Abstract
Rationale: Although observational studies suggest that clofazimine-containing regimens are highly active against drug-resistant tuberculosis, the contribution of clofazimine for the treatment of this disease has never been systematically evaluated.
Objectives: Our goal was to directly compare the activity of a standard second-line drug regimen with or without the addition of clofazimine in a mouse model of multidrug-resistant tuberculosis. Our comparative outcomes included time to culture conversion in the mouse lungs and the percentage of relapses after treatment cessation.
Methods: Mice were aerosol-infected with an isoniazid-resistant (as a surrogate of multidrug-resistant) strain of Mycobacterium tuberculosis. Treatment, which was administered for 5 to 9 months, was initiated 2 weeks after infection and comprised the following second-line regimen: daily (5 d/wk) moxifloxacin, ethambutol, and pyrazinamide, supplemented with amikacin during the first 2 months. One-half of the mice also received daily clofazimine. The decline in lung bacterial load was assessed monthly using charcoal-containing agar to reduce clofazimine carryover. Relapse was assessed 6 months after treatment cessation.
Measurements and Main Results: After 2 months, the bacillary load in lungs was reduced from 9.74 log10 at baseline to 3.61 and 4.68 in mice treated with or without clofazimine, respectively (P < 0.001). Mice treated with clofazimine were culture-negative after 5 months, whereas all mice treated without clofazimine remained heavily culture-positive for the entire 9 months of the study. The relapse rate was 7% among mice treated with clofazimine for 8 to 9 months.
Conclusions: The clofazimine contribution was substantial in these experimental conditions.
Keywords: tuberculosis, multidrug-resistant tuberculosis, clofazimine, inbred BALB/c mice
At a Glance Commentary
Scientific Knowledge on the Subject
Clofazimine is an antimycobacterial drug used for the treatment of leprosy. Although often used for the treatment of drug-resistant tuberculosis in patients, clofazimine has never been evaluated in humans or in experimental models for its possible contribution in tuberculosis treatment.
What This Study Adds to the Field
Using a mouse model of multidrug-resistant tuberculosis, we directly assessed the activity of a second-line regimen with or without the addition of clofazimine. Compared to its clofazimine-free counterpart, the clofazimine-containing regimen was significantly more active in achieving culture conversion and preventing relapse. Considering that clofazimine is a drug safe for long-term use in humans, our work suggests that clofazimine should be more closely evaluated for use in the treatment of drug-resistant tuberculosis.
Clofazimine, also known as B.663 or Lamprene, is a fat-soluble riminophenazine dye developed in the 1950s by Vincent Barry and colleagues (1, 2) as a drug for the treatment of tuberculosis. The drug was shown to be even more bactericidal than isoniazid when tested in a mouse model of tuberculosis chemotherapy (2). For reasons that are not well documented, clofazimine was not advanced for the treatment of human tuberculosis but was used for the treatment of leprosy and later, unsuccessfully, for the treatment of Mycobacterium avium complex infection in patients with AIDS (3, 4). The unfortunate development of multidrug- and extensively drug-resistant tuberculosis has kindled a worldwide push for the development of new therapy options for this disease, including a renewed interest in clofazimine (5). Although several groups have characterized clofazimine activity for in vitro and animal models of tuberculosis (6), a seminal publication by van Deun and colleagues in 2010 reported for the first time that a 9-month drug regimen including gatifloxacin, ethambutol, pyrazinamide, and clofazimine throughout plus a 4-month initial supplement of kanamycin, prothionamide, and a high dose of isoniazid achieved a relapse-free cure of 87.9% (95% confidence interval [CI], 82.7–91.6) among 206 patients in Bangladesh (7). Such a rate is higher than the 62% (95% CI, 57–67%) rate of successful outcomes reported in a recent systematic review of multidrug-resistant tuberculosis treatment (8), and it is also better than the 61% (95% CI, 53–69%) efficacy of the World Health Organization (WHO)-recommended two year-long retreatment regimen (9, 10).
Although exciting and promising, the findings reported by van Deun and colleagues (7) were obtained from observational studies and as such were not appropriate for assessment of the impact of individual drugs in the regimen. Therefore, we designed an experiment in a mouse model of multidrug-resistant tuberculosis chemotherapy to specifically investigate the contribution of clofazimine and to address the hypothesis that clofazimine could shorten the duration of treatment for this disease. These data have been partially reported in a joint National Institute of Allergy and Infectious Disease/Johns Hopkins University webinar on clofazimine (11).
Methods
All experimental work was performed at the Johns Hopkins University School of Medicine Center for Tuberculosis Research in Baltimore, Maryland. All work with live bacteria was performed in a biosafety level-3 (BSL-3) laboratory, and all work with animals was performed in an animal BSL-3 facility.
Mice and Aerosol Infection
Six-week-old female BALB/c mice were purchased from Charles River. They were aerosol infected with M. tuberculosis H37Rv (parent strain ATCC 27294) with a KatG W149R mutation; this isoniazid-resistant strain was isolated in our laboratory from mice treated with isoniazid monotherapy at 6.25 mg/kg/d. Although the strain has weak catalase activity (compared with the parent strain), it has retained full virulence in the mouse (12). The minimum inhibitory concentrations (MICs) of each drug (except pyrazinamide) for this strain, and the parent strain, are presented in Table E1 in the online supplement. Aerosol infection was performed as previously described (12). All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Chemotherapy.
The experimental scheme is presented in Table 1. Treatment began 18 days after infection. Amikacin was injected subcutaneously at 0.2 ml, and all other drugs were administered orally by gavage. Oral drugs were given together in a single dose of 0.2 ml in water, with the exception of clofazimine, which was given separately in 0.2 ml of 0.05% agar suspension. Dosages (described in Table 1) were chosen to produce area under the (serum) concentration curve values in mice estimated to be close to those obtained with currently recommended human dosages (12–16).
TABLE 1.
SCHEME OF THE EXPERIMENT TO TEST THE CONTRIBUTION OF CLOFAZIMINE IN A SECOND-LINE REGIMEN IN A MOUSE MODEL OF MULTIDRUG-RESISTANT TUBERCULOSIS CHEMOTHERAPY
| Drug Regimen | Number of Mice to Be Killed at the Given Time Points |
Total Mice | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D −17 | D 0 | Mo. 2 | Mo. 3 | Mo. 4 | Mo. 5 | Mo. 6 | Mo. 7 | Mo. 8 | Mo. 9 | ||
| Untreated |
9 |
9 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
58 |
| 2AMEZ/7MEZ |
— |
— |
5 |
5 |
5 |
5 (15) |
5 (15) |
5 (15) |
5 (15) |
5 (15) |
115 |
| 2AMEZC/7MEZC |
— |
— |
5 |
5 |
5 |
5 (15) |
5 (15) |
5 (15) |
5 (15) |
5 (15) |
115 |
| Total mice | 9 | 9 | 15 | 15 | 15 | 15 (30) | 15 (30) | 15 (30) | 15 (30) | 15 (30) | 288 |
Definition of abbreviations: A = amikacin 100 mg/kg; C = clofazimine 25 mg/kg; D = day; E = ethambutol 100 mg/kg; M = moxifloxacin 100 mg/kg; Mo. = month of treatment; Z = pyrazinamide 150 mg/kg.
The numbers in parentheses represent the number of mice withdrawn from treatment and kept for the 6-month relapse assessment. The numbers in the treatment regimen descriptions represent the number of months the drug combination was administered.
Assessment of treatment efficacy.
Treatment efficacy was assessed on the basis of (1) lung cfu counts during treatment, and (2) the proportion of mice with culture-positive relapse 6 months after treatment completion (to allow for as much clearance of clofazimine as possible within the approximately 18-month life span of the mice). Mice were killed according to the schedule outlined in Table 1. After killing of the mice, the lungs were dissected and homogenized in 2.5 ml phosphate-buffered saline. The appropriate dilutions of the homogenates were plated on 7H11 agar enriched with 10% oleic acid-albumin-dextrose-catalase and rendered selective by supplementation with cycloheximide (10 mg/ml), carbenicillin (50 mg/ml), polymyxin B (25 mg/ml), and trimethoprim (20 mg/ml) to prevent contamination with minimal impact on M. tuberculosis growth (17). To detect and limit the consequences of clofazimine carryover, lung homogenates from the clofazimine-treated mice were plated in duplicate on 7H11 selective agar with and without 0.4% (weight/volume) activated charcoal, as Tasneen and colleagues have demonstrated that supplementation of the agar with this percentage of charcoal is effective in adsorbing clofazimine and minimizing carryover of the drug (18). For assessing relapse after treatment cessation in mice that received clofazimine, the lung homogenate was inoculated onto plain and charcoal-containing 7H11 agar plates. All plates were incubated for 28 days at 37°C before the cfu were enumerated.
Drug susceptibility testing.
During treatment, isolates from any mouse yielding a positive culture after most mice in the same treatment group converted were subjected to indirect drug susceptibility testing on 7H11 agar, as was each isolate obtained from relapsing mice. Drug susceptibility testing was performed using the proportion method (19); the drug concentrations used for testing are presented in Table E2.
Statistical analysis.
cfu counts (x) were log-transformed as (x + 1) before analysis. Group means were compared by one-way analysis of variance with Dunnett posttest to control for multiple comparisons. Relapse proportions were compared using Fisher exact test, adjusting for multiple comparisons.
Results
Establishment of Infection
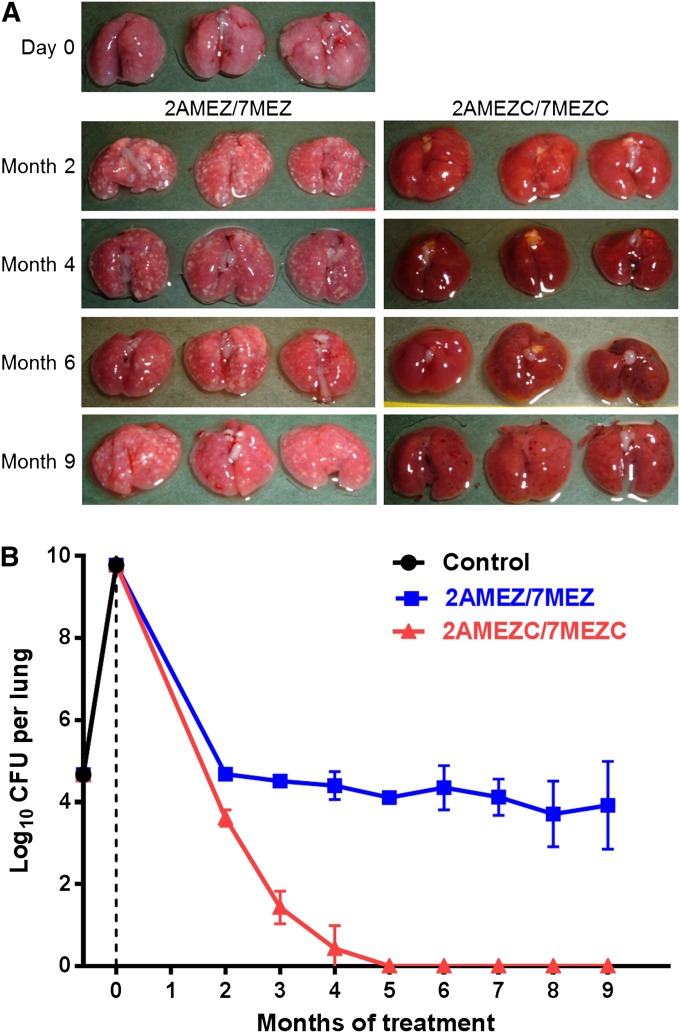
The day after infection (Day −17), the mean lung log10 cfu count (SD) was 4.67 (0.07), a much higher cfu count than expected. At Day 0, the time of treatment initiation, all mice were sick, and at killing their lungs exhibited extensive inflammation and whitish foci of consolidation (Figure 1A). The mean lung log10 cfu counts had increased to 9.74 (0.08), indicating that the isoniazid-resistant strain used for infection was not attenuated (Figure 1B). All remaining untreated mice were moribund during the fourth week of infection and were killed in accordance with animal care regulations.
Figure 1.
The effect of clofazimine when added to a second-line drug regimen in a mouse model of multidrug-resistant tuberculosis chemotherapy. (A) Gross pathology of lungs dissected from three mice in each of the treatment groups after 2 to 9 months of treatment. Day 0 is the day of treatment initiation. (B) Lung cfu counts during the course of the experiment. cfu data were obtained from the lungs of five mice per time point, and the error bars represent the SD. 2AMEZ/7MEZ = two months of amikacin (A, 100 mg/kg), moxifloxacin (M, 100 mg/kg), ethambutol (E, 100 mg/kg), and pyrazinamide (Z, 150 mg/kg), followed by 7 months of moxifloxacin, ethambutol, and pyrazinamide. 2AMEZC/7MEZC = the same drug regimen as described above with the addition of clofazimine (C, 25 mg/kg). Control mice did not receive any treatment.
Response during Treatment
During the first 2 months of treatment, no treated mice died. After 2 months of treatment, the ears, tail, and subcutaneous tissue of clofazimine-treated mice were red-brown discolored, reflecting the diffuse accumulation of the drug in these mice (Figure E1). Mice treated without clofazimine exhibited numerous gross lung lesions, whereas the lung lesions in mice treated with clofazimine were barely visible either because they were masked by the intense red discoloration of the lungs or because they were reduced in size (Figure 1A). The accumulation of clofazimine in the lungs was associated with significant carryover of the drug from the mouse lungs to the culture medium that resulted in culture negativity of all undiluted lung homogenates inoculated onto plain 7H11 agar, whereas there were numerous cfu on the 0.4% charcoal-containing agar (Table 2). Taking into account the cfu isolated on charcoal-containing agar, the lung cfu counts were reduced from 9.74 (0.08) log10 at baseline to 4.68 (0.12) and 3.61 (0.20) in mice treated with clofazimine-free and clofazimine-containing regimens, respectively (P < 0.001) (Figure 1B).
TABLE 2.
COMPARISON OF THE CARRYOVER EFFECT OF CLOFAZIMINE IN LUNG HOMOGENATES PLATED ON SELECTIVE 7H11 AGAR PLATES WITH AND WITHOUT 0.4% CHARCOAL
| Mouse | Dilution of Lung Homogenate | No. of cfu on Selective 7H11 Agar Plates |
|
|---|---|---|---|
| Without Charcoal | With 0.4% Charcoal | ||
| Mouse 1 |
Undiluted |
0, 0 |
+++ |
| |
10−1 |
2, 5 |
90, 100 |
| Mouse 2 |
Undiluted |
0, 0 |
+++ |
| |
10−1 |
7, 5 |
43, 46 |
| Mouse 3 |
Undiluted |
0, 0 |
+++ |
| |
10−1 |
18, 27 |
160, 150 |
| Mouse 4 |
Undiluted |
0, 0 |
+++ |
| |
10−1 |
4, 0 |
65, 66 |
| Mouse 5 |
Undiluted |
0, 0 |
+++ |
| 10−1 | 2, 1 | 65, 100 | |
cfu counts are from the lungs of mice receiving 2 months of the clofazimine-containing regimen. Each lung homogenate sample was plated in duplicate, and the number of cfu from each plate is provided. +++ indicates too many to count.
After Month 2 of treatment, after stopping the administration of amikacin, the difference in the decline of lung cfu counts was significantly in favor of mice treated with the clofazimine-containing regimen. In these mice, lung cultures became close to negative at Month 4 of treatment, with two mice being culture negative and three mice having a single cfu. All lung cultures (five out of five) from mice receiving clofazimine were negative at Month 5. At later time points, a single mouse was culture positive with 31 cfu on charcoal-containing agar at Month 6, another mouse was culture positive with 35 cfu on plain agar and 160 cfu on charcoal-containing agar at Month 8, and two mice were culture positive each with a single cfu on charcoal-containing agar at Month 9. All of the cfu remained drug susceptible and may be considered persisters, with the notable exception of the cfu isolated from the mouse that was culture positive at Month 8. These cfu were resistant to clofazimine with an MIC of 1.0 μg/ml, significantly higher than the 0.25 μg/ml MIC of the original strain. The substantial cfu decline in clofazimine-treated mice was associated with an intense brown-reddish staining of the lungs and internal tissues of mice (Figure E1) and also with progressive reduction in the size of the lungs and clearance of the gross lung lesions (Figure 1A), related to the antimicrobial and/or the antiinflammatory effect of clofazimine.
In strong contrast to the findings in clofazimine-treated mice, the lungs of mice treated without this drug remained full of gross lesions, and lung cfu counts slightly decreased to 4.68 (0.12) at Month 2, to 3.92 (1.07) at Month 9, and then plateaued at the level of 4 log10 cfu. The mainly bacteriostatic activity of the moxifloxacin-ethambutol-pyrazinamide combination in mice treated with the clofazimine-free regimen is rather surprising but fits with the full susceptibility to ethambutol and moxifloxacin of isolates from these mice.
Relapse after Treatment Cessation
Mice treated with the clofazimine-free regimen were still heavily culture positive at treatment cessation, and a proportion of mice survived the 6-month follow-up period after treatment cessation (11, 8, 7, 9, and 8 of the 15 mice treated for 5, 6, 7, 8, and 9 mo, respectively). All mice that did survive had gross lung lesions worse than at treatment cessation (Figure E2), and the lungs were heavily culture positive.
Mice treated with the clofazimine-containing regimen survived during the 6-month follow-up period after treatment cessation in apparent good health, with the exception of two mice treated for 8 months that died 2 months after treatment cessation of unknown causes (they were found dead after a weekend). Culture of lungs was possible only in one of these mice. At regular sacrifice, 6 months after treatment cessation, the intense red discoloration of the lungs had totally faded away and no gross lesions were apparent in all mice, even in those that had been treated for 5 months only. As shown in Table 3, culture-positive relapses were observed in 3 out of the 15 mice treated for 5 and 6 months, for 5 out of the 15 mice treated for 7 months, for 1 of the 14 mice treated for 8 months, and in 1 of the 15 mice treated for 9 months. On average, the relapse rate was 17.6%, and the differences in the relapse rates between the treatment duration groups were not statistically significant (P > 0.1). The isolated cfu were equally distributed on charcoal-containing plates and on charcoal-free plates, indicating there was no more clofazimine carryover. The number of cfu isolated from the relapsing mice was relatively limited, with a trend toward less numerous cfu in mice treated longer, likely reflecting the sustained activity of clofazimine after treatment cessation and the highest accumulation of clofazimine in mice treated longer. All isolates from relapsing mice had the same drug susceptibility as the H37Rv isoniazid-resistant mutant used for infection.
TABLE 3.
CULTURE RESULTS 6 MONTHS AFTER TREATMENT CESSATION OF THE CLOFAZIMINE-CONTAINING SECOND-LINE REGIMEN IN A MOUSE MODEL OF MULTIDRUG-RESISTANT TUBERCULOSIS CHEMOTHERAPY
| Treatment Duration, mo | No. of Culture-Positive Mice/Total No. of mice (% Culture-Positive) | Total cfu Isolated per Culture-Positive Mouse |
|---|---|---|
| 5 |
3/15 (20) |
414, 407, 2 |
| 6 |
3/15 (20) |
153, 447, 30 |
| 7 |
5/15 (33) |
1, 1, 3, 4, 5 |
| 8 |
1/14 (7) |
50 |
| 9 |
1/15 (7) |
2 |
| Total | 13/74 (18) | — |
Relapse is defined as culture positivity. Mice receiving the non–clofazimine-containing regimen were unable to reduce the lung bacterial load to undetectable levels, precluding relapse assessment in these mice.
Discussion
The present experiment was performed to assess the contribution of clofazimine to the activity of a 9-month second-line regimen for tuberculosis close to the one that has been used with success by van Deun and colleagues in Bangladesh (7). It not only provides a positive response but also demonstrates that the contribution of clofazimine was obtained without any apparent damage to the mice. The only drawback was the intense brownish discoloration of internal tissues, tail, and ears, a phenomenon that is similar to the skin discoloration of patients with leprosy on clofazimine treatment. It may be considered the short-lived cosmetic price for antimicrobial benefit.
Because the huge extent of the benefit brought by the addition of clofazimine was not expected, the validity of our findings should be examined and even challenged. First, we have to consider the virulence of the isoniazid-resistant M. tuberculosis strain that was used in the present experiment, because if it had been attenuated, the bacilli could have responded excessively well to the treatment, and consequently the value of the treatment could have been overestimated. Actually, the aerosol infective load was very high, and all untreated negative control mice died by the fourth week after infection, a relevant sign of virulence. In addition, none of the mice treated with the clofazimine-free drug regimen were cured, and a proportion of them died of tuberculosis during the 6-month follow-up after treatment cessation, indicating that there was no spontaneous clearance of the isoniazid-resistant bacilli, and consequently the strain may be considered virulent.
A second issue is the validity of the rapid culture conversion of the mouse lungs during clofazimine-containing treatment. Had charcoal-containing plates not been used and appropriate dilutions of the lung homogenates not been done, the culture conversion would have been recorded as having taken place as soon as at the second month of treatment, when in fact it occurred only after 5 months. But there is no absolute certainty that the clofazimine carryover has been totally neutralized in the charcoal-containing plates and that the growth of some remaining viable bacilli was prevented by the amount of clofazimine that had accumulated in the lungs during the course of treatment. In fact, it is the relapse rate studied 6 months after treatment cessation that allows for the conclusion that culture conversion was the result of antimicrobial activity of the treatment and not the result of clofazimine carryover. After such a 6-month follow-up, the lung discoloration had faded, no more gross lung lesions were apparent, and from the few mice that relapsed the cfu grew as well on plain agar as on charcoal-containing agar, indicating there was no residual clofazimine able to interfere with bacillary growth. Hence, one may consider that the culture conversion during treatment was a reality and not an artifact resulting from clofazimine carryover.
The fading away of tissue discoloration within the 6-month follow-up period after treatment cessation was rather unexpected, because the discoloration should have persisted if the half-life of clofazimine in mice was as long as the 70-day half-life in humans (20). Likely, it is much shorter than the half-life in humans (21), and for this reason there was no more sustained clofazimine exposure at the time of sacrifice.
In sharp contrast with the activity of the clofazimine-containing drug regimen was the poor activity of the clofazimine-free drug regimen. In spite of the substantial microbial killing of the 2-month initial phase that included amikacin, the continuation phase with the three-drug regimen moxifloxacin-ethambutol-pyrazinamide had only bacteriostatic activity. The potent activity of amikacin explains and indirectly supports the WHO recommendation that the 6 to 8 months of initial intensive phase should include an injectable drug. As for the limited activity of the moxifloxacin-ethambutol-pyrazinamide combination, it gives support to the WHO recommendation of an overall duration of at least 20 months and the use of ethionamide or prothionamide for the treatment of multidrug-resistant tuberculosis (10). The limited antimicrobial activity of the moxifloxacin, ethambutol, and pyrazinamide combination is illustrated by the fact that, at Month 8 of treatment with the clofazimine-containing regimen, one mouse yielded on plain plates and on charcoal-containing plates more than 100 cfu resistant to clofazimine. In this particular mouse, the moxifloxacin, ethambutol, and pyrazinamide combination was not able to prevent the selection of mutants resistant to clofazimine, a very negative consideration for the three-drug combination. However, the corollary of this is that the addition of clofazimine did eliminate the clofazimine-susceptible organisms and selected the resistant ones, and therefore this drug had contributed to bactericidal activity, a very promising finding.
Although the clofazimine-containing regimen had strong activity compared with the clofazimine-free regimen, its activity was far from perfect because it resulted in a mean relapse-free cure rate of only 82.4% among the 74 mice maintained for a 6-month follow-up after completion of treatment. On the positive side, the percentage of relapse-free cure was very close to the 87.9% (95% CI, 82.7–91.6%) relapse-free cure observed in 206 patients with multidrug-resistant tuberculosis treated for 9 months with comparable regimens (7), and all relapsing mice did so with bacilli having the same drug susceptibility as the infective strain. On the negative side, the relapse rate in mice treated for 5 or 6 months was not significantly different from the relapse rate in mice treated for longer duration (i.e., 7, 8, and 9 mo) even though the cfu isolated were more numerous in those treated for shorter durations. This suggests that the sterilizing process was achieved by the fifth month, an extremely positive observation, which may mean that clofazimine has potent bactericidal activity but limited sterilizing activity. If that were the case, the ideal would be to combine clofazimine with the most potent available sterilizing companion drugs. In that respect, one can wonder whether the best companion drugs with clofazimine should be rifampin and pyrazinamide. In other words, perhaps clofazimine could be included in the standard first-line regimen, for example, as a substitution for ethambutol. We should remember that the mode of action of clofazimine is poorly understood and probably pleiotropic and that previous studies have reported an association of clofazimine with DNA function, membrane disruption, and inhibition of potassium transport (23, 24). However, a recent study in Mycobacterium smegmatis has demonstrated that enzymatic reduction of clofazimine by NDH-2, the primary respiratory chain nicotinamide adenine dinucleotide reduced (NADH):quinone reductase, is followed by nonenzymatic oxidation of reduced clofazimine, generating reactive oxygen species and ultimately hydrogen peroxide (22). This would explain the early observation that clofazimine was more active against catalase-negative isoniazid-resistant strains (22) and may also account for the dramatic effect of adding clofazimine to our multidrug-resistant tuberculosis treatment regimen, as an isoniazid monoresistant strain with a mutation in katG was used. More work is therefore needed to optimize the use of clofazimine.
Overall, our study results indicate that the addition of clofazimine to a second-line regimen tremendously increased the efficacy of the regimen. When combined with the reported clinical data, our findings make the old drug clofazimine a promising new antituberculosis drug, especially considering that clofazimine is a US Food and Drug Administration–approved drug that is already known to be safe for long-term use, as documented by its use for the treatment of leprosy.
Footnotes
Supported by National Institutes of Health National Institute of Allergy and Infectious Disease contract HHSN272201000015I (J.H.G). The drug moxifloxacin was provided as a gift from Bayer via the Global Alliance.
Author Contributions: All authors contributed to the conception and design of the study, the acquisition of data, and analysis and interpretation of the data. J.H.G. drafted the manuscript, which was then reviewed and revised by all other authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201304-0753OC on July 3, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barry VC, Conalty ML, Gaffney EE. Antituberculosis activity in the phenazine series; isomeric pigments obtained by oxidation of o-phenylenediamine derivatives. J Pharm Pharmacol. 1956;8:1089–1096. doi: 10.1111/j.2042-7158.1956.tb12238.x. [DOI] [PubMed] [Google Scholar]
- 2.Barry VC, Belton JG, Conalty ML, Denneny JM, Edward DW, O’Sullivan JF, Twomey D, Winder F. A new series of phenazines (rimino-compounds) with high antituberculosis activity. Nature. 1957;179:1013–1015. doi: 10.1038/1791013a0. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson RE, Keiser P, Pierce M, Fessel WJ, Ruskin J, Lahart C, Benson CA, Meek K, Siepman N, Craft JC. Clarithromycin and ethambutol with or without clofazimine for the treatment of bacteremic Mycobacterium avium complex disease in patients with HIV infection. AIDS. 1997;11:311–317. doi: 10.1097/00002030-199703110-00008. [DOI] [PubMed] [Google Scholar]
- 4.Shafran SD, Singer J, Zarowny DP, Phillips P, Salit I, Walmsley SL, Fong IW, Gill MJ, Rachlis AR, Lalonde RG, et al. A comparison of two regimens for the treatment of Mycobacterium avium complex bacteremia in AIDS: rifabutin, ethambutol, and clarithromycin versus rifampin, ethambutol, clofazimine, and ciprofloxacin. Canadian HIV Trials Network Protocol 010 Study Group. N Engl J Med. 1996;335:377–383. doi: 10.1056/NEJM199608083350602. [DOI] [PubMed] [Google Scholar]
- 5.Global Alliance for TB Drug Development. Clofazimine. Tuberculosis (Edinb) 2008;88:96–99. [Google Scholar]
- 6.Dooley KE, Obuku EA, Durakovic N, Belitsky V, Mitnick C, Nuermberger EL Efficacy Subgroup, RESIST-TB. World Health Organization group 5 drugs for the treatment of drug-resistant tuberculosis: unclear efficacy or untapped potential? J Infect Dis. 2012;207:1352–1358. doi: 10.1093/infdis/jis460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 8.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Switzerland: World Health Organization Press; 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB) 2010 global report on surveillance and response. [Google Scholar]
- 10.World Health Organization. Switzerland: World Health Organization Press; 2011. Guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. [PubMed] [Google Scholar]
- 11.Grosset J.Does clofazimine contribute to the activity of second-line regimens for tuberculosis? Presented at Joint NIAID/Johns Hopkins University Webinar, Resurrecting clofazimine - new indications for an old drug; 10 Dec 2012
- 12.Almeida D, Nuermberger E, Tasneen R, Rosenthal I, Tyagi S, Williams K, Peloquin C, Grosset J. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal IM, Zhang M, Williams KN, Peloquin CA, Tyagi S, Vernon AA, Bishai WR, Chaisson RE, Grosset JH, Nuermberger EL. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosset J, Ji B. Experimental chemotherapy of mycobacterial diseases. Mycobacteria. Vol. II. Chemotherapy. In: Gangadharam PRJ, Jenkins PA, editors. New York: Chapman & Hall; 1998. pp. 51–97. [Google Scholar]
- 15.Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, Grosset JH, Karakousis PC. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother. 2010;65:729–734. doi: 10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta NK, Alsultan A, Gniadek TJ, Belchis DA, Pinn ML, Mdluli KE, Nuermberger EL, Peloquin CA, Karakousis PC. Potent rifamycin-sparing regimen cures guinea pig tuberculosis as rapidly as the standard regimen. Antimicrob Agents Chemother. 2013;57:3910–3916. doi: 10.1128/AAC.00761-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchison DA, Allen BW, Carrol L, Dickinson JM, Aber VR. A selective oleic acid albumin agar medium for tubercle bacilli. J Med Microbiol. 1972;5:165–175. doi: 10.1099/00222615-5-2-165. [DOI] [PubMed] [Google Scholar]
- 18.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods GL, Warren NG, Inderlied CB.Susceptibility test methods: mycobacteria, nocardia, and other actinomycetes. In: Baron EJ and Murray PR, editors. Manual of clinical microbiology, 9th ed. Washington: ASM Press; 2007. 1223–1247. [Google Scholar]
- 20.Mansfield RE. Tissue concentrations of clofazimine (B663) in man. Am J Trop Med Hyg. 1974;23:1116–1119. doi: 10.4269/ajtmh.1974.23.1116. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Zheng M, Wang B, Fu L, Zhao W, Li P, Xu J, Zhu H, Jin H, Yin D, et al. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob Agents Chemother. 2011;55:5185–5193. doi: 10.1128/AAC.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, Schechter NM, Rubin H. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011;286:10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bopape MC, Steel HC, Cockeran R, Matlola NM, Fourie PB, Anderson R. Antimicrobial activity of clofazimine is not dependent on mycobacterial C-type phospholipases. J Antimicrob Chemother. 2004;53:971–974. doi: 10.1093/jac/dkh215. [DOI] [PubMed] [Google Scholar]
- 24.Cholo MC, Boshoff HI, Steel HC, Cockeran R, Matlola NM, Downing KJ, Mizrahi V, Anderson R. Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. J Antimicrob Chemother. 2006;57:79–84. doi: 10.1093/jac/dki409. [DOI] [PubMed] [Google Scholar]