Abstract
Background
To compare the efficacy of glycemic control and insulin secretion of alpha glucosidase inhibitors (AGI) on type 2 diabetes patients between Asian and Caucasian.
Methodology/Principal Findings
The MEDLINE®, EMBASE®, CENTRAL were searched and qualified studies in Asian and Caucasian population comparing AGI treatment with placebo or other oral anti-diabetic drugs in type 2 diabetic patients were included. Totally 58 qualified studies were included. When AGI treatment was compared with placebo, a significant difference in HbA1c decline from baseline favoring AGI treatment was found in Asian (weighted mean difference (WMD), −0.50%; 95% CI, −0.66% to −0.34%) and in Caucasian a significant difference in HbA1c decline favoring AGI treatment was also found (WMD, −0.68%; 95% CI, −0.76% to −0.60%). In Asian, fasting plasma glucose was reduced with AGI treatment compared with placebo (WMD, −0.53 mmol/L; 95% CI, −0.91 to −0.14 mmol/L) and in Caucasian there was also a significant difference in FPG changes favoring AGI therapy (WMD, −0.88 mmol/L; 95% CI, −1.00 to −0.77 mmol/L). Studies in Asian showed a significant difference in fasting insulin changes favoring AGI treatment (WMD, −0.78 uU/ml; 95% CI, −0.96 to −0.59 uU/ml). While in Caucasian fasting insulin was decreased without significance with AGI treatment (WMD-1.24 uU/ml; 95% CI, −2.51 to 0.04 uU/ml). Body weight was decreased with AGI treatment in Asian (WMD, −1.00 kg; 95% CI, −1.69 to −0.31 kg) and was also decreased with AGI treatment in Caucasian (WMD, −0.73 kg; 95% CI, −1.13 to −0.33 kg).
Conclusions/Significance
According to results from this meta-analysis, the efficacy in glucose lowering, body weight reduction and insulin secretion decreasing of AGI treatment in Asian were comparable with those in Caucasian.
Introduction
In the treatment of type 2 diabetes, alpha-glucosidase inhibitors (AGIs; including acarbose, miglitol, voglibose) were recommend by guidelines for glucose control in type 2 diabetes. AGIs delay the absorption of carbohydrates by the gut, by inhibiting alpha-glucosidase in the small intestine, and thus have an effect on lowering postprandial blood glucose and insulin levels [1]–[3]. It was postulated that due to its mode of action, AGIs might be more efficacious in Asian population following an eastern diet with higher carbohydrate content than Caucasian population following a western diet [4], [5]. Although this is a reasonable assumption, it was not evidence based. Recently, there are some systemic reviews or meta-analysis evaluating the efficacy and safety of alpha-glucosidase inhibitors mainly in Caucasians [6], [7]. The present meta-analysis evaluated the clinical evidence of efficacy of AGIs in Asians and Caucasians and made a comparison of efficacy of AGIs between Asians and Caucasians.
Methods
Search Strategy
The following databases for primary studies during the stipulated period of time were searched: MEDLINE® (1966 to June 2012), EMBASE® (1974 to June 2012), the Cochrane Central Register of Controlled Trials (CENTRAL; 1966 to June 2012). The electronic search was first conducted in January 2012 and then repeated in June 2012. The main search concepts were type 2 diabetes, alpha-glucosidase inhibitors, acarbose, miglitol, voglibose, randomized controlled trials, Asian, Chinese, Japanese, Korean, Indian, etc. The PubMed strategy formed the basis for the strategies developed for the other electronic databases. We searched for additional trials in the prescribing information documents of approved medications, at relevant Web sites (http://www.clinicalstudyresults.org and http://www.clinicaltrials.gov).
Study Selection
We defined anti-hyperglycemia efficacy of AGIs as placebo adjusted HbA1c changes from baseline after administration of AGIs treatment in placebo control in randomized clinical trials when we made a comparison of efficacy of AGIs between Asians and Caucasians. We also try to summarize the information on the relative efficacies of AGIs against other hypoglycemic agent by comparing the absolute reduction of HbA1c from baseline after administration of AGIs and other active oral hypoglycemia comparators in randomized clinical trials. Based on these analysis goal, we defined the inclusion criteria of studies as following: 1) placebo or active oral hyperglycemic agents controlled randomized controlled trials carried out in Asian countries as Asians; 2) placebo or active oral hyperglycemic agent controlled randomized controlled trials carried out in western countries as Caucasians; 2) The length of study was at least 12 weeks; 3) The index of glucose changes was change of HbA1c and fasting plasma glucose during the clinical trial from the baseline in the comparative groups. The contents of 441 abstracts or full-text manuscripts identified through the literature search were reviewed independently by two investigators (CXL, LYY) in duplicate to determine whether the study met eligibility criteria for inclusion. Where discrepancies between investigators occurred for inclusion or exclusion, a third investigator (HXY) was involved to conduct additional assessment of the study and discrepancies were resolved by discussion.
A validated 3-item scale was used to evaluate the overall reporting quality of the trials selected for inclusion in the present review. This scale provided scoring for randomization (0–2 points), double blinding (0–2 points), and withdrawals (1 point). Scores ranged from 0 to 5, and scores≥3 indicated a study of high quality [8].
Data Abstraction
Similar to study selection, data abstraction was completed by two independent investigators (CXL, LYY). Discrepancies between the results of the abstraction were resolved by discussion and simultaneous reference to the relevant articles. Using a standardized form, the following data were collected: title, primary author’s name, year and source of publication, country of origin, study design, treatment allocation procedures, baseline characteristics of the study population (sample size, age, diabetic duration, HbA1c), description of the drug therapies, duration of the treatment, outcomes of diabetes (changes of HbA1c, fasting plasma glucose, fasting insulin, body weight, and hypoglycemic events). If data concerning the outcome were missing from an article, then the investigators attempted to contact the primary author in order to obtain the missing data.
Statistical Analysis
This meta-analysis was conducted according to the PRISMA guidelines for the conduct and reporting of meta-analyses of RCTs [9]. Heterogeneity of the effect across studies was assessed by Q 2 statistics, which is distributed as χ2 statistics. A value of P<0.10 was used to indicate lack of homogeneity among effects. I 2 statistics were provided to quantify the percentage of total variation across studies that was attributable to heterogeneity rather than to chance. A value >50% represented substantial variability. A fixed-effect model was used when no significant heterogeneity was detected among subgroups. When significant heterogeneity was detected, its sources were first analyzed. In the absence of obvious clinical or other sources of heterogeneity, a random-effect model was used. We assessed publication bias by visual inspection of the funnel plot. All analyses were conducted in Review Manager, version 5.1. For continuous variables (HbA1c, fasting plasma glucose, weight, etc.), we calculated weighted mean differences (WMD) and 95% confidence intervals (CIs) for change from baseline in AGI vs comparator (placebo or active hypoglycemic agent) groups. Analyses were also made to test the subgroup differences between different ethnic groups. Randomized controlled trials carried out in Asian countries were defined as Asians, randomized controlled trials carried out in western countries were defined as Caucasians.
Results
Flow of Included Studies
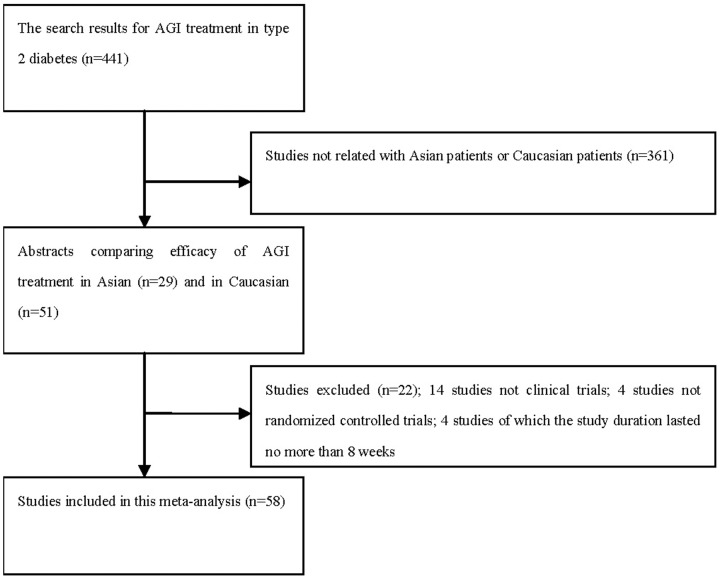
From the search, after a review of the titles and abstracts, 441 abstracts were deemed eligible for further review, among which 29 studies were carried out in Asian population and 51 studies were in Caucasian population, and these studies were retrieved for more detailed evaluation. Then, 22 studies were excluded, in which, 14 studies were not clinical trials, 4 studies were not randomized controlled trials, and 4 studies lasted no more than 8 weeks. Figure 1 showed the flowchart of included studies in this analysis. According to the criteria of inclusion, a total of 58 studies were included in this meta-analysis. (Table S1 shows the characteristics of RCTs included.).
Figure 1. The flowchart of included studies.
Study Characteristics
There were totally 19 studies in Asian(13 for acarbose, 3 for voglibose, 3 for miglitol),in which 12 published trials comparing an AGI with placebo, given as monotherapy [10]–[13] or add-on therapy to other hypoglycemic agents [14]–[21], 8 published studies comparing an AGI with an active agents, given as monotherapy [12], [22]–[26] or add-on thearapy [27], [28]. There were totally 39 RCTs in Caucasians, in which 35 studies comparing an AGI with placebo (28 for acarbose, 7 for miglitol), given as monotherapy [29]–[48], [70], [71] or add-on thearapy [31], [42], [49]–[60], and 15studies comparing an AGI with an active agent (13 for acarbose, 2 for miglitol), given as monotherapy [32], [35], [37], [38], [46], [61]–[63], [70] or add-on therapy [57], [58], [64]–[68].
Methodological Quality
All studies were randomized controlled trials including a control group (placebo or oral hypoglycemic agents). Eligibility criteria were clearly reported in all trials. The studies that were funded by pharmaceutical companies were clearly disclosed. Studies were restricted to randomized controlled trials to ensure the inclusion of only high-quality evidence.
Changes in HbA1c for AGI Treatment vs Placebo or Active Agents in Asian
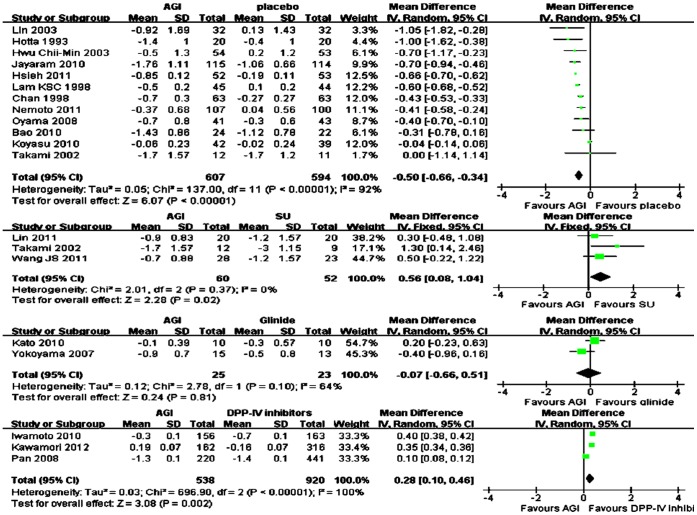
Overall, AGI treatment (n = 607) was significantly associated with more reduction of mean HbA1c from baseline compared with placebo (n = 594) in Asians (WMD in change from baseline, −0.50%; 95% CI, −0.66% to −0.34%) (Figure 2). For monotherapy, compared with placebo (n = 133), AGI treatment (n = 137) was also significantly associated with more mean HbA1c change from baseline (WMD, −0.35%; 95% CI, −0.69% to 0.00%), and for add-on therapy, the reduction of mean HbA1c from baseline was also significantly more in AGI treatment (n = 470) compared with placebo (n = 461) (WMD, −0.59%; 95% CI, −0.67% to −0.50%). Among these studies, there were nine studies comparing arcabose with placebo, two studies comparing miglitol with placebo. Compared with placebo, arcabose treatment and miglitol treatment both were associated with more reduction of mean HbA1c from baseline (WMD for arcabose, −0.51%; 95% CI, −0.72% to −0.30%; WMD for miglitol, −0.64%; 95% CI, −0.69% to −0.60% respectively). Compared with sulfonylurea (SU) treatment (n = 52), AGI treatment (n = 60) was significantly associated with less reduction of mean HbA1c from baseline (WMD, 0.56%; 95% CI, 0.08% to 1.04%. Compared with DPP-IV inhibitors treatment (n = 920), AGI treatment (n = 538) was significantly associated with less reduction of mean HbA1c from baseline (WMD, 0.28%; 95% CI, 0.27% to 0.29%). Compared with glinide treatment (n = 23), the reduction of mean HbA1c from baseline was comparable with AGI treatment (n = 25) (WMD, −0.02%; 95% CI, −0.36% to 0.32%) (Figure 2).
Figure 2. Weighted mean difference in change in HbA1c for AGI treatment versus placebo or active control in Asian.
Changes in HbA1c for AGI Treatment vs Placebo or Active Agents in Caucasian
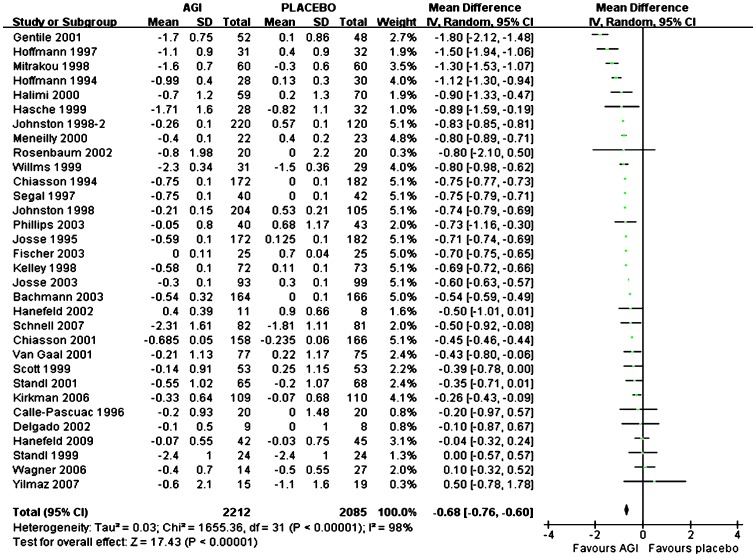
Overall, mean HbA1c reduction from baseline was significantly more with AGI treatment (n = 2212) compared with placebo (n = 2085) in Caucasians (WMD, −0.68%; 95% CI, −0.76% to −0.60%) (Figure 3). For monotherapy, compared with placebo (n = 1112), mean HbA1c reduction from baseline was significantly more with AGI treatment (n = 1275) (WMD, −0.68%; 95% CI, −0.77% to −0.58%), and for add-on therapy, mean HbA1c reduction from baseline was also significantly more with AGI treatment (n = 899) compared with placebo (n = 934) (WMD, −0.66%; 95% CI, −0.74% to −0.58%). Among these studies, there were twenty-five studies comparing arcabose with placebo, seven studies comparing miglitol with placebo. Compared with placebo, arcabose treatment and miglitol treatment both were associated with more reduction of mean HbA1c from baseline (WMD for arcabose, −0.68%; 95% CI, −0.74% to −0.61%; WMD for miglitol, −0.71%; 95% CI, −0.90% to −0.52% respectively). Compared with SUs treatment (n = 258), the reduction of mean HbA1c from baseline was comparable with AGI treatment (n = 259) (WMD, 0.29%; 95% CI, −0.37% to 0.95%). Compared with metformin treatment (n = 182), the reduction of mean HbA1c from baseline was comparable with AGI treatment (n = 168) (WMD, 0.35%; 95% CI, −0.15% to 0.86%). Compared with TZDs treatment (n = 336), AGI treatment (n = 326) was associated with significantly less reduction of mean HbA1c from baseline (WMD, 0.52%; 95% CI, 0.41% to 0.62%).
Figure 3. Weighted mean difference in change in HbA1c for AGI treatment versus placebo in Caucasian.
Changes in Fasting Plasma Glucose (FPG) for AGI Treatment vs Placebo or Active Agents in Asian
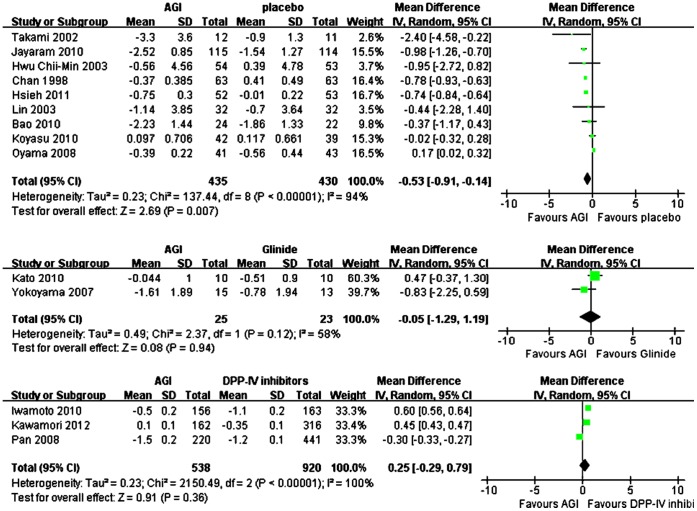
Overall, mean FPG reduction from baseline was significantly more with AGI treatment (n = 435) compared with placebo (n = 430) in Asians (WMD, −0.53 mmol/L; 95% CI, −0.91 to −0.14 mmol/L) (Figure 4). For monotherapy, compared with placebo (n = 113), mean FPG reduction from baseline was comparable with AGI treatment (n = 117) (WMD, −0.59 mmol/L; 95% CI, −1.32 to 0.14 mmol/L), and for add-on therapy, mean FPG reduction from baseline was also comparable with AGI treatment (n = 318) compared with placebo (n = 317) (WMD, −0.51 mmol/L; 95% CI, −1.07 to 0.04 mmol/L). Among these studies, there were seven studies comparing arcabose with placebo, and compared with placebo, arcabose treatment was associated with comparable reduction of mean FPG from baseline (WMD for arcabose, −0.43 mmol/L; 95% CI, −0.92 mmol/L to 0.07 mmol/L). Compared with DPP-IV inhibitors treatment (n = 920), AGI treatment (n = 538) was associated with comparable reduction of mean FPG from baseline (WMD, 0.25 mmol/L; 95% CI, −0.29 mmol/L to 0.79 mmol/L). Compared with glinide treatment (n = 23), the reduction of mean FPG from baseline was also comparable with AGI treatment (n = 25) (WMD, −0.05 mmol/L; 95% CI, −1.29 mmol/L to 1.19 mmol/L) (Figure 4).
Figure 4. Weighted mean difference in changes in FPG for AGI treatment versus placebo or active control in Asian.
Changes in Fasting Plasma Glucose (FPG) for AGI Treatment vs Placebo or Active Agents in Caucasian
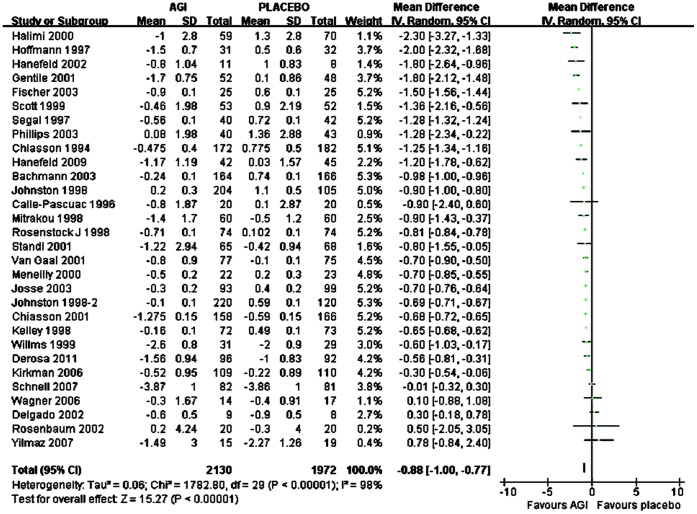
Overall, mean FPG reduction from baseline was significantly more with AGI treatment (n = 2130) compared with placebo (n = 1972) in Caucasians (WMD, −0.88 mmol/L; 95% CI, −1.00 to −0.77 mmol/L) (Figure 5). For monotherapy, compared with placebo (n = 1026), mean FPG reduction from baseline was significantly more with AGI treatment (n = 1220) (WMD, −0.94 mmol/L; 95% CI, −1.18 to −0.71 mmol/L), and for add-on therapy, mean FPG reduction from baseline was also significantly more with AGI treatment (n = 949) compared with placebo (n = 984) (WMD, −0.84 mmol/L; 95% CI, −0.98 to −0.69 mmol/L). Among these studies, there were twenty-three studies comparing arcabose with placebo and seven studies comparing miglitol with placebo. Compared with placebo, arcabose treatment and miglitol treatment both were associated with more reduction of mean FPG from baseline (WMD for arcabose, −0.90 mmol/L; 95% CI, −1.04 mmol/L to −0.75 mmol/L; WMD for miglitol, −0.86 mmol/L; 95% CI, −1.08 mmol/L to −0.63 mmol/L respectively). Compared with SUs treatment (n = 231), the reduction of mean FPG from baseline was comparable with AGI treatment (n = 231) (WMD, 0.67 mmol/L; 95% CI, −0.65 mmol/L to 2.00 mmol/L). Compared with metformin treatment (n = 182), the reduction of mean FPG from baseline was comparable with AGI treatment (n = 168) (WMD, −0.15 mmol/L; 95% CI, −1.11 mmol/L to 0.81 mmol/L). Compared with TZDs treatment (n = 336), AGI treatment (n = 326) was associated with comparable reduction of mean FPG from baseline (WMD, 0.70 mmol/L; 95% CI, −0.41 mmol/L to 1.81 mmol/L).
Figure 5. Weighted mean difference in changes in FPG for AGI treatment versus placebo in Caucasian.
Changes in Fasting Insulin for AGI Treatment vs Placebo or Active Agents in Asian
Overall, mean fasting insulin reduction from baseline was significantly more with AGI treatment (n = 242) compared with placebo (n = 241) in Asians (WMD, −0.78 uU/ml; 95% CI, −0.96 to −0.59 uU/ml). For monotherapy, compared with placebo (n = 113), mean fasting insulin reduction from baseline was more with AGI treatment (n = 117) but without significance (WMD, −0.31 uU/ml; 95% CI, −1.13 to 0.51 uU/ml), and for add-on therapy, mean fasting insulin reduction from baseline was significantly more with AGI treatment (n = 125) compared with placebo (n = 128) (WMD, −0.80 uU/ml; 95% CI, −1.00 to −0.61 uU/ml). Among these studies, there were four studies comparing arcabose with placebo, and compared with placebo, arcabose treatment was associated with significantly more reduction of mean fasting insulin from baseline (WMD for arcabose, −0.78 uU/ml; 95% CI, −0.97 uU/ml to −0.59 uU/ml). Compared with DPP-IV inhibitors treatment (n = 479), AGI treatment (n = 318) was associated with significantly more reduction of mean fasting insulin from baseline (WMD, −0.54 uU/ml; 95% CI, −0.78 uU/ml to −0.30 uU/ml). Compared with glinide treatment (n = 23), the reduction of mean fasting insulin from baseline was comparable with AGI treatment (n = 25) (WMD, −0.65 uU/ml; 95% CI, −4.42 uU/ml to 3.12 uU/ml).
Changes in Fasting Insulin for AGI Treatment vs Placebo or Active Agents in Caucasian
Overall, mean fasting insulin reduction from baseline was more with AGI treatment (n = 1293) compared with placebo (n = 1125) in Caucasians but without significance (WMD, −1.24 uU/ml; 95% CI, −2.51 to 0.04 uU/ml). For monotherapy, compared with placebo (n = 674), mean fasting insulin reduction from baseline was more with AGI treatment (n = 860) but without significance (WMD, −1.25 uU/ml; 95% CI, −2.71 to 0.20 uU/ml), and for add-on therapy, mean fasting insulin reduction from baseline was more with AGI treatment (n = 433) compared with placebo (n = 451) without significance (WMD, −1.11 uU/ml; 95% CI, −4.11 to 1.89 uU/ml). Among these studies, there were fifteen studies comparing arcabose with placebo and five studies comparing miglitol with placebo. Compared with placebo, arcabose treatment was associated with more reduction of mean fasting insulin from baseline (WMD for arcabose, −1.62 uU/ml; 95% CI, −3.21 uU/ml to −0.03 uU/ml), and miglitol treatment was associated with comparable reduction of mean fasting insulin from baseline (WMD for miglitol, −0.11 uU/ml; 95% CI, −1.20 uU/ml to 0.98 uU/ml). Compared with SUs treatment (n = 92), the reduction of mean fasting insulin from baseline was significantly more with AGI treatment (n = 91) (WMD, −0.38 uU/ml; 95% CI, −0.51 uU/ml to −0.26 uU/ml). Compared with metformin treatment (n = 123), the reduction of mean fasting insulin from baseline was comparable with AGI treatment (n = 122) (WMD, 1.99 uU/ml; 95% CI, −2.84 uU/ml to 6.81 uU/ml).
Changes in Body Weight for AGI Treatment vs Placebo or Active Agents in Asian
Body weight reduction from baseline was significantly more with AGI treatment (n = 216) compared with placebo (n = 210) in Asians (WMD, −1.00 kg; 95% CI, −1.69 kg to −0.31 kg). Among these studies, there were four studies comparing arcabose with placebo, and compared with placebo, arcabose treatment was associated with significantly more reduction of mean weight from baseline (WMD for arcabose, −1.02 kg; 95% CI, −1.73 kg to −0.31 kg). Compared with DPP-IV inhibitors treatment (n = 604), AGI treatment (n = 376) was associated with significantly more reduction of mean weight from baseline (WMD, −1.00 kg; 95% CI, −1.59 kg to −0.40 kg). Compared with SUs treatment (n = 52), the reduction of mean weight from baseline was comparable with AGI treatment (n = 60) (WMD, −1.61 kg; 95% CI, −6.59 kg to 3.37 kg).
Changes in Body Weight for AGI Treatment vs Placebo or Active Agents in Caucasian
In Caucasians, body weight reduction from baseline was significantly more with AGI treatment (n = 653) compared with placebo (n = 671) (WMD, −0.73 kg; 95% CI, −1.13 to −0.33 kg). Among these studies, there were ten studies comparing arcabose with placebo and four studies comparing miglitol with placebo. Compared with placebo, arcabose treatment was associated with comparable reduction of mean weight from baseline (WMD for arcabose, −0.33 kg; 95% CI, −0.67 kg to 0.01 kg), and miglitol treatment was associated with significantly more reduction of mean weight from baseline (WMD for miglitol, −1.17 kg; 95% CI, −1.63 kg to −0.7 kg). Compared with SUs treatment (n = 175), the reduction of mean weight from baseline was significantly more with AGI treatment (n = 173) (WMD, −2.77 kg; 95% CI, −3.3 kg to −2.24 kg). Compared with metformin treatment (n = 142), the reduction of mean weight from baseline was comparable with AGI treatment (n = 128) (WMD, −0.45 kg; 95% CI, −2.04 kg to 1.14 kg).
Comparisons between Asian and Caucasian
Comparisons of HbA1c changes from baseline between Asian and Caucasian showed that when AGI was compared with placebo, the between-group difference was 0.08% without significance (95% CI, −0.21% to 0.38%, P>0.05), and when arcabose or miglitol was compared with placebo, the between-group difference was also without significance, respectively. Comparison of FPG changes between groups showed that when AGI was compared with placebo, the between-group difference was 0.31 mmol/L (95% CI, −0.22 to 0.84 mmol/L, P>0.05). When AGI was compared with placebo, the between-group difference of fasting insulin level was not significantly different (0.39 uU/ml (95% CI, −1.83 to 2.61 uU/ml, P>0.05), and the between-group difference of body weight was also comparable (0.51 kg (95% CI, −2.46 to 3.47 kg, P>0.05). Details were shown in Table 1.
Table 1. Comparisons between Asian and Caucasian in the WMD in changes of efficacy of AGI treatment versus placebo or active control treatment.
| Asian | Caucasian | Difference between two groups | ||||
| WMD | 95% CI | WMD | 95% CI | Difference | 95% CI | |
| HbA1c(%) | ||||||
| AGI vs placebo in monotherapy | −0.35 | −0.69 to 0.00 | −0.68 | −0.77 to −0.58 | 0.28 | −0.26 to 0.82 |
| AGI vs placebo in add-on therapy | −0.59 | −0.67 to −0.50 | −0.66 | −0.74 to −0.58 | −0.06 | −0.43 to 0.31 |
| AGI vs placebo (total) | −0.50 | −0.66 to −0.34 | −0.68 | −0.76 to −0.60 | 0.08 | −0.21 to 0.38 |
| Arcabose vs placebo | −0.51 | −0.72 to −0.30 | −0.68 | −0.74 to −0.61 | 0.01 | −0.35 to 0.37 |
| Miglitol vs placebo | −0.64 | −0.69 to −0.60 | −0.71 | −0.90 to −0.52 | 0.16 | −0.43 to 0.74 |
| AGI vs SU | 0.56 | 0.08 to 1.04 | 0.29 | −0.37 to 0.95 | 0.54 | −1.01 to 2.10 |
| AGI vs glinide | −0.02 | −0.36 to 0.32 | / | / | / | / |
| AGI vs DPP-IV inhibitors | 0.28 | 0.27 to 0.29 | / | / | / | / |
| AGI vs metformin | / | / | 0.35 | −0.15 to 0.86 | / | / |
| AGI vs TZDs | / | / | 0.52 | 0.41 to 0.62 | / | / |
| FPG(mmol/L) | ||||||
| AGI vs placebo in monotherapy | −0.59 | −1.32 to 0.14 | −0.94 | −1.18 to −0.71 | 0.46 | −0.66 to 1.58 |
| AGI vs placebo in add-on therapy | −0.51 | −1.07 to 0.04 | −0.84 | −0.98 to −0.69 | 0.23 | −0.43 to 0.89 |
| AGI vs placebo (total) | −0.53 | −0.91 to −0.14 | −0.88 | −1.00 to −0.77 | 0.31 | −0.22 to 0.84 |
| Arcabose vs placebo | −0.43 | −0.92 to 0.07 | −0.90 | −1.04 to −0.75 | 0.32 | −0.31 to 0.99 |
| Miglitol vs placebo | / | / | −0.86 | −1.08 to −0.63 | / | / |
| AGI vs SU | / | / | 0.67 | −0.65 to 2.00 | / | / |
| AGI vs glinide | −0.05 | −1.29 to 1.19 | / | / | / | / |
| AGI vs DPP-IV inhibitors | 0.25 | −0.29 to 0.79 | / | / | / | / |
| AGI vs metformin | / | / | −0.15 | −1.11 to 0.81 | / | / |
| AGI vs TZDs | / | / | 0.70 | −0.41 to 1.81 | / | / |
| Fasting insulin (uU/ml) | ||||||
| AGI vs placebo in monotherapy | −0.31 | −1.13 to 0.51 | −1.25 | −2.71 to 0.20 | 1.40 | −2.01 to 4.81 |
| AGI vs placebo in add-on therapy | −0.80 | −1.00 to −0.61 | −1.11 | −4.11 to 1.89 | −0.70 | −4.01 to 2.61 |
| AGI vs placebo (total) | −0.78 | −0.96 to −0.59 | −1.24 | −2.51 to 0.04 | 0.39 | −1.83 to 2.61 |
| Arcabose vs placebo | −0.78 | −0.97 to −0.59 | −1.62 | −3.21 to −0.03 | 0.13 | −2.78 to 3.04 |
| Miglitol vs placebo | / | / | −0.11 | −1.20 to 0.98 | / | / |
| AGI vs SU | / | / | −0.38 | −0.50 to −0.26 | / | / |
| AGI vs glinide | −0.65 | −4.42 to 3.12 | / | / | / | / |
| AGI vs DPP-IV inhibitors | −0.54 | −0.78 to −0.30 | / | / | / | / |
| AGI vs metformin | / | / | 1.99 | −2.84 to 6.81 | / | / |
| Weight(kg) | ||||||
| AGI vs placebo (total) | −1.00 | −1.69 to −0.31 | −0.73 | −1.13 to −0.33 | 0.51 | −2.46 to 3.47 |
| Arcabose vs placebo | −1.02 | −1.73 to −0.31 | −0.33 | −0.67 to 0.01 | 0.37 | −3.51 to 4.24 |
| Miglitol vs placebo | / | / | −1.17 | −1.63 to −0.70 | / | / |
| AGI vs SU | −1.61 | −6.59 to 3.37 | −2.77 | −3.30 to −2.24 | 1.13 | −1.75 to 4.02 |
| AGI vs DPP-IV inhibitors | −1.00 | −1.59 to −0.40 | / | / | / | / |
| AGI vs metformin | / | / | −0.45 | −2.04 to 1.14 | / | / |
Discussion
In this meta-analysis, we had analysis the effect of AGIs on glucose, insulin secretion and weight against placebo or other active oral hypoglycemic agents in Asian and Caucasian type 2 diabetes patients. The analysis suggested that treatment with AGIs (acarbose, voglibose and miglitol) lead to comparable changes of HbA1c and body weight in type 2 diabetes patients in Asian and Caucasian population when compared with placebo and other active oral hypoglycemic agents. In this analysis, we also observed a comparable change of insulin level between Asian and Caucasian population associated with AGIs treatment.
In terms of clinical efficacy, as measured by the reduction in HbA1c and FPG from baseline after adjusting placebo effect, across trials of 12–52 weeks, AGI treatment produced a mean HbA1c reduction difference of 0.50% and a mean FPG reduction difference of 0.53 mmol/L, respectively, in comparison with placebo in Asian. But compared with active agents (mainly sulphonylurea), HbA1c was reduced not favoring AGI treatment. In Caucasians, AGI treatment produced comparable HbA1c and FPG reduction from baseline (0.68% and 0.88 mmol/L respectively) after adjusting placebo effect. The results of our meta-analysis are in accordance with some results concluded mainly in Caucasians previously. As one meta-analysis reported by Van de Laar [6] indicated that in clinical trials (36 trials in Caucasians and 5 trials in Asians), after adjusting placebo effect, acarbose decreased HbA1c by 0.77% (95% CI 0.64–0.90%) and miglitol by 0.68% (95% CI 0.44–0.93%), voglibose, yielded a difference of 0.47% in favor of voglibose (95% CI 0.31–0.63%). For FPG, after adjusting for placebo effects, acarbose treatment is associated with a mean FPG reduction of 1.09 mmol/l (28 comparisons; 95% CI 0.83–1.36), miglitol 0.52 mmol/l (2 comparisons; 95% CI 0.16–0.88), and voglibose 0.60 mmol/l (1 comparison; 95% CI 0.23–0.97). And the overall comparison of acarbose with sulfonylurea yielded a non-significant HbA1c reduction of 0.38% (95% CI −0.02–0.77%) favoring sulfonylurea treatment. Derosa [7] in a systemic review (15 trials in Caucasians and 4 trials in Asians) concluded that treatment with acarbose was more effective than placebo in improving HbA1c levels and in reducing FPG levels after 7 months of therapy, but did not give the exact weighted mean difference.
In terms of insulin secretion, compared with placebo or active agents, treatment with AGI in Asian showed a more reduction in fasting insulin from baseline of 0.78 uU/ml and 0.55 uU/ml respectively. While in Caucasians, AGI treatment showed a decrease in fasting insulin of 1.24 uU/ml when compared with placebo and a trend of increase when compared with active agents. However, there was no significant difference between Asian and Caucasian in fasting insulin level changes in response to AGI treatment. Van de Laar [6] reported that compared with placebo, acarbose had no effect on fasting insulin levels and a lowering effect on 1-h postload insulin levels of 40.8 pmol/l (95% CI 21.0–50.6pmol/l) mainly in Caucasians. For systemic review in Asian patients, no comparison is available.
In terms of weight changes, treatment of AGI produced a weight reduction of 1.0 kg either when compared with placebo or active agents in Asian. In Caucasians, AGI treatment produced a weight decrease of 0.73 kg and 1.79 kg respectively compared with placebo and active agents. Weight changes between Asian and Caucasian after adjusting placebo effect were also comparable. However, what we found seems to be different from previous studies. Van de Laar [6] found in a meta analysis concluded mainly in Caucasians (36 trials in Caucasians and 5 trials in Asians) that acarbose had a statistically significant decreasing effect on BMI of 0.17 kg/m2, but the effect on the outcome “body weight” was not statistically significant.
In dis-concordance with the result of our meta analysis, A recent meta-analysis [69] had shown that acarbose achieved a greater absolute reduction of HbA1c levels in the Eastern diet type 2 diabetes population than in the Western diet type 2 diabetes population. Based on this observation, the author suggested that AGIs are more efficacious in type 2 diabetes of eastern population. Although this was an interesting observation, we had noticed that qualities of some studies in Eastern diet group in this article were low level and should not be included in the meta-analysis for reason of publication bias and performance bias.
As a meta-analysis, we should admit that there are several potential limitations. The glycemic control of the AGI group and the control group was not optimal in several studies. The included studies used different targets for HbA1c or FPG to guide the titration of hypoglycemic agents. The including criteria and the baseline characteristics of selected studies were different. Most of the trials were not long term, generally lasting less than 1 year, and few evaluated important clinical outcomes, such as cardiovascular events and death. Reporting bias may also be a concern. Whatever, we pooled the results of a group of trials with the aim of evaluating the efficacy and other non-glycemic effects of AGI treatment in Asian, and drawing comparisons between Asian and Caucasians of these effects.
This article may be the first research that made a whole systemic review of AGI treatment in Asian and also the first research that made comparisons of efficacy of AGI treatment between Asian and Caucasian. The observation made from this study might provide evidence for guideline development and clinical treatment. According to this meta-analysis, what we have found is that the efficacy in glucose lowering, body weight reduction and insulin secretion decreasing of AGI treatment in Asian is comparable with that in Caucasian.
Supporting Information
Characteristics of Randomized Controlled Trials of Alpha glucosidase Inhibitors Included in the Systematic Review.
(DOCX)
A PRISMA checklist for this meta-analysis.
(DOC)
Acknowledgments
We are grateful to the doctors, nurses as well as assistants for their practical work during the study at Peking University People’s Hospital Endocrinology & Metabolism Department.
Funding Statement
This meta-analysis was supported by National Natural Science Foundation of China (NSFC) 81000334. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, et al. (1994) The efficacy of acarbose in the treatment of patients with NIDDM: a multicenter controlled clinical trial. Ann Intern Med 121: 928–935. [DOI] [PubMed] [Google Scholar]
- 2. Lebovitz HE (1998) α-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev 6 (2): 132–45. [Google Scholar]
- 3. Laube H (2002) Acarbose: an update of its therapeutic use in diabetes treatment. Clin Drug Invest 22 (3): 141–56. [Google Scholar]
- 4. Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 13: 3–9. [DOI] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations (2013) Dimensions of need- Staple foods: what do people eat? http://www.fao.org/docrep/u8480e/u8480e07.htm.
- 6. Van De Laar FA, Lucassen PL, Akkermans RP, et al. (2005) α-Glucosidase Inhibitors for Patients With Type 2 Diabetes. Results from a Cochrane systematic review and meta-analysis. Diabetes Care 28: 166–175. [DOI] [PubMed] [Google Scholar]
- 7. Derosa G, Maffioli P (2012) Efficacy and Safety Profile Evaluation of Acarbose Alone and in Association With Other Antidiabetic Drugs: A Systematic Review Clin Ther. 34: 1221–1236. [DOI] [PubMed] [Google Scholar]
- 8. Jadad AR, Moore RA, Carroll D, et al. (1996) Assessing the quality of randomized controlled trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 9. Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P (2009) Comparison of Registered and Published Primary Outcomes in Randomized Controlled Trials. JAMA 302(9): 977–984. [DOI] [PubMed] [Google Scholar]
- 10. Chan JC, Chan KW, Ho LL, Fuh MM, Horn LC, et al. (1998) An Asian multicenter clinical trial to assess the efficacy and tolerability of acarbose compared with placebo in type 2 diabetic patients previously treated with diet. Asian Acarbose Study Group. Diabetes Care 21(7): 1058–61. [DOI] [PubMed] [Google Scholar]
- 11. Hotta N, Kakuta H, Sano T, Matsumae H, Yamada H, et al. (1993) Long-term effect of acarbose on glycaemic control in non-insulin-dependent diabetes mellitus: a placebo-controlled double-blind study. Diabet Med 10(2): 134–8. [DOI] [PubMed] [Google Scholar]
- 12. Takami K, Takeda N, Nakashima K, Takami R, Hayashi M, et al. (2002) Effects of Dietary Treatment Alone or Diet With Voglibose or Glyburide on Abdominal Adipose Tissue and Metabolic Abnormalities in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes Care 25: 658–662. [DOI] [PubMed] [Google Scholar]
- 13. Koyasu M, Ishii H, Watarai M, Takemoto K, Inden Y, et al. (2010) Impact of Acarbose on Carotid Intima-Media Thickness in Patients With Newly Diagnosed Impaired Glucose Tolerance or Mild Type 2 Diabetes Mellitus: A One-Year, Prospective, Randomized, Open-Label, Parallel-Group Study in Japanese Adults With Established Coronary Artery Disease.Clin Ther. 32: 1610–1617. [DOI] [PubMed] [Google Scholar]
- 14. Jayaram S, Hariharan RS, Madhavan R, Periyandavar I, Samra SS (2010) A Prospective, Parallel Group, Open-Labeled, Comparative, Multi-centric, Active Study to Evaluate the Safety, Tolerability and Benefits of Fixed Dose Combination o and Metformin versus Metformin Alone in Type 2 Diabetes. Journal of Association Physicians of India 58: 12–16. [PubMed] [Google Scholar]
- 15. Bao YQ, Zhou J, Zhou M, Cheng YJ, Lu W, et al. (2010) Glipizide controlled-release tablets, with or without acarbose, improve glycaemic variability in newly diagnosed Type 2 diabetes. Clinical and Experimental Pharmacology and Physiology 37: 564–568. [DOI] [PubMed] [Google Scholar]
- 16. Hwu CM, Ho LT, Fuh MMT, Siu SC, Sutanegara D, et al. (2003) The Asian Acarbose Study Group. Acarbose improves glycemic control in insulin-treated Asian type 2 diabetic patients: Results from a multinational, placebocontrolled study. Diabetes Research and Clinical Practice 60: 111–118. [DOI] [PubMed] [Google Scholar]
- 17. Lin BJ, Wu HP, Huang HS, Huarng J, Sison A, et al. (2003) The Writing Group for the Asian Study of Acarbose with Sulfonylureas. Efficacy and tolerability of acarbose in Asian patients with type 2 diabetes inadequately controlled with diet and sulfonylureas. Journal of Diabetes and Its Complications 17: 179–185. [DOI] [PubMed] [Google Scholar]
- 18. Lam KSL, Tiu SC, Tsang MW, IP TR, Tam SCF (1998) Acarbose in NIDDM Patients With Poor Control on Conventional Oral Agents. A 24-week placebo-controlled study. Dabetes Care 21(7): 1154–58. [DOI] [PubMed] [Google Scholar]
- 19. Oyama T (2008) Effect of acarbose, an alpha-glucosidase inhibitor, or serum lipoprotein lipase mass levels and common carotid artery intima-media thickness in type 2 diabetes mellitus treated by sulfonylurea. Journal of atherosclerosis and thrombosis 15: 154–159. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh SH, Shih KC, Chou CW, Chu CH (2011) Evaluation of the efficacy and tolerability of miglitol in Chinesepatients with type 2 diabetes mellitus inadequately controlled by diet and sulfonylureas. ActaDiabetol 48: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nemoto M, Tajima N, Kawamori R (2011) Efficacy of combined use of miglitol in Type 2 diabetes patients receiving insulin therapy-placebo-controlled double-blind comparative study. Acta Diabetol 48: 15–20. [DOI] [PubMed] [Google Scholar]
- 22. Kato T, Inoue T, Node K (2010) Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: an acarbose and nateglinide comparative study. Cardiovascular Diabetology 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yokoyama H, Kannno S, Ishimura I, Node K (2007) Miglitol increases the adiponectin level and decreases urinary albumin excretion in patients with type 2 diabetes mellitus. Metabolism Clinical and Experimental 56: 1458–1463. [DOI] [PubMed] [Google Scholar]
- 24. Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, et al. (2012) Linagliptinmonotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes, Obesity and Metabolism 14: 348–357. [DOI] [PubMed] [Google Scholar]
- 25.Pan C, Yang W, Barona JP, Wang Y, Niggli M, et al. (2008) Comparison of vildagliptin and acarbosemonotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet. Med 25, 435–441. [DOI] [PubMed]
- 26. Iwamoto Y, Tajima N, Kadowaki T, Nonaka K, Taniguchi T, et al. (2010) Efficacy and safety of sitagliptinmonotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind trial. Diabetes, Obesity and Metabolism 12: 613–622. [DOI] [PubMed] [Google Scholar]
- 27. Wang JS, Lin SD, Lee WJ, Su SL, Lee IT, et al. (2011) Effects of Acarbose Versus Glibenclamide on Glycemic Excursion and Oxidative Stress in Type 2 Diabetic Patients Inadequately Controlled by Metformin: A 24-Week, Randomized, Open-Label, Parallel-Group Comparison. ClinTher 33: 1932–1942. [DOI] [PubMed] [Google Scholar]
- 28. Lin SD, Wang JS, Hsu SR, Sheub WHH, Tu ST, et al. (2011) The beneficial effect of α-glucosidase inhibitor on glucose variability compared with sulfonylurea in Taiwanese type 2 diabetic patients inadequately controlled with metformin: preliminary data. Journal of Diabetes and Its Complications 25: 332–338. [DOI] [PubMed] [Google Scholar]
- 29. Calle-Pascuac A (1996) Influence of 16-week monotherapy with acarbose on cardiovascular risk factors in obese subjects with non-insulin-dependent DM: a controlled, double-blind comparison study with placebo. Diabetes & Metabolism 22: 201–202. [PubMed] [Google Scholar]
- 30. Derosa G, Maffioli P, D’Angelo A, Fogari E, Bianchi L, et al. (2011) Acarbose on insulin resistance after an oral fat load: a double-blind, placebo controlled study. Journal of Diabetes and Its Complications 25: 258–266. [DOI] [PubMed] [Google Scholar]
- 31. Chiasson JL (1994) The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus: a multicenter, controlled clinical trial. Ann Intern Med 121: 928–935. [DOI] [PubMed] [Google Scholar]
- 32. Chiasson JL, Naditch L (2001) For the Miglitol Canadian University Investigator group (2001) The Synergistic Effect of Miglitol Plus Metformin Combination Therapy in the Treatment of Type 2 Diabetes. Diabetes Care 24: 989–994. [DOI] [PubMed] [Google Scholar]
- 33. Delgado H (2002) Acarbose improves indirectly both insulin resistance and secretion in obese type 2 diabetic patients. Diabetes Metab 28: 195–200. [PubMed] [Google Scholar]
- 34. Gentile S, Turco S, Guarino G, Oliviero B, Annunziata S, et al. (2001) Effect of treatment with acarbose and insulin in patients with non-insulin-dependent diabetes mellitus associated with non-alcoholic liver cirrhosis. Diabetes, Obesity and Metabolism 3: 33–40. [DOI] [PubMed] [Google Scholar]
- 35. Hanefeld M, Haffner SM, Menschikowski M, Koehler C, Temelkova-Kurktschiev T, et al. (2002) Different effects of acarbose and glibenclamide on proinsulin and insulin profiles in people with Type 2 diabetes. Diabetes Research and Clinical Practice 55: 221–227. [DOI] [PubMed] [Google Scholar]
- 36. Hanefeld M, Schaper F, Koehler C, Bergmann S, Ugocsai P, et al. (2009) Effect of Acarbose on Postmeal Mononuclear Blood Cell Response in Patients with Early Type 2 Diabetes: The AI(I)DA Study. HormMetab Res 41: 132–136. [DOI] [PubMed] [Google Scholar]
- 37. Hoffmann J, Spengler M (1997) Efficacy of 24-Week Monotherapy with Acarbose, Metformin, or Placebo in Dietary-treated NIDDM Patients: The Essen-II Study. Am J Med 103: 483–490. [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann J, Spengler M (1994) Efficacy of 24-Week Monotnerapy With Acarbose, Ghbenclamide, or Placebo in NIDDM Patients. The Essen Study. Diabetes Care 17(6): 561–566. [DOI] [PubMed] [Google Scholar]
- 39. Kirkman MS, Shankar RR, Shankar S, Shen C, Brizendine E, et al. (2006) Treating Postprandial Hyperglycemia Does Not Appear to Delay Progression of Early Type 2 Diabetes. The Early Diabetes Intervention Program. Diabetes Care 29: 2095–2101. [DOI] [PubMed] [Google Scholar]
- 40. Meneilly GS, Ryan EA, Radziuk J, Lau DCW, Yale JF, et al. (2000) Effect of Acarbose on Insulin Sensitivity in Elderly Patients With Diabetes. Diabetes Care 23: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 41. Rosenbaum P, Peres RB, Zanella MT, Ferreira SRG (2002) Improved glycemic control by acarbose therapy in hypertensive diabetic patients: effects on blood pressure and hormonal parameters. Brazilian journal of medical and biological Research 35: 877–884. [DOI] [PubMed] [Google Scholar]
- 42. Josse RG (1995) Acarbose for the treatment of Type II diabetes: the results of a Canadian multi-centre trial. Diabetes Research and Clinical Practice 28 Suppl: S167–S172 [DOI] [PubMed] [Google Scholar]
- 43. Josse RG, Chiasson JL, Ryan EA, Lau DCW, Ross SA, et al. (2003) Acarbose in the treatment of elderly patients with type 2 Diabetes. Diabetes Research and Clinical Practice 59: 37–42. [DOI] [PubMed] [Google Scholar]
- 44. Scott R, Lintott CJ, Zimmet P, Campbell L, Bowen K, et al. (1999) Will acarbose improve the metabolic abnormalities of insulin-resistant type 2 diabetes mellitus? Diabetes Research and Clinical Practice 43: 179–185. [DOI] [PubMed] [Google Scholar]
- 45. Wagner H, Degerblad M, Thorell A, Nygren J, Stahle A, et al. (2006) Combined Treatment With Exercise Training and Acarbose Improves Metabolic Control and Cardiovascular Risk Factor Profile in Subjects With Mild Type 2 Diabetes. Diabetes Care 29: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 46. Segal P, Feig PU, Schernthaner G, Ratzmann KP, Rybka J, et al. (1997) The Efficacy and Safely of Miglitol Therapy Compared With Glibenclamide in Patients With NIDDM Inadequately Controlled by Diet Alone. Diabetes Care 20(5): 687–691. [DOI] [PubMed] [Google Scholar]
- 47. Johnson PS, Feig PU, Coniff RF, Krol A, Kelley DE, et al. (1998) Chronic Treatment of African-American Type 2 Diabetic Patients With a-Glucosidase Inhibition. Diabetes Care 21(3): 416–422. [DOI] [PubMed] [Google Scholar]
- 48. Johnson PS, Feig PU, Coniff RF, Krol A, Davidson JA, et al. (1998) Long-Term Titrated-Dose a-Glucosidase Inhibition in Non-Insulin-Requiring Hispanic NIDDM Patients. Diabetes Care 21(3): 409–415. [DOI] [PubMed] [Google Scholar]
- 49. Bachmann W, Petzinna D, Raptis SA, Wascher T, Westermeier T (2003) The European Acarbose Study Group (2003) Long-Term Improvement of Metabolic Control by Acarbose in Type 2 Diabetes Patients Poorly Controlled with Maximum Sulfonylurea Therapy. Clin Drug Invest 23 (10): 679–686. [DOI] [PubMed] [Google Scholar]
- 50. Halimi S, Le Berre MA, Grange V (2000) Efficacy and safety of acarbose add-on therapy in the treatment of overweight patients with Type 2 diabetes inadequately controlled with metformin: a double-blind, placebo-controlled study. Diabetes Research and Clinical Practice 50: 49–56. [DOI] [PubMed] [Google Scholar]
- 51. Kelley DE, Bidot P, Freedman Z, Haag B, Podlecki D, et al. (1998) Efficacy and Safety of Acarbose in Insulin-teated Patients With Type 2 Diabetes. Diabetes Care 21: 2056–2061. [DOI] [PubMed] [Google Scholar]
- 52. Phillips P, Karrasch J, Scott R, Wilson D, Moses R (2003) Acarbose Improves Glycemic Control in Overweight Type 2 Diabetic Patients Insufficiently Treated With Metformin. Diabetes Care 26: 269–273. [DOI] [PubMed] [Google Scholar]
- 53. Rosenstock J, Brown A, Fischer J, Adeshjain, LittleJohn T, et al. (1998) Efficacy and Safety of Acarbose in Metformin-Treated Patients With Type 2 Diabetes. Diabetes Care 21: 2050–2055. [DOI] [PubMed] [Google Scholar]
- 54. Schnell O, Mertes G (2007) Standl E on behalf of the Acarbose–Insulin Combination Study Group (2007) Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy. Diabetes, Obesity and Metabolism 9: 853–858. [DOI] [PubMed] [Google Scholar]
- 55. Standl E (1999) Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes, Obesity and Metabolism 1: 215–220. [DOI] [PubMed] [Google Scholar]
- 56. Standl E, Schernthaner G, Rybka J, Hanefeld M, Raptis SA, et al. (2001) Improved glycaemic control with miglitol in inadequately-controlled type 2 diabetics. Diabetes Research and Clinical Practice 52: 205–213. [DOI] [PubMed] [Google Scholar]
- 57. Willms B, Ruge D (1999) Comparison of acarbose and metformin in patients with type 2 diabetes mellitus insufficiently controlled with diet and sulphonylureas: a randomized, placebo-controlled study. Diabetic Medicine 16: 755–761. [DOI] [PubMed] [Google Scholar]
- 58. Yilmaz H, Gursoy A, Sahin M, Demirag NG (2007) Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and rosiglitazone or insulin and acarbose in type 2 diabetes. Acta Diabetol 44: 187–192. [DOI] [PubMed] [Google Scholar]
- 59. Van Gaal L, Maislos M, Schernthaner G, Rybka J, Segal P (2001) Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diabetes, Obesity and Metabolism 3: 326–331. [DOI] [PubMed] [Google Scholar]
- 60. Mitrakou A, Tountas N, Raptis AE, Bauer RJ, Schulz H, et al. (1998) Long-term Effectiveness of a New a-Glucosidase Inhibitor (BAY m1099-Miglitol) in Insulin-treated Type 2 Diabetes Mellitus. Diabet. Med 15: 657–660. [DOI] [PubMed] [Google Scholar]
- 61. Feinböck C, Luger A, Klingler A, Egger T, Bielesz GK, et al. (2003) Austrian Glimepiride Study Group (2003) Prospective multicentre trial comparing the efficacy of, and compliance with, glimepiride or acarbose treatment in patients with type 2 diabetes not controlled with diet alone. Diabetes Nutr Metab 16(4): 214–21. [PubMed] [Google Scholar]
- 62. Göke B (2002) on Behalf of the German Pioglitazone Study Group (2002) Improved Glycemic Control and Lipid Profile in a Randomized Study of Pioglitazone Compared with Acarbose in Patients with Type 2 Diabetes Mellitus. Treat Endocrinol 1(5): 329–336. [DOI] [PubMed] [Google Scholar]
- 63. Salman S, Salman F, Satman I, Yılmaz Y, Özer E, et al. (2001) Comparison of Acarbose and Gliclazide as First-line Agents in Patients with Type 2 Diabetes. Current Medical Research and Opinion 16(4): 296–306. [DOI] [PubMed] [Google Scholar]
- 64. Bayraktar M (1996) A comparison of acarbose versus metformin as an adjuvant therapy in sulfonylurea-treated NIDDM patients. Diabetes Care 19(3): 252–254. [DOI] [PubMed] [Google Scholar]
- 65. Giuseppe Derosa, Angela D’Angelo, Sibilla A.T Salvadeo, Ilaria Ferrari, Elena Fogari, et al. (2009) Modulation of adipokines and vascular remodelling markers during OGTT with acarbose or pioglitazone treatment. Biomedicine & Pharmacotherapy 63: 723e733. [DOI] [PubMed] [Google Scholar]
- 66. Derosa G, Mereu R, D’Angelo A, Salvadeo SA, Ferrari I, et al. (2010) Effect of pioglitazone and acarbose on endothelial inflammation biomarkers during oral glucose tolerance test in diabetic patients treated with sulphonylureas and metformin. Journal of Clinical Pharmacy and Therapeutics 35: 565–579. [DOI] [PubMed] [Google Scholar]
- 67. Duran C (2009) The investigation of the efficacy of insulin glargine on glycemic control when combined with either repaglinide or acarbose in obese type 2 diabetic patients. J Endocrinol Invest 32: 69–73. [DOI] [PubMed] [Google Scholar]
- 68. Güvener N, Gedik O (1999) Effects of combination of insulin and acarbose compared with insulin and gliclazide in type 2 diabetic patients. Acta Diabetol 36: 93–97. [DOI] [PubMed] [Google Scholar]
- 69. Zhu Q, Tong Y, Wu T, Li J, Tong N (2013) Comparison of the Hypoglycemic Effect of Acarbose Monotherapy in Patients With Type 2 Diabetes Mellitus Consuming an Eastern or Western Diet: A Systematic Meta-analysis. Clinical Therapeutics 35(6): 880–899. [DOI] [PubMed] [Google Scholar]
- 70. Fischer S (2003) Influence of treatment with acarbose or glibenclamide on insulin sensitivity in type 2 diabetic patients. Diabetes Obesity and Metabolsim 5: 38–44. [DOI] [PubMed] [Google Scholar]
- 71. Hasche H (1999) Effects of acarbose treatment in type 2 diabetic patients under dietary training: A multicenter, double-blind, placebo-controlled, 2 year study. Diab Nutr Metab 12: 277–285. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of Randomized Controlled Trials of Alpha glucosidase Inhibitors Included in the Systematic Review.
(DOCX)
A PRISMA checklist for this meta-analysis.
(DOC)