Abstract
Background
The prognostic importance of B-type natriuretic peptide (BNP) or N-terminal pro BNP (NT-proBNP) in patients with end-stage renal disease (ESRD) remains controversial.
Methodology/Principal Findings
We conducted an unrestricted search from the MEDLINE and EMBASE in all languages that were published between 1966 and Augest2013. Twenty-seven long-term prospective studies met our inclusion criterias. From the pooled analysis, elevated BNP/NT-proBNP was significantly associated with increased all cause mortality [odds ratio (OR), 3.85; 95% CI, 3.11 to 4.75], cardiovascular mortality (OR, 4.05; 95% CI, 2.53 to 6.84), and cardiovascular events (OR, 7.02; 95% CI, 2.21 to 22.33). The funnel plot showed no evidence of publication bias. The corresponding pooled positive and negative likelihood ratio for prediction of all cause mortality were 1.86 (95% CI, 1.66 to 2.08) and 0.48 (95% CI, 0.42 to 0.55), respectively.
Conclusions/Significance
BNP/NT-proBNP is a promising prognostic tool to risk-stratify the patients with ESRD. Further investigations are warranted to elucidate the specific pathogenic mechanisms and the impact of other potential prognostic factors.
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in patients with end-stage renal disease (ESRD), accounting for approximately 50% of the deaths [1]. Early identification of ESRD patients at high risk for future events may facilitate more aggressive and focused treatments.
B-type natriuretic peptide (BNP) is a 32-amino acid polypeptide secreted by ventricles of the heart in response to excessive stretching of cardiomyocytes. This peptide is believed to play an important role in regulating blood pressure and volume through direct effects on the kidney and systemic vasculature. BNP is co-secreted along with a 76-amino acid polypeptide, NT-proBNP, which is more stable and biologically inactive [2]. Measurement of circulating natriuretic peptide (NPs), BNP or NT-proBNP has been recommended in the diagnosis and prognosis of patients with acute or chronic heart failure [3]. Given the high prevalence of left ventricular (LV) hypertrophy and systolic dysfunction in patients with ESRD [4], [5], [6], [7], it has been proposed that the NPs may assist in risk stratification for cardiovascular events among patients with ESRD.
The past few years have seen a rapidly growing interest in testing this hypothesis. Many prospective studies have investigated the link between NPs and the adverse outcomes in ESRD patients, and most found a positive association. However, the magnitudes of the association varied between studies and most of them have not been systematically assessed. Several previous relevant reviews involved only about one-fourth of the currently available data [8], [9]. In addition, interpretation of the evidence has been complicated by studies that have involved different markers (ie, BNP, NT-proBNP, or both), different disease outcomes (eg, all-cause mortality, cardiovascular mortality or events) and the impact of other prognostic variables besides NPs. To help resolve this uncertainty of BNP/NT-proBNP as a prognostic tool, our goal, therefore, was to quantify the association between BNP/NT-proBNP and long-term adverse outcomes by conducting a meta-analysis of these prospective studies.
Methods
The methods used in this review are in accordance with the Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting [10].and follows PRISMA guidelines (Checklist S1).
Research Objectives
The primary research objective was to determine, by use of systematic review techniques, whether elevated circulating BNP and/or NT-proBNP predicted long-term risks of all-cause mortality, cardiovascular mortality or cardiovascular events (defined as any fatal or nonfatal myocardial infarction, stroke, transient ischemic attack, or heart failure) among patients with ESRD. The secondary objective was to determine the ability of BNP or NT-proBNP to discriminate the patients who do and do not experience subsequent events.
Data Sources and Selection
To identify relevant studies, MEDLINE (1966- Aug., 2013), EMBASE (1980- Aug., 2013) were reviewed without language restrictions. The electronic searches were completed by manual search of the reports' reference lists. The search strategy, developed with an experienced librarian, included the following terms: 1) end stage, kidney disease, renal disease, dialysis, hemodialysis, peritoneal dialysis, renal replacement therapy; 2) natriuretic peptide, B-type natriuretic peptide, brain natriuretic peptide, N-terminal pro B-type natriuretic peptide, BNP, NT-proBNP); 3) mortality, all-cause mortality, cardiac death, cardiovascular diseases, myocardial ischemia, myocardial infarction, coronary stenosis, cerebrovascular disorders, stroke; and 4) cohort studies, prospective studies, and follow-up studies.
Study Selection
We first performed an initial screening of titles or abstracts. The second screening was based on full-text review. Studies were considered eligible if they met the following criteria: 1) prospective observational study design; and studies that 2) evaluated the prognosis of patients with abnormal levels of either BNP or NT-proBNP in ESRD patients, 3) examined all cause mortality, cardiovascular mortality or cardiovascular events. We exclude studies that did not report tabular data for the outcomes separately.
Quality Review
All studies included for methodological and reporting quality were evaluated according to Egger's quality checklist for prognostic studies [11], adapting the checklist for the purposes of this review because all the included studies were prognostic in nature. Two authors (YJ Cheng and FJ Yao) independently rated study quality. Percentage agreement between the 2 reviewers on the items on the quality review ranged from 84% to 100%. Disagreements were resolved by consensus.
Data Abstraction
Data on the following characteristics were independently extracted with standardized data extraction protocols and corresponded with study authors to obtain supplementary tabular data: study size; geographical location; year of baseline survey; age range of participants at baseline; percentage of female participants; mean duration of follow-up; storage temperature; NPs assay methods; the number and type (all cause death, cardiovascular death and event) of outcome event. In instances of multiple publications, the most up-to-date or comprehensive information was used.
Data Synthesis and Analysis
Summary estimates of the univariate odds ratio (OR) and likelihood ratios were calculated using the random effects model because fixed and random-effects model results were similar and random-effects models tend to produce more conservative estimates. In the case of papers that gave multiple cut-off points for BNP analysis, the score that gave the maximum overall accuracy was chosen. As studies used different cut-off points for defining raised BNP or NT-proBNP, we calculated Spearman's correlation coefficient between sensitivity and specificity [12]. This looks for the presence of a threshold effect, where variations in sensitivity and specificity are related to differences in the cut-off point used to define an abnormal result. If there is no evidence of a threshold effect then likelihood ratios can be pooled. Consistency of findings across studies was assessed by Cochran's Q and the I2 statistic [13]. Heterogeneity was assssed by comparing results from studies grouped according to prespecified study-level characteristics with meta-regression. Small study bias, consistent with publication bias was assessed with funnel plot (i.e. a plot of study results against precision), by Begg's adjusted rank correlation test, and by Egger's regression asymmetry test [14].
All analyses were performed using STATA version 11.0 (Stata Corp LP, College Station, Texas, USA). A P value<0.05 was considered statistically significant.
Results
Literature Search
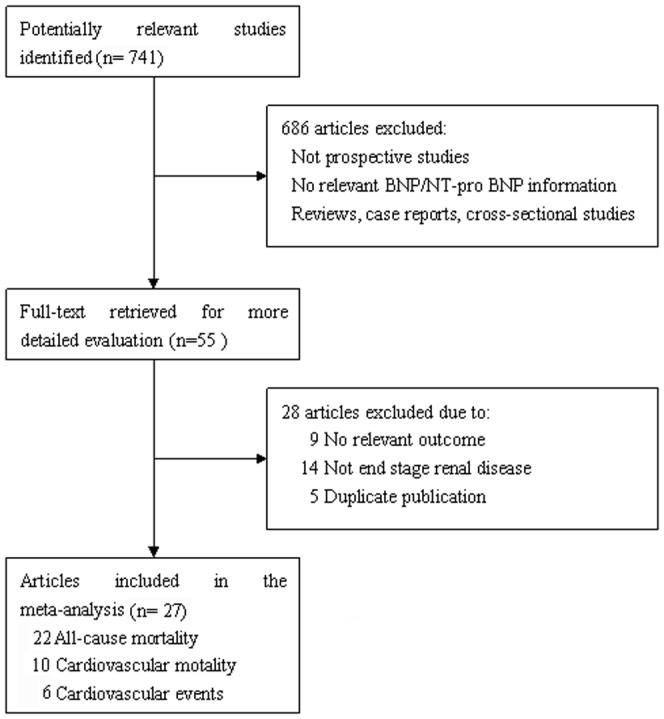
From the search strategy, 741 unique citations were initially retrieved. Of these, the majority were excluded after the first screening based on abstracts or titles, mainly because they were reviews, case-control studies, not BNP/NT-proBNP associated, or not relevant to our analysis. After full-text review of 55 papers, 14 studies were excluded because they recruited non-ESRD patients, and 5 were excluded due to duplicate publications. An additional 9 studies in which the outcomes of interest were not evaluated or not reported separately were also excluded. Finally, 27 studies were included in our meta-analysis ( Figure 1 ).
Figure 1. Flow chart of study selection. Flow chart shows literature search for prospective studies of BNP/NT-ProBNP in relation to all-cause mortality, cardiovascular mortality and events in end stage renal disease.
Methodological Quality
The 27 prospective studies were determined to be of fair quality. Of the primary studies, 59% reported details of exclusion criteria. However, few studies (30%) had explicitly ascertained that patients did not have any acute symptoms of congestive heart failure. The majority of studies (96%) reported at least 1 cardiac risk factor, and 77% reported baseline cardiovascular history. However, only 37% of studies indicated the participants' baseline medication use. All the studies described the BNP/NT-proBNP assays and reported cutoff values to define normal and abnormal levels. BNP/NT-proBNP values were known for all patients. All the studies described treatments or management of patients during follow-up. Only a minority (30%) of primary studies specified the number of patients lost to follow-up, with 22% reporting that no patients were lost to follow-up.
Study Characteristics
Twenty-seven relevant studies reporting on 8,666 individuals were identified from 16 countries. Thirteen studies were based in Europe [4], [7], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], 5 in North America [5], [26], [27], [28], [29], [30], 2 in Australia [31], [32] and and 6 in East Asia [6], [33], [34], [35], [36], [37]. From all primary studies, over 63.4% of patients were receiving hemodialysis (HD). Of the total population, 57.1% were males and the median (or mean) ages of the cohorts ranged from 48.6 to 70 years. The mean duration on dialysis was 36 months and the patients followed for an average of 23 months (range, 12 to 60 months). Fifteen studies reported associations with NT-proBNP only, 8 with BNP only, and 4 with both NPs. The median value of NT-proBNP and BNP levels varied substantially across available studies (NT-proBNP values ranged from 288 to 9761 pg/mL; BNP values ranged from 116.8 to 570 pg/mL), with a tendency toward higher values in individuals with higher incidence of cardiovascular disease at entry. Table 1 and Table 2 demonstrates variability in prevalence of diabetes mellitus (or diabetic nephropathy), prior history of cardiovascular disease, length of follow-up of individual studies and mean number of years spent on dialysis, characteristics of the assays and outcomes across the individual studies.
Table 1. Summary of Available Prospective Studies Included in the Present Meta-analysis.
| Source Study | Location | No. n | Mean Age, y | Male, % | patient type | Mean time On HD/PD†, mo | Prior History of CAD or MI* | Diabetes Mellitus | Duration of Follow-up | Lost to Follow-up |
| % | % | Mo | % | |||||||
| Kim YK [33] | Korea | 72 | 49 | 52.8 | HD | 38 | … | 15 | 45 | 0 |
| Koch M [15] | Gernany | 255 | 69.6 | 60 | HD/PD | … | 52.2 | 45 | 41 | 0 |
| Roberts MA [31] | Australia | 108 | 62.3 | 64 | HD/PD | 30 | 31 | 20 | 33.6 | 0 |
| Zoccali C [7] | Italy | 246 | 60.2 | 53.9 | HD/PD | 43 | 47.56 | 15 | 37 | 0 |
| Codognotto M [16] | Italy | 50 | 68 | 72 | HD | … | … | … | 36 | 0 |
| Goto T [34] | Japan | 53 | 61 | 56.6 | HD | … | … | 34 | 12 | 0 |
| Hallen J [17] | Norway | 107 | 62 | 75 | HD | 20 | 51 | 35 | 48 | 0 |
| Apple FS [26] | USA | 399 | 61 | 58 | HD | 24 | 30 | 46 | 24 | 0 |
| Paniagua R [27] | Mexico | 753 | 48.6 | 55.1 | HD/PD | … | … | … | 16 | 0 |
| Svensson M [18] | Denmark | 206 | 67 | 27 | HD/PD | 30 | 68.5 | 22 | 24 | 0 |
| Trape J [19] | Spain | 52 | 74 | 46 | HD | 42.5 | 48 | 28 | 36 | 0 |
| Wang AY [6] | Hongkong | 230 | 56 | 51 | PD | … | 22.6 | 30 | 36 | 0 |
| Gutierrez OM [28] | USA | 2990 | 63.2 | 55.2 | HD | … | 21.1 | 50 | 12 | 0 |
| Guo Q [20] | Sweden | 222 | 63.8 | 55.7 | HD | 27.9 | 64 | 26 | 31 | 0 |
| Madsen L [4] | Denmark | 109 | 61.8 | 75.2 | HD | 20 | 50.5 | 34.9 | 32 | 0 |
| Hickman PE [32] | Australia | 143 | 59.7 | 63 | HD/PD | 40.6 | 37.8 | 26.6 | 30 | 0 |
| Sun L [35] | China | 217 | 65.4 | 62.2 | HD | … | 56.7 | 47.5 | 24 | 0 |
| Paniagua R [29] | Mexico | 965 | 47.3 | 58.3 | PD | … | … | 43.32 | 32 | 9.3 |
| Satyan S [5] | USA | 150 | 56 | 52 | HD | … | 47 | 31 | 34 | 0 |
| Sharma R [21] | UK | 140 | 52 | 64.3 | HD/PD | … | 29 | 38 | 39 | 0 |
| Ishii J [36] | Japan | 100 | 58 | 61 | HD/PD | 48 | 22 | 41 | 24 | 0 |
| Biasioli S [22] | Italy | 52 | 58.7 | 73.1 | HD | 60 | 62.5 | 19.2 | 28 | 0 |
| Naganuma T [37] | Japan | 164 | 58.8 | 70.1 | HD | … | 77.4 | 36.6 | 36 | 0 |
| Sivalingam M [23] | UK | 103 | 50.2 | 68 | HD | 57.5 | 48.5 | … | 48 | 4.85 |
| Selim G [24] | Macedonia | 125 | 53 | 57.6 | HD | 75.2 | … | 13.6 | 24 | 0 |
| Westenbrink BD [25] | Netherlands | 59 | 70 | 52.5 | HD | 25 | 25.4 | 30.5 | 35 | 0 |
| Foley RN [30] | Canada | 596 | 51.5 | 60.4 | HD | 9 | … | 17.8 | 24 | 0 |
HD indicates hemodialysis, PD, peritoneal dialysis.
CAD indicates coronary artery disease, MI indicates myocardial infarction
Table 2. Characteristics of the Assays and Outcomes.
| Source Study | Peptides | Median | Assay | All-cause | Cardiovascular | Cardiovascular |
| Assessed | Peptide, pg/ml | Source | Mortality n/100 patient-years | Mortality n/100 patient-years | Events n/100 patient-years | |
| Kim YK [33] | NT-proBNP | 6165 | Roche | 2.96 | 0.37 | 4.07 |
| Koch M [15] | BNP | 340 | Biosite | 9.64 | 1.38 | 8.15 |
| Roberts MA [31] | BNP/NT-proBNP | 191/3233 | Biosite/Roche | 6.29 | 0.99 | 7.95 |
| Zoccali C [7] | BNP | 361 | Peninsula | 8.30 | 4.61 | 9.75 |
| Codognotto M [16] | NT-proBNP | 9719 | Dade Behring | 8.67 | … | … |
| Goto T [34] | BNP | 390 | Shionogi | … | 11.3 | 24.5 |
| Hallen J [17] | NT-proBNP | 3912 | Roche | 12.15 | … | … |
| Apple FS [26] | NT-proBNP | 4032 | Roche | 12.66 | … | … |
| Paniagua R [27] | NT-proBNP | 5700¶ | Roche | 18.13 | 8.47 | … |
| Svensson M [18] | NT-proBNP | 12200‡ | Roche | 21.84 | … | … |
| Trape J [19] | NT-proBNP | 33314‡ | Roche | 17.95 | 6.41 | … |
| Wang AY [6] | NT-proBNP | 5698 | Roche | 9.56 | 6.23 | 11.30 |
| Gutierrez OM [28] | NT-proBNP | 5100 | Roche | 14.78 | 8.29 | … |
| Guo Q [20] | NT-proBNP | 9761 | Siemens | 14.81 | 5.92 | 24.74 |
| Madsen L [4] | NT-proBNP | 4079 | Roche | 11.68 | 3.09 | … |
| Hickman PE [32] | BNP/NT-proBNP | 116.8/591 | In house/Roche | 7.82 | … | … |
| Sun L [35] | BNP/NT-proBNP | 570/725 | Roche/in house | … | 5.99 | 28.11 |
| Paniagua R [29] | NT-proBNP | 6198 | Roche | 12.27 | 4.24 | … |
| Satyan S [5] | NT-proBNP | 3276 | Roche | 10.82 | 6.12 | … |
| Sharma R [21] | NT-proBNP | 350‡ | Roche | 4.62 | 3.08 | … |
| Ishii J [36] | BNP | 200 | Shionogi | 9.50 | 6.00 | … |
| Biasioli S [22] | BNP | 335 | Meia | 7.44 | ||
| Naganuma T [37] | BNP | 450 | Shionogi | … | 2.64 | … |
| Sivalingam M [23] | BNP/NT-proBNP | 447/677‡ | Biomed/Roche | 8.49 | … | … |
| Selim G [24] | BNP | 1200‡ | In house | 11.20 | 7.60 | … |
| Westenbrink BD [25] | BNP | 303 | Biosite | 14.53 | … | … |
| Foley RN [30] | NT-proBNP | 288 | Roche | 2.77 | 1.76 | 6.54 |
Best cut-off;
Mean
Association with All-cause Mortality
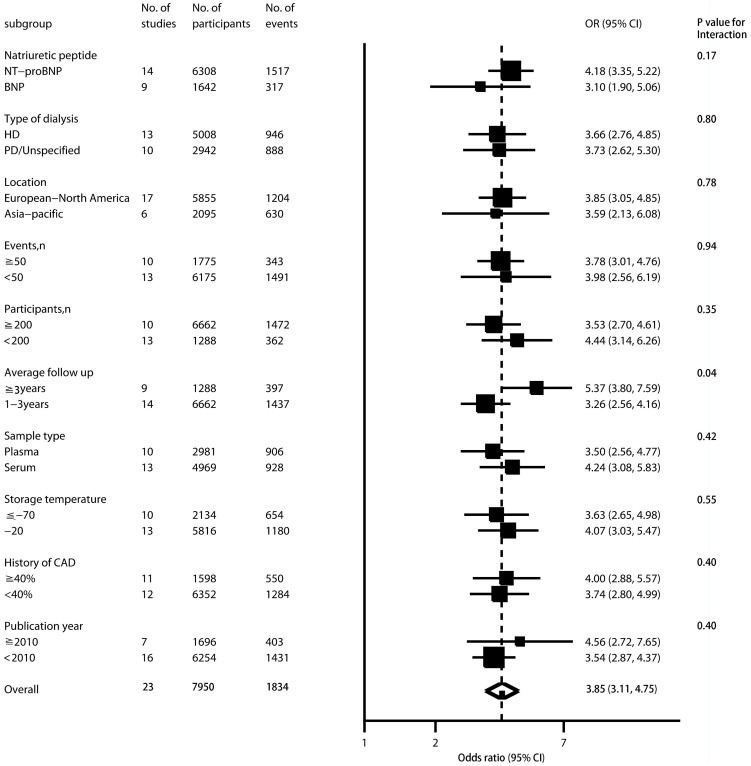
There were 22 primary studies evaluating the association between BNP and all-cause mortality. In the forest plot of individual prognostic effect sizes in Table 3, Figure 2, Figure S1 and Table S1 the lower boundaries of the 95% CIs of almost trials were greater than 1, suggesting a consistent association between BNP and all cause mortality. From the pooled analysis, elevated BNP was significantly associated with increased all-cause mortality (OR, 3.57; 95% CI, 3.17 to 4. 02). There was statistical heterogeneity between studies (I2 = 55.81%, 95% CI, 29.45% to 72.33%, P = 0.001). However, little of the heterogeneity was explained by type of NP assayed, type of dialysis, location, number of participants and events, sample type and storage temperature, percentage of coronary artery disease at baseline, publication year. Studies that followed up more than 3 years tended to report somewhat higher ORs than studies with shorter follow-up duration (P = 0.04; Figure 2).
Table 3. Summary estimates of odds ratios and likelihood ratios to predict all cause mortality, cardiovascular mortality or cardiovascular events.
| Summary estimates | No. of studies | No. of participants | No. of events | OR (95% CI) | Sensitivity (95% CI) | Specitivity (95% CI) | PLR (95% CI) | NLR (95% CI) |
| All cause mortality | 23 | 7,950 | 1,834 | 3.85 (3.11, 4.75) | 0.70 (0.65, 0.74) | 0.63 (0.58, 0.67) | 1.86 (1.66, 2.08) | 0.48 (0.42, 0.55) |
| Cardiovascular mortality | 10 | 6,396 | 689 | 4.05 (2.53, 6.84) | 0.75 (0.67, 0.81) | 0.60 (0.53, 0.66) | 1.87 (1.52, 2.30) | 0.42 (0.30, 0.58) |
| Cardiovascular events | 6 | 1,463 | 425 | 7.02 (2.21, 22.33) | 0.80 (0.65, 0.89) | 0.64 (0.49, 0.76) | 2.21 (1.48, 3.30) | 0.32 (0.17, 0.58) |
CI, confidence interval; OR, odds ratio; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Figure 2. Association between elevated BNP and all cause mortality in patients with end stage renal disease, according to different study level characteristics.
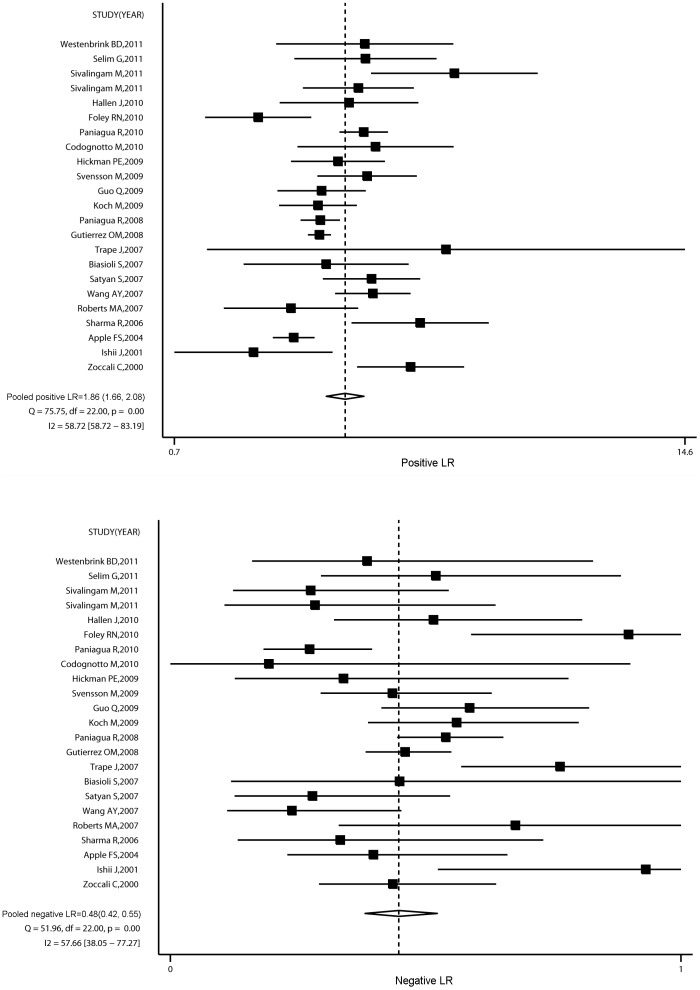
The sensitivity and specitivity of increased BNP or NT-proBNP levels to predict all cause mortality were 0.70 (95% CI, 0.65 to 0.74) and 0.63 (95% CI, 0.58 to 0.67), respectively. Spearman's correlation between sensitivity and specificity was 0.08 (P = 0.70), suggesting no evidence of a threshold effect. We therefore calculated pooled positive (PLR) and negative likelihood ratios (NLR). The summary PLR was 1.86 (95% CI, 1.66 to 2.08) and NLR was 0.48 (95% CI, 0.42 to 0.55). There was statistical heterogeneity for the PLR (I2 = 58.72%, 95% CI, 58.72% to 83.19%, P = 0.001) and NLR (I2 = 57.66%, 95% CI, 38.05% to 77.27%, P = 0.001) (Figure 3).
Figure 3. Summary of likelihood ratios of an elevated BNP to predict all cause mortality.
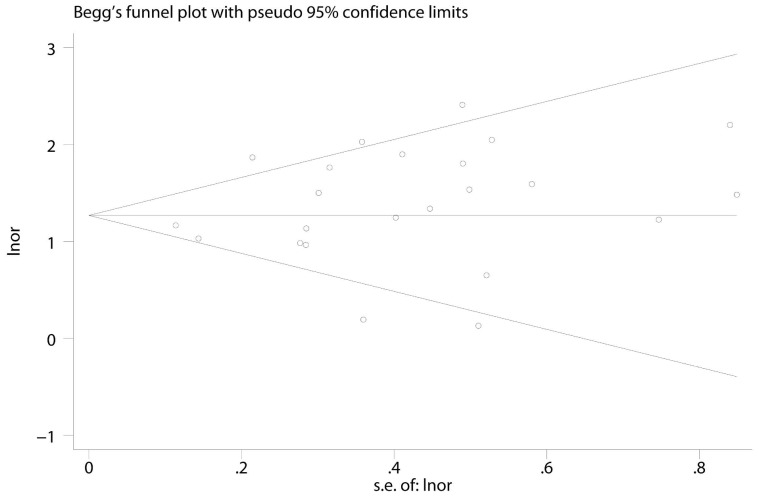
Visual inspection of the Begg funnel plot did not identify substantial asymmetry. The Begg rank correlation test and Egger linear regression test also indicated no evidence of publication bias among studies of either NP and all-cause mortality (Begg, P = 0.49; Egger, P = 0.22) (Figure 4).
Figure 4. Begg's funnel plots with 95% CI for BNP or NT-proBNP primary studies.
Association with Cardiovascular Mortality or Events
The elevated BNP/NT-proBNP was associated with increased cardiovascular mortality (OR: 4.05; 95% CI, 2.53 to 6.84) with high heterogeneity (I2 = 80.38%, 95% CI, 64.77% to 89.07%, P<0.001) (Table 3, Figure S2 and Table S1). When we removed the study by Foley and colleagues that reported a lower OR in a large population [30], the OR for the remaining studies did not materially change (4.58; 95% CI: 3.40 to 6.17; P<0.001), but heterogeneity was decreased to 36.56% (95% CI: 0.00% to 70.81%; P = 0.13).
There were 6 studies that reported the association with cardiovascular events. Elevated BNP/NT-proBNP was strongly associated with a significant increase in long-term cardiac events (OR:7.02; 95% CI: 2.21 to 22.33; P<0.001) (Table 3, Figure S3 and Table S1). BNP/NT-proBNP had better pooled sensitivity (0.80 vs 0.75), specificity (0.64 vs 0.60), PLR (2.21 vs 1.87) and NLR (0.32 vs 0.42) for cardiovascular events prediction than for cardiovascular mortality prediction (figure S4, figure S5).
Discussion
The present meta-analysis of more than 8000 patients with ESRD from 27 long-term prospective studies provides evidence that elevated BNP/NT-proBNP predicted approximately a four-fold increase in the risk of all cause mortality and cardiovascular mortality, and over a seven-fold increase in the risk of cardiovascular events.
Although NT-proBNP is more stable with longer half-life than BNP (≈120 minutes versus ≈20 minutes, respectively) [2] and has theoretical superiority for prognostication, the present data suggest that a given proportional increment in each marker is similarly associated with increased risk of all-cause mortality. On the basis of indirect comparisons with previous meta-analysis of several established risk factors, the magnitude of death risk with BNP/NT-proBNP concentration appears to be stronger than those with C-reactive protein, albumin, troponin or homocysteine [38], [39], [40].
Understanding the mechanisms that underlie elevation in BNP/NT-proBNP levels among ESRD patients is of particular importance to help frame appropriate therapeutic decisions. Previous studies have suggested that the mechanism for a rise in NPs among ESRD patients was a result of LV structural and functional abnormalities [6], [41], enabling NPs to be a potential marker of LV hypertrophy in ESRD [42], [43]. In addition,there is emerging evidence that increases in circulating NPs may indicate myocardial ischemia or necrosis, due to the coronary atherosclerosis prevalent in patients with ESRD [6], [44], supported by a strong relationship between elevated BNP/NT-proBNP and cardiovascular mortality/events in our analysis. Furthermore, given that BNP and NT-pro-BNP are released in response to increased myocardial wall stress, it is tempting to hypothesize that circulating NPs levels may be a useful marker of volume status in ESRD patients [45], [46]. The fluid overload may affect the intensity of chronic inflammation through the presence of intestinal wall edema, which allows a translaocation of bacteria endotoxin from intestinal lumen to bloodstream and contribute to an increased mortality in patients with ESRD [47]. However, other studies failed to detect a positive association between fluid removed and change of BNP in patients receiving HD [41], [48], suggesting that this mechanism needs to be further elucidated.
There is one factor that may confound the interpretation of elevated NPs in ESRD patients. Previous authors have suggested that BNP and NT-proBNP concentrations are strongly correlated with residual renal function [49], [50], making both NPs potentially lose prognostic value in ESRD patients. Nevertheless, several latest studies have reported both NPs are only 20% or less dependent on renal function for their clearance [51], [52], [53], indicating BNP/NT-proBNP to be a prognostic marker for patients with ESRD.
Along with the strict inclusion criteria, strengths of this meta-analysis include the large number of patients analyzed, the robustness of the findings at sensitivity analyses, the fact that all subgroup analyses were prespecified a priori. The absence of important publication bias supports the robustness of the study findings. Although the present study involves over 4 times as much information as in previous reviews [8], [9], it has been limited by the moderate amount of available data from primary studies. For example, of the 27 studies, risk estimates for cardiovascular mortality and events were only available from 10 and 6 studies, respectively. The studies included in our analysis used different cut-off points to define high-risk, which varied significantly between studies. In the analysis with all cause mortality as the end-point, the ‘best-fit’ cut-off point ranged from surprisingly low to expectedly high in different studies. This variation in cut-off point is at least in part related to the significant heterogeneity in patient population between the different studies. From the currently available data it is not possible to draw any firm conclusions regarding the most appropriate cut-off point for risk stratification. Another acknowledged limitation to the meta-analysis is the inability to adjust statistically for identical covariates or prognostic factors that could have influenced the outcomes of ESRD. In addition, although serum concentration of BNP is associated with residual renal function and left ventricular dysfunction, the limited data of glomerular filtration rate and left ventricular ejection fraction reported in the original studies made it difficult to conduct a further statistical investigation.
BNP/NT-proBNP is a promising prognostic tool to risk-stratify stable, asymptomatic ESRD patients, as elevated levels identify a subset of ESRD patients who have poor survival and higher risk of cardiovascular events. This study corroborates previous postulates that a cardiovascular cause underlies the association between mortality and elevated BNP or NT-proBNP in ESRD patients. Further studies, including well-designed clinical trials, are warranted to elucidate the specific pathogenic mechanisms, and the impact of other potential prognostic factors.
Supporting Information
Association between elevated BNP and all cause mortality in patients with end stage renal disease.
(TIF)
Association between elevated BNP and cardiovascular mortality in patients with end stage renal disease.
(TIF)
Association between elevated BNP and cardiovascular events in patients with end stage renal disease.
(TIF)
Summary of likelihood ratios of an elevated BNP to predict cardiovascular mortality.
(TIF)
Summary of likelihood ratios of an elevated BNP to predict cardiovascular events.
(TIF)
Data set of tabular data and odds ratio calculations for all cause mortality, cardiovascular mortality and cardiovascular events.
(XLS)
PRISMA Checklist.
(DOC)
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 81370285), Guangdong Province Natural Science Foundation (No. 06021338); Guangdong Province Science and Technology Program (No. 2012B031800091, 2007B031508003) and National Ministry of Education Scholarly Exchanges Foundation (No. 200724). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Herzog CA, Ma JZ, Collins AJ (1998) Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med 339: 799–805. [DOI] [PubMed] [Google Scholar]
- 2. Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol 50: 2357–2368. [DOI] [PubMed] [Google Scholar]
- 3. Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, et al. (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 10: 933–989. [DOI] [PubMed] [Google Scholar]
- 4. Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, et al. (2007) N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int 71: 548–554. [DOI] [PubMed] [Google Scholar]
- 5. Satyan S, Light RP, Agarwal R (2007) Relationships of N-terminal pro-B-natriuretic peptide and cardiac troponin T to left ventricular mass and function and mortality in asymptomatic hemodialysis patients. Am J Kidney Dis 50: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang AY, Lam CW, Yu CM, Wang M, Chan IH, et al. (2007) N-terminal pro-brain natriuretic peptide: an independent risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic peritoneal dialysis patients. J Am Soc Nephrol 18: 321–330. [DOI] [PubMed] [Google Scholar]
- 7. Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, et al. (2001) Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol 12: 1508–1515. [DOI] [PubMed] [Google Scholar]
- 8. Dastoor H, Bernieh B, Boobes Y, Abouchacra S, Eltayeb E, et al. (2005) Plasma BNP in patients on maintenance haemodialysis: a guide to management? J Hypertens 23: 23–28. [DOI] [PubMed] [Google Scholar]
- 9. Mark PB, Petrie CJ, Jardine AG (2007) Diagnostic, prognostic, and therapeutic implications of brain natriuretic peptide in dialysis and nondialysis-dependent chronic renal failure. Semin Dial 20: 40–49. [DOI] [PubMed] [Google Scholar]
- 10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 11. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, et al. (2002) Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koch M, Trapp R, Kohnle M, Aker S, Haastert B, et al. (2010) B-type natriuretic peptide and severe heart failure at baseline predict overall mortality in incident dialysis patients. Clin Nephrol 73: 21–29. [DOI] [PubMed] [Google Scholar]
- 16. Codognotto M, Piccoli A, Zaninotto M, Mion MM, Ruzza L, et al. (2010) Effect of a dialysis session on the prognostic values of NT-proBNP, troponins, endothelial damage and inflammation biomarkers. J Nephrol 23: 465–471. [PubMed] [Google Scholar]
- 17. Hallen J, Madsen L, Ladefoged S, Fagerland MW, Serebruany VL, et al. (2011) Incremental value of a combination of cardiac troponin T, N-terminal pro-brain natriuretic peptide and C-reactive protein for prediction of mortality in end-stage renal disease. Scand J Urol Nephrol 45: 151–158. [DOI] [PubMed] [Google Scholar]
- 18. Svensson M, Gorst-Rasmussen A, Schmidt EB, Jorgensen KA, Christensen JH (2009) NT-pro-BNP is an independent predictor of mortality in patients with end-stage renal disease. Clin Nephrol 71: 380–386. [DOI] [PubMed] [Google Scholar]
- 19. Trape J, Perez A, Naval I, Escudero J, Comerma I, et al. (2008) Nt-proBNP in haemodialysis patients: a preliminary study. Scand J Clin Lab Invest 68: 415–420. [DOI] [PubMed] [Google Scholar]
- 20. Guo Q, Barany P, Qureshi AR, Snaedal S, Heimburger O, et al. (2009) N-terminal pro-brain natriuretic peptide independently predicts protein energy wasting and is associated with all-cause mortality in prevalent HD patients. Am J Nephrol 29: 516–523. [DOI] [PubMed] [Google Scholar]
- 21. Sharma R, Gaze DC, Pellerin D, Mehta RL, Gregson H, et al. (2006) Raised plasma N-terminal pro-B-type natriuretic peptide concentrations predict mortality and cardiac disease in end-stage renal disease. Heart 92: 1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biasioli S, Zamperetti M, Borin D, Guidi G, De Fanti E, et al. (2007) Significance of plasma B-type natriuretic peptide in hemodialysis patients: blood sample timing and comorbidity burden. ASAIO J 53: 587–591. [DOI] [PubMed] [Google Scholar]
- 23. Sivalingam M, Suresh M, Farrington K (2011) Comparison of B-type natriuretic peptide and NT proBNP as predictors of survival in patients on high-flux hemodialysis and hemodiafiltration. Hemodial Int 15: 359–365. [DOI] [PubMed] [Google Scholar]
- 24. Selim G, Stojceva-Taneva O, Spasovski G, Georgievska-Ismail L, Zafirovska-Ivanovska B, et al. (2011) Brain natriuretic peptide between traditional and nontraditional risk factors in hemodialysis patients: analysis of cardiovascular mortality in a two-year follow-up. Nephron Clin Pract 119: c162–c170. [DOI] [PubMed] [Google Scholar]
- 25. Westenbrink BD, Hovinga TK, Kloppenburg WD, Veeger NJ, Janssen WM (2011) B-type natriuretic peptide and interdialytic fluid retention are independent and incremental predictors of mortality in hemodialysis patients. Clin Nephrol 76: 373–379. [DOI] [PubMed] [Google Scholar]
- 26. Apple FS, Murakami MM, Pearce LA, Herzog CA (2004) Multi-biomarker risk stratification of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and cardiac troponin T and I in end-stage renal disease for all-cause death. Clin Chem 50: 2279–2285. [DOI] [PubMed] [Google Scholar]
- 27. Paniagua R, Ventura MD, Avila-Diaz M, Hinojosa-Heredia H, Mendez-Duran A, et al. (2010) NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant 25: 551–557. [DOI] [PubMed] [Google Scholar]
- 28. Gutierrez OM, Tamez H, Bhan I, Zazra J, Tonelli M, et al. (2008) N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations in hemodialysis patients: prognostic value of baseline and follow-up measurements. Clin Chem 54: 1339–1348. [DOI] [PubMed] [Google Scholar]
- 29. Paniagua R, Amato D, Mujais S, Vonesh E, Ramos A, et al. (2008) Predictive value of brain natriuretic peptides in patients on peritoneal dialysis: results from the ADEMEX trial. Clin J Am Soc Nephrol 3: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foley RN, Curtis BM, Randell EW, Parfrey PS (2010) Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roberts MA, Srivastava PM, Macmillan N, Hare DL, Ratnaike S, et al. (2008) B-type natriuretic peptides strongly predict mortality in patients who are treated with long-term dialysis. Clin J Am Soc Nephrol 3: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hickman PE, McGill DA, Talaulikar G, Hiremagalur B, Bromley J, et al. (2009) Prognostic efficacy of cardiac biomarkers for mortality in dialysis patients. Intern Med J 39: 812–818. [DOI] [PubMed] [Google Scholar]
- 33. Kim YK, Shin SJ, Ihm SH, Park CS, Kim HY, et al. (2010) Association between N-terminal pro-brain natriuretic peptide and acute ischemic stroke in patients on chronic hemodialysis. Int Urol Nephrol 42: 537–543. [DOI] [PubMed] [Google Scholar]
- 34. Goto T, Takase H, Toriyama T, Sugiura T, Kurita Y, et al. (2002) Increased circulating levels of natriuretic peptides predict future cardiac event in patients with chronic hemodialysis. Nephron 92: 610–615. [DOI] [PubMed] [Google Scholar]
- 35. Sun L, Sun Y, Zhao X, Xu C, Chen D, et al. (2008) Predictive role of BNP and NT-proBNP in hemodialysis patients. Nephron Clin Pract 110: c178–c184. [DOI] [PubMed] [Google Scholar]
- 36. Ishii J, Nomura M, Okuma T, Minagawa T, Naruse H, et al. (2001) Risk stratification using serum concentrations of cardiac troponin T in patients with end-stage renal disease on chronic maintenance dialysis. Clin Chim Acta 312: 69–79. [DOI] [PubMed] [Google Scholar]
- 37. Naganuma T, Sugimura K, Wada S, Yasumoto R, Sugimura T, et al. (2002) The prognostic role of brain natriuretic peptides in hemodialysis patients. Am J Nephrol 22: 437–444. [DOI] [PubMed] [Google Scholar]
- 38. Herselman M, Esau N, Kruger JM, Labadarios D, Moosa MR (2010) Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition 26: 10–32. [DOI] [PubMed] [Google Scholar]
- 39. Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A (2005) Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation 112: 3088–3096. [DOI] [PubMed] [Google Scholar]
- 40. Heinz J, Kropf S, Luley C, Dierkes J (2009) Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis 54: 478–489. [DOI] [PubMed] [Google Scholar]
- 41. Safley DM, Awad A, Sullivan RA, Sandberg KR, Mourad I, et al. (2005) Changes in B-type natriuretic peptide levels in hemodialysis and the effect of depressed left ventricular function. Adv Chronic Kidney Dis 12: 117–124. [DOI] [PubMed] [Google Scholar]
- 42. Dastoor H, Bernieh B, Boobes Y, Abouchacra S, Eltayeb E, et al. (2005) Plasma BNP in patients on maintenance haemodialysis: a guide to management? J Hypertens 23: 23–28. [DOI] [PubMed] [Google Scholar]
- 43. Flemmer M, Rajab H, Mathena T, Paulson J, Perkins S, et al. (2008) Blood B-type natriuretic peptide and dialysis: present assessment and future analyses. South Med J 101: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 44. Nishikimi T, Futoo Y, Tamano K, Takahashi M, Suzuki T, et al. (2001) Plasma brain natriuretic peptide levels in chronic hemodialysis patients: influence of coronary artery disease. Am J Kidney Dis 37: 1201–1208. [DOI] [PubMed] [Google Scholar]
- 45. Lee SW, Song JH, Kim GA, Lim HJ, Kim MJ (2003) Plasma brain natriuretic peptide concentration on assessment of hydration status in hemodialysis patient. Am J Kidney Dis 41: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 46. Booth J, Pinney J, Davenport A (2010) N-terminal proBNP–marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin J Am Soc Nephrol 5: 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ritz E (2011) Intestinal-renal syndrome: mirage or reality? Blood Purif 31: 70–76. [DOI] [PubMed] [Google Scholar]
- 48. Paniagua R, Ventura MD, Avila-Diaz M, Hinojosa-Heredia H, Mendez-Duran A, et al. (2010) NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant 25: 551–557. [DOI] [PubMed] [Google Scholar]
- 49. de Lemos JA, McGuire DK, Drazner MH (2003) B-type natriuretic peptide in cardiovascular disease. Lancet 362: 316–322. [DOI] [PubMed] [Google Scholar]
- 50. Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, et al. (2005) Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension 46: 118–123. [DOI] [PubMed] [Google Scholar]
- 51. Palmer SC, Richards AM (2009) Does renal clearance differ between the B-type natriuretic peptides (BNP versus NT-proBNP)? J Am Coll Cardiol 53: 891–892. [DOI] [PubMed] [Google Scholar]
- 52. van Kimmenade RR, Januzzi JJ, Bakker JA, Houben AJ, Rennenberg R, et al. (2009) Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol 53: 884–890. [DOI] [PubMed] [Google Scholar]
- 53. Schou M, Alehagen U, Goetze JP, Gustafsson F, Dahlstrom U (2009) Effect of estimated glomerular filtration rate on plasma concentrations of B-type natriuretic peptides measured with multiple immunoassays in elderly individuals. Heart 95: 1514–1519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between elevated BNP and all cause mortality in patients with end stage renal disease.
(TIF)
Association between elevated BNP and cardiovascular mortality in patients with end stage renal disease.
(TIF)
Association between elevated BNP and cardiovascular events in patients with end stage renal disease.
(TIF)
Summary of likelihood ratios of an elevated BNP to predict cardiovascular mortality.
(TIF)
Summary of likelihood ratios of an elevated BNP to predict cardiovascular events.
(TIF)
Data set of tabular data and odds ratio calculations for all cause mortality, cardiovascular mortality and cardiovascular events.
(XLS)
PRISMA Checklist.
(DOC)