Abstract
Microorganisms play a critical role in the decomposition of organic matter, which contributes to energy and nutrient transformation in every ecosystem. Yet, little is known about the functional activity of epinecrotic microbial communities associated with carrion. The objective of this study was to provide a description of the carrion associated microbial community functional activity using differential carbon source use throughout decomposition over seasons, between years and when microbial communities were isolated from eukaryotic colonizers (e.g., necrophagous insects). Additionally, microbial communities were identified at the phyletic level using high throughput sequencing during a single study. We hypothesized that carrion microbial community functional profiles would change over the duration of decomposition, and that this change would depend on season, year and presence of necrophagous insect colonization. Biolog EcoPlates™ were used to measure the variation in epinecrotic microbial community function by the differential use of 29 carbon sources throughout vertebrate carrion decomposition. Pyrosequencing was used to describe the bacterial community composition in one experiment to identify key phyla associated with community functional changes. Overall, microbial functional activity increased throughout decomposition in spring, summer and winter while it decreased in autumn. Additionally, microbial functional activity was higher in 2011 when necrophagous arthropod colonizer effects were tested. There were inconsistent trends in the microbial function of communities isolated from remains colonized by necrophagous insects between 2010 and 2011, suggesting a greater need for a mechanistic understanding of the process. These data indicate that functional analyses can be implemented in carrion studies and will be important in understanding the influence of microbial communities on an essential ecosystem process, carrion decomposition.
Introduction
Decomposition is an important component of nutrient and organic matter cycling in ecosystems [1]–[3] and forms the base of many food webs [4]. Microbial communities are ubiquitous in the biosphere [5]–[7]. Identifying the functional activity of microbial communities involved in the decomposition process can provide a mechanistic understanding into how detrital community ecology influences ecosystem processes [8]–[10]. Microbes are an important, but often understudied, trophic level in decomposition ecology. For instance, microbes contribute to the decay and recycling of plant organic matter and nutrients [11], which accounts for approximately 99% of the organic matter on Earth [12]. Microbes are also a major component of large carrion resource pulses such as salmon runs [13], [14] and cicada emergences [15], which are important in mediating ecosystem structure and function [16], [17]. However, often in such studies, decay rates or nutrient fluxes provide a measure of the overall decomposition process [10], with little attention given to the discrete function, specifically the carbon source use, of the associated microbial communities and their influence on decomposition rates [18]–[20].
Microbial communities associated with carrion are part of the necrobiome, sensu Benbow et al. [21]. These communities can be from the pre-existing living flora of the organism [22] and the environmental communities existing where carrion falls [23]. Those microbial communities associated with external surfaces of carrion are considered the epinecrotic microbial community, which has been hypothesized to regulate ecological interactions occurring during carrion decomposition through several mechanisms [24].
Prokaryotes can compete with eukaryotes by producing toxic compounds that affect resource “appeal” to consumers and thus reduce competition from eukaryotes [25]. Undisturbed microbial proliferation can deter consumption from higher trophic arthropods such as crustaceans [19] and insects [26]. Additionally, carrion microbial communities influence the physiology and behavior of necrophagous insects through the production of metabolically derived volatile molecules [9], [27]–[29]. Recent studies have documented potential interkingdom interactions with insects eavesdropping on microbial community communication; specifically, in laboratory studies insects have detected and behaviorally responded to quorum sensing and swarming associated molecules produced by bacteria [30], [31]. Yet, there is little understanding of how these interactions may occur in the natural environment, and how they may change during carrion decomposition. If metabolic by-products affect the arrival sequence of necrophagous insects (often the primary consumers of carrion) then microbial functional succession may define consumer community assembly and therefore mediate decomposition and the pathways of nutrient and energy recycling.
There are several factors influencing microbial community assembly and function during vertebrate carrion decomposition: temperature [32]–[34], moisture and humidity [35], tissue type [20], surrounding vegetation [36], [37] and soil pH [38]. However, there are a variety of challenges for researchers studying microbial assemblages during decomposition. Metabolic or physiological profiling is a practical and affordable approach to understand the functional role of microbes within the environment. The study of metabolomics uses a systematic approach to assess metabolic by-products for understanding biological processes [39]. The function of entire microbial communities can be assessed using techniques like Biolog EcoPlates™ [40], [41]. These phenotypic microarrays provide microbial metabolic community profiles (MMCPs) determined by the differential use of carbon sources from environmental samples [42], [43]. Plates similar to Biolog EcoPlates™ were originally developed for the medical field, and have had successful application in many ecological studies [42]–[46]. This technique has been used to study culturable microbial community functional activity in terrestrial [47]–[49] and aquatic habitats [45], [50], [51]. Biolog EcoPlates™ are ideal for evaluating the functional response of entire microbial communities, not individual species or taxa within the community, based on differential utilization of carbon sources (i.e., carbohydrates, polymers, carboxylic and acetic acids, amino acids, and amines/amides). Thus, this approach provides a community-level functional profile (i.e., MMCP) that can be compared between sample types or over time [51]. However, there are limitations to using this technique that have been previously highlighted (see review Preston-Mafham et al. 2011). Some of these limitations for heterogeneous environmental samples are related to the difficulty of quantifying and consistently maintaining the inoculation amount for each carbon source, the potential bias of dominant taxa outcompeting rare taxa present in the community and sensitivity to oxygen concentrations [52].
There are several emerging areas of research focused on a better understanding of microbial community functional ecology importance in carrion decomposition: 1) how does the functional activity of microbial communities change during decomposition; 2) the role of temperature driving this functional succession; 3) how microbial function is mediated by necrophagous insect colonization and succession; and 4) intra- and inter-seasonal variation in these processes [9], [27]. The overall aim of this study was to use Biolog EcoPlate™ MMCPs to characterize microbial community function throughout carrion decomposition, which is the first time the microbial metabolic activity will be characterized in carrion decomposition systems and assess that function with one data set of bacterial metagenomic sequence from matched samples. We tested three hypotheses: 1) microbial functional activity would be dependent on seasonal variation related to local ambient environmental (e.g., temperature) conditions; 2) microbial functional diversity would vary between years depending on temperatures; and 3) that necrophagous insect colonization would have a significant impact on microbial community functional change throughout carrion decomposition. The effects of necrophagous insects on microbial succession were further assessed during one field season by describing the structure of bacterial communities using 454-pyrosequencing with the objective to identify key phyla associated with community functional changes.
Materials and Methods
Seasonal Variation
Seasonal variation in MMCPs was studied within Morris Bean Reserve of Greene County, Ohio, USA (39° 45′53.67″ N, 83° 54′ 41.12″W), a Midwest temperate forest of 12.2 hectares surrounded by agricultural fields with a small tributary stream running adjacent to the reserve that empties into the Little Beavercreek River. The predominant trees were honey locust (Gleditsia triacanthos) and a variety of maples (Acer spp.), while the most common sub-canopy cover was Amur honeysuckle (Lonicera maackii). Sus scrofa (swine) carcasses weighing 14–18 kg were used as models of vertebrate carrion decomposition [53] during four seasonal trials: spring (March until June 2009), summer (July until August 2009), autumn (November 2009 until March 2010) and winter (February until March 2010).
Carcasses purchased from a local farm were double bagged during transport and prior to field placement to prevent insect colonization. Using the same experimental design of Benbow et al. [21], six replicate carcasses were used in the spring and summer trials whereas three were used for the autumn and winter trials; the latter trials only included three replicates because of cost constraints and longer decomposition time expected during the colder months. One carcass was placed randomly along five-1 m2 plots, along each of six transects (50 m) for a total of 30 plots within 10–80 m from each other as previously described [21], [54]. All carcasses were placed under anti-scavenging cages constructed of poultry netting wrapping a wooden frame (0.6×0.9×0.6 m) to prevent disturbance by large vertebrate scavengers (e.g., coyotes, vultures). Temperature was recorded every 0.25 h using NexSens DS1921G micro-T data loggers (Fondriest Environmental, Inc., Alpha, Ohio, USA). Temperature data were converted into accumulated degree hours (ADH), which accounts for temperature variation over decomposition time [55].
Annual Variation and Necrophagous Insect Effects
Annual variation of microbial community activity and effects of necrophagous insect colonization was studied in a Midwestern temperate forest habitat surrounded by agricultural fields in Xenia, Ohio, USA (39° 38′14.83″ N, 83° 1′ 37.82″W). The dominant tree fauna consisted of oak (Quercus spp.) and maple. Carcasses ranging from 5–30 kg were sampled from 5 August 2010 until 14 August 2010 and 26 July 2011 until 2 August 2011. Carcasses purchased from the same farm as described above were double bagged during transport and prior to field placement to prevent insect colonization. In 2010, six male carcasses were randomly placed at a minimum of 20 m apart along three transects. In 2011, using the same methods as described in 2010, six carcasses (three females and three males) were purchased from the same farm, double bagged during transport to the field and randomly placed along three new transects.
During each year, three random carcasses were enclosed in individual 1.8 m3 Lumite® screen (18×14 mesh size) portable field cages (BioQuip Products, Rancho Dominguez, California, USA) to exclude insect colonization. These carcasses were considered the necrophagous insect exclusion treatment (EXC), while the three remaining carcasses were the insect access treatment (ACC) with insects immediately allowed access to the carcasses. As with the previous seasonal study, all carcasses were covered with anti-scavenging cages with NexSens DS1923 micro-T temperatures loggers measuring local ambient temperature every 0.25 h. Temperature data were converted into ADH.
Permission was granted by the Green County Park District for the seasonal trials at Morris Bean Reserve, while no permits were necessary for the annual and necrophagous insect colonization studies, as the landowners granted permission to use the privately owned property. The field studies did not involve endangered or protected species.
Epinecrotic Microbial Community Sampling
During the seasonal trials, sterile cotton applicators were used to sample the microbial communities of the buccal cavity (the top area of the mouth and under the tongue), skin and the interior anal cavity. Epinecrotic microbial communities were sampled from the carcasses every three days, weather and field conditions permitting, until the dry stage of decomposition as defined by Payne [56].
In both the annual and necrophagous insect effect studies, sterile cotton applicators were used to sample microbial communities from two regions on each carcass for 60s: the buccal cavity and the skin, which consisted of combining three areas (approximately 2.54×15.24 cm) along a single transect of a carcass. Care was taken to assure that new areas were swabbed at subsequent samplings. Duplicate samples were collected from the carcasses for 16S rRNA analysis of the bacterial communities using 454-pyrosequencing during the first necrophagous insect effect study (2010). Carcasses were sampled until the dry stage of decomposition as described by Payne [56].
Epinecrotic Microbial Function
Microbial community function was evaluated using phenotype Biolog EcoPlates™ (Biolog Inc., Hayward, California, USA) to provide MMCPs in all experiments [57], [58]. Biolog EcoPlates™ provide quantifiable functional responses of environmental microbial communities using 29 carbon sources (Table S1 in Materials S1); the tweens were used as a positive control to assess microbial growth on the plate and were excluded from analyses [58], [59]. The samples taken for seasonal microbial assessments were stored at −20°C for several months, slowly thawed and then processed using a modified protocol described by Insam and Goberna [60]. All samples from the annual and necrophagous insect effect studies were stored at 4°C and processed within 12 h. Briefly, samples were added individually to 50 ml Falcon tubes containing 40 ml of 25% Ringer solution and 15 sterilized 3 mm glass beads. All samples were homogenized using a Burrell Wrist-Action® shaker (Burrell Scientific, Pittsburg, Pennsylvania, USA) at the power ranking 9 for 10 min. Samples were centrifuged at 500×g for 2 min and the supernatant was retained. The plates were inoculated with 100 µl supernatant aliquots per well and incubated at 25°C in darkness based on preliminary experiments for these types of samples (unpublished data). Absorbance, or overall plate functional activity, was measured at 590 nm every 12 h up to 120 h or until the average plate absorbance reached 0.7 OD using a Wallac 1420 VICTOR2™ with Wallac 1420 Workstation software version 2.0 (Perkin Elmer, Inc., Waltham, Massachusetts, USA) during 2009 and 2010, and a Tecan Sunrise™ with Magellan™ software version 7.0 (Tecan Group Ltd., Männedorf, Switzerland) during 2011. A two-tailed paired t-test was used to test for any differences between the plate reader models, and there was not a significant difference in data between plate readers (t = 0.4620, df = 95, P = 0.645).
Data Analysis
After all seasonal samples were collected, three dates representing three different phases of decomposition (i.e., early, middle, and late) were chosen for analysis. The spring and summer trials followed the same sampling regime, with the first sampling date corresponding with the initial oviposition of Calliphoridae (Insecta: Diptera) signifying the early phase of decomposition (e.g., fresh and bloat). The second sampling date took place when the carcasses reached the middle phase of decomposition (e.g., active decay), while the last sampling date represented the late phase of decomposition (e.g., advanced decay and dry). For the autumn trial, the first sampling date corresponded with initial Calliphoridae oviposition; however, the second sampling date occurred prior to the first snow of the season. It was decided at the beginning of the study that carcasses would not be disturbed once they were covered with snow. The third sampling date took place in January of 2011, which corresponded with increased temperatures and snowmelt. For the winter trial, there were four sampling dates instead of three because of little insect activity during the beginning of the trial. The first sampling date took place immediately after carcass exposure since a delay in initial oviposition was expected during winter temperature conditions. The second date coincided with the next snowmelt. Both a third and fourth sampling date were chosen to represent different decomposition time points, even though there appeared to be negligible visible change to the carcasses and little adult or larval blow fly activity on the carcasses during the entire trial. Sampling for the annual and necrophagous insect colonizer effects studies occurred at initial field placement and on days 1, 3 and 5 during 2010. While in 2011, sampling occurred at initial field placement and then daily until 5 days of decomposition had occurred.
Analyses were performed according to Stefanowicz [44] and Weber and Legge [40]. To generate MMCPs, data were standardized by using the mean metabolic activity that approached or met 0.7 OD or after 10 consecutive readings. This was done to account for possible cell density differences among samples and provided a means to assess overall average microbial activity [40]. First, the average well color development, as determined by the absorbance measurement at 590 nm, was used to calculate the average carbon use of each plate. These data were then normalized using methods modified from Thottathil et al. [45] and Calbrix [46] by subtracting the average water well (background) activity for each plate, then subtracting that value from each carbon source usage, and then dividing by the average of all normalized carbon resources of the plate (Weber et al. 2010). The mean normalized metabolic activity for each carbon source was used for statistical analyses. A minimum base temperature of 0°C was assumed for these microbial community analyses as previously described [24].
454-Pyrosequencing of Communities
Samples collected in 2010 were used to identify microbial communities through 454-pyrosequencing using methods described in Pechal et al. [24]; a modified chloroform-phenol protocol was used for DNA extractions from each sample [24]. Samples were sent to Molecular Research Laboratories (Lubbock, TX) for bacterial tagged encoded FLX amplicon pyrosequencing. PCR amplification of V1–3 regions of 16S rRNA was performed using bacterial primers: Gray28F (5′ TTTGATCNTGGCTCAG) and Gray519r (5′GTNTTAC NGCGGCKGCTG) [61], [62]. The sequences have been deposited in the Sequence Read Archive at the European Bioinformatics Institute (accession number ERP001998). Taxonomic classification of the sequences were conducted using Naïve Bayesian rRNA classifier version 2.2 in the Ribosomal Database Project [63], [64]. The data from the ACC treatment in this study were presented in Pechal et al. [24], with a focus and analysis on family level modeling to estimate decomposition time. Here, we present phyletic data for both the ACC and EXC treatments as a qualitative comparison to functional profile changes over decomposition. For more specific (family) information on the ACC communities see Pechal et al. [24].
Statistical Analyses
Bray-Curtis distance with nonmetric multidimensional scaling (NMDS) and 999 permutations using vegan 2.0–7 library in R [65] was used to visualize MMCP differences among seasons, sample dates, replicate carcasses, sample location (i.e., anal, buccal, skin) and treatment (EXC or ACC). Outliers were identified and removed prior to ordination using Jackknife distances in JMP 9.0.0 (SAS Institute Inc., Cary, NC, USA) as recommended by McCune and Grace [66]. For each of the ordinations, the two axes that explained the most variation with the strongest orthogonality (lowest stress) were used for representing the data in multidimensional space [66].
Differences in microbial function and variation over decomposition were tested using Bray-Curtis dissimilarity values with permutational multivariate analysis of variance (PERMANOVA) with the adonis function using the vegan 2.0–7 library in the R statistical package [65], [67]. PERMANOVA is a nonparametric technique used to differentiate groups of data based on a dissimilarity matrix [67].
Results
Seasonal Variation
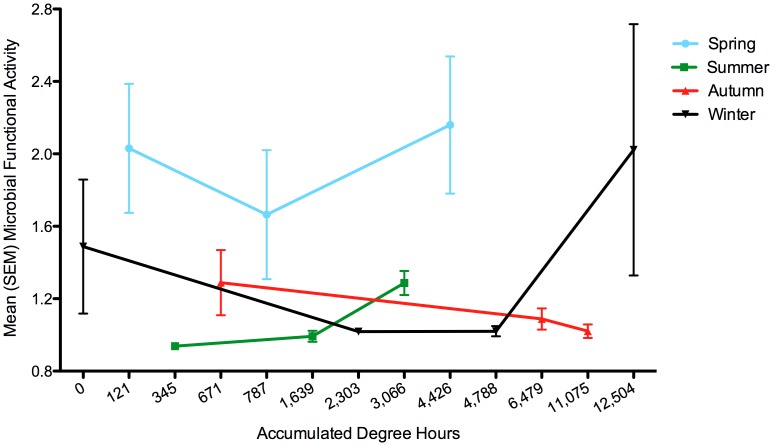
Spring overall microbial functional use of the carbon resources was highest of all seasons (Fig. 1) and increased by 6.0% from the first (121 ADH) to the last sampling day (4,426 ADH); the greatest functional activity change occurred during the summer with an increase of 27.1% from the first (345 ADH) to last (3,066 ADH) sampling day; autumn activity decreased by 20.9% over decomposition from 671 to 11,075 ADH; and winter microbial activity increased by 26.2% from 0 to 12,504 ADH.
Figure 1. Seasonal microbial metabolic activity.
The mean microbial functional activity for spring (blue circle), summer (green square), autumn (red triangle), and winter (black inverted triangle) over accumulated degree hours (ADH), which was used as a surrogate of decomposition time.
Using PERMANOVA we found no significant differences in normalized MMCPs among the sample locations (buccal, skin, anus) of each carcass; therefore, data were pooled for the remainder of analyses. A two-dimensional NMDS ordination (stress = 0.198, R2 = 0.88) was sufficient to describe normalized carcass MMCPs among seasons (Fig. S1). There were significant differences (PERMANOVA: P = 0.001) in MMCPs among all seasons (Table 1; Fig. 1). Seasons were then separated to assess MMCP differences among the phases (early, middle and late) of decomposition (Fig. S2). There were significantly different MMCPs among decomposition phases in spring and summer (PERMANOVA: P = 0.001), but no differences during the autumn (PERMANOVA: P = 0.226) or winter studies (PERMANOVA: P = 0.101).
Table 1. PERMANOVA results testing microbial community functional responses among seasons; and among carcass decomposition phases (i.e., early, middle and late), among seasons including the interaction with significant results indicated by an asterisk.
| Factor | Source | d.f. | SS | MS | F | P |
| Season | Seasons | 3 | 2.662 | 0.887 | 4.642 | 0.001* |
| Residuals | 162 | 30.970 | 0.191 | |||
| Total | 165 | 33.633 | ||||
| Decomposition Phase | Seasons | 3 | 2.662 | 0.887 | 5.595 | 0.001* |
| DecompositionPhase | 2 | 2.584 | 1.292 | 8.144 | 0.001* | |
| Season × DecompositionPhase | 6 | 3.957 | 0.659 | 4.157 | 0.001* | |
| Residuals | 154 | 24.430 | 0.159 | |||
| Total | 165 | 33.633 |
Annual Variation
There were significant differences in carcass MMCPs over decomposition, a significant difference between years, and no significant interaction (Table 2). Overall mean functional activity was significantly higher (PERMANOVA: P<0.001) in 2011 (Fig. S3). A two-dimensional NMDS ordination (stress = 0.199, R2 = 0.87) described the variation in carcass MMCPs between years (Fig. S4).
Table 2. PERMANOVA results testing microbial community functional responses between years and among carcass decomposition days including the interaction with significant results indicated by an asterisk.
| Factor | Source | d.f. | SS | MS | F | P |
| Year | Year | 1 | 1.6909 | 1.69088 | 9.1259 | 0.001* |
| Decomposition Day | 5 | 1.5231 | 0.30462 | 1.6441 | 0.007* | |
| Season × Decomposition Day | 3 | 0.7220 | 0.24067 | 1.2989 | 0.113 | |
| Residuals | 93 | 17.2313 | 0.18528 | |||
| Total | 102 | 21.1673 |
Annual Variation and Necrophagous Insect Effects
2010
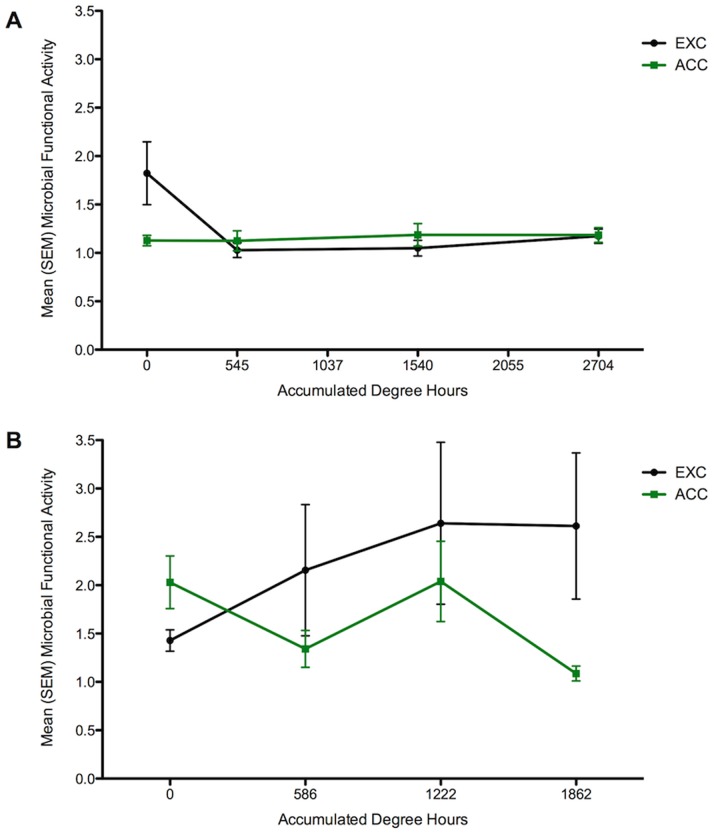
There was a significant difference in carcass MMCPs over decomposition, no difference between treatments (EXC and ACC) and a significant interaction effect (Table 3). Overall, mean microbial functional activity of ACC carcasses increased by 4.2% over decomposition; however, it decreased by 35.7% in EXC carcasses (Fig. 2). There were significant differences in MMCPs over decomposition and between sampling regions (buccal and skin), with no significant interaction effects (Table S2 in Materials S1); buccal community microbial activity initially decreased on sampling day 1 followed by a slight increase in activity until the fifth sampling day with an overall net decrease from the initial to last sampling day (Fig. S5). Skin communities exhibited a similar pattern of decreased overall microbial activity until sampling day 3 followed by increased activity as decomposition progressed with an overall net decrease in microbial activity from the initial to last sampling day (Fig. S5).
Table 3. PERMANOVA results testing microbial community functional responses between treatments (EXC and ACC) and among carcass decomposition days including the interaction for both 2010 and 2011 field seasons with significant results indicated by an asterisk.
| Factor | Source | d.f. | SS | MS | F | P |
| 2010 | Treatment | 1 | 0.1417 | 0.14170 | 1.0998 | 0.353 |
| Decomposition Day | 3 | 0.8350 | 0.27833 | 2.1603 | 0.001* | |
| Treatment × Decomposition Day | 3 | 0.5906 | 0.19687 | 1.5280 | 0.017* | |
| Residuals | 40 | 5.1537 | 0.12884 | |||
| Total | 47 | 6.7211 | ||||
| 2011 | Treatment | 1 | 0.0743 | 0.0743 | 0.3249 | 0.991 |
| Decomposition Day | 5 | 1.4353 | 0.2871 | 1.2550 | 0.139 | |
| Treatment × Decomposition Day | 3 | 0.9527 | 0.3176 | 1.3884 | 0.104 | |
| Residuals | 45 | 10.2931 | 0.2285 | |||
| Total | 54 | 12.7554 |
Figure 2. Epinecrotic microbial metabolic activity with delayed initial insect colonization.
The mean microbial functional activity in the A) 2010 field trial for carcasses excluded from insect access (EXC-black circle) and carcasses allowed to have insect access (ACC-green square), and in B) 2011 field trial for carcasses excluded from insect access (EXC-black circle) and carcasses allowed to have insect access (ACC-green square) over accumulated degree hours (ADH), which was used as a surrogate of decomposition time.
A two-dimensional NMDS ordination (stress = 0.177, R2 = 0.91) was sufficient to describe the variation in carcass MMCPs between ACC and EXC carcasses (Fig. S6). There were not significantly different MMCPs between insect exclusion and insect access carcasses, but there was a significant difference in MMCPs over decomposition time and a significant interaction (Table 3); thus data from each treatment were pooled for further analysis. Pair-wise comparisons indicated significantly different MMCPs between the initial day (Day 0) and each subsequent day of decomposition (Days 1, 3 and 5).
454-Pyrosequencing of Communities
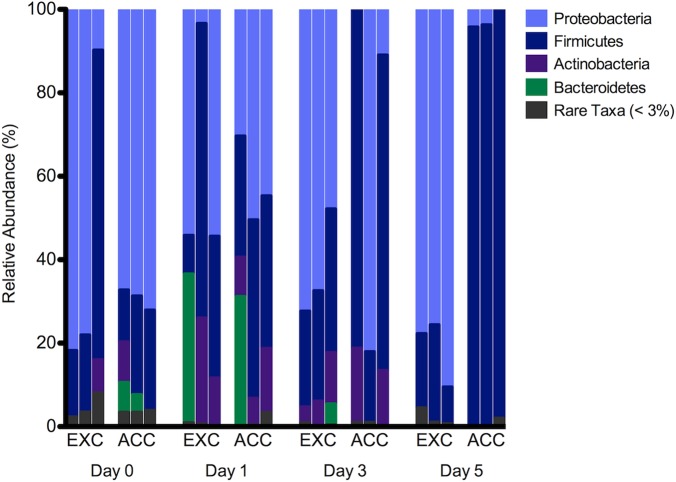
A total of 378,904 sequences were obtained throughout carcass decomposition. At initial field placement, Proteobacteria was the predominant phyla for EXC (62%) and ACC (70%) carcasses with Firmicutes being the next predominant phyla at 33% and 20% of EXC and ACC communities, respectively. Proteobacteria remained predominant on the first (35%), third (63%), and fifth (82%) sampling days for EXC carcasses. While Firmicutes decreased as decomposition progressed on the first (43%), third (28%), and fifth (16%) day. However, ACC displayed an inverse trend as Proteobacteria decreased from the first (42%) and third (44%) days to only 3% total abundance on the fifth day, but with some inter-carcass variation as discussed in detail in Pechal et al. [24]. Firmicutes was the predominant phyla as decomposition progressed comprising of 36, 48, and 96% on the first, third and fifth sampling day, respectively. Rare phyla accounted for less than 0.5% of the total relative abundance across decomposition (Fig. 3).
Figure 3. Identification of carcass microbial communities over decomposition using 454-pyrosequencing.
Phyletic level taxonomic relative abundance of the microbial communities based on 454-pyrosequencing between treatments (EXC and ACC) over decomposition days during 2010. Day 0 is the initial field placement followed by subsequent sample collections on days 1, 3 and 5 post-field placement. Rare taxa includes any phyla with <3% of the total relative abundance.
2011
Over decomposition, mean microbial functional activity of ACC carcasses decreased by 46.3% while there was a 45.2% increase on EXC carcasses (Fig. 2). There were no significant differences in carcass MMCPs over decomposition, between treatments nor was there a significant interaction (Table 3). However, there was a significant difference in microbial functional activity between the buccal and skin communities and the composite sample but there was no significant difference over decomposition and no significant interaction. The buccal communities increased on day 1, decreased by day 3, and increased on day 5 with an overall net increase in mean microbial activity from the initial to last sampling day (Fig. S5). Skin communities exhibited an inverse trend of decreased activity on day 1, increased by day 3, and finally decreased on day 5 with an overall net decrease in mean microbial activity from the initial to last sampling day (Fig. S5). However, there were no significant differences in MMCPs between sampling regions (buccal, skin), but a significant interaction (Table S2 in Materials S1).
A two-dimensional NMDS ordination (stress = 0.166, R2 = 0. 89) was sufficient to describe the variation in carcass MMCPs between ACC and EXC carcasses (Fig. S6).
Abiotic Conditions
Mean daily ambient temperature among carcasses was 23.2±2.1°C during 2010 and 25.1±1.0°C in 2011. Mean ADH associated with the carcasses in 2011 was significantly higher (F1,7 = 432.9, P<0.001) by 8–18% than 2010 (data not shown) throughout decomposition, except at initial placement in the field when ADH for all carcasses was zero.
Discussion
Here we report the use of Biolog EcoPlates™ for describing microbial community metabolic profiles throughout carrion decomposition as a representation of the overall functional activity and differentiated carbon source use of epinecrotic communities. The results demonstrated 1) seasonal variation of microbial community function, with overall higher and more variable activity during spring when compared to the other seasons; 2) annual differences in metabolic profiles; and 3) inconsistent effects of necrophagous insect activity (e.g., blow fly oviposition and subsequent larval development) on microbial functional profiles and overall activity.
Fungi and bacteria have been documented as facilitators of the initial decomposition processes of carrion [19], [68]. The inherent stochastic spatial and temporal nature of microbial communities on ephemeral resources, such as carrion, may contribute to the variation both within and across carcasses [69]. We demonstrated that microbial metabolic community profiles varied by season, and within a season they were highly variable during the carcass decomposition process. We also found that when initial insect access and colonization was delayed the metabolic profile changes that occurred during decomposition depended on the year of study, while overall metabolic activity was more highly variable for microbial communities of insect exclusion carcasses during both years. These results suggest that microbial abundance and activity of epinecrotic communities are patchy and sensitive to environmental factors such as temperature and habitat. This variability in metabolic profile responses both within and between seasons and years may be tied to corresponding shifts in the microbial community composition throughout the decomposition process, but this requires additional study. There are limitations associated with sole-carbon source utilization profiles [52]; however, in this study metabolic profiles were used as a potential surrogate for differentiating microbial community changes on the resource over time (e.g., decomposition).
Seasonal Variation
Seasonal variance in abiotic conditions was hypothesized to significantly impact microbial community function. For the spring and winter trials, microbial functional activity decreased during early decomposition followed by an increase in later phases of decomposition. The increase in functional activity during the winter trial most likely results from a lack of competition with invertebrates (e.g., blow fly larvae) because of the colder temperatures (i.e., <5°C). Summer had an increase in microbial activity throughout decomposition. Autumn was the only season that demonstrated a decreasing trend in overall activity throughout decomposition, which can be due to competition with invertebrates, the depletion of the carrion resource, or both. Competition between microbes and insects for a resource occurs by microbes producing toxins that deter eukaryotic consumers [25], while insects can produce antimicrobial peptides that inhibit microbial proliferation [70].
We found significantly different metabolic profiles over decomposition for carcasses in spring and summer but not autumn and winter. However, why did microbial functional activity increase in the winter trial as temperatures decreased? Carter and Tibbett [33] demonstrated when chicken carcasses were subjected to different temperatures, the efficiency of the microbial metabolic activity (respiration) during decomposition varied. They reported that when carcasses decomposed under 2, 12, and 22°C, the process was most efficient at 2°C. This may explain the increased functional activity between the second and last sampling dates for the winter trial, but this requires additional investigation into the optimal metabolic temperatures of multi-species assemblages of microbes. This could also aide in explaining microbial functional variability in different seasons. For the spring and summer trials, large Calliphoridae larval masses colonized and consumed the carcass throughout the decomposition process. Carter and Tibbett [33] also speculated that higher ambient temperatures might have negative impacts on microbial community catabolic reactions. Dipteran larval masses have the potential to generate increased temperatures up to 50°C higher than the surrounding ambient temperature through collective thermogenesis [71]. The depletion of the carrion resource by an invertebrate competitor, but also increased aggregate temperatures resulting from calliphorid larval masses may be affecting microbial community assembly and overall activity during carrion decomposition. Additional studies are warranted to further understand this process.
Annual Variation
Qualitative phases of decomposition could be differentiated for carcasses based on microbial functional activity, and insects appeared to be reducing the microbial community throughout the decomposition process in 2010. However, these trends were not consistent between years. The lack of similarities between years may result from abiotic (e.g., temperature) and biotic factors (e.g., pre-existing microbial communities), as was demonstrated in our seasonal study. Abiotic factors including temperature may account for microbial community function variation between years and throughout decomposition. Ambient temperatures were up to 18% higher in 2011. Previous studies, however, have reported conflicting microbial species response to changing temperature. For instance, soil bacteria abundance increased with temperature (+3°C) in the presence of elevated carbon dioxide, but decreased under conditions of similar temperature conditions without elevated carbon dioxide [72]. Therefore, during 2011, higher average temperature may have been associated with increased available carbon and overall activity of the carcass microbial communities regardless of treatment. Finally, the variation may be associated with other parameters or interactions (e.g., pH changes occurring as part of the carrion or soil) that were not assessed during this study [73].
Necrophagous Insect Effects
Primary insect colonizers utilize the carrion resource as nutrition, mating or an oviposition site. Subsequent larval development may disrupt established microbial communities through direct or indirect competitive interactions on the carcass [25], [70]. Blow flies may directly impact microbial species through chemical secretions while consuming carrion tissue as changes in the microbial community have been demonstrated when using calliphorid larvae for wound debridement therapy [74], [75]. Insects arriving to colonize carrion could introduce their own exogenous microbial community [76], such as Musca domestica (Diptera: Muscidae), which carries over 100 pathogenic microbes [77], [78]. The introduction of insect associated microbial communities may influence carrion microbial community function through microbially mediated competitive mechanisms, which can alter metacommunity dynamics and biogeographic patterns of microbial communities in the landscape [79], [80]. For example, one of the Biolog EcoPlates™ carbon sources, putrescine, is a well know volatile associated with decomposing remains [81]–[84] and is a blow fly attractant but repellant for male carrion beetles (Coleoptera: Silphidae) [85]. Putrescine is also a molecule required for the swarming behavior of bacteria such as Proteus mirabilis, which is commonly associated with blow flies [30], [31]. Alternatively, specific microbes or functional groups may be outcompeting other species present during later phases of undisturbed decomposition, which has been found in other systems [86], [87]. In this study, we found that overall functional activity was affected by the initial access or exclusion of insect colonizers, but the response depended on the year of study, and presumably associated temperature conditions. These functional responses were also associated with the phyletic differences in communities associated with insect exclusion, suggesting 1) there is variability in the structure and function of carrion microbial communities and 2) that necrophagous flies have important effects on microbial community assembly during decomposition. A similar pattern has been observed for undisturbed vernal rain pools that produced significantly higher species richness of protozoan and metazoan species [88] and higher protozoan and rotifer richness in undisturbed artificial container communities filled by rain than that found in disturbed vernal pools [89], [90]. While the variation of microbial metabolic profiles among years and in the presence of necrophagous insects is not surprising, these insect-microbe interactions are still poorly understood in carrion decomposition systems.
Additionally, we documented four major phyla associated with the carcasses throughout decomposition using 454-pyrosequencing that included Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes. These phyla have been reported in one other study where epinecrotic communities were described using 454-pyrosequencing (Pechal et al. [24]). Taxa within Proteobacteria are commonly associated with the spoiling of meat and are found on the hides of slaughtered animals [91]. While taxa in the remaining three phyla are associated with the human microbiome [92] and soil communities [93]. Thus, shifts in functional profiles exhibited throughout the 2010 field trial may have resulted from the changes in the relative abundance of these four main phyla. It is unknown at this time whether or not there is a difference of species composition or functional groups based on taxonomic identification of bacteria.
Conclusions
These results demonstrate for the first time the use of metabolic profiling to assess carrion decomposition, and that there is great potential to use this technique for carrion decomposition research. Current research in this field has been aimed at determining what microorganisms compose communities involved in carrion decomposition [19], [20]. Our results demonstrate that the microbial metabolic profiles describe significant functional changes in the community during decomposition both within and among seasons, similar to other studies in aquatic habitats [19], [20]. This demonstrates community-level functional changes occurring over decomposition; however, more detailed research involving which species are changing over time is needed using high throughput metagenomic sequencing approaches [94], [95]. Specifically, using microbial functional data in conjunction with metagenomic and entomological data is needed to better understand the complexity and importance of the necrobiome to decomposition.
Overall, empirical data are sparse within the microbe-insect-carrion model in terrestrial ecosystems. We have demonstrated that insects may have moderating effects on decomposition by mediating microbial structure and function. It is important to further investigate the role of microbes and their importance in determining underlying mechanisms controlling community assembly, biomass turnover and nutrient cycling of ephemeral resources.
Supporting Information
Seasonal Bray-Curtis dissimilarity based non-metric multidimensional scaling. Using Bray-Curtis dissimilarity values, MMCPs for seasonal carrion communities were plotted using a two dimensional non-metric multidimensional scaling model (stress = 0.198, R2 = 0.88). The MMCPs were significantly different (PERMANOVA: P = 0.001) among seasons. The circles indicate 95% standard error of each season.
(TIFF)
Decomposition phase within season Bray-Curtis dissimilarity based non-metric multidimensional scaling. Non-metric multidimensional scaling ordinations of MMCPs were plotted on two dimensions for carrion communities of decomposition phases (early, middle and late) during A) spring (stress = 0.122, R2 = 0.94), B) summer (stress = 0.098, R2 = 0. 98), C) autumn (stress = 0.149, R2 = 0.91), and D) winter (stress = 0.158, R2 = 0.91) seasons. There were significant differences among decomposition phases in spring and summer (PERMANOVA: P = 0.001), but no significant difference among decomposition phases in autumn (PERMANOVA: P = 0.226) or winter (PERMANOVA: P = 0.011). The circles indicate 95% standard error of each decomposition phases.
(TIFF)
Mean functional activity over decomposition between years. The mean (SEM) microbial functional activity between the 2010 (gray circle) and 2011 (black square) field trials at initial field placement (Day 0) and subsequent sampling on days 1, 3, and 5.
(TIFF)
Annual Bray-Curtis dissimilarity based non-metric multidimensional scaling. Using Bray-Curtis dissimilarity values, MMCPs from both 2010 and 2011 field seasons were plotted using a two dimensional non-metric multidimensional scaling model (stress = 0.199, R2 = 0.87). There were significantly different MMCPs (PERMANOVA: P = 0.001) between years. The circles indicate 95% standard error of each year.
(TIFF)
Mean functional activity over decomposition between sampling regions in both field seasons. The mean microbial functional activity for the buccal (orange circle) and skin (yellow square) sampling region in the A) 2010 and B) 2011 field trials at initial field placement (Day 0) and subsequent sampling on days 1, 3, and 5.
(TIFF)
Treatment within field season Bray-Curtis dissimilarity based non-metric multidimensional scaling. Non-metric multidimensional scaling ordinations of MMCPs were plotted on two dimensions for ACC and EXC carrion communities during A) 2010 (stress = 0.177, R2 = 0.91), and B) 2011 (stress = 0.166, R2 = 0. 86) field seasons.
(TIFF)
Supplementary Tables S1–S2. Table S1, Carbon sources and their respective groupings (i.e., amines/amides, amino acid, carbohydrate, and carboxylic and acetic acid) in a Biolog EcoPlate™. Table S2, PERMANOVA results testing MMCPs between sampling region (buccal and skin) communities and among carcass decomposition days including the interaction for both 2010 and 2011 field seasons with significant results indicated by an asterisk.
(DOCX)
Acknowledgments
The authors thank T. Blair and J. White for help during the study. The Blair family is gratefully acknowledged for allowing access to their property for this research. We thank B. Singh for assistance in the metagenomic data sequence classification processing. The authors thank P. Barton and two anonymous reviewers for their critical reviews and useful comments to improve this manuscript.
Funding Statement
The authors (AJL and MEB) would like to thank the University of Dayton Research Council and the Department of Biology for financial support of this project. JLP was funded by the Department of Entomology and the Whole Systems Genomics Initiative for Improved Human, Animal, and Environmental Wellbeing at Texas A & M University. JKT and AMT are funded by the Department of Entomology at Texas A & M University and Texas A&M University AgriLife Research. This project was also funded (TLC, AMT, JKT and MEB), in part, by the National Institute of Justice, Office of Justice Programs, United States Department of Justice through Grant 2010-DN-BX-K243. Points of view in this document are those of the authors and do not necessarily represent the official position or policies of the United States Department of Justice. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Campbell NA, Reece JB, Urry LA, Cain ML, Wasserman SA, et al.. (2008) Biology; Wilbur B, editor. San Francisco, CA: Pearson Benjamin Cummings.
- 2. Barton PS, Cunningham SA, Macdonald BC, McIntyre S, Lindenmayer DB, et al. (2013) Species Traits Predict Assemblage Dynamics at Ephemeral Resource Patches Created by Carrion. PLoS ONE 8: e53961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore JC, Berlow EL, Coleman DC, Ruiter PCd, Dong Q, et al. (2004) Detritus, trophic dynamics and biodiversity. Ecology Letters 7: 584–600. [Google Scholar]
- 4. Putman RJ (1978) Flow of energy and organic matter from a carcass during decomposition. Decomposition of small mammal carrion in temperate systems. Oikos 31: 58–68. [Google Scholar]
- 5. Ellis-Evan JC, Layboum J, Bayliss PR, Perriss SJ (1998) Physical, chemical and microbial community characteristics of lakes of the Larsemann Hills, Continental Antarctica. Archiv fur Hydrobiologie 141: 209–230. [Google Scholar]
- 6.Brock TD (1978) Thermophilic microorganisms and life at high temperatures. New York: Springer.
- 7. Skirnisdottir S, Hreggvidsson GO, Hjorleifsdottir S, Marteinsson VT, Petursdottir SK, et al. (2000) Influence of Sulfide and Temperature on Species Composition and Community Structure of Hot Spring Microbial Mats. Applied Environmental Microbiology 66: 2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janetski DJ, Chaloner DT, Tiegs SD, Lamberti GA (2009) Pacific Salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 159: 583–595. [DOI] [PubMed] [Google Scholar]
- 9. Tomberlin JK, Mohr R, Benbow ME, Tarone AM, VanLaerhoven S (2011) A roadmap for bridging basic and applied research in forensic entomology. Annual Review of Entomology 56: 401–421. [DOI] [PubMed] [Google Scholar]
- 10. Parmenter R, MacMahon J (2009) Carrion decomposition and nutrient cycling in a semiarid shrub-steppe ecosystem. Ecological Monographs 79: 637–661. [Google Scholar]
- 11. Jenkinson DS (1977) The soil biomass. NZ Soil News 25: 213–218. [Google Scholar]
- 12.Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems; Anderson DJ, Greig-Smith P, Pitelka FA, editors. Berkely and Los Angeles: Blackwell Scientific Publications.
- 13. Hocking M, Ring R, Reimchen T (2009) The ecology of terrestrial invertebrates on Pacific salmon carcasses. Ecological Research 24: 1091–1100. [Google Scholar]
- 14. Chaloner DT, Wipfli MS, Caouette JP (2002) Mass loss and macroinvertebrate colonization of Pacific salmon carcassess in southeastern Alaskan streams. Freshwater Biol 47: 263–274. [Google Scholar]
- 15. Yang LH (2004) Periodical cicadas as resource pulses in North American forests. Science 306: 1565–1567. [DOI] [PubMed] [Google Scholar]
- 16. Yang LH, Bastow JL, Spence KO, Wright AN (2008) What can we learn from resource pulses? Ecology 89: 621–634. [DOI] [PubMed] [Google Scholar]
- 17. Yang LH, Edwards KF, Byrnes JE, Bastow JL, Wright AN, et al. (2010) A meta-analysis of resource pulse–consumer interactions. Ecological Monographs 80: 125–151. [Google Scholar]
- 18. Kominoski JS, Marczak LB, Richardson JS (2011) Riparian forest composition affects stream litter decomposition despite similar microbial and invertebrate communities. Ecology 92: 151–159. [DOI] [PubMed] [Google Scholar]
- 19. Burkepile DE, Parker JD, Woodson CB, Mills HJ, Kubanek J, et al. (2006) Chemically mediated competition between microbes and animals: microbes as consumers in food webs. Ecology 87: 2821–2831. [DOI] [PubMed] [Google Scholar]
- 20. Dickson GC, Poulter RTM, Maas EW, Probert PK, Kieser JA (2011) Marine bacterial succession as a potential indicator of postmortem submersion interval. Forensic Science International 209: 1–10. [DOI] [PubMed] [Google Scholar]
- 21. Benbow ME, Lewis AJ, Tomberlin JK, Pechal JL (2013) Seasonal Necrophagous Insect Community Assembly During Vertebrate Carrion Decomposition. Journal of Medical Entomology 50: 440–450. [DOI] [PubMed] [Google Scholar]
- 22.Janaway RC (1996) The decay of human buried remains and their associated materials. In: Hunter J, Roberts C, Martin A, editors. Studies in crime: An introduction to forensic archaeology. London: B.T. Batsford Ltd. 58–85.
- 23.Wilson M (2005) Microbial inhabitants of humans: their ecology and role and health and disease. New York: Cambridge University Press.
- 24.Pechal JL, Crippen TL, Benbow ME, Tarone AM, Dowd S, et al.. (2013) The potential use of bacterial community succession in forensics as described by high throughput metagenomic sequencing. International Journal of Legal Medicine. [DOI] [PubMed]
- 25. Janzen DH (1977) Why fruits rot, seeds mold, and meat spoils. American Naturalist 111: 691–713. [Google Scholar]
- 26.Trienens M, Keller NP, Rohlfs M (2010) Fruit, flies and filamentous fungi – experimental analysis of animal–microbe competition using Drosophila melanogaster and Aspergillus mould as a model system. Oikos: 1765–1775.
- 27. Tomberlin JK, Benbow ME, Tarone AM, Mohr R (2011) Basic research in evolution and ecology enhances forensics. Trends in Ecology and Evolution 26: 53–55. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc H, Logan J (2010) Exploiting insect olfaction in forensic entomology. In: Amendt J, Goff M, Campobasso C, Grassberger M, editors. Current concepts in forensic entomology. London: Springer Dordrecht. 205–221.
- 29.LeBlanc HN (2008) Olfactory stimuli associated with the different stages of vertebrate decomposition and their role in the attraction of the blowfly Calliphora vomitoria (Diptera: Calliphoridae) to carcasses [PhD]. Derby, United Kingdom: The University of Derby.
- 30. Ma Q, Fonseca A, Liu W, Fields AT, Pimsler ML, et al. (2012) Proteus mirabilis interkingdom swarming signals attract blow flies. The ISME Journal 6: 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomberlin JK, Crippen TL, Tarone AM, Singh B, Adams K, et al. (2012) Interkingdom responses of flies to bacteria mediated by fly physiology and bacterial quorum sensing. Animal Behaviour 84: 1449–1456. [Google Scholar]
- 32. Ward DM, Ferris MJ, Nold SC, Bateson MM (1998) A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Micrbiology and Molecular Biology Review 62: 1353–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter DO, Tibbett M (2006) Microbial decomposition of skeletal muscle tissue (Ovis aries) in a sandy loam soil at different temperatures. Soil Biology and Biochemistry 38: 1139–1145. [Google Scholar]
- 34. Barton P, Cunningham S, Lindenmayer D, Manning A (2012) The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 171: 761–772. [DOI] [PubMed] [Google Scholar]
- 35. Schimel JP, Gulledge JM, Clein-Curley JS, Lindstrom JE, Braddock JF (1999) Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biology and Biochemistry 31: 831–838. [Google Scholar]
- 36. Ibekwe AM, Kennedy AC, Frohne PS, Papiernik SK, Yang CH, et al. (2002) Microbial diversity along a transect of agronomic zones. FEMS Microbiology Ecology 39: 183–191. [DOI] [PubMed] [Google Scholar]
- 37. Kuske CR, Ticknor LO, Miller ME, Dunbar JM, Davis JA, et al. (2002) Comparison of soil bacterial communities in rhizospheres of three plant species and the interspaces in an arid grassland. Applied Environmental Microbiology 68: 1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haslam TCF, Tibbett M (2009) Soils of contrasting pH affect the decomposition of buried mammalian (Ovis aries) skeletal muscle tissue. Journal of Forensic Sciences 54: 900–904. [DOI] [PubMed] [Google Scholar]
- 39. Daviss B (2005) Growing pains for metabolomics. The Scientist 19: 25–28. [Google Scholar]
- 40. Weber KP, Legge RL (2010) Community-Level Physiological Profiling. Bioremediation, Methods in Molecular Biology 599: 263–281. [DOI] [PubMed] [Google Scholar]
- 41. Fiehn O (2002) Metabolomics - the link between genotypes and phenotypes. Plant Molecular Biology 48: 155–171. [PubMed] [Google Scholar]
- 42. Insam H, Goberna M (2004) Use of Biolog for the Community Level Physiological Profiling (CLPP) of Environmental Samples. Mol Micro Ecol Man 5 3.2: 1–8. [Google Scholar]
- 43. Bucher AE, Lanyon LE (2005) Evaluating soil management with microbial community-level physiological profiles. Applied Soil Ecology 29: 59–71. [Google Scholar]
- 44. Stefanowicz A (2006) The biolog plates technique as a tool in ecological studies of microbial communities. Polish J Environ Stu 15: 669–676. [Google Scholar]
- 45. Thottathil SD, Balachandran KK, Jayalakshmy KV, Gupta GVM, Nair S (2008) Tidal switch on metabolic activity: Salinity induced responses on bacterioplankton metabolic capabilities in a tropical estuary. Est Coastal Shelf Sci 78: 665–673. [Google Scholar]
- 46. Calbrix R, Laval K, Barray S (2005) Analysis of the potential functional diversity of the bacterial community in soil: a reproducible procedure using sole-carbon-source utilization profiles. European Journal of Soil Biology 41: 11–20. [Google Scholar]
- 47. Miller CD, Child R, Hughes JE, Benscai M, Der JP, et al. (2007) Diversity of soil mycobacterium isolates from three sites that degrade polycyclic aromatic hydrocarbons. J App Micro 102: 1612–1624. [DOI] [PubMed] [Google Scholar]
- 48. Papatheodorou EM, Efthimiadou E, Stamou GP (2008) Functional diversity of soil bacteria as affected by management practices and phenological stage of Phaseolus vulgaris. Euro J Soil Biol 44: 429–436. [Google Scholar]
- 49. Ros M, Gobema M, Pascual JA, Larnmer S, Insain H (2008) 16S rDNA analysis reveals low microbial diversity in community level physiological profile assays. J Microbiol Methods 72: 221–226. [DOI] [PubMed] [Google Scholar]
- 50. Sala MM, Pinhassi J, Gasol JM (2006) Estimation of bacterial use of dissolved organic nitrogen compounds in aquatic ecosystems using Biolog plates. Aqu Micro Ecol 42: 1–5. [Google Scholar]
- 51. Richardson NF, Ruesink JL, Naeem S, Hacker SD, Tallis HM, et al. (2008) Bacterial abundance and aerobic microbial activity across natural and oyster aquaculture habitats during summer conditions in a northeastern Pacific estuary. Hydrobiol 596: 269–278. [Google Scholar]
- 52. Preston-Mafham J, Boddy L, Randerson PF (2002) Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles – a critique. FEMS Microbiology Ecology 42: 1–14. [DOI] [PubMed] [Google Scholar]
- 53. Schoenly KG, Haskell NH, Hall RD, Gbur JR (2007) Comparative Performance and Complementarity of Four Sampling Methods and Arthropod Preference Tests from Human and Porcine Remains at the Forensic Anthropology Center in Knoxville, Tennessee. Journal of Medical Entomology 44: 881–894. [DOI] [PubMed] [Google Scholar]
- 54. Lewis AJ, Benbow ME (2011) When entomological evidence crawls away: Phormia regina en masse larval dispersal. Journal of Medical Entomology 48: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 55. Megyesi MS, Nawrocki SP, Haskell NH (2005) Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. Journal of Forensic Sciences 50: 618–626. [PubMed] [Google Scholar]
- 56. Payne JA (1965) A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 46: 592–602. [Google Scholar]
- 57. Garland JL, Mills AL (1991) Classification and characterizaiton of heterotropic microbial communiites on the basis of patterns of community-level sole-carbon-source utilization. Applied and Environmental Microbiology 57: 2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garcia-Villaraco Velasco A, Probanza A, Gutierrez Maoero FJ, Cruz Trevioo A, Moreno JM, et al. (2009) Effect of fire and retardant on soil microbial activity and functional diversity in a Mediterranean pasture. Geoderma 153: 186–193. [Google Scholar]
- 59. Garland JL (1997) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiology Ecology 24: 289–300. [Google Scholar]
- 60.Insam H, Goberna M (2004) Use of Biolog for the community level physiological profiling (CLPP) of environmental samples. In: Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans ADL, van Elsas JD, editors. Molecular Microbial Ecology Manual. Netherlands: Kluwer Academic Publishers. 853–860.
- 61.Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, et al.. (2008) Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiology 8. [DOI] [PMC free article] [PubMed]
- 62. Dowd SF, Sun Y, Wolcott RD, Domingo A, Carroll JA (2008) Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: Bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathogens and Disease 5: 459–472. [DOI] [PubMed] [Google Scholar]
- 63.Garrity GM, Bell JA, Lilburn TG (2004) Taxonomic outline of the prokaryotes. In: Garrity GM, editor. Bergey’s manual of systematic bacteriology. 2nd ed. New York, New York: Springer-Verlag. 4–23.
- 64. Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology 73: 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.R Development Core Team (2010) R: A language and environment for statistical computing. Vienna, Austria ISBN 3–900051–07–0: http://www.R-project.org.
- 66.McCune B, Grace J (2002) Analysis of Ecological Communities. Gleneden Beach, OR: MjM.
- 67. Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. [Google Scholar]
- 68. Jiron LF, Cartin VM (1981) Insect succession in the decomposition of a mammal in Costa Rica. Journal of the New York Entomological Society 89: 158–165. [Google Scholar]
- 69. Ramette A, Tiedje JM (2007) Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proceedings of the National Academy of Science of the United States of America 104: 2761–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rozen DE, Engelmoer DJP, Smiseth PT (2008) Antimicrobial strategies in burying beetles breeding on carrion. Proceedings of the National Academy of Sciences 105: 17890–17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anderson G, VanLaerhoven S (1996) Initial studies on insect succession on carrion in southwestern British Columbia. Journal of Forensic Sciences 41: 617–625. [PubMed] [Google Scholar]
- 72. Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW (2010) Soil microbial community responses to multiple experimeintal climate change drivers. Applied and Environmental Microbiology 76: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Salt GW (1979) A comment on the use of the term emergent properties. The American Naturalist 113: 145–148. [Google Scholar]
- 74. Sherman RA, Hall MJR, Thomas S (2000) Medicinal maggots: An ancient remedy for some contemporary afflictions. Annual Review of Entomology 45: 55–81. [DOI] [PubMed] [Google Scholar]
- 75. Mumcuoglu KY, Miller J, Mumcuoglu M, Friger M, Tarshis M (2001) Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae). Journal of Medical Entomology 38: 161–166. [DOI] [PubMed] [Google Scholar]
- 76. Nayduch D, Noblet GP, Stutzenberger FJ (2002) Vector potential of houseflies for the bacterium Aeromonas caviae . Medical and Veterinary Entomology 16: 193–198. [DOI] [PubMed] [Google Scholar]
- 77. Alam MJ, Zurek L (2004) Association of Escherichia coli O157:H7 with houseflies on a cattle farm. Applied and Environmental Microbiology 70: 7578–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Greenberg B, Klowden M (1972) Enteric bacterial interactions in insects. American Journal of Clinical Nutrition 25: 1459–1466. [DOI] [PubMed] [Google Scholar]
- 79. Langenheder S, Szekely AJ (2011) Species sorting and neutral processes are both important during the initial assembly of bacterial communities. The ISME Journal 5: 1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jones SE, McMahon KD (2009) Species-sorting may explain an apparent minimal effect of immigration on freshwater bacterial community dynamics. Environmental Microbiology 11: 905–913. [DOI] [PubMed] [Google Scholar]
- 81. Curran AM, Rabin SI, Prada PA, Furton KG (2005) Comparison of the Volatile Organic Compounds Present in Human Odor Using Spme-GC/MS. Journal of Chemical Ecology 31: 1607–1619. [DOI] [PubMed] [Google Scholar]
- 82. Frederickx C, Dekeirsschieter J, Brostaux Y, Wathelet JP, Verheggen FJ, et al. (2012) Volatile organic compounds released by blowfly larvae and pupae: New perspectives in forensic entomology. Forensic Science International 219: 215–220. [DOI] [PubMed] [Google Scholar]
- 83. Frederickx C, Dekeirsschieter J, Verheggen FJ, Haubruge E (2012) Responses of Lucilia sericata Meigen (Diptera: Calliphoridae) to cadaveric volatile organic compounds. Journal of Forensic Sciences 57: 386–390. [DOI] [PubMed] [Google Scholar]
- 84. Statheropoulos M, Spiliopoulou C, Agapiou A (2005) A study of volatile organic compounds evolved from the decaying human body. Forensic Science International 153: 147–155. [DOI] [PubMed] [Google Scholar]
- 85.Dekeirsschieter J, Frederickx C, Lognay G, Brostaux Y, Verheggen F, et al.. (2013) Electrophysiological and behavioural responses of Thanatophilus sinuatus F.(Coleoptera: Silphidae) to selected cadaveric volatile organic compounds. Journal of Forensic Sciences. [DOI] [PubMed]
- 86. Setälä H, McLean M (2004) Decomposition rate of organic substrates in relation to the species diversity of soil saprophytic fungi. Oecologia 139: 98–107. [DOI] [PubMed] [Google Scholar]
- 87. Hattenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics 36: 191–218. [Google Scholar]
- 88. McGrady-Steed J, Morin PJ (1996) Distrubance and the species composition of rain pool microbial commnities. Oikos 76: 93–102. [Google Scholar]
- 89. Kneitel JM, Chase JM (2004) Disturbance, predator, and resource interactions alter container community composition. Ecology 85: 2088–2093. [Google Scholar]
- 90. Kneitel JM, Perrault D (2006) Distrubance-induced changes in community composition increase species invasion sucess. Community Ecology 7: 245–252. [Google Scholar]
- 91. Gill CO, Newton KG (1978) The ecology of bacterial spoilage of fresh meat at chill temperatures. Meat Science 2: 207–217. [DOI] [PubMed] [Google Scholar]
- 92. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. (2008) A core gut microbiome in obese and lean twins. Nature 457: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Applied and Environmental Microbiology 75: 5111–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kirk JL, Beaudette LA, Hart M, Moutoglis P, Klironomos JN, et al. (2004) Methods of studying soil microbial diversity. Journal of Microbiological Methods 58: 169–188. [DOI] [PubMed] [Google Scholar]
- 95. Mardis ER (2008) Next-Generation DNA Sequencing Methods. Annual Review of Genomics and Human Genetics 9: 387–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seasonal Bray-Curtis dissimilarity based non-metric multidimensional scaling. Using Bray-Curtis dissimilarity values, MMCPs for seasonal carrion communities were plotted using a two dimensional non-metric multidimensional scaling model (stress = 0.198, R2 = 0.88). The MMCPs were significantly different (PERMANOVA: P = 0.001) among seasons. The circles indicate 95% standard error of each season.
(TIFF)
Decomposition phase within season Bray-Curtis dissimilarity based non-metric multidimensional scaling. Non-metric multidimensional scaling ordinations of MMCPs were plotted on two dimensions for carrion communities of decomposition phases (early, middle and late) during A) spring (stress = 0.122, R2 = 0.94), B) summer (stress = 0.098, R2 = 0. 98), C) autumn (stress = 0.149, R2 = 0.91), and D) winter (stress = 0.158, R2 = 0.91) seasons. There were significant differences among decomposition phases in spring and summer (PERMANOVA: P = 0.001), but no significant difference among decomposition phases in autumn (PERMANOVA: P = 0.226) or winter (PERMANOVA: P = 0.011). The circles indicate 95% standard error of each decomposition phases.
(TIFF)
Mean functional activity over decomposition between years. The mean (SEM) microbial functional activity between the 2010 (gray circle) and 2011 (black square) field trials at initial field placement (Day 0) and subsequent sampling on days 1, 3, and 5.
(TIFF)
Annual Bray-Curtis dissimilarity based non-metric multidimensional scaling. Using Bray-Curtis dissimilarity values, MMCPs from both 2010 and 2011 field seasons were plotted using a two dimensional non-metric multidimensional scaling model (stress = 0.199, R2 = 0.87). There were significantly different MMCPs (PERMANOVA: P = 0.001) between years. The circles indicate 95% standard error of each year.
(TIFF)
Mean functional activity over decomposition between sampling regions in both field seasons. The mean microbial functional activity for the buccal (orange circle) and skin (yellow square) sampling region in the A) 2010 and B) 2011 field trials at initial field placement (Day 0) and subsequent sampling on days 1, 3, and 5.
(TIFF)
Treatment within field season Bray-Curtis dissimilarity based non-metric multidimensional scaling. Non-metric multidimensional scaling ordinations of MMCPs were plotted on two dimensions for ACC and EXC carrion communities during A) 2010 (stress = 0.177, R2 = 0.91), and B) 2011 (stress = 0.166, R2 = 0. 86) field seasons.
(TIFF)
Supplementary Tables S1–S2. Table S1, Carbon sources and their respective groupings (i.e., amines/amides, amino acid, carbohydrate, and carboxylic and acetic acid) in a Biolog EcoPlate™. Table S2, PERMANOVA results testing MMCPs between sampling region (buccal and skin) communities and among carcass decomposition days including the interaction for both 2010 and 2011 field seasons with significant results indicated by an asterisk.
(DOCX)