Abstract
The impact of combining epidermal growth factor receptor tyrosine kinase inhibitors (EGFR–TKIs) and chemotherapy as first-line therapy for patients with advanced non-small-cell lung cancer (NSCLC) remains controversial. Therefore, randomized trials that compared this combined regimen with chemotherapy or EGFR–TKIs monotherapy were included for this meta-analysis. We used published hazard ratios (HRs), if available, or derived treatment estimates from other survival data. Pooled estimates of treatment efficacy of the combined regimen in the entire unselected population and selected patients by EGFR-mutation status and smoking history were calculated. Eight trials eventually entered into this meta-analysis, including 4585 patients. Overall, the combined regimen significantly delayed disease progression (HR = 0.81, 95% CI 0.69–0.95, P = 0.01); subgroup analysis showed significantly higher progression free survival advantages in Asian patients (P<0.001), with sequential combination of TKIs and chemotherapy (P = 0.02). In selected patients by EGFR-mutation, both mutation positive (HR = 0.48, 95% CI 0.28–0.83, P = 0.009) and negative (HR = 0.84, 95% CI 0.72–0.98, P = 0.02) patients gained progression free survival benefit from the combined regimen, albeit the magnitude of benefit was marginally larger in mutation positive patients (P = 0.05). In selected patients by smoking history, never/light smokers achieved a great progression free survival benefit from the combined regimen (HR = 0.51, 95% CI 0.35–0.74, P = 0.0004). Unfortunately, the combined regimen had no significant impact on overall survival, irrespective of ethnicity, dose schedules or EGFR-mutation status. Severe anorexia (RR = 2.01, 95% CI 1.11–3.63; P = 0.02) and diarrhea (RR = 2.70, 95% CI 1.94–3.76; P<0.001) were more frequent in the combined regimen arm. This strategy of combining EGFR–TKIs and chemotherapy deserved to be considered in the future, although it is not approved for advanced NSCLC at the moment.
Introduction
For advanced non-small-cell lung cancer (NSCLC) patients, platinum-based chemotherapy was recommended as standard regimen for initial treatment. Advances in genetic testing allowed the discovery and clinical application of driver oncogenes, such as activating epidermal growth factor receptor (EGFR) mutations, as a therapeutic target. Several randomized controlled trials [1]–[4] and meta-analyses [5], [6] have demonstrated that EGFR-tyrosine-kinase inhibitor (EGFR–TKI), erlotinib or gefitinib, is superior to chemotherapy as first-line treatment for patients with EGFR mutations. However, patients may still have unknown EGFR mutation status at the time when first-line treatments decisions are made, due to limited high-quality tumor samples or insufficient testing facilities. Treating patients with unknown EGFR-mutations with a combination of chemotherapy and an EGFR–TKI is an applicable option. Unfortunately, previous randomized trials showed no significant improvement of survival by combining EGFR–TKIs and chemotherapy [7]–[12]. But another phase II trial of sequential combination of erlotinib and chemotherapy as first-line treatment for advanced NSCLC showed a significant improvement in progression-free survival (PFS) [13]. And the interesting findings are recently confirmed in the phase III trial – FASTACT–II [14]. Controversy continues regarding the role of the addition of EGFR–TKIs in patients receiving chemotherapy. Therefore, we conducted this meta-analysis to comprehensively estimate the treatment effect of the combined regimen on PFS and overall survival (OS) based on characteristics of patients.
Materials and Methods
Search Strategy and Selection Criteria
All randomized trials evaluating the effect of the combined regimen of EGFR–TKIs and chemotherapy were eligible for inclusion. Two investigators (P. Y. OuYang and Z. Su) independently searched PubMed database, Cochrane Controlled Trials Register via Cochrane Library and ClinicalTrials.gov with the terms “erlotinib OR tarceva”, “gefitinib OR iressa”, “chemotherapy”, “first-line”, and “non-small-cell lung cancer OR NSCLC”. The search was limited to randomized controlled trials or clinical trials. We also searched the conference proceedings of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology and the International Association for the Study of Lung Cancer for relevant clinical trials.
Eligible trials satisfying the following requirements were eventually included: (a) prospective randomized controlled trials (phase II or III), (b) chemotherapy-naïve patients with cancer were randomly assigned to first-line treatment with chemotherapy or an EGFR–TKI monotherapy or the combined regimen of EGFR–TKI and chemotherapy, (c) adequate survival data available for calculation or estimation of a hazard ratio (HR) with a 95% confidence interval (CI). Phase I study and phase II study with only one single arm were excluded because of either drug dosage difference or the missing control group.
Data Extraction and Study Quality Assessment
Two authors (P. Y. OuYang and Z. Su) independently identified eligible trials and extracted information on trial name, year of publication, name and dosage of EGFR–TKI, trial design and treatment protocol, number of patients in investigational and control arms, median age (range), sex (female), race (Asian), never/light smoker and severe toxicities. Mutational analysis data was also extracted. Patients were classified as EGFR mutation–positive if a mutation was detected using molecular assessment tools such as Sanger sequencing, polymerase chain reaction clamp, and amplification refractory mutation system. Patients were classified as EGFR mutation–negative if no mutation was detected. We did not classify the EGFR mutation status based on immunohistochemistry and fluorescent in situ hybridization for EGFR gene copy numbers.
To award study quality, we examined the randomization procedure, estimation of sample size, blinding, loss to follow-up, dropout and if the intention-to-treat analysis was followed.
Statistical Analysis
We extracted HRs and associated 95% CIs for PFS and OS outcomes to assess treatment efficacy. If HR and CI were not reported, these were estimated using the methods of Parmar [15]. Risk ratio (RR) with 95% CI was used for results of comparing severe toxicities in both arms.
Heterogeneity across studies was estimated by Chi-square test and I2 statistic and correct effects models were chosen accordingly. Statistically significant heterogeneity was defined as a Chi-square P value less than 0.1 or an I2 statistic greater than 50% [16]. If heterogeneity was not observed, we just reported the summary estimation results on the basis of fixed-effects model. If heterogeneity was observed, the summary estimation was based on random-effects model. Subgroup analysis was conducted to detect evident heterogeneities.
Potential publication bias was assessed with the Begg’s test and Egger’s test, and graphically presented by funnel plots. All statistical analysis was performed by Review Manager Version 5.2 (Revman; the Cochrane Collaboration; Oxford, England) and STATA version12.0. A two-sided P value of less than 0.05 was considered significant for all analysis except heterogeneity tests.
Results
Eligible Studies
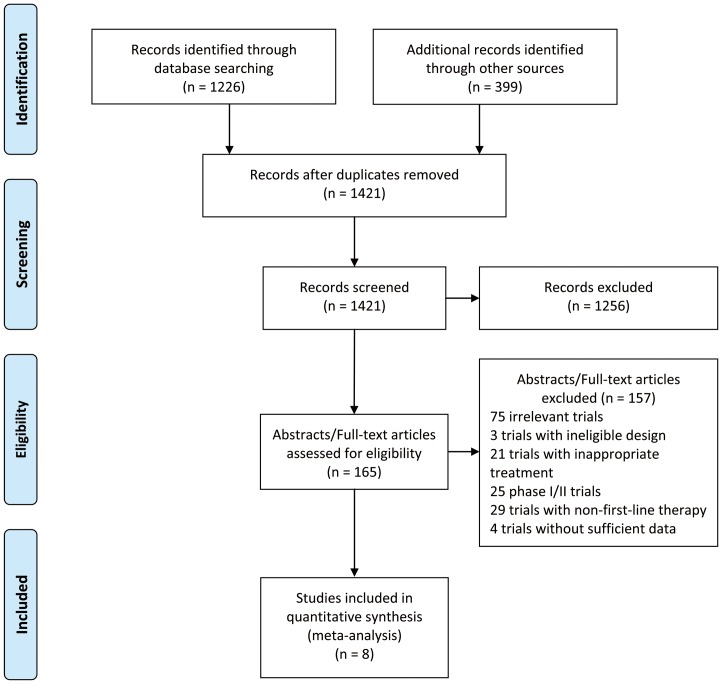
Overall, eight trials [7]–[14] were highly eligible for inclusion in this meta-analysis (Figure 1). Six trials (INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], FASTACT [13] and FASTACT–II [14]) compared the combined regimen with chemotherapy alone, while the other two trials (trial by Hirsch et al [11] and CALGB 30406 trial [12]) compared this combination with EGFR–TKIs monotherapy. Participants in the FASTACT [13], FASTACT–II [14] and trial by Hirsch et al [11] were administered with platinum-based chemotherapy sequentially followed by erlotinib or placebo, whereas patients in the other trials were delivered with concurrent dosing schedules. The baseline characteristics of ethnicity, adenocarcinoma histology, never/light smoking history, female gender and EGFR mutation were presented in Table 1. However, survival information was only available in selected patients by smoking history and EGFR mutation status.
Figure 1. Flow diagram of identifying trials.
Table 1. Baseline characteristics of the included trials in the meta-analysis.
| Trials(year) | TKIs | chemotherapy (dose*cycles) | Patientsanalyzed | Median age(range) | Female | Race (% Asian) | Never/light smoker | EGFR mutation positive |
| FASTACT(2009) [13] | E† | DDP(75 mg/m2,d1)/CBP(AUC = 5,d1)+GEM1250(mg/m2,d1,8),q4w*6 | 76vs78 | 57.5(33–79) vs57.0(27–79) | 22vs24 | 93vs95 | 24vs28 | 2vs5 |
| FASTACT–II (2013) [14] | E† | DDP(75 mg/m2,d1)/CBP(AUC = 5,d1)+GEM1250(mg/m2,d1,8),q4w*6 | 226vs225 | 59.0(31–96)vs57.3(37–88) | 94vs85 | 100vs100 | 112vs107 | 49vs48 |
| INTACT 1(2004) [7] [17] | G‡ | DDP(80 mg/m2,d1)+GEM(1250 mg/m2d1,8),q3w*6 | 365vs363 | 59(34–83)vs61(33–81) | 85vs101 | 1.6vs0.8 | NA | 23vs9& |
| INTACT 2(2004) [8] [17] | G‡ | CBP(AUC = 6)+TAX(225 mg/m2),q3w*6 | 345vs345 | 61(27–86)vs63(31–85) | 146vs133 | NA | NA | |
| TALENT(2007) [9] | E | DDP(80 mg/m2,d1)+GEM(1250 mg/m2d1,8),q3w*6 | 580vs579 | 61(26–82)vs60(28–84) | 125vs142 | 3vs4 | 8vs10 | NA |
| TRIBUTE(2005) [10] [18] | E | CBP(AUC = 6)+TAX(200 mg/m2),q3w*6 | 539vs540 | 63(24–84)vs63(26–84) | 217vs207 | 3.9vs2.4 | 72vs44 | 15vs14 |
| CALGB30406(2012) [12] | E | CBP(AUC = 6)+TAX(200 mg/m2),q3w*6 | 100vs81 | 60(34–81)vs58(32–78) | 58vs49 | 8vs6 | 100vs81 | 33vs33 |
| Hirsch et al.2011 [11] | E | CBP(AUC = 6)+TAX(200 mg/m2),q3w*4 | 71vs72 | NA | 31vs44 | 6vs12 | NA | 6vs9 |
Note: TKIs = tyrosine kinase inhibitors, PS = performance status, E = erlotinib, G = gefitinib, DDP = cisplatin, CBP = carboplatin, AUC = area under the curve, GEM = gemcitabine, q4w = every four weeks, vs = the combined regimen versus chemotherapy or TKIs monotherapy, NA = not available, TAX = paclitaxel.
Sequential administration of erlotinib following gemcitabine/platinum chemotherapy, rather than concurrent administration as the other trials.
Only included patients treated with gefitinib 250 mg/d.
Data from trials INTACT 1and 2 together.
Overall, these studies were of high quality – blinding, showing randomization procedure, conducting estimation of sample size, mostly reporting dropout and following the principle of intention-to-treat analysis. (Table S1).
Effect of the Combined Regimen on PFS and OS in Unselected Patients
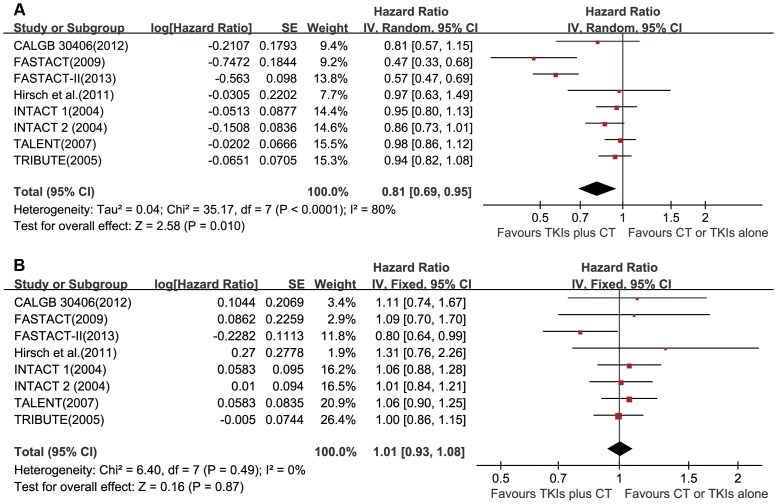
Data of four trials [9], [10], [13], [14] was directly available, while the information was estimated from survival curves in the other trials. Significant PFS benefit was observed from the combined regimen of TKIs and chemotherapy (HR = 0.81, 95% CI 0.69–0.95, P = 0.01; Figure 2a) based on random-effects model, due to significant heterogeneity (Chi2 = 35.17, P<0.001; I2 = 80%). Unfortunately, there was no evidence of improvement in OS with the combined regimen (HR = 1.01, 95% CI 0.93–1.08, P = 0.87, fixed-effects model; Figure 2b).
Figure 2. Forest plots in unselected patients.
HRs and 95% CIs of (a) progression-free survival and (b) overall survival. TKIs = tyrosine kinase inhibitors, CT = chemotherapy, SE = standard error.
Subgroup analysis was conducted according to the regimen in the control group, ethnicity and dose schedules (Table 2). Significant associations between PFS improvement and ethnicity or dose schedules were observed. There were significant higher PFS advantages in Asian patients (P<0.001), with sequential combination of TKIs and chemotherapy (P = 0.02). Interestingly, the combined regimen was superior over chemotherapy alone in PFS (P = 0.01), whereas it was similar to TKIs monotherapy (P = 0.32). However, this discrepancy was non-significant (P = 0.58), which may be caused by relatively small number of patients in the subgroup of the combined regimen versus TKIs monotherapy. As observed in the entire unselected population, there was no significant OS improvement in subgroups.
Table 2. Subgroup analysis in unselected patients.
| Subgroup | Included trials | HR (95%CI) | P values for heterogeneity | HR (95%CI) | P values for heterogeneity |
| Regimen in control | |||||
| TKIs | Hirsch [11] and CALGB 30406 [12] | 0.87 [0.66, 1.14] | 0.58 | 1.18 [0.85, 1.63] | 0.33 |
| Chemotherapy | INTACT 1 [7], INTACT 2 [8], TALENT [9],TRIBUTE [10], FASTACT [13], FASTACT–II [14] | 0.79 [0.66, 0.96] | 1.00 [0.92, 1.08] | ||
| Ethnicity | |||||
| Asian | FASTACT [13] and FASTACT–II [14] | 0.55 [0.46, 0.65] | <0.001 | 0.85 [0.70, 1.03] | 0.06 |
| Non-Asian | INTACT 1 [7], INTACT 2 [8], TALENT [9],TRIBUTE [10], Hirsch [11], CALGB 30406 [12] | 0.93 [0.87, 1.00] | 1.04 [0.96, 1.12] | ||
| Dose schedule | |||||
| Sequential | Hirsch [11], FASTACT [13], FASTACT–II [14] | 0.62 [0.44, 0.87] | 0.02 | 0.89 [0.74, 1.07] | 0.15 |
| Concurrent | INTACT 1 [7], INTACT 2 [8], TALENT [9],TRIBUTE [10], CALGB 30406 [12] | 0.93 [0.87, 1.00] | 1.03 [0.95, 1.12] |
Effect of the Combined Regimen on PFS and OS in Selected Patients by EGFR-Mutation Status
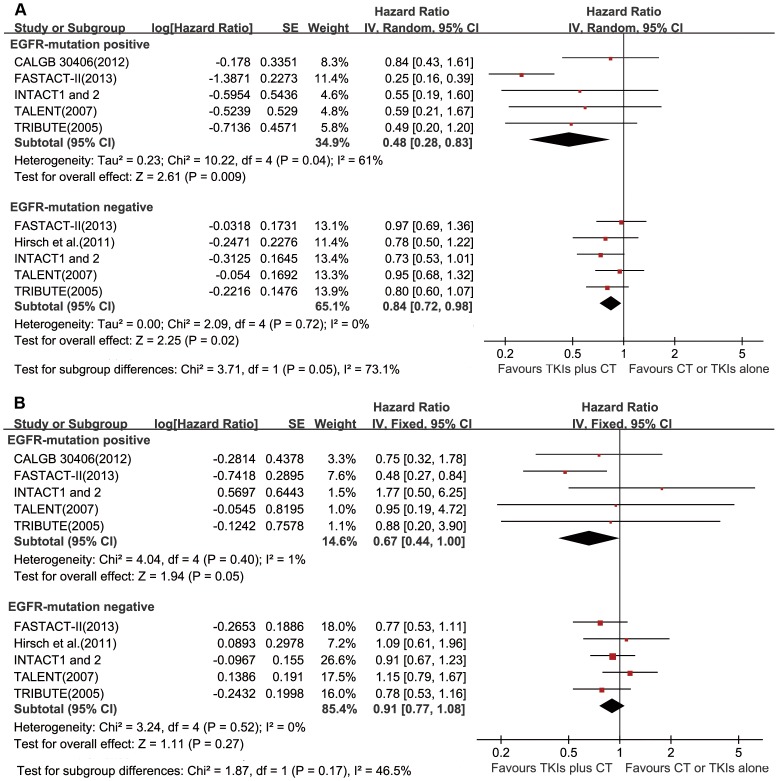
Survival data of EGFR-mutation positive patients was only available in the FASTACT–II [14], INTACT 1 and 2 [17], TALENT [9], TRIBUTE [18] and CALGB30406 [12]. Estimates of PFS and OS in EGFR-mutation negative patients could only be calculated in the FASTACT–II [14], INTACT 1 and 2 [17], TALENT [9], TRIBUTE [18] and trial by Hirsch et al [11]. In the EGFR-mutation positive cohort, the combined regimen was superior over chemotherapy or TKIs monotherapy with a significant improvement in PFS (HR = 0.48, 95% CI 0.28–0.83, P = 0.009; Figure 3a). Interestingly, the combined regimen also showed significant PFS benefit in the EGFR-mutation negative cohort, compared with chemotherapy or TKIs monotherapy (HR = 0.84, 95% CI 0.72–0.98, P = 0.02; Figure 3a). Certainly, the magnitude of PFS improvement resulted from the combined regimen in the EGFR-mutation positive cohort was marginally larger than that in the EGFR-mutation negative cohort (P = 0.05).
Figure 3. Forest plots in selected patients by EGFR-mutation status.
HRs and 95% CIs of (a) progression-free survival and (b) overall survival. TKIs = tyrosine kinase inhibitors, CT = chemotherapy, SE = standard error.
In terms of OS, the combined regimen marginally enhanced OS of EGFR-mutation positive patients (HR = 0.67, 95% CI 0.44–1.00, P = 0.05), but not EGFR-mutation negative patients (HR = 0.91, 95% CI 0.77–1.08, P = 0.27). (Figure 3b).
Effect of the Combined Regimen on PFS in Never/light Smokers
We pooled analysis of FASTACT [13], FASTACT–II [14], TRIBUTE [10] and CALGB30406 [12], and found an improvement of PFS in never/light smokers with the combined regimen (HR = 0.51, 95% CI 0.35–0.74, P = 0.0004). (Figure 4).
Figure 4. Forest plots in never/light smokers.
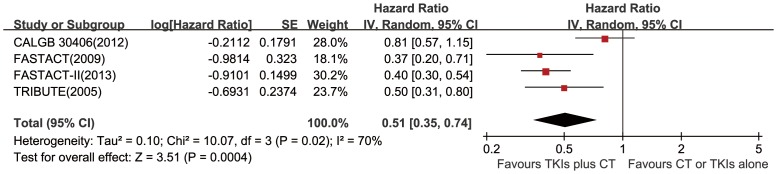
HRs and 95% CIs of progression-free survival. TKIs = tyrosine kinase inhibitors, CT = chemotherapy, SE = standard error.
Grade 3 and Higher Toxicities
Compared with chemotherapy or TKIs monotherapy, the combined regimen caused more grade 3 and higher anorexia (RR = 2.01, 95% CI 1.11–3.63; P = 0.02) and diarrhea (RR = 2.70, 95% CI 1.94–3.76; P<0.001). And there were no differences of other severe toxicities between the two arms. (Table 3).
Table 3. Grade 3 and higher toxicities by meta-analysis for the combined regimen versus chemotherapy or TKI monotherapy.
| Subgroup | Trials with data | Risk ratio (95%CI) | P values |
| Hematologic | |||
| Anemia | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], FASTACT [13],FASTACT–II [14] and CALGB 30406 [12] | 0.98 [0.63, 1.53] | 0.93 |
| Leukopenia | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10] and FASTACT–II [14] | 0.97 [0.74, 1.27] | 0.84 |
| Thrombocytopenia | INTACT 1 [7], TALENT [9], TRIBUTE [10], CALGB 30406 [12],FASTACT [13] and FASTACT–II [14] | 1.15 [0.93, 1.41] | 0.20 |
| Neutropenia | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], Hirsch [11],CALGB 30406 [12], FASTACT [13] and FASTACT–II [14] | 1.23 [0.88, 1.73] | 0.23† |
| Non-hematologic | |||
| Rash | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], Hirsch [11],CALGB 30406 [12], FASTACT [13] and FASTACT–II [14] | 2.08 [0.60, 7.16] | 0.25† |
| Nausea | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], Hirsch [11],CALGB 30406 [12], FASTACT [13] and FASTACT–II [14] | 0.95 [0.40, 2.23] | 0.90† |
| Vomiting | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], Hirsch [11],CALGB 30406 [12], FASTACT [13] and FASTACT–II [14] | 1.09 [0.81, 1.48] | 0.57 |
| Anorexia | INTACT 1 [7], INTACT 2 [8], TALENT [9], Hirsch [11] and FASTACT [13] | 2.01 [1.11, 3.63] | 0.02 |
| Fatigue/Asthenia | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], Hirsch [11],CALGB 30406 [12], FASTACT [13] and FASTACT–II [14] | 1.53 [0.78, 2.99] | 0.21† |
| Diarrhea | INTACT 1 [7], INTACT 2 [8], TALENT [9], TRIBUTE [10], Hirsch [11],CALGB 30406 [12], FASTACT [13] and FASTACT–II [14] | 2.70 [1.94, 3.76] | <0.001 |
| Dyspnea | INTACT 2 [8], TALENT [9], TRIBUTE [10], and FASTACT–II [14] | 0.88 [0.62, 1.23] | 0.45 |
Using random-effects model for heterogeneity.
Publication Bias
No publication bias was observed in the meta-analysis (Begg’s test P≥0.108, Egger’s test P≥0.134). We showed funnel plot of PFS in unselected patients (Figure S1).
Discussion
Petrelli et al [19] in their meta-analysis collected data of patients with EGFR-mutation from INTACT 1, INTACT 2, TRIBUTE and other 10 trials, and found that NSCLCs harboring EGFR mutations derived greater benefit from erlotinib or gefltinib than from chemotherapy; however, they did not include data from the most recent trials [13], [14], and main results of OS and PFS were based on all trials irrespective of the line of treatment. Another recent meta-analysis [20] compared TKIs plus platinum-based doublet chemotherapy (PBDC) with PBDC alone, and showed marginally improved PFS from the combined regimen; but importantly, it did not explore the effect in selected patients by EGFR-mutation status or demographic factors, nor did it compare survival differences in subgroups according to ethnicity or dose schedules of TKIs and chemotherapy.
Single agent of EGFR–TKIs, either erlotinib or gefitinib, has been demonstrated to be superior to chemotherapy [2]–[6], [13] and recommended by NCCN guideline for first-line treatment of EGFR-mutation positive patients. But it is common that the EGFR-mutation status of the majority of patients is still unknown at the time of making a first-line treatment decision. This meta-analysis incorporates results of eight trials in nearly 4600 patients, and supports the point that combining EGFR–TKIs and chemotherapy is superior in delaying disease progression for advanced NSCLC. The ethnicity and dose schedules of TKIs and chemotherapy greatly influenced the efficacy of the combined regimen in PFS. The magnitude of PFS benefit was larger for the Asian patients, with sequential administration of TKIs and chemotherapy. This study also showed that EGFR mutation was an important predictive biomarker of treatment benefit in terms of PFS. The magnitude of PFS benefit was not similar between EGFR-mutation positive and negative subgroups, although the combined regimen showed significant improvement in both.
As we know, somatic mutations in the EGFR kinase domain had been discovered in a subset of NSCLC [21]–[25]. The two most frequent mutations were the exon 19 deletion that removed residues 746–750 of the expressed protein and the exon 19 point substitution that replaced leucine 858 with arginine (L858R) [24], [25]. Structurally, these mutations clustered around the active site cleft of the tyrosine kinase domain. Comparison of the structures of the mutant kinases with the inactive wild-type EGFR indicated that the mutations were expected to destabilize the inactive conformation, and therefore to promote the active conformation of the kinase. In particular, the L858R mutation was clearly incompatible with the inactive conformation. [26]. Direct measurement of the binding affinity of gefitinib to the wild type and mutant kinases revealed that gefitinib binds the L858R mutant 20-fold more tightly than the wild-type kinase [26]. Based on the kinetics of the wild type and mutant kinases in vitro, the L858R mutant was 50-fold more active than the wild-type kinase [26]. Additionally, cells bearing the mutant EGFR were in general more sensitive to EGFR–TKIs than cells expressing the wild type kinase. The L858R mutant was 10–100 fold more sensitive to erlotinib and gefitinib than the wild type kinase [23], [27]. These basic researches, randomized controlled clinical trials [1]–[4] and meta-analyses [5], [6] thoroughly demonstrated the better treatment outcomes of the EGFR–TKIs in mutation positive patients. Moreover, preclinical studies [28], [29] had clarified a synergistic effect of combining EGFR–TKIs with chemotherapy. Therefore, it is not unexpected that the combined therapy of EGFR–TKIs and chemotherapy shows higher benefit in EGFR-mutation positive cohort.
Remarkably, erlotinib and chemotherapy such as gemcitabine/cisplatin had different mechanisms of action (cytostatic and cytotoxic, respectively). The antiproliferative effects of erlotinib, arising from cell-cycle arrest [30], might render tumor cells less sensitive to cytotoxic agents, as suggested by recent preclinical studies of combinations of EGFR TKIs with chemotherapy [31], [32]. That is, the concurrent schedule might have the potential issue of cell cycle–based antagonism between TKIs and chemotherapy, while the special combination of sequential administration of erlotinib following chemotherapy in FASTACT [13] and FASTACT–II [14] appeared to be successful in that respect, and led to a significant improvement in PFS. Furthermore, considering the heterogeneity of EGFR-mutation in intratumor tissue [33], the sequential schedule had its advantage in theory. When comparing sequential dose schedules with concurrent, we did observe significant differences in effects (P = 0.02).
In spite of a large PFS benefit, this meta-analysis did not demonstrate OS advantage with the combined regimen. Regardless of EGFR-mutation status, the overall treatment effects on OS were similar. Limited number of patients with mutational analysis possibly underpowered the effect of the combined regimen in EGFR-mutation positive patients. Additionally, appropriate dose schedules of the combined regimen might contribute to OS benefit, as OS benefit was noted in EGFR-mutation positive patients with sequential combination of chemotherapy and erlotinib in a single trial – the FASTACT–II [14]. More importantly, differences in OS are potentially affected not only by treatment allocation, but also by differences of second or third-line treatment given to patients in both arms after disease progression. A recent systematic review of chemotherapy trials indicated that PFS advantage was unlikely to be associated with OS advantage with increasing impact of salvage therapy and that the prolongation of survival postprogression might limit the role of OS for assessing true efficacy derived from front-line therapy [34].
This meta-analysis had several limitations. Firstly, all data was extracted from published studies, which might result in publication bias and selection bias. Secondly, EGFR-mutation status was only assessed in approximately 20% patients enrolled in eligible trials, with treatment efficacy estimated from small numbers of EGFR-mutation positive patients identified in many of these trials. The potential influence on the results of restricting our analysis to this subset of patients is unknown. Additionally, albeit evidences showed that the distinct EGFR mutations could differ markedly in their EGFR–TKIs susceptibilities [26], [35], [36], it was difficult to perform stratification analysis by the EGFR mutations due to absence of original data and small sample size in respective strata. Thirdly, we performed analysis of ethnicity on the assumption that all patients in FASTACT and FASTACT–II [13], [14] were Asian, while the others were non-Asian according to the actual percent of race (Table 1).
In conclusion, on the basis of this meta-analysis, combination of EGFR–TKIs and chemotherapy leads to PFS benefit as first-line treatment for advanced NSCLC, regardless of EGFR-mutation status, but has no demonstrable impact on OS. And there is a larger magnitude of PFS benefit for Asian patients, with sequential administration of EGFR–TKIs and chemotherapy. EGFR-mutation status is still a predictive biomarker of benefit with the combined regimen, for a larger magnitude of improvement in EGFR-mutation positive patients. This strategy deserved to be considered in the future although it is not approved for advanced NSCLC at the moment.
Supporting Information
Funnel plot of progression-free survival of unselected patients. TKIs = tyrosine kinase inhibitors, CT = chemotherapy, SE = standard error.
(TIF)
Quality assessment of the included studies.
(DOCX)
PRISMA checklist.
(DOC)
Acknowledgments
We greatly thank Xu-Yu Zhou, Wei-Jia Wang and Yun-Yun Liao (Professors of medical library, Sun Yat-sen University) for assistance in searching literature.
Funding Statement
The authors have no support or funding to report.
References
- 1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 2. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG–0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 5. Bria E, Milella M, Cuppone F, Novello S, Ceribelli A, et al. (2011) Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: a meta-analysis. Ann Oncol 22: 2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao G, Ren S, Li A, Xu J, Xu Q, et al. (2012) Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer 131: E822–E829. [DOI] [PubMed] [Google Scholar]
- 7. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, et al. (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol 22: 777–784. [DOI] [PubMed] [Google Scholar]
- 8. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, et al. (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol 22: 785–794. [DOI] [PubMed] [Google Scholar]
- 9. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, et al. (2007) Phase III Study of Erlotinib in Combination With Cisplatin and Gemcitabine in Advanced Non-Small-Cell Lung Cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol 25: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, et al. (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI–774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23: 5892–5899. [DOI] [PubMed] [Google Scholar]
- 11. Hirsch FR, Kabbinavar F, Eisen T, Martins R, Schnell FM, et al. (2011) A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer. J Clin Oncol 29: 3567–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, et al. (2012) Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 30: 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mok TS, Wu YL, Yu CJ, Zhou C, Chen YM, et al. (2009) Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 27: 5080–5087. [DOI] [PubMed] [Google Scholar]
- 14. Wu Y–L, Lee JS, Thongprasert S, Yu C–J, Zhang L, et al. (2013) Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 14: 777–786. [DOI] [PubMed] [Google Scholar]
- 15. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, et al. (2005) Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 23: 8081–8092. [DOI] [PubMed] [Google Scholar]
- 18. Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, et al. (2005) Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23: 5900–5909. [DOI] [PubMed] [Google Scholar]
- 19. Petrelli F, Borgonovo K, Cabiddu M, Barni S (2012) Efficacy of EGFR Tyrosine Kinase Inhibitors in Patients With EGFR–Mutated Non–Small-Cell Lung Cancer: A Meta-Analysis of 13 Randomized Trials. Clin Lung Cancer 13: 107–114. [DOI] [PubMed] [Google Scholar]
- 20. Chen P, Wang L, Liu B, Zhang H–Z, Liu H–C, et al. (2011) EGFR-targeted therapies combined with chemotherapy for treating advanced non-small-cell lung cancer: a meta-analysis. Eur J Clin Pharmacol 67: 235–243. [DOI] [PubMed] [Google Scholar]
- 21. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 22. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 23. Pao W, Miller V, Zakowski M, Doherty J, Politi K, et al. (2004) EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101: 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shigematsu H, Gazdar AF (2006) Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 118: 257–262. [DOI] [PubMed] [Google Scholar]
- 25. Chan SK, Gullick WJ, Hill ME (2006) Mutations of the epidermal growth factor receptor in non-small cell lung cancer – search and destroy. Eur J Cancer 42: 17–23. [DOI] [PubMed] [Google Scholar]
- 26. Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, et al. (2007) Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, et al. (2005) Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst 97: 1185–1194. [DOI] [PubMed] [Google Scholar]
- 28. Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, et al. (2000) Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD–1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res 6: 2053–2063. [PubMed] [Google Scholar]
- 29. Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG (2000) Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res 6: 4885–4892. [PubMed] [Google Scholar]
- 30. Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, et al. (1997) Induction of apoptosis and cell cycle arrest by CP–358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 57: 4838–4848. [PubMed] [Google Scholar]
- 31. Davies AM, Ho C, Lara PN Jr, Mack P, Gumerlock PH, et al. (2006) Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer 7: 385–388. [DOI] [PubMed] [Google Scholar]
- 32. Solit DB, She Y, Lobo J, Kris MG, Scher HI, et al. (2005) Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continuous dosing for sensitizing tumors to paclitaxel. Clin Cancer Res 11: 1983–1989. [DOI] [PubMed] [Google Scholar]
- 33. Bai H, Wang Z, Chen K, Zhao J, Lee JJ, et al. (2012) Influence of Chemotherapy on EGFR Mutation Status Among Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 30: 3077–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hotta K, Kiura K, Fujiwara Y, Takigawa N, Hisamoto A, et al. (2011) Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: a systematic review. PLoS One 6: e26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, et al. (2006) Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res 66: 8163–8171. [DOI] [PubMed] [Google Scholar]
- 36. Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, et al. (2005) Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med 2: e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot of progression-free survival of unselected patients. TKIs = tyrosine kinase inhibitors, CT = chemotherapy, SE = standard error.
(TIF)
Quality assessment of the included studies.
(DOCX)
PRISMA checklist.
(DOC)