats was significantly higher than that of lean rats at both 3.5 weeks and 9 months of age (Table 1). Small intestine length and weight, colon length and weight, and liver weight were significantly greater in obese vs. lean animals at both age time-points (Table 1). Taking into account the greater overall body mass of the obese animals, the young obese rats still showed a proportionally greater small intestine, colon, and liver mass when adjusted for body weight compared to lean rats (Table 2). In adult rats, adjustment for body weight resulted in a significantly lower small intestine length and weight and colon length and weight in obese rats compared to lean rats (Table 2). While the absolute mass of the liver was double in adult obese rats compared to lean rats, after adjustment for body weight, no significant differences were seen. Stomach weight was increased in the well-established group; however, adjustment for body weight revealed a significantly smaller stomach weight in proportion to body size with established obesity. There were no differences detected in stomach weight at the onset of obesity.
Table 1.
Physical characteristics of the intestine, stomach, and liver of lean and obese rats at the onset and in well-established obesity
| Obesity onset
|
Well-established obesity
|
|||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| Body weight (g) | 87.9 ± 3.7* | 106.6 ± 4.1 | 389.5 ± 12.4* | 807.5 ± 18.7 |
| Small intestine length (cm) | 90.7 ± 1.0* | 103.7 ± 1.5 | 111.4 ± 1.9* | 136.1 ± 1.7 |
| Small intestine weight (g) | 3.6 ± 0.18* | 5.2 ± 0.16 | 5.9 ± 0.2* | 10.8 ± 0.3 |
| Colon length (cm) | 12.2 ± 0.31* | 14.1 ± 0.40 | 17.7 ± 0.7* | 21.1 ± 0.3 |
| Colon weight (g) | 0.58 ± 0.05* | 0.88 ± 0.02 | 1.21 ± 0.05* | 1.59 ± 0.06 |
| Stomach weight (g) | 0.73 ± 0.05 | 0.77 ± 0.04 | 1.88 ± 0.13* | 2.43 ± 0.14 |
| Liver weight (g) | 3.9 ± 0.2* | 5.3 ± 0.3 | 11.1 ± 0.4* | 23.0 ± 0.7 |
Results represent the mean ± s.e.m.
P < 0.01.
Table 2.
Body weight-adjusted physical characteristics of the intestine, stomach, and liver of lean and obese rats at the onset and in well-established obesity
| Obesity onset
|
Well-established obesity
|
|||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| Small intestine length (cm) | 104.2 ± 3.7 | 98.3 ± 4.2 | 287.0 ± 5.1* | 169.0 ± 3.0 |
| Small intestine weight (g) | 4.1 ± 0.07* | 4.9 ± 0.12 | 1.5 ± 0.02** | 1.3 ± 0.02 |
| Colon length (cm) | 14.0 ± 0.45 | 13.3 ± 0.58 | 45.7 ± 2.0* | 26.3 ± 0.5 |
| Colon weight (g) | 0.66 ± 0.05* | 0.83 ± 0.02 | 0.31 ± 0.01* | 0.20 ± 0.01 |
| Stomach weight (g) | 0.82 ± 0.03 | 0.75 ± 0.02 | 0.54 ± 0.03* | 0.43 ± 0.02 |
| Liver weight (g) | 4.4 ± 0.09* | 5.0 ± 0.09 | 2.4 ± 0.4 | 2.9 ± 0.1 |
Results represent the mean ± s.e.m. of weight and lengths adjusted using body weight as a denominator.
P < 0.01;
P < 0.05.
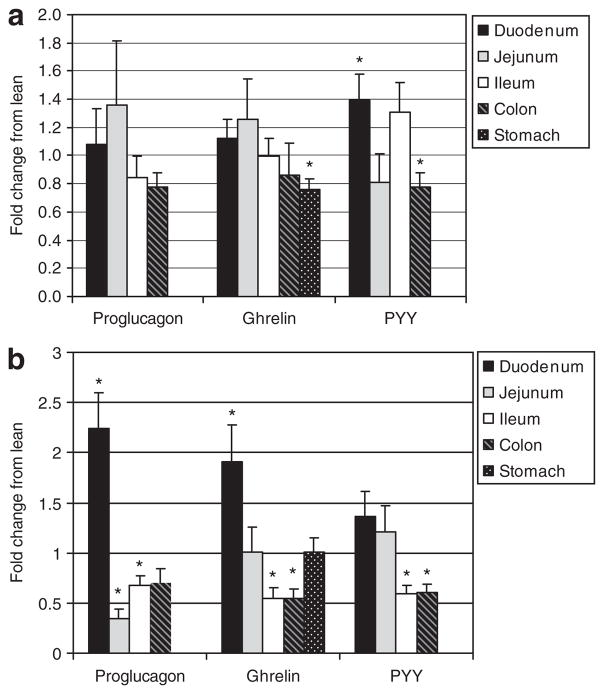
In young rats, there was no significant difference in intestinal proglucagon expression between lean and obese rats; however, ghrelin mRNA levels in the stomach and PYY mRNA levels in the colon were significantly lower in the obese rats (Figure 1a). Conversely, the young, obese rats demonstrated significantly higher levels of PYY mRNA in the duodenum. After the accumulation of massive adipose tissue, the adult obese rats had significantly higher expression of proglucagon and ghrelin mRNA in the proximal intestine (Figure 1b). This pattern was reversed in the distal small intestine where adult obese rats had significantly lower expression of proglucagon, ghrelin, and PYY mRNA. Additionally, there was a significant decrease in ghrelin and PYY mRNA in the colon and a trend toward decreased proglucagon expression (P = 0.067).
Figure 1.
mRNA level of satiety hormones at the onset and in well-established obesity, as determined by real-time PCR. (a) Young 3.5-week-old rats. (b) Adult 9-month-old rats. Results represent the mean fold change ± s.e.m. compared to lean controls. An ANOVA was done to determine significance. Asterisk represents a significant difference between lean and obese within a specific tissue (P ≤ 0.05). PYY, peptide YY.
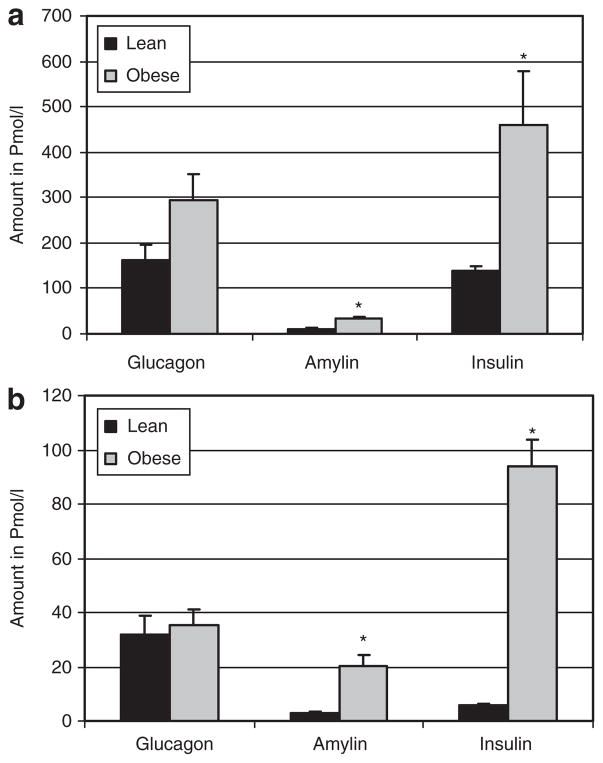
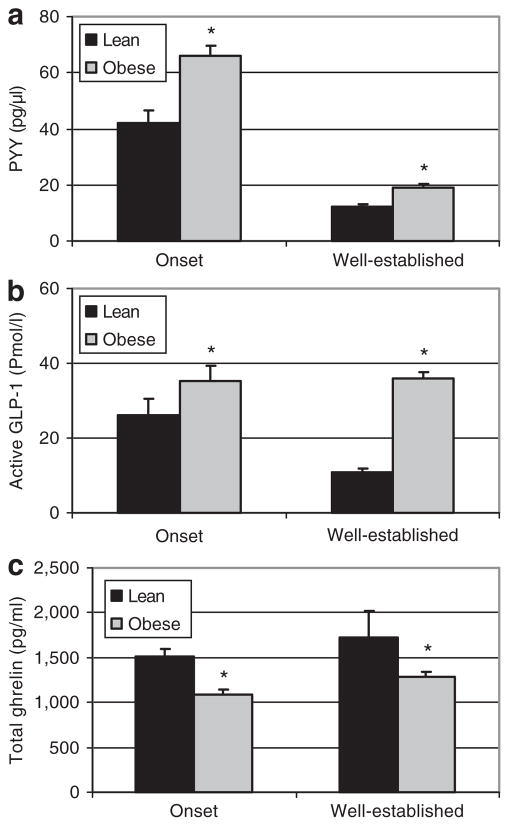
The pattern observed in plasma levels of satiety hormones was similar at the onset of obesity and in well-established obesity. In both young and adult rats, the pancreatic hormones, insulin and amylin were significantly higher in obese compared to lean rats (Figure 2). Plasma concentrations of the gut hormones, GLP-1 and PYY, were also significantly higher in obese compared to lean rats at both the onset and in well-established obesity (Figure 3). Plasma total ghrelin diverged in both the young and adult rats with levels significantly lower in obese vs. lean rats (Figure 3).
Figure 2.
Plasma glucagon, amylin and insulin for lean and obese rats at the onset and in well-established obesity. (a) Young 3.5-week-old rats. (b) Adult 9-month-old rats. Plasma, obtained via a cardiac bleed, was analyzed using a LINCOplex kit to determine glucagon, total amylin, and insulin. Results represent mean ± s.e.m. Due to the marked hyperinsulinemia of adult rats, actual insulin concentrations in (b) are tenfold higher than represented on the graph for presentation purposes (i.e., lean were 55.9 ± 7.1 and obese were 938.1 ± 99.2). Asterisk represents a significant difference between lean and obese (P ≤ 0.05).
Figure 3.
Plasma peptide YY (PYY), glucagon like peptide-1 (GLP-1), and total ghrelin levels for lean and obese rats at the onset and in well-established obesity. (a) PYY and was quantified using an ELISA kit (Diagnostic Systems Laboratories). (b) Active GLP-1 and was quantified using a LINCOplex kit (Linco Research Inc.). (c) Total ghrelin and was quantified using an RIA kit (Linco Research Inc). Results represent mean ± s.e.m. Asterisk represents a significant difference between lean and obese rats in either onset or well-established obesity (P < 0.05).
Feeding status clearly has a direct effect on satiety hormone levels; thus comparisons were not made between the young and old rats as only the 9-month-old rats were fasted.
Discussion
Obesity is a multifaceted disease with a complex and undefined pathophysiology. Our characterization of obesity at both the onset of obesity and in well-established obesity provides novel evidence that several defects in the obese gut occur early on in the disease before accumulation of large excesses of body fat mass. These defects may be potential targets for obesity prevention. Our major findings suggest that (i) both young and adult obese rats have larger absolute gut size; (ii) when adjusted for body weight, young rats have a proportionally larger and adults rats a proportionally smaller gut size; (iii) larger gut size is associated with higher plasma GLP-1 and PYY but lower ghrelin; and (iv) given the decreased mRNA expression of proglucagon and PYY in the distal gut, the increased expression of these genes in the proximal gut may account for the higher plasma levels observed.
The increase in gut mass and length seen in young rats appears early in the disease before the accumulation of large excesses of body fat. A greater functional area and increased physical capacity of the intestine may permit greater food and energy intake. In our JCR:LA-cp model, the increased gut mass prevails on an absolute weight basis into adulthood; however, given the marked increase in fat mass, it is no longer proportionally larger. This proportionately smaller gut mass in adult rats may have implications for the secretion of satiety hormones produced in the gut. In the young rats, the increase in gut size may permit gains in excessive body weight early on in the disease, as greater holding capacity of the gut allows increased intake before satiation due to gastric distension is recognized. Indeed, Delgado-Aros et al. (38) report that humans with increased BMI require increased caloric intake to achieve maximum satiation. Previously, increased gut size in response to dietary energy dilution, in the form of non-nutrient diluents or cellulose, has been reported (39,40); however, to our knowledge, our observations are the first to associate elevated total gut size with the obese state. This novel observation may help explain increased caloric intake in the absence of increased gastric capacity with obesity noted in the literature (38,41). Potentially, by focusing solely on stomach capacity, rather than total gut size, a viable mechanism to explain the increased food intake required to obtain satiation in obese individuals has been overlooked. The factors responsible for this increased gut size are not known but may involve gut trophic hormones, including GLP-2 which is cosecreted from the L-cells (42).
The increased gut size and, therefore, greater endocrine productive capacity may also explain the augmented circulating PYY and GLP-1 observed in the obese rats. Additional work measuring GLP-1 and PYY concentrations, as well as meal size over a 24-h period would further clarify our observations. It might be suggested that increased gut size, which develops with obesity, overrides the “ileal break” actions of these hormones and facilitates the consumption of larger meals. The larger meal size could thus account for the increased PYY and GLP-1 concentrations, as both these hormones are released in response to nutrient stimulation.
In this specific monogenic model of obesity, it is interesting to consider the role of leptin and the lack of a functional receptor in regard to the increased GLP-1 we observed. Leptin has been demonstrated to stimulate GLP-1 secretion in mouse, rat, and human intestinal L-cells (43). In rodents, both intracerebroventricular and peripheral injections of leptin stimulated GLP-1 neurons in the hypothalamus (44). JCR:LA-cp rats are markedly hyperleptinemic with male cp/cp rats demonstrating leptin levels 30-fold higher than lean rats by 4 weeks of age (45). It is plausible that our results are distinct to this model and due, in part, to over-stimulation of GLP-1 secretion via leptin. Further support for this theory comes from the fact that significant levels of leptin have been found in the duodenum (46), and proglucagon expression in our rats was strictly upregulated in this portion of the gut. This may be a critical observation in explaining why our results do not coincide with other reports, where the majority find decreased levels of GLP-1 in obesity (47). It may also explain the apparent lack of insulin- independent changes that have been observed when portal GLP-1, acting via the hepato-portal sensors, is infused (48).
PYY is produced in the same endocrine cells as GLP-1 in the gut and levels are generally reduced in obesity (24,25). While initial studies with infusion of PYY3–36 resulted in decreased caloric intake and a reduced weight gain (26), others have found no effect with PYY administration particularly in rodents (49). Part of the controversy appears to relate to the stress response of rodents and to the complexities associated with two species of PYY and multiple receptors (50). In humans, despite lower fasting levels of PYY3–36, obesity does not appear to be associated with resistance to the effects of PYY3–36 (24). Our findings of increased plasma PYY mirror the increased GLP-1 levels seen in both young and adult rats. While these results may be specific to our model and reflect the notable differences in leptin levels, it is also possible that the increased PYY concentrations reflect chronic increased caloric intake as PYY secretion has been positively correlated with caloric intake (51). While a recent study examining the interaction between leptin and PYY determined that a pharmaceutical dose of leptin did not influence PYY concentrations, this study utilized subcutaneous injections of leptin rather than direct exposure of gut cells to leptin (52). Further research is required to determine whether direct exposure of PYY-producing cells to leptin alters secretion.
The reduced levels of ghrelin observed in our obese rats at both the onset and in well-established obesity are in agreement with the lower fasting ghrelin levels seen in obese subjects (33). High levels of GLP-1 have been shown to inhibit ghrelin secretion (27). Exendin 4, a GLP-1 agonist, also inhibited total ghrelin levels (53), although these same researchers were unable to find an inhibitory effect of GLP-1 administration on total ghrelin. If GLP-1 is indeed able to suppress ghrelin levels, it is possible that the low ghrelin levels found in our obese rats could be due to suppression caused by the high levels of GLP-1. In addition, although the attenuation of acylated ghrelin, an orexigen, appears contradictory in obesity, recent interest in the des-acyl form of ghrelin may provide insight. Evidence is emerging to suggest des-acyl ghrelin antagonizes some of the effects of the acylated form and in fact decreases food intake (30,54). This implies that the des-acyl form of ghrelin may be physiologically relevant to the development of obesity with decreased levels promoting increased food intake. In our study, we did not assay specifically for des-acyl ghrelin; however, it has been reported that des-acyl ghrelin represents 90–95% of total circulating ghrelin (55) and, thus, a decrease in total ghrelin likely reflects a decrease in des-acyl ghrelin. If des-acyl ghrelin has anorexigenic properties, a downregulation of this hormone could theoretically promote weight gain.
Experimentally, both gene expression and plasma levels of the satiety hormones were quantified. Although mRNA is translated into protein, changes in mRNA expression do not necessarily reflect protein levels or activity. In our work, mRNA expression did not reflect plasma levels of the related hormone in all instances. While the circulating hormone undoubtedly has the ability to affect physiological change, mRNA expression data provides support for plasma concentrations and also reflects transcriptional changes which may influence regional production of satiety hormones along the length of the gut. The significantly higher expression of proglucagon mRNA in the proximal intestine coupled with a significant decrease in proglucagon mRNA in the distal small intestine in our obese rats suggests that the normally larger production of GLP-1 from the distal gut (56) could be shifted in favor of the proximal gut. This is plausible given that increased proximal absorption rates have been reported in obese adults (57), and this phenomenon could result in reduced exposure of the distal gut to nutrients. This may explain the commonly observed decrease in GLP-1 levels in obesity.
Despite the increase in overall circulating PYY, the downregulation of PYY mRNA that occurred in the more distal intestine at the onset and in well-established obesity may play a role in promoting the development of obesity. PYY3–36 has been shown to inhibit food intake through the vagal nerve, which acts as a communication pathway between the alimentary tract and brain (58). A decrease in colonic PYY may potentially lessen the postprandial satiety effects of the vagal nerve resulting in body weight gain. Additionally, the lower expression of PYY mRNA seen in the distal small intestine and colon prevails in well-established obesity, perhaps acting with many other factors, which enable the maintenance of a high body weight and inhibit weight loss. Although there is experimental evidence implicating attenuated PYY levels in obesity, there is enough conflicting evidence to suggest that these observations cannot be applied to the obese population as a whole. Further research will be required to establish that diminished PYY is responsible for the development or maintenance of obesity.
Finally, in addition to insulin, obese rats had significantly higher plasma concentrations of amylin, an anorexigenic peptide cosecreted with insulin from pancreatic β-cells. These changes were seen at both the onset and in well-established obesity, implying that these adaptations occur early in the disease.
In conclusion, this study demonstrates that the obese state is characterized by both physical and hormonal abnormalities. While many defects have been described for the obese state, our work provides novel insight into early defects occurring in the intestine that may promote dysregulation of body weight. Many of these changes occur early in the progression of the disease before the accumulation of large excesses in body fat mass and could play a role in the promotion of obesity. Although our findings are likely unique to this monogenic form of obesity, there is clearly a genetic component to many cases of obesity, and a greater understanding of the pathophysiology of the disease is required to match an appropriate treatment with the individual. Future studies should examine changes in intestinal physiology and endocrinology in response to diet-induced obesity using high fat/high sucrose diets in rats in both fasted and fed states. Additionally, we provide evidence for interactions between the various satiety hormones, suggesting that hormonal treatments for obesity need to focus on the integrated response to fully elucidate the impact on whole body energy homeostasis. Identification of these early defects and hormonal interactions will ultimately lead to novel therapies aimed at the prevention of obesity and the avoidance of significant health care costs and loss of quality of life.
Acknowledgments
This research was funded by a grant from the Canadian Institutes for Health Research.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 2.Larsen PJ, Vrang N, Tang-Christensen M. Central pre-proglucagon derived peptides: opportunities for treatment of obesity. Curr Pharm Des. 2003;9:1373–1382. doi: 10.2174/1381612033454775. [DOI] [PubMed] [Google Scholar]
- 3.Huda MSB, Wilding JPH, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev. 2006;7:163–182. doi: 10.1111/j.1467-789X.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Dhanvantari S, Izzo A, Jansen E, Brubaker PL. Coregulation of glucagon-like peptide-1 synthesis with proglucagon and prohormone convertase 1 gene expression in enteroendocrine GLUTag cells. Endocrinology. 2001;142:37–42. doi: 10.1210/endo.142.1.7870. [DOI] [PubMed] [Google Scholar]
- 6.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide 1 in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 7.Reimer RA. Meat hydrolysate and essential amino acid-induced glucagon like peptide-1 secretion in the human enteroendocrine NCI-H716 cell line is regulated by extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases. J Endocrinol. 2006;191:159–170. doi: 10.1677/joe.1.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker PL, Schloos J, Drucker DJ. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology. 1998;139:4108–4114. doi: 10.1210/endo.139.10.6228. [DOI] [PubMed] [Google Scholar]
- 9.Iritani N, Sugimoto T, Fukuda H, Komiya M, Ikeda H. Oral triacylglycerols regulate plasma glucagon-like peptide-1(7-36) and insulin levels in normal and especially in obese rats. J Nutr. 1999;129:46–50. doi: 10.1093/jn/129.1.46. [DOI] [PubMed] [Google Scholar]
- 10.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–3956. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- 11.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 12.Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 13.Verdich C, Toubro S, Buemann B, et al. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 16.Aponte GW, Fink AS, Meyer JH, Tatemoto K, Taylor IL. Regional distribution and release of peptide YY with fatty acids of different chain length. Am J Physiol. 1985;249(6 Pt 1):G745–G750. doi: 10.1152/ajpgi.1985.249.6.G745. [DOI] [PubMed] [Google Scholar]
- 17.Greeley GH, Jr, Hashimoto T, Izukura M, et al. A comparison of intraduodenally and intracolonically administered nutrients on the release of peptide-YY in the dog. Endocrinology. 1989;125:1761–1765. doi: 10.1210/endo-125-4-1761. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Brubaker PL, Thompson JC, Greeley GH. Characterization of peptide-YY release in response to intracolonic infusion of amino acids. Endocrinology. 1993;132:553–557. doi: 10.1210/endo.132.2.8093875. [DOI] [PubMed] [Google Scholar]
- 19.Park A, Bloom SR. Peptides and obesity: the PYY 3-36 story. Regul Pept. 2004;119:1–2. doi: 10.1016/j.regpep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Grandt D, Schimiczek M, Beglinger C, et al. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 21.Neary NM, Small CJ, Bloom SR. Gut and mind. Gut. 2003;52:918–921. doi: 10.1136/gut.52.7.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- 23.Degen L, Oesch S, Casanova M, et al. Effect of peptide YY3-36 on food intake in humans. Gastroenterology. 2005;129:1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Batterham RL, Cohen MA, Ellis SM. Inhibition of food intake in obese subjects by peptide YY 3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 25.le Roux CW, Batterham RL, Aylwin SJB, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 26.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY (3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 27.Tschop M, Castaneda TR, Joost HG, et al. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004;430:1565–1567. doi: 10.1038/nature02665. [DOI] [PubMed] [Google Scholar]
- 28.Challis BG, Pinnock SB, Coll AP, et al. Acute effects of PYY 3-36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun. 2003;311:915–919. doi: 10.1016/j.bbrc.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 29.Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96:201–226. doi: 10.1079/bjn20061787. [DOI] [PubMed] [Google Scholar]
- 30.Chen C-Y, Inui A, Asakawa A, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Date Y, Kojima M, Hosoda H. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tract of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 32.Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50:260–269. doi: 10.1159/000091684. [DOI] [PubMed] [Google Scholar]
- 33.Tschop M, Weyer C, Tataranni AP, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 34.Hansen TK, Dall R, Hosoda H. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56:203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 35.Guo ZF, Zheng X, Qin YW, et al. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. J Clin Endocrinol Metab. 2007;92:1875–1880. doi: 10.1210/jc.2006-2306. [DOI] [PubMed] [Google Scholar]
- 36.Koletsky S. Pathologic findings and laboratory data in a new strain of obese hypertensive rats. Am J Pathol. 1975;80:129–142. [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Delgado-Aros S, Cremonini F, Castillo JE, et al. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–440. doi: 10.1053/j.gastro.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Peterson AD, Baumgardt BR. Influence of level of energy demand on the ability of rats to compensate for diet dilution. J Nutr. 1971;101:1069–1074. doi: 10.1093/jn/101.8.1069. [DOI] [PubMed] [Google Scholar]
- 40.Peterson AD, Baumgardt BR. Food and energy intake of rats fed diets varying in energy concentration and density. J Nutr. 1971;101:1057–1068. doi: 10.1093/jn/101.8.1057. [DOI] [PubMed] [Google Scholar]
- 41.Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiol Behav. 2001;74:743–746. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- 42.Drucker DJ. Glucagon like peptide 2. Endocrinology. 2001;86:1759–1764. doi: 10.1210/jcem.86.4.7386. [DOI] [PubMed] [Google Scholar]
- 43.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 44.Goldstone AP, Morgan I, Mercer JG, et al. Effect of leptin on hypothalamic GLP-1 peptide and brain-stem pre-glucagon mRNA. Biochem Biophys Res Commun. 2000;269:331–335. doi: 10.1006/bbrc.2000.2288. [DOI] [PubMed] [Google Scholar]
- 45.Mantha L, Russell JC, Brindley DN, Deshaies Y. Developmental changes in adipose and muscle lipoprotein lipase activity in the atherosclerosis-prone JCR: La-corpulent rat. International J Obes. 2002;26:308–317. doi: 10.1038/sj.ijo.0801882. [DOI] [PubMed] [Google Scholar]
- 46.Guilmeau S, Buyse M, Tsocas A, Laigneau JP, Bado A. Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes. 2003;52:1664–1672. doi: 10.2337/diabetes.52.7.1664. [DOI] [PubMed] [Google Scholar]
- 47.Ranganath LR, Beety JM, Morgan LM, et al. Attenuated GLP-1 secretion in obesity: cause or consequence. Gut. 1996;38:916–919. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dardevet D, Moore MS, Neal D, et al. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am J Physiol Endocrinol Metab. 2004;287:E75–E81. doi: 10.1152/ajpendo.00035.2004. [DOI] [PubMed] [Google Scholar]
- 49.Boggiano MM, Chandler PC, Oswald KD, et al. PYY3-36 as an anti-obesity drug target. Obes Rev. 2005;6:307–322. doi: 10.1111/j.1467-789X.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 50.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams SH, Lei C, Jodka CM, et al. PYY[3-36] administration decreases the respiratory quotient and reduces adiposity in diet-induced obese mice. J Nutr. 2006;136:195–201. doi: 10.1093/jn/136.1.195. [DOI] [PubMed] [Google Scholar]
- 52.Chan JL, Stoyneva V, Kelesidis T, Riciti P, Mantzoros CS. Peptide YY levels are decreased by fasting and elevated following caloric intake but are not regulated by leptin. Diabetologia. 2006;49:169–173. doi: 10.1007/s00125-005-0041-2. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, et al. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes. 2007;56:143–151. doi: 10.2337/db05-0996. [DOI] [PubMed] [Google Scholar]
- 54.Chen CY, Chao Y, Chang FY, et al. Intracisternal des-acyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. Int J Mol Med. 2005;16:695–699. [PubMed] [Google Scholar]
- 55.Ariyasu H, Takaya K, Hosoda H. Delayed short term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology. 2002;143:3341–3350. doi: 10.1210/en.2002-220225. [DOI] [PubMed] [Google Scholar]
- 56.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res. 2005;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 57.Wisen O, Johannson C. Gastrointestinal function in obesity: motility, secretion, and absorption following a liquid test meal. Metabolism. 1992;41:390–395. doi: 10.1016/0026-0495(92)90073-j. [DOI] [PubMed] [Google Scholar]
- 58.Koda SY, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY 3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]