Abstract
Cell-cell communication mediated by the secreted Hedgehog (Hh) and Wnt signaling molecules is essential to the coordination of cell fate decision making throughout the metazoan lifespan. From decades of genetically based interrogation, core components constituting the Hh and Wnt signal transduction pathways have been assembled, and a deep appreciation of how these signals elaborate distinct bodily tissues during development has been established. On the other hand, our incapacity to leverage similar genetic approaches to study adult organ systems has limited our understanding of how these molecules promote tissue renewal and regeneration through stem cell regulation. We discuss recent progress in the use of chemically based approaches to achieve control of these pathway activities in a broad range of biological studies and therapeutic contexts. In particular, we discuss the unique experimental opportunities that chemical modulators of these pathways afford in exploring the cancer stem cell hypothesis.
Keywords: cancer therapeutics, Tankyrase, Porcupine, Smoothened
INTRODUCTION
Recent developments in our understanding of fundamental mechanisms that support adult stem cell self-renewal have transformed our approach to regenerative medicine and anticancer therapy. Now evident is the reliance of all metazoans on a handful of cell-cell signaling systems for both the elaboration and maintenance of distinct tissues that collaboratively sustain phyla diversity (1, 2). Studies in two of these systems—the Hedgehog and Wnt signal transduction pathways—have enabled milestone achievements in stem cell biology research and constitute the forefront in stem cell–based experimentation (3–5).
Initially described as signaling molecules essential to body-pattern formation in Drosophila, the Hh and Wnt proteins have since been studied in a broad range of organisms (6, 7). Almost invariably, these molecules influence all aspects of development and tissue homeostasis. For the most part, components that enable the production of and cellular response to these molecules have been ascertained from decades of genetically based studies that used either classical approaches or more recent RNAi-based technology (8–11).
In most respects, these pathways have enjoyed histories that parallel one another, with a notable exception. The remarkable chance discovery of a natural teratogen named cyclopamine and the subsequent assignment of its activity to the Hh pathway paved the way for rapid development of synthetic Hh pathway inhibitors with drug-like properties and improved bioactivity (12). Whereas efforts to develop similar antagonists in the Wnt pathway have seen more limited success in the past decade, Hh inhibitors have transformed approaches used to study Hh signaling and the treatment of associated tumors such as basal cell carcinoma and medulloblastoma (13, 14).
Chemically based tools can effectively overcome many of the experimental limitations imposed by routine molecular biology approaches. Indeed, the need for such tools to study stem cell biology is readily apparent given the unique considerations associated with gene manipulation in adult stem cells. First, the mutual dependency of developmental processes and stem cell self-renewal on the same core set of signaling pathways has limited the utility of standard genetic strategies typically applied to the study of embryonic patterning and tissue morphogenesis. Second, the availability of more refined techniques such as inducible gene targeting has been inadequate given the scarcity of well-validated, tissue-specific stem cell markers.
Studies aimed at understanding the relevance of the putative cancer stem cell compartment to tumorigenesis pose exceptional challenges akin to those associated with normal stem cells. The dichotomous roles of Hh and Wnt signaling in the maintenance of normal stem cells and possibly cancer stem cells (CSCs) make these pathways uniquely relevant to our understanding of how normal stem cells may transform into tumor-initiating cells. Thus in many ways, our ability to realize the promise and peril of attacking the cancer stem cell compartment as an anticancer treatment modality depends on the success of chemically based strategies to manipulate the activity of these pathways.
In this review, we discuss the discovery of naturally occurring and synthetic small molecules that modulate Hh and Wnt pathway responses and how they have yielded important insights into underlying mechanisms of signal transduction. Additionally, we mine existing data related to preclinical and clinical chemical agents for insights into potential challenges associated with disruption of these pathways as an anticancer therapeutic strategy. Lastly, we discuss the pivotal role these chemical agents occupy in our quest to understand the contribution of deviant stem cells in supporting tumor progression.
MECHANISMS OF SIGNAL TRANSDUCTION
Wnt-Mediated Pathway Responses
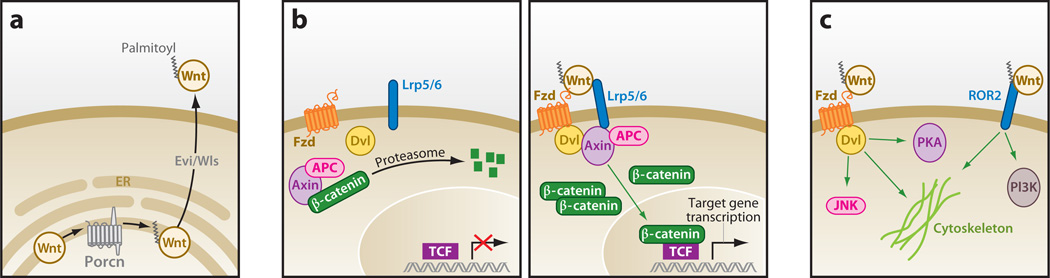
The mammalian Wnt family consists of 19 cysteine-rich proteins that contain a signature (C-K-C-H-G-X-S-G-X-C) motif (15, 16). Successful biosynthesis of Wnt proteins depends on the activity of Porcupine (Porcn), a member of the membrane-bound O-acyltransferase (MBOAT) protein family that adds a fatty acyl adduct, typically to an N-terminal cysteine of Wnt molecules (17) (Figure 1a). These acylation events are indispensable to subsequent Wnt production; in addition to promoting transport from the endoplasmic reticulum (ER), palmitoylation facilitates interaction with Evenness interrupted or Wntless (Evi/Wls), a multipass G protein–coupled receptor (GPCR)-like protein responsible for chaperoning Wnt proteins from the secretory pathway to the cell surface (18, 19). Not only do these fatty acyl adducts make an essential contribution to Wnt biosynthesis, but the modification is also necessary for engagement of Wnt molecules with their receptors, possibly by promoting multimerization or packaging into lipoprotein particles (20, 21).
Figure 1.
Wnt-dependent pathway responses. (a) Production of Wnt proteins. Production of active Wnt molecules is dependent on the extracellular acyltransferase Porcupine (Porcn) that adds a palmitoyl adduct to Wnts in the endoplasmic reticulum (ER). Acylated Wnt is then chaperoned by a seven-transmembrane protein—Evenness interrupted/Wntless (Evi/Wls)—to the extracellular milieu. (b) Activation of the Wnt/β-catenin pathway. A Wnt protein, Lrp5/6, and a Fzd receptor complex (right) recruit the APC/Axin complex, β-catenin, and a Disheveled (Dvl) scaffolding protein to the membrane, thus abrogating destruction of β-catenin (left). Accumulated β-catenin activates members of the TCF/LEF transcription factor family. (c) Examples of β-catenin-independent (noncanonical) Wnt pathway responses include the Wnt/JNK, Wnt/PKA, and Wnt/ROR2 pathways. Other pathway responses include Wnt-mediated control of cytoskeletal rearrangement. Like the Wnt/β-catenin pathway, many of these pathways utilize Dvl as an intracellular signaling molecule. Abbreviations: APC, adenomatous polyposis coli; JNK, c-Jun NH2-terminal kinase; LEF, lymphocyte enhancement factor; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; ROR2, receptor tyrosine kinase-like orphan receptor 2; TCF, T cell factor.
Canonical (or β-catenin-dependent) pathway response is initiated by the formation of a tertiary complex consisting of a Wnt protein, a member of the Frizzled (Fzd) family of GPCRs, and one of two low-density lipoprotein (LDL)-related proteins (either Lrp5 or Lrp6) (reviewed in References 7 and 10) (Figure 1b). This membrane-associated complex, in turn, engages two cytoplasmic components: phosphorylated Lrp5/6 and Fzd proteins recruit the Axin and Disheveled (Dvl) signaling molecules, respectively. These biochemical events preclude an alternate function of Axin; in the absence of Wnt ligand, Axin and its associated kinases glycogen synthase kinase 3β (GSK3β) and casein kinase 1 (CK1) partner with the tumor suppressor adenomatous polyposis coli (APC), producing a destruction complex responsible for the phosphorylation of the transcriptional coactivator β-catenin. In turn, the E3 ligase βTrCP recognizes this modification and ubiquitinylates β-catenin, leading to its proteasome-dependent degradation. Under conditions of pathway stimulation, phosphorylation and thus degradation of β-catenin is abrogated. In response to other Wnt-dependent instructive cues that remain unclear, accumulated β-catenin translocates to the nucleus, where it associates with the T cell factor/lymphocyte enhancement factor (TCF/LEF) family of transcriptional coactivators, culminating in target gene activation.
Forms of Wnt signaling that do not utilize β-catenin additionally occur and are collectively considered “noncanonical” (reviewed in Reference 10) (Figure 1c). These include, among others, Wnt-induced signal transduction through intracellular calcium, and intermediaries associated with the c-Jun NH2-terminal kinase (JNK), protein kinase A (PKA), Nemo-like kinase (NLK), and mechanistic target of rapamycin (mTOR) pathways. Wnt proteins functioning in this manner were initially identified based on an inability to induce differentiation of the mouse mammary epithelial cell line C57MG; they include members 2, 4, 5a, 5b, 6, 7b, and 11 (22). Subsequent research has shown this distinction to be imprecise, as several noncanonical associated Wnt proteins are capable of inducing β-catenin accumulation within certain contexts. The divergence of canonical and noncanonical pathways may instead occur at the level of Dvl regulation following receptor activation, although a mechanistic understanding of these events remains largely unknown. The output of these pathways is diverse and includes changes in cytoskeletal organization associated with cell polarity and migration, as well as changes in gene transcription (23).
The Hedgehog Signal Transduction Pathway
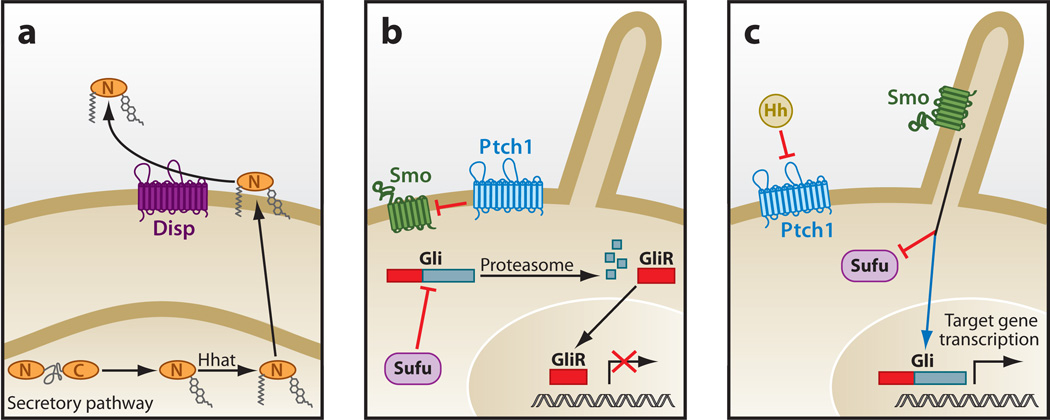
The production of active Hh protein is initiated with the autocatalytic cleavage of a precursor molecule to yield a cholesterol-modified amino-terminal signaling domain named HhN (Hedgehog ligand N-terminal fragment) (24). A dually lipidated HhN emerges from the secretory pathway following palmitoylation by the acyltransferase Skinny/Hhat/Rasp, another member of the MBOAT gene family (25). Release of fully processed HhN from the cell membrane requires active input from Dispatched (Disp), a 12-transmembrane protein that is structurally similar to the Hh receptor Patched1 (Ptch1) (26–28) (Figure 2a). Disp and Ptch likely act as small-molecule transporters, given their structural similarity to members of the resistance nodulation division (RND) superfamily of proteins that have demonstrated transporter activity (29).
Figure 2.
Hh signaling in mammals. (a) Hh protein production. Autocatalytic cleavage of the Hh precursor protein yields a cholesterol-modified signaling peptide (HhN) that is further palmitoylated by Hh acyltransferase (Hhat). Lipophilic HhN released from the plasma membrane by Dispatched (Disp) is distributed to other cells. (b, c) Hh pathway response. The suppressive action of Patched 1 (Ptch1) on Smoothened (Smo) (b) is inhibited by binding of Hh to Ptch1, a 12-transmembrane protein with structural similarities to the resistance nodulation division (RND) family of small-molecule transporters (c). Smo controls activity of Ci/Gli transcription by regulating nuclear localization and proteolytic processing into repressor molecules, subsequent to Smo localization to the primary cilium. Suppressor of fused homolog (Sufu) appears to be the primary pathway suppressor in mammals, although its regulation by Smo has not been demonstrated. Abbreviations: GliR, glioma-associated oncogene family zinc finger, repressor domain; Hh, Hedgehog signaling molecule.
The engagement of HhN with Ptch lifts the normal suppressive activity that Ptch imposes on the seven-transmembrane protein Smoothened (Smo) (Figure 2b). Although the underlying mechanism of Ptch-mediated Smo inhibition remains unclear, an intermediary small molecule regulated by Ptch may modulate Smo activity (30). Strong candidates for such small molecules are metabolites with steroid-like chemical structures that are similar to known Smo modulators such as oxysterols and cyclopamine (31). Although not discussed here, a number of other Hh receptors—including those belonging to the CAM-related/downregulated by oncogenes (Cdo) or glypican protein families—additionally facilitate Hh binding to Ptch (32–34).
Activation of Smo entails a conformational switch that promotes engagement of cytoplasmic regulators, which ultimately influences the activity ofCi/Gli zinc finger proteins (35, 36). Whereas the intermediary molecules that bridge Smo to Gli signaling are well interrogated in Drosophila, our understanding of similar processes in vertebrates is less clear (8, 11, 37). Complicating the translation of historic findings from fruit flies to mammals is the unique dependency of Hh signal transduction in the latter case on the primary cilium—an antenna-like, microtubule-scaffolded organelle found on the surface of most cells (38, 39) (Figure 2). Almost all Hh signaling components in mammals, including Smo and the Gli molecules, have been shown to exhibit dynamic changes in ciliary localization upon Hh pathway activation (40, 41). Despite these observations and those from other recent Hh-related studies, a requirement for the primary cilium in Hh response beyond its role as an assembly point for Hh pathway components remains unclear.
Regardless of species-specific variations on how Hh signals are relayed from Smo to cytoplasmic components, ultimately the cellular changes associated with Gli protein activity are conserved—namely Gli accumulation in the nucleus and the abrogation of proteasome-mediated proteolytic processing into GliR (glioma-associated oncogene family zinc finger, repressor domain) molecules (Figure 2c). Whereas the accumulation of Gli proteins in the nucleus likely entails disengagement of the suppressive actions of Suppressor of fused homolog (Sufu) (42) andKif7 (43–45) on Gli proteins, inhibition of GliR formation upon pathway activation requires disruption of PKA-mediated phosphorylation of Gli proteins, the initiating event of this biochemical process (6). Thus the overall transcriptional output of Hh response rests in part on the ratio of activator molecules (Gli1–3) to repressor molecules (Gli2R and Gli3R) in addition to absolute levels of Gli proteins that amass in the nucleus upon Hh pathway activation (42). Additional biochemical changes that have remained elusive are likely required to activate the Gli molecules.
THE ROLE OF Hh AND Wnt PATHWAYS IN TISSUE HOMEOSTASIS AND DISEASE
The identification of tissue-specific stem cells has radically reshaped the conceptual framework that guides the development of therapeutic strategies targeting cancers, degenerative diseases, and post-traumatic injuries. Initially characterized in the hematopoietic system, cells able to elaborate some or all cell types in a given cellular compartment have now been identified in many other tissue types (3). Experimental approaches to study these cells have centered on the Hh and Wnt pathways, which bring with them an impressive set of genetic and biochemical observations that can be leveraged into mechanistic models amenable to interrogation. In this respect, our understanding of the Hh and Wnt pathways continues to influence almost all aspects of stem cell research.
The role of Wnt proteins in tissue homeostasis is best characterized in the intestinal epithelium, where signaling promotes expansion and differentiation of stem cells located within the crypt base. Genetically mediated disruption of pathway response at the level of ligand/receptor interactions (46) or transcription (47) effectively inhibits formation of downstream epithelial lineages, resulting in a loss of the epithelial lining and altered tissue architecture. On the other hand, 80–90% of all colorectal cancer incidents harbor mutations in the APC gene that give rise to a truncated APC protein and aberrant pathway activation (48). Biallelic loss of APC induces aberrant induction of β-catenin-mediated transcription of growth-promoting programs, including upregulation of the proto-oncogene c-Myc (49, 50). The convergence of phenotypic changes in the gastrointestinal (GI) epithelium that stem from perturbations of the Wnt/β-catenin pathway support a pivotal function of this pathway in controlling the behavior of a multipotent cell population relevant in both normal and disease contexts. The Wnt/β-catenin target gene Lgr5 (leucine-rich repeat-containing G protein–coupled receptor 5) was found to function as a marker that can be used to isolate cells with the capacity to elaborate all of the distinct epithelial cell types in the GI and skin. This discovery solidifies the role that essential Wnt molecules play in stem cell self-renewal in some tissues (51).
The waning capacity of tissues to elaborate normal cell types following injury is an inevitable condition associated with aging (52). In lieu of normal tissue regeneration, fibrosis constitutes a common response to injury in aged animals. The selection of a transcriptional program that favors fibrogenesis rather than normal cell lineage recapitulation in muscle repair, for example, is observed with age-related increases in humoral Wnt protein activity (53). In the same vein, changes in the function of the type II diabetes gene Tcf7l2/Tcf4, encoding a DNA-binding protein that regulates transcriptional responses to Wnt proteins, likely accelerate age-dependent erosion of pancreatic β-cell function (54). These observations, in addition to many others reviewed in References 10 and 55, support a broad role for homeostatic levels of Wnt pathway responses in preventing degenerative diseases.
The inception and maintenance of some tissues are similarly dependent on Hh pathway activity. The phenotypic outcomes from targeted deletion of the Sonic Hedgehog (Shh) gene include derangements in body patterning and a range of neurological defects such as cyclopia and neural tube malformation (56). A role for Hh in neuronal tissue appears to be retained in adults, where Hh secreted by Purkinje cells promotes granule-cell precursor differentiation into neurons; at the same time, Hh inhibits stem cell apoptosis within the external granular layer (57–59). Loss of function of the Hh pathway suppressor Ptch frequently gives rise to medulloblastoma development both in sporadic incidents and in hereditary Gorlin’s tumor syndrome (60). Patients afflicted with the latter disease are often additionally diagnosed with basal cell carcinoma and rhabdomyosarcoma, reflecting the roles of Hh in skin and muscle homeostasis.
Additional observations relating to functions of Hh and Wnt proteins in tissue maintenance that directly bear on therapeutic efforts to target them are noted here. First, these two pathways function collaboratively and with other signaling pathways to achieve developmental, regenerative, and disease-associated outcomes (61–70). Thus Hh and Wnt pathway-modulating agents that are typically defined by simplistic in vitro readouts of pathway activity will most certainly exhibit unanticipated effects within in vivo settings. Particularly in cancerous contexts, the main lines of communication between tumor and stroma are mediated by the concerted actions of the Hh and Wnt ligands, which are often difficult to model in vitro (71). Second, consideration must be given to so-called noncanonical responses to Wnt and Hh ligands that do not involve the well-documented gene transcription changes historically associated with ligand engagement (10, 72). These include Wnt- and Hh-mediated responses that influence cytoskeletal behavior, cell polarity, and cell migration. Likely, the roles of these activities in tissue homeostasis and disease are underestimated, given the dearth of readily accessible readouts for monitoring their status. Thus, despite the wealth of knowledge that relates to the biology of the Hh and Wnt molecules, unanticipated challenges certainly will arise.
THE PIPELINE
The yield from screening large numbers of molecular libraries by many independent groups is a portfolio of agents that collectively target different underlying mechanisms of signal transduction in the Hh and Wnt pathways (73). In many cases, the mechanism of action for these molecules is still unknown, with only a handful of bona fide druggable components identified in both pathways. Of the agents that have been discovered, the Smo antagonists are the most advanced in development, with several candidates in clinical studies. We focus our discussion here on small molecules that inhibit pathway responses by targeting components that engage endogenous small molecules, either as substrates or as ligands, and that likely represent the most readily druggable components within each pathway.
Antagonists of the Hh Pathway Effector Smo
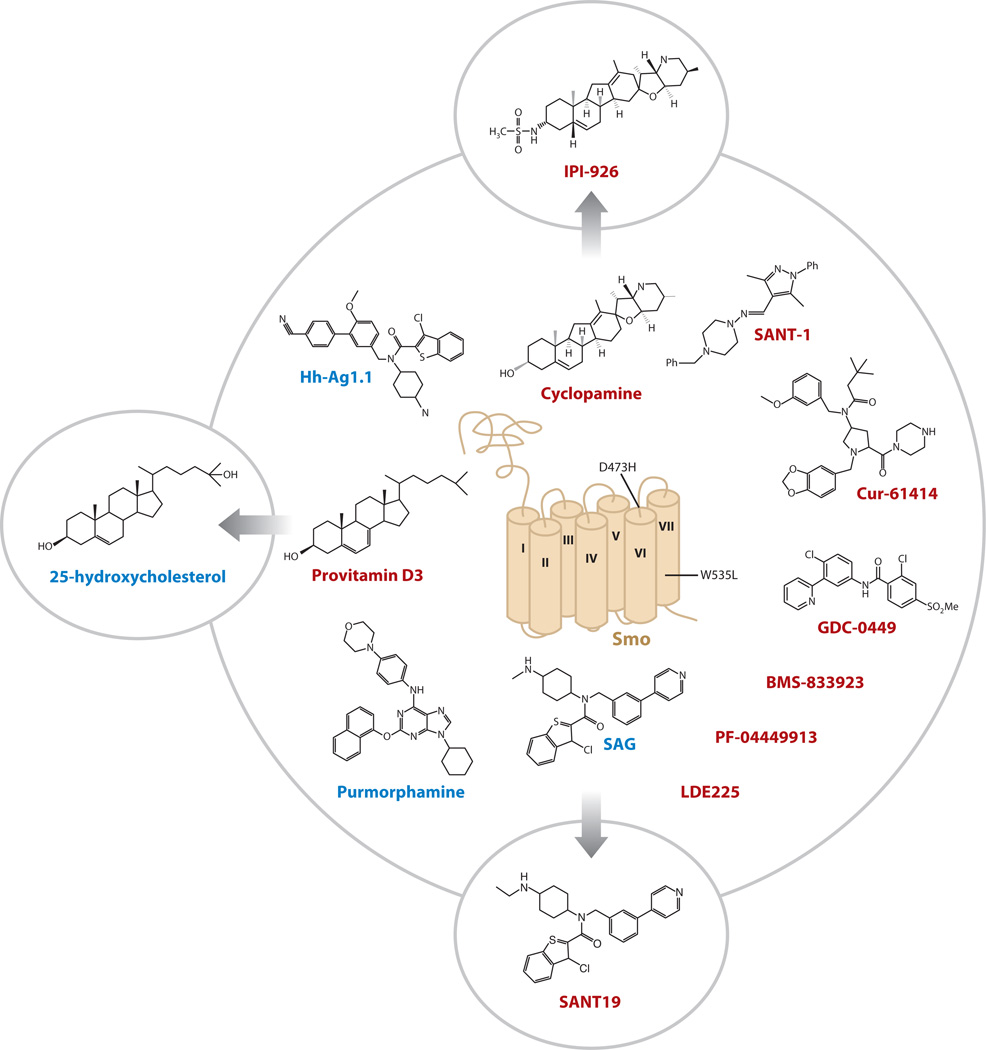
Serendipity played a significant role in the identification of Smo as a druggable component in the Hh pathway: Pregnant ewes consuming significant quantities of wild corn lily (Veratrum californicum) due to drought conditions produced offspring that exhibited cyclopia, a phenotype similar to those later seen in animals lacking Shh (74). Isolation of the active component from the plant, a steroidal jerveratrum alkaloid named cyclopamine, and the subsequent demonstration that it directly inhibits Hh signaling in cultured cells, set the stage for biochemical testing of cyclopamine interaction with Smo, the anticipated target of the compound based on an understanding of Smo action within the pathway (75, 76) (Figure 3). Emboldened by the identification of a druggable Hh pathway element, two groups screened diverse synthetic chemical libraries for Hh antagonists and identified some of the first Smo antagonists with drug-like properties, the Smo antagonists (SANTs) and Cur-61414 (75, 76). The realization that synthetic small molecules can outperform cyclopamine kicked off the race to identify Smo antagonists with favorable in vivo behavior that may prove useful against Hh-related tumors. The outcome of these efforts was the entry of several Smo antagonists into clinical trials, including Cur-61414, GDC-0449, and BMS-833923 (12). Also in clinical testing is a derivative of cyclopamine termed IPI-926, which features improved drug-like properties and potency (77).
Figure 3.
Natural and synthetic Smoothened (Smo) antagonists. Smo antagonist 1 (SANT-1) and Cur-61414 represent the first synthetic compounds identified with Smo inhibitory activity. IPI-926 improves on the naturally occurring cyclopamine with increased potency and favorable pharmacokinetic properties. GDC-0449, BMS-833923, PF-04449913, and LDE225, in addition to other synthetic small molecules and IPI-926, are in clinical testing. SAG, Hh-Ag1.1, purmorphamine, and oxysterols (25-OHC is shown) are Smo agonists. Oxysterols and SAG can be transformed into Smo antagonists with relatively conserved chemical changes, suggesting that Smo agonists and antagonists can induce opposing conformational changes by occupying the same small-molecule binding pocket. The cancer-associated Smo mutation (SmoW535L, also known as SmoA1) or the GDC-0449-induced resistance mutation (SmoD473H) is noted. Smo antagonists generally exhibit weakened activity against SmoA1 as compared with the wild-type counterpart. Molecules that resemble oxysterols are candidate metabolites that may regulate cellular Smo activity. Smo inhibitors are in red; activators are in blue. Transmembrane domains are numbered. Abbreviations: SAG, Smo agonist; SANT, Smo antagonist.
Pathway inhibition by cyclopamine occurs by direct compound binding to the heptahelical region of Smo (75). Surprisingly, virtually all subsequently identified Hh pathway modulators, both natural and synthetic, have targeted Smo in this manner, suggesting that Smo possesses a small-molecule binding domain that influences its conformation on the basis of the nature of the occupying molecule. Indeed, Smo agonists, including the purine derivative purmorphamine and the chlorobenzothiophene Smo agonist (SAG), also appear to bind to the same site to which Smo inhibitors bind (78, 79) (Figure 3). Remarkably, SAG can be readily converted into a Smo antagonist with minor chemical modifications, thus elegantly demonstrating that different Smo conformations can be achieved by small-molecule engagement to the same binding site (80). Direct evidence that Smo undergoes a conformational change in response to chemical agonists/antagonists was more recently provided by studies using Smo molecules engineered to report changes in conformation by fluorescence resonance energy transfer (FRET) (80).
The existence of a small molecule–binding domain in Smo suggests that its activity is gated by Hh-dependent access to a cellular metabolite with either Smo-inhibitory or Smo-activating properties. This model is buttressed by the observation that Ptch1 is structurally similar to members of the RND family of small-molecule transporters (see above), and that cyclopamine and steroid-like molecules, some of which influence Smo activity, share a similar chemical scaffold (Figure 3). Although the identity of this molecule has not been defined, conceivably the primary cilium represents a compartment enriched for a Smo-activating molecule or devoid of a Smo-inhibitory molecule. Indeed, this organelle has been shown to compartmentalize certain lipids (81).
Of all the Hh and Wnt pathway antagonists, the most developed are those targeting Smo. Thus Smo inhibitors in clinical studies provide an early glimpse into the promise of targeting the Hh pathway. Despite early success in clinical studies on metastatic basal cell carcinoma (14), the next phases in the development of this therapeutic program will see tremendous challenges and potential rewards. The rapid development of drug resistance to Smo antagonists and the possibility of irreversible bone growth defects associated with transient inhibition of Hh pathway response during adolescence are confounding issues that could interfere with the realization of these inhibitors’ full therapeutic potential (82). A large repertoire of Smo antagonists used on a rotational basis may limit drug resistance, thus justifying the clinical development of multiple Smo antagonists in parallel. On the other hand, efforts to identify small molecules that target components functioning downstream of Smo are in their infancy but may offer an alternative strategy for attacking Hh-dependent tumors with drug-induced mutations in Smo (83–85). Lastly, the identification of Smo antagonists with pharmacokinetic properties that isolate them within the central nervous system and away from bone tissue may be a useful approach to address current limitations on the use of Hh inhibitors in developing young adults, who experience the majority of medulloblastoma incidents.
Tankyrase: A Novel Drug Target in the Wnt/β-Catenin Pathway
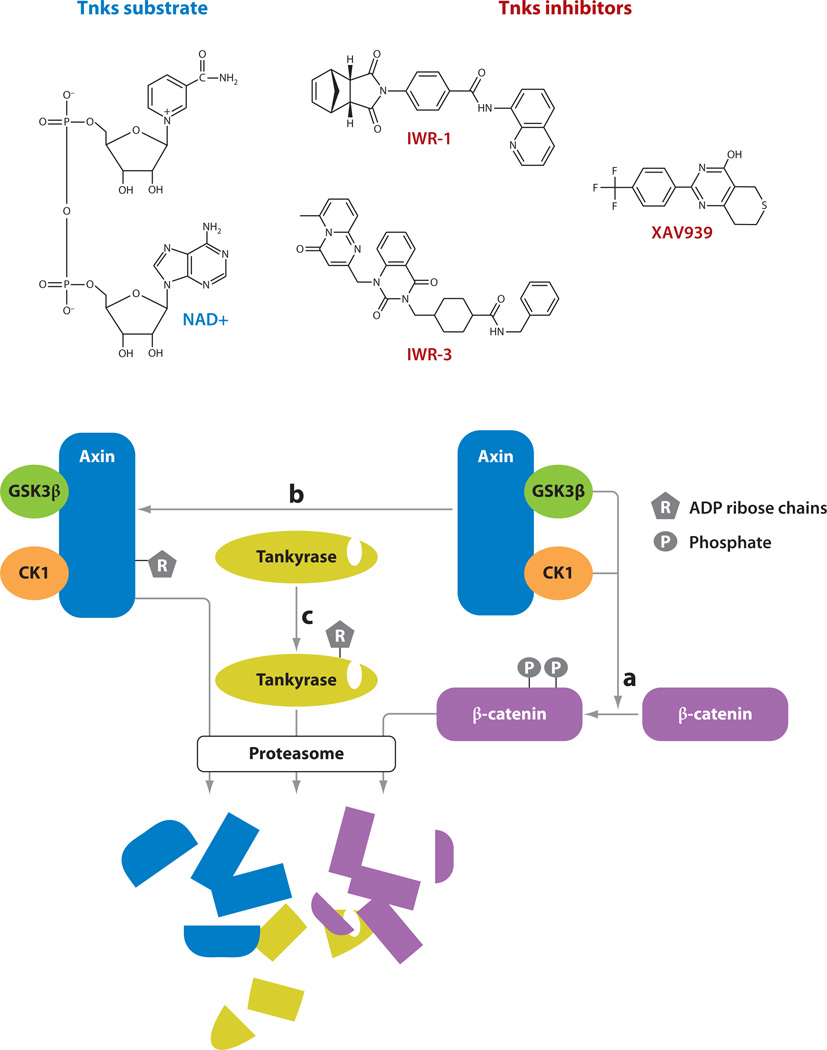
A tremendous amount of resources has been allocated to the identification of small molecules capable of disrupting aberrant Wnt/β-catenin pathway responses induced by loss of APC with the promise that such agents would be therapeutically effective against colorectal cancer (CRC) and other tumors. The simultaneous discovery of Tankyrase (Tnks) enzymes as critical regulators of Axin and β-catenin protein levels that can additionally be drugged has opened new opportunities for achieving this goal, the success of which had depended primarily on efforts to develop inhibitors of TCF/β-catenin interactions (86). Disruption of Tnks-mediated ADP-ribosylation activity results in increased Axin protein stability by mechanisms that are still unclear, but they likely involve changes in Axin ubiquitinylation status (87) (Figure 4). Given that Axin proteins are the rate-limiting factor for the formation of the β-catenin destruction complex, stabilization of this scaffolding component by blocking Tnks function results in Wnt pathway inhibition.
Figure 4.
Small-molecule modulators of Tankyrase (Tnks) enzymes. (a) Axin scaffolding of glycogen synthase kinase 3β (GSK3β) and casein kinase 1 (CK1) facilitates phosphorylation of β-catenin, the initiating biochemical event in its proteasome-mediated destruction. (b) Tnks enzymes elaborate highly branched poly(ADP-ribose) (PAR) chains. Axin is ADP-ribosylated by Tnks, which influences Axin ubiquitinylation and destruction. (c) Tnks enzymes also undergo auto-PARsylation, presumably resulting in proteasome-mediated destruction. IWR-1 and IWR-3, representing two classes of the same Inhibitor of Wnt Response compound series, and XAV939 inhibit Tnks enzymatic activity.
Small molecules that target Tnks fall into two general classes, represented by XAV939 and the Inhibitors of Wnt Response (IWR compounds) (87, 88) (Figure 4). Affinity derivatives of both lead structures associate with Tnks in pull-down assays; additionally, XAV939 was observed binding directly to the enzyme’s active site in a crystal structure. The chemical makeup of these two compounds is notably different with respect to the presence of a norbornyl group in IWR. Intriguingly, this chemical adduct is the source of diastereomeric configuration sensitivity associated with the IWR-1 compound (88). The fact that the differential activity of the endo but not exo forms of IWR-1 observed in vivo (with IWR-1-exo exhibiting a tenfold decrease in activity) is mirrored in in vitro tests that directly measure Tnks activity suggests that the basis for this difference lies in the ability of the compounds to occupy Tnks’ active site (87). Although not discussed here, additional chemical scaffolds different from those exemplified by XAV939 and IWR-1 and -3 have been identified (89; X. Wu & L. Lum, unpublished data).
Stabilization of Axin induced by compound treatment is particularly efficacious in suppressing cancer cell lines dependent on APC truncations for growth potential (e.g., DLD1 cells). Although these results await clinical translation, studies in zebrafish show early in vivo potential for targeting stem cell functions; Wnt-dependent caudal fin regeneration is inhibited upon introduction of either XAV939 or IWR compounds into the aquarium water. Additionally, IWR induces loss of the presumptive crypt stem cells in the GI epithelium, as measured using bromodeoxyuridine (BrdU) labeling (88). Likely as a consequence, fish treated for more than a week exhibit GI tissue damage and loss of appetite. Removal of IWR from the aquarium water after chemical treatment periods sufficient to induce these histological changes allows resumption of tailfin regeneration and normal feeding behavior, suggesting that the stem/progenitor cell function in both appendage and GI tissues is not permanently altered by transient Wnt/β-catenin pathway inhibition. Given the shared role of Wnt/β-catenin signaling in homeostatic renewal of the GI epithelium in mammals and fish, it seems likely that replacement of the epithelial lining following IWR treatment is supported by a stem cell population that is spared chemically induced cell death rather than by the dedifferentiation of mature cells into those with stem cell–like properties as proposed for myocytes during repair of zebrafish cardiac tissue (90, 91). In this regard, the resilience to IWR treatment of stem/progenitor cells in the zebrafish GI tract may hold true in human tissues.
The first Tnks enzyme substrate identified was TRF1 (telomere repeat-binding factor 1), a component of the telomere nucleoprotein complex (Tankyrase gets its name from TRF1-interacting ankyrin-related ADP-ribose polymerase) (92). TRF1 binds to double-stranded TTAGGG sequences within chromosome ends, precluding telomerase (Tert) access and subsequent strand extension. Tnks PARsylation of TRF1 in this context derepresses Tert by preventing TRF1 binding to DNA. Consequently, Tnks inhibitors may have important implications for countering the widespread Tert upregulation/activation observed in cancer. For example, genetically mediated silencing of Tnks in Brca-deficient background has been found to induce cell death and correlates with increased genetic abnormalities (93). Further studies have found that the use of general poly(ADP-ribose) polymerase (PARP) inhibitors can synergize with compounds directly targeting Tert, increasing cell death by attacking regulation at multiple nodes. These observations, taken together, suggest that Tnks inhibitors such as XAV939 and IWR compounds may have additional therapeutic potential in targeting CSCs that lack Wnt hyperactivation.
Porcupine: An Unconventional Drug Target
Chemical screening efforts to target signal transduction pathways have historically focused on molecules that function at or below the level of ligand/receptor interactions rather than ligand production for reasons that include the following: (a) the majority of proteins known to be druggable, such as kinases, typically participate in cellular responses; (b) classic models of tumorigenesis typically involve oncogenic lesions that result in the engagement of ligand-independent signaling mechanisms to promote cell growth; and (c) few proteins with specific regulatory activities in signaling ligand production have been identified in the first place. In the case of Wnt and Hh pathways, the first two arguments mostly hold true. However, unlike in other signaling systems, the activity of Wnt and Hh proteins is governed by two relatively dedicated post-translational modification enzymes, Porcn and Hhat, respectively.
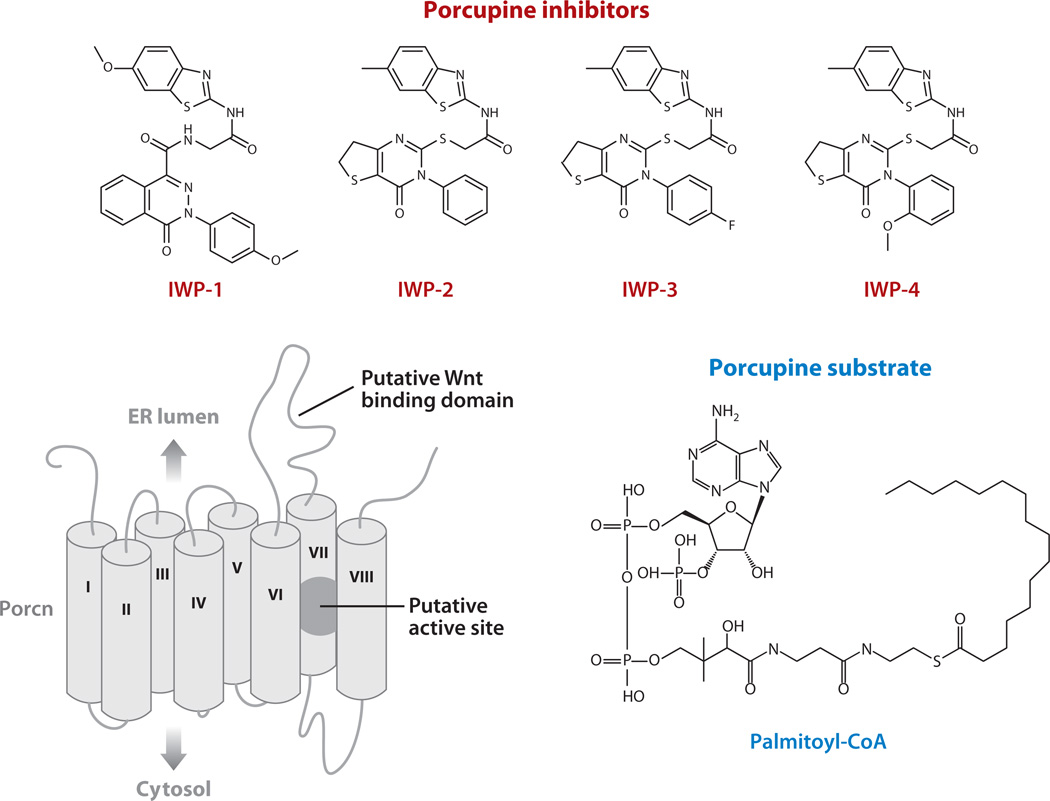
A recently completed chemical screen using cultured cells that report ligand-dependent, cell-autonomous Wnt/β-catenin pathway response yielded a class of small molecules, known as Inhibitors of Wnt Production (IWPs), that disrupt Wnt ligand palmitoylation and secretion (88) (Figure 5). The reversibility of IWP-associated effects on Wnt/β-catenin pathway response with Porcn overexpression suggests that these inhibitors directly target Porcn acyltransferase activity. Furthermore, results from chemical-target interaction studies support this conclusion and have additionally revealed specific residues within Porcn’s putative active site necessary for IWP interaction (M. Dodge & L. Lum, unpublished data).
Figure 5.
Porcupine inhibitors. Several inhibitors of Porcn (Inhibitors of Wnt Production, or IWPs) are shown. A model of Porcn with putative Wnt binding and catalytic active site are shown. Palmitoyl-CoA is a substrate of Porcn necessary for the modification of Wnt proteins (98). ER, endoplasmic reticulum.
Because most if not all mammalian Wnt proteins are palmitoylated, loss of this adduct upon IWP exposure has the potential to inhibit all downstream ligand-dependent events, including those within noncanonical signaling pathways (15). Given the large number of Wnt proteins likely capable of eliciting a range of cellular responses in a cell-context dependent manner, our understanding of these noncanonical responses is probably in its infancy. The potentially far-ranging effects of IWP compounds on Wnt-mediated responses may pose exceptional challenges to their deployment in therapeutic contexts. On the other hand, the ability to shut down most if not all Wnt-mediated responses may prove to be a reliable approach to stop deviant cancer cell behavior in a broad range of cancer types. For example, the ability of IWP compounds to inhibit the growth of CRC cells lacking APC is mostly unexpected but may highlight cell growth–promoting signals provided by cell-autonomous Wnt production that does not engage β-catenin (88).
The functions of Porcn in adult tissues are largely unknown. In humans, heterozygous deletions and nonsense mutations mapping within the gene’s Xp11.23 loci are associated with several developmental disorders, including focal dermal hypoplasia (Goltz syndrome), Van Allen-Myhre syndrome, and osteopathia striata (94–96). Complete loss of Porcn function is presumed to be embryonic lethal, with corresponding loss-of-function disorders in males attributable to postzygotic mosaicism (95). In adults, overexpression of Porcn has been documented in a significant number of lung cancer cell lines, as well as in primary tumor samples. RNAi-mediated targeting of Porcn in these cases results in significant cell death, with minimal apoptosis induced in normal human lung samples (97).
The chemical tractability of Porcn as demonstrated by the discovery of IWP compounds has additional therapeutic implications for related MBOAT family members. Similar to the case of Wnt ligands, palmitoylation of Hh proteins by the acyltransferase Hhat (98) ensures its graded distribution in developing tissues and may additionally contribute to its cancer-supporting activity. The functional dependency of the appetite-controlling ghrelin peptide upon an octanoyl adduct provided by the action of ghrelin-O-acyltransferase (GOAT), another member of the MBOAT family, points to a potential utility of GOAT inhibitors in the control of obesity (99). Essential to the future identification of other MBOAT inhibitors is a further understanding of IWP specificity for Porcn as well as an understanding of mechanisms generally underlying MBOAT protein activity.
THE PROMISE OF TARGETING THE CANCER STEM CELL COMPARTMENT
Growing support for the existence of rare CSCs that initiate and sustain tumors has galvanized efforts to identify novel therapeutic strategies for selectively attacking these cells. At stake with the investment in these approaches is the potential to decrease the recurrence frequency associated with currently available anticancer treatment regimens. Indeed, CSCs have been shown to be resistant to some frontline chemotherapeutic agents (100). Thus success in identifying chemicals that target signal transduction pathways controlling CSC self-renewal, such as the Hh and Wnt pathways, would open up new possibilities for monotherapeutic or combinatorial therapeutic options that may improve on current strategies that target general mechanisms of rapid cell growth.
Two major strategies aimed at destroying the CSC compartment have emerged. In one case, small molecules with the ability to counter the effects of underlying genetic derangements associated with a tumor would be deployed with the assumption that at least some of the cellular processes identified by genomic sequencing would be essential for CSC survival. In another case, agents targeting the major signaling pathways that maintain homeostatic renewal of the tissue from which the tumor is derived would exhibit great biological effect on cell viability in CSCs as compared with their normal stem cell counterparts. We discuss the strengths and weaknesses of each strategy using lessons learned from chemically based studies of the Hh and Wnt signal transduction pathways.
Targeting Genetically Defined Derangements in the Hh and Wnt Pathways
The high frequency of Hh and Wnt pathway mutations found in cancers arising from tissues dependent on these pathways for homeostatic renewal advocates for the use of comprehensive genome sequencing efforts to uncover mechanisms that support CSC biogenesis (48, 101). Arguments that such mutations also sustain tumor growth are largely derived from Hh-related studies in which patients harboring tumors with loss of Ptch1 function are responsive to Smo inhibitors. Most strikingly, the majority of patients with Ptch1-deficient locally invasive and metastatic basal cell carcinomas exhibited disease stabilization with GDC-0449, which supports a role for Hh pathway response in late-stage tumor growth (14). Similar success has been observed in an adult case of late-stage medulloblastoma using Smo antagonists (13), suggesting the Hh pathway represents a common Achilles heel in both medulloblastoma and locally invasive and metastatic basal cell carcinomas.
Whereas testing of Smo inhibitors in humans is in its infancy, two observations from these early studies are notable. First, monotherapeutic deployment of a Smo antagonist yielded impressive tumor shrinkage despite the near certainty that other oncogenic lesions in addition to those found in Ptch1 were present in the tumors. Indeed, mutations in Ptch1 are frequently accompanied by others that alter the function of p53 or K-ras in medulloblastomas and basal cell carcinomas (102). The responsiveness of tumors either induced by targeted deletion of these genes in mice or found in late stages of malignancy in humans to a single agent that attacks Smo suggests that the Hh pathway represents a major vulnerability in these tumor types (13, 103, 104). Additional cancer genome sequencing of tumors shown to be responsive to Smo antagonists will refine our capability to predict the therapeutic efficacy of such inhibitors based on genetic parameters of disease. Furthermore, sequencing efforts will reveal other cellular derangements that could be targeted along with the Hh pathway to achieve combinatorially based drug regimens that may be, in some instances, desirable over monotherapeutic options.
The second striking observation from the first clinical tests of Smo inhibitors is that the associated adverse effects—including loss of weight, hair, and sense of taste—were relatively mild as compared with other chemotherapeutic agents that generally target rapidly dividing cells. For example, in contrast to agents such as taxol that are typically associated with neutropenia and other insufficiencies of blood cell formation, GDC-0449 has not exhibited widespread impact on adult hematopoiesis (14). Indeed, these findings are consistent with the lack of genetic evidence for Hh signaling in adult hematopoiesis in mice (105). However, given that these clinical tests are relatively small, ongoing trials with larger patient populations will yield a more complete spectrum of adverse effects that may be associated with Hh pathway inactivation.
The frequency of APC mutations associated with familial adenomatous polyposis (FAP) and sporadic CRC incidents underscores the importance of APC in maintaining the integrity of the GI epithelium. Evidence from molecular studies has attributed these disease phenotypes to loss in APC governance of genomic integrity and Wnt/β-catenin pathway activity (106). Strong support for the latter phenomenon is seen in the absence of adenomatous tissue arising from targeted deletion of both APC and the Wnt/β-catenin target gene c-Myc; it is also seen in the capacity of increased β-catenin levels to phenocopy the effects of APC loss (107). The demonstration that targeted deletion of APC in the Lgr5+ crypt stem cell population in mice can recapitulate many aspects of CRC in humans suggests that APC loss in this disease impacts the normal functions of crypt stem cells (108).Therefore, as in the many cases of medulloblastoma and basal cell carcinoma, cancer genome sequencing data in CRC cells faithfully reflect deviant cellular processes and thus vulnerabilities in the CSC compartment.
Despite important advances in our understanding of Wnt biology in CSCs, we have few clues regarding the potential therapeutic benefits of targeting the Wnt/β-catenin pathway in cancer or, for that matter, in any disease. As the use of genetic approaches to address this question in vivo poses exceptional technical challenges, we are resigned, for the moment, to discuss potential outcomes based on in vitro findings. Like so many other important observations on the role of the Wnt/β-catenin pathway in disease, these were derived from studies in CRC cell lines. For example, targeted deletion of a mutant form of β-catenin resistant to proteolysis in the CRC cell line HCT116 abrogates clonogenicity in defined growth conditions but unexpectedly showed little change in cell growth under normal cell culturing conditions (109). These observations raised questions as to the importance of sustained β-catenin activity in promoting the growth of CRC cells following transformation events induced by APC loss. Ultimately, the effectiveness of targeting the Wnt/β-catenin pathway to achieve therapeutic outcomes may be faithfully measured not through the use of cell culture systems, but through the use of in vivo models of disease. Indeed, disrupting β-catenin expression in Apc+/− (Min) mice using antisense oligonucleotides abrogates the formation of adenomas, suggesting therapeutic utility for chemicals that can achieve the same end (110).
The Hh and Wnt Signal Transduction Pathways as Cancer Stem Cell Vulnerabilities
A number of cancer types exhibit growth susceptibility to perturbations in Hh and Wnt pathway activity despite the lack of somatic or hereditary mutations in pathway components. This understanding may simply reflect the incompleteness of genomic sequencing information relating to these diseases, or the lack of insight into other molecular derangements that influence pathway activity (e.g., changes in gene promoter methylation or microRNA expression levels); nevertheless, a body of evidence supports the therapeutic potential in targeting the Wnt and Hh pathways in such diseases. Underlying this strategy is the presumption that CSCs exhibit a heightened dependency on these pathways to maintain self-renewal as compared with normal tissue counterparts.
Therapeutic exploitation of CSC addiction to the Hh and Wnt pathways has been most rigorously pursued in leukemic stem cells (LSCs). In chronic myelogenous leukemia (CML), LSCs positive for the oncogenic fusion protein Bcr-Abl appear to be vulnerable to targeted deletion of Smo or exposure to cyclopamine (105, 111). Similarly, β-catenin may support CML progression by promoting Bcr-Abl protein stability or expression (112). Thus small molecules that decrease β-catenin levels may be useful for attacking LSCs that support CML. More recently, acute myeloid leukemia (AML) induced either by coexpression of Hoxa9 and Meis1a oncogenes or by the fusion oncoprotein MLL-AF9 has been shown to depend on β-catenin activity (113). Aberrant β-catenin signaling engaged by the MLL-AF9 oncogene in granulocyte/macrophage progenitors (GMPs) that are normally quiescent for Wnt/β-catenin pathway activity further supports the frequent exploitation in LSCs of normal developmental pathways that are pivotal to hematopoietic stem cell self-renewal. As evidence that disrupting β-catenin function in AML may be therapeutically useful, exposure of LSCs in this disease to a Cox2 inhibitor known to disrupt the Wnt/β-catenin pathway response is sufficient to decrease cell number (113). The lack of evidence supporting normal hematopoiesis as a Hh or Wnt/β-catenin pathway–dependent process suggests that the deployment of these molecules in leukemias may be a viable therapeutic option, particularly in cases where drug resistance is observed, such as in Gleevec®-resistant CML incidents.
Aberrant engagement of the Wnt/β-catenin pathway has also been observed to support metastasis in lung tumors that have not previously been associated with somatic pathway mutations in either primary or metastatic tumors (114). Here, a gain in presumably ligand-mediated pathway responses results in the expression of Lef1 and Hoxb9, which in turn activate a transcriptional program that supports metastatic cell behavior. Although the mechanism underlying activation of Wnt signaling in this scenario is unclear, the preponderance of lung cancer cells exhibiting excessive Porcn expression may be a contributing factor (97). Thus in cases in which tumor initiation may not be dependent on aberrant activation of the Wnt and Hh pathways, such as those well established in CRC or medulloblastoma, small-molecule inhibitors of these pathways may nevertheless be useful in blocking the dissemination of cancer cells from the primary site.
Although best understood in cancer contexts as forms of cell-autonomous signaling induced by somatic mutations, the Wnt and Hh pathways are now additionally established as lines of communication between stromal and cancerous compartments that function together to support a niche for CSCs (52). Response of stromal cells to aberrantly high levels of Hh ligand expression in a broad range of tumor types includes the expression of various molecules that promote cancer cell growth (115). Thus the efficacy of Smo antagonists observed against a broad range of xenografts that do not exhibit responsiveness to Hh ligand or Smo antagonists in vitro may, in many cases, be explained by the disruption of this communication loop (115, 116). Modulation of stroma-tumor interactions using Smo antagonists may also facilitate the delivery of traditional chemotherapeutics to the tumor by increasing local vascularization (117). In the same study, the authors also observed sensitivity of xenograft pancreatic tumors, but not tumors initiated in pancreatic tissue, to Smo antagonists; this finding suggests that the utility of these inhibitors against tumors that are not driven by cell-autonomous Hh signaling may be more limited than initially anticipated.
CONCLUDING REMARKS
There is much to be optimistic about with respect to the potential use of Hh and Wnt inhibitors in cancer. Smo antagonists have shown remarkable success against some tumors known to be associated with the Hh pathway, including medulloblastoma and basal cell carcinoma. Our deep understanding of these pathways in development and tissue homeostasis has provided invaluable insight into ongoing clinical translations. Certainly, this knowledge has already averted the use of such molecules in contexts that may promote developmental aberrations, such as those seen with the use of thalidomide in childbearing women, and that have since overshadowed its potential as an anticancer agent.
At stake during clinical testing of Wnt inhibitors will be not only the tremendous investments devoted to the study of Wnt signaling, but also the hopes placed on therapeutic possibilities not yet realized from the targeting of the CSC compartment. Indeed, the Wnt/β-catenin pathway constitutes a unique and pivotal battleground for advocates of CSC targeting, given that no other signal transduction pathway has been as strongly associated with a particular cancer type and corresponding stem cell maintenance. Also important in moving forward is a greater understanding of β-catenin-independent Wnt responses that would facilitate the development of molecules attacking Wnt function, such as Porcn inhibitors or proteins that effectively inhibit Wnt protein binding to receptors. Indeed, our understanding of Wnt support of cancerous cell behavior outside the canonical pathway is limited. Lastly, one interpretation from cancer genome sequencing efforts and the entire body of research focused on cancer is that cancerous cells exhibit a limited range with respect to mechanisms exploited for deviant purposes. Assigning additional associations between the Hh and Wnt pathways in various diseases will broaden the relevance of these exciting therapeutic efforts discussed here to otherwise unanticipated contexts.
SUMMARY POINTS.
The morphogenic Wnt and Hedgehog pathways are indispensable to metazoan development and subsequent tissue homeostasis. Co-opting of pathway responses frequently occurs in diverse diseases, necessitating the development of small molecule–based interventions.
Utilizing Wnt/Hh antagonists to target putative cancer stem cells (CSCs) offers several advantages over standard chemotherapeutics, principally by exploiting the unique survival dependency that certain tumors exhibit for these pathways.
Almost all known Hedgehog antagonists disrupt the activity of the GPCR Smoothened (Smo), often preventing ciliary localization in the process. Several promising candidate compounds are being tested in clinical trials related to basal cell carcinoma and medulloblastoma.
The discovery of novel Wnt inhibitors targeting Tankyrase (Tnks) and Porcupine (Porcn) activity supplement earlier efforts to disrupt protein-protein interactions, principally at the level of TCF/β-catenin.
FUTURE ISSUES.
The mechanisms of noncanonical Wnt/Hh ligand–mediated responses, as well as their contribution to disease states, remain largely unknown.
Previously defined “druggable space” within canonical signaling pathways awaits further elaboration, including Tnks/Axin Wnt regulatory events and signal transduction coupling among Ptch, Smo, and/or Gli within the Hedgehog pathway. Advances in these areas will serve to aid future small-molecule discovery efforts, including potential endogenous small-molecule regulators.
A greater understanding of chemically targeted inhibition of ligand production is required, particularly with respect to Porcupine. Discoveries in this area may aid future MBOAT chemical-targeting efforts, with implications for corresponding pathways.
Direct targeting of cancer stem cells may influence nominal somatic stem cell function with potential deleterious consequences to patient outcomes. Consequently, factors differentiating tumor cells from normal stem cells need to be explored.
ACKNOWLEDGMENTS
We would like to thank Dr. Chuo Chen for critical reading of this manuscript and Leni Jacob for help with illustrations. This work was supported by the National Institutes of Health (5R01GM076398), the American Cancer Society (RSG-07-062-01-GMC), and the Welch Foundation (I-1665).
Glossary
- Cancer stem cells (CSCs)
a subpopulation of cancer cells with stem cell–like properties, including the capacity for self-renewal, asymmetric division, and the production of daughter cells that undergo further differentiation
- MBOAT
membrane-bound O-acyltransferase
- Acylation
the covalent attachment of an RCO group to a molecular substrate, where R represents an alkyl subunit of varying length; in this review, acylation refers to the addition of fatty acids such as palmitate
- GPCR
G protein–coupled receptor
- APC
adenomatous polyposis coli
- β-catenin
the principal regulatory transcription factor of the canonical Wnt pathway; under nonstimulating conditions, it is constitutively degraded in a proteasome-dependent manner, preventing signaling
- HhN
Hedgehog ligand N-terminal fragment
- RND
resistance nodulation division
- Smoothened (Smo)
a GPCR intermediary component of the Hedgehog pathway responsible for activation of Gli transcription factors; the most common target of Hh pathway antagonists
- Primary cilium
an organelle projecting into the extracellular space formed principally from the cellular membrane, microtubule bundles, and associated assembly machinery; involved in sequestering mammalian Hh signal transduction components and gating pathway activation
- GliR
glioma-associated oncogene family zinc finger, repressor domain
- Lgr5
leucine-rich repeat-containing G protein–coupled receptor 5
- Tnks
Tankyrase
Footnotes
DISCLOSURE STATEMENT
The authors are listed as inventors on a patent application describing the IWR and IWP classes of Wnt inhibitors (WO/2009/155001).
LITERATURE CITED
- 1.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev. Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 8.Jacob L, Lum L. Deconstructing the Hedgehog pathway in development and disease. Science. 2007;318:66–68. doi: 10.1126/science.1147314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat. Cell Biol. 2007;9:1005–1009. doi: 10.1038/ncb435. [DOI] [PubMed] [Google Scholar]
- 12.Peukert S, Miller-Moslin K. Small-molecule inhibitors of the Hedgehog signaling pathway as cancer therapeutics. ChemMedChem. 2010;5:500–512. doi: 10.1002/cmdc.201000011. [DOI] [PubMed] [Google Scholar]
- 13.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 15.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 16.Nusse R. The Wnt homepage. 2009 http://www.stanford.edu/~rnusse/wntwindow.html.
- 17.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 18.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 19.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form ofWnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem. J. 2007;402:515–523. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol. Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 24.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 25.Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 26.Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–815. doi: 10.1016/s0092-8674(00)81677-3. [DOI] [PubMed] [Google Scholar]
- 27.Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, et al. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr. Biol. 2002;12:1628–1632. doi: 10.1016/s0960-9822(02)01147-8. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Erkner A, Gong R, Yao S, Taipale J, et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 29.Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, et al. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999;1:107–125. [PubMed] [Google Scholar]
- 30.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–896. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 31.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. USA. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, et al. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299:2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- 33.Yao S, Lum L, Beachy P. The Ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 2006;125:343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X, Mann RK, Sever N, Beachy PA. Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 2010;24:57–71. doi: 10.1101/gad.1870310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252–258. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- 37.Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- 38.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 40.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 41.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates Hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 42.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian Hedgehog signaling. Sci. Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 44.Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr. Biol. 2009;19:1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 45.Liem KF, Jr, He M, Ocbina PJR, Anderson KV. Mouse Kif 7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA. 2009;106:13377–13382. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 48.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 49.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, et al. Myc deletion rescuesApc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 50.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 51.Barker N, van Es JH, Jaks V, Kasper M, Snippert H, et al. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb. Symp. Quant. Biol. 2008;73:351–356. doi: 10.1101/sqb.2008.72.003. [DOI] [PubMed] [Google Scholar]
- 52.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Habener JF. Wnt signaling in pancreatic islets. Adv. Exp. Med. Biol. 2010;654:391–419. doi: 10.1007/978-90-481-3271-3_17. [DOI] [PubMed] [Google Scholar]
- 55.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 56.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 57.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 58.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 59.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 60.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 61.Akiyoshi T, Nakamura M, Koga K, Nakashima H, Yao T, et al. Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving Wnt signalling activation. Gut. 2006;55:991–999. doi: 10.1136/gut.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borycki A, Brown AM, Emerson CP., Jr Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127:2075–2087. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- 63.Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, et al. Sonic Hedgehog–dependent proliferation in a series of patients with colorectal cancer. Surgery. 2006;139:665–670. doi: 10.1016/j.surg.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Li X, Deng W, Lobo-Ruppert SM, Ruppert JM. Gli1 acts through Snail and E-cadherin to promote nuclear signaling by β-catenin. Oncogene. 2007;26:4489–4498. doi: 10.1038/sj.onc.1210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maeda O, Kondo M, Fujita T, Usami N, Fukui T, et al. Enhancement of GLI1-transcriptional activity by β-catenin in human cancer cells. Oncol. Rep. 2006;16:91–96. [PubMed] [Google Scholar]
- 66.Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–3963. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- 67.Mullor JL, Dahmane N, Sun T, Ruiz i Altaba A. Wnt signals are targets and mediators of Gli function. Curr. Biol. 2001;11:769–773. doi: 10.1016/s0960-9822(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 68.van den Brink GR, Bleuming SA, Hardwick JCH, Schepman BL, Offerhaus GJ, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004;36:277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 69.Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol. Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang SH, Andl T, Grachtchouk V, Wang A, Liu J, et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β3-catenin signaling. Nat. Genet. 2008;40:1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–6010. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 72.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell. Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 73.Firestone AJ, Chen JK. Controlling destiny through chemistry: small-molecule regulators of cell fate. ACS Chem. Biol. 2010;5:15–34. doi: 10.1021/cb900249y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hovhannisyan A, Matz M, Gebhardt R. From teratogens to potential therapeutics: natural inhibitors of the Hedgehog signaling network come of age. Planta Med. 2009;75:1371–1380. doi: 10.1055/s-0029-1185979. [DOI] [PubMed] [Google Scholar]
- 75.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J. Med. Chem. 2009;52:4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 78.Rominger CM, Bee WL, Copeland RA, Davenport EA, Gilmartin A, et al. Evidence for allosteric interactions of antagonist binding to the Smoothened receptor. J. Pharmacol. Exp. Ther. 2009;329:995–1005. doi: 10.1124/jpet.109.152090. [DOI] [PubMed] [Google Scholar]
- 79.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 80.Yang H, Xiang J, Wang N, Zhao Y, Hyman J, et al. Converse conformational control of Smoothened activity by structurally related small molecules. J. Biol. Chem. 2009;284:20876–20884. doi: 10.1074/jbc.M807648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J. Cell Sci. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura H, Ng JMY, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 83.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc. Natl. Acad. Sci. USA. 2009;106:14132–14137. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahindroo N, Connelly MC, Punchihewa C, Kimura H, Smeltzer MP, et al. Structure-activity relationships and cancer-cell selective toxicity of novel inhibitors of glioma-associated oncogene homologue 1 (Gli1) mediated transcription. J. Med. Chem. 2009;52:4277–4287. doi: 10.1021/jm900106f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 87.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, et al. Tankyrase inhibition stabilizes axin and antagonizesWnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 88.Chen B, Dodge ME, Tang W, Lu J, Ma Z, et al. Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atwood F, Cheung C. Patent No. 60/985,454. U.S. Provisional. 2008
- 90.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisúua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 93.McCabe N, Cerone MA, Ohishi T, Seimiya H, Lord CJ, Ashworth A. Targeting Tankyrase 1 as a therapeutic strategy for BRCA-associated cancer. Oncogene. 2009;28:1465–1470. doi: 10.1038/onc.2008.483. [DOI] [PubMed] [Google Scholar]
- 94.Behninger C, Rott HD. Osteopathia striata with cranial sclerosis: literature reappraisal argues for X-linked inheritance. Genet. Couns. 2000;11:157–167. [PubMed] [Google Scholar]
- 95.Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat. Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 96.Hancock S, Pryde P, Fong C, Brazy JE, Stewart K, et al. Probable identity of Goltz syndrome and Van Allen-Myhre syndrome: evidence from phenotypic evolution. Am. J. Med. Genet. A. 2002;110:370–379. doi: 10.1002/ajmg.10456. [DOI] [PubMed] [Google Scholar]
- 97.Chen Z, Li J, Li QS, Fan JQ, Dong XM, et al. Suppression of PPN/MG61 attenuates Wnt/β-catenin signaling pathway and induces apoptosis in human lung cancer. Oncogene. 2008;27:3483–3488. doi: 10.1038/sj.onc.1211006. [DOI] [PubMed] [Google Scholar]
- 98.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J. Biol. Chem. 2008;283:22076–22088. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 100.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 102.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, et al. Medulloblastoma growth inhibition by Hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 104.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1+/− p53−/− mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 105.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 107.Phelps RA, Broadbent TJ, Stafforini DM, Jones DA. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 108.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 109.Chan TA, Wang Z, Dang LH, Vogelstein B, Kinzler KW. Targeted inactivation of CTNNB1 reveals unexpected effects of β-catenin mutation. Proc. Natl. Acad. Sci. USA. 2002;99:8265–8270. doi: 10.1073/pnas.082240999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foley PJ, Scheri RP, Smolock CJ, Pippin J, Green DW, Drebin JA. Targeted suppression of β-catenin blocks intestinal adenoma formation in APC Min mice. J. Gastrointest. Surg. 2008;12:1452–1458. doi: 10.1007/s11605-008-0519-6. [DOI] [PubMed] [Google Scholar]
- 111.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, et al. The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 116.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]