Abstract
Background
In 2010, the Italian Society of Immunohaematology and Transfusion Medicine (SIMTI) carried out a survey of the incidence of haemolytic disease of the newborn (HDN) and the prevention of HDN caused by anti-Rh(D) in Italian Transfusion Structures (TS).
Materials and methods
A questionnaire divided into the following five sections was administered: (i) types of services provided and maintenance of legally required registers, (ii) immunoprophylaxis (IP), (iii) red cell typing and searches for irregular antibodies, (iv) evaluation of foetal-maternal haemorrhage (FMH), and (v) incidence of HDN in 2010. Of the 280 TS sent the questionnaire, 176 (63%) replied.
Results
A HDN register was available in 55.5% of the TS (n =91). Immunoprophylaxis with a dose of anti-D IgG was given to all Rh(D) negative and Rh(D) variant puerpera with Rh(D) positive newborns: in more than 93% of cases the dose was between 1,500 IU (300 μg) and 1,250 IU (250 μg). Antenatal IP between the 25th and 28th week was proposed by 42 TS (26%). Seventy percent of the TS (n =115) did not make any evaluation of FMH. The number of births surveyed in 2010 was 203,384, the number of Rh(D) negative pregnancies was 13,569, while anti-D antibodies were present in 245 pregnancies. There were 111 cases of HDN due to anti Rh(D) incompatibility and in 40 of these, intrauterine transfusion (n =8) or exchange transfusion (n =32) was necessary. In 94 cases HDN was due to other irregular antibodies: in 4 of these cases intrauterine transfusion was needed and in 11 other recourse was made of exchange transfusion. Finally, there were 1,456 newborns with ABO HDN of whom 13 underwent exchange transfusion.
Discussion
The data collected give a picture of the incidence of HDN in Italy and of the methods of managing IP and could form the basis for an update of the SIMTI recommendations on the management and prevention of this disease.
Keywords: anti-D immunoprophylaxis, haemolytic disease of the newborn, anti-D immunoglobulins, FMH
Introduction
Before the introduction of immunoprophylaxis (IP), haemolytic disease of the newborn (HDN) was an important cause of neonatal morbidity and mortality1–3. Starting from the end of the 1960s, the administration of anti-D immunoglobulins to Rh(D) negative women immediately after delivery greatly reduced the incidence of the disease and the mortality rate has decreased from 1.2 cases every 1,000 newborns to the current level of 0.02 cases every 1,000 newborns4. The rate of immunisation also decreased notably, from 12–13% to about 1.2% and a further reduction was achieved following the introduction of prophylaxis during the third trimester of pregnancy, bringing the final rate to values between 0.17 and 0.28%5–11. Thus, the use of IP has led to both a decrease in the incidence of the disease and a lessening of its severity12.
Despite the excellent results achieved with IP, cases of HDN do still occur and engage transfusion doctors, gynaecologists and neonatologists. There are several reasons why cases of HDN still occur:
- possible errors in typing the pregnant woman and the newborn;
- lack of administration of prophylaxis (particularly in women from countries with lower levels of health care);
- ineffectiveness of the prophylaxis because the dose is too small for the amount of foetal-maternal haemorrhage (FMH);
- immunisation secondary to the transfusion of blood components.
The Italian law n. 219 of 21 October 2005, New regulations on transfusion activities and national production of blood derivatives14, sets out the essential levels of health care with regards to transfusion activities, including among these that Transfusion Structures (TS) carry out all the antenatal investigations aimed at preventing immunohaematological problems and HDN. Furthermore, the TS are obliged to keep a register of individuals to be given prophylaxis. Unfortunately, a considerable number of TS often cannot meet these obligations in full because of organisational problems resulting from the frequent lack of collaboration with birthing centres (private or public), which are the centres which actually administer the prophylaxis in almost all cases.
A survey15 carried out by the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) in 2004 and published 2007, to which only 69 TS replied out of a total of 300 surveyed, found out that only four centres gave IP at the 28th week, that only 30 TS were able to determine variants of the D antigen as part of the immunohaematological tests that they carried out on newborn and mother and that the data on the techniques used to evaluate FMH were very fragmented.
In order to update knowledge on the prevention of HDN in Italy, in 2011 SIMTI proposed a new survey to collect information from Italian TS. The results of the survey, presented during the Congress of Transfusion Services in Pisa in May 2011, led us to focus on the most critical and controversial aspects of HDN. It is also hoped that these data can form the basis for an update of the SIMTI recommendations on the management of HDN16 published in 2006 in collaboration with the Italian Society of Obstetrics and Gynaecology.
Materials and methods
The SIMTI set up a Working Group to design a multiple choice questionnaire with the purpose of obtaining a real, current view of the Italian situation concerning the prevention of HDN. The questionnaire comprised 37 questions, most of which had closed multiple choice answers, and was divided into five sections:
Types of services provided and legally required registers.
Immunoprophylaxis.
Red blood cell typing and investigations for irregular antibodies.
Evaluation of FMH.
Incidence of HDN in 2010.
The questionnaire was sent via the SIMTI website to the 280 Italian TS surveyed. It was possible to respond to the questionnaire via the web, but it was also possible to print it and send it back to the SIMTI offices by fax or e-mail. The data were analysed by the Working Group and provide a realistic “photograph” of the management of HDN in Italy.
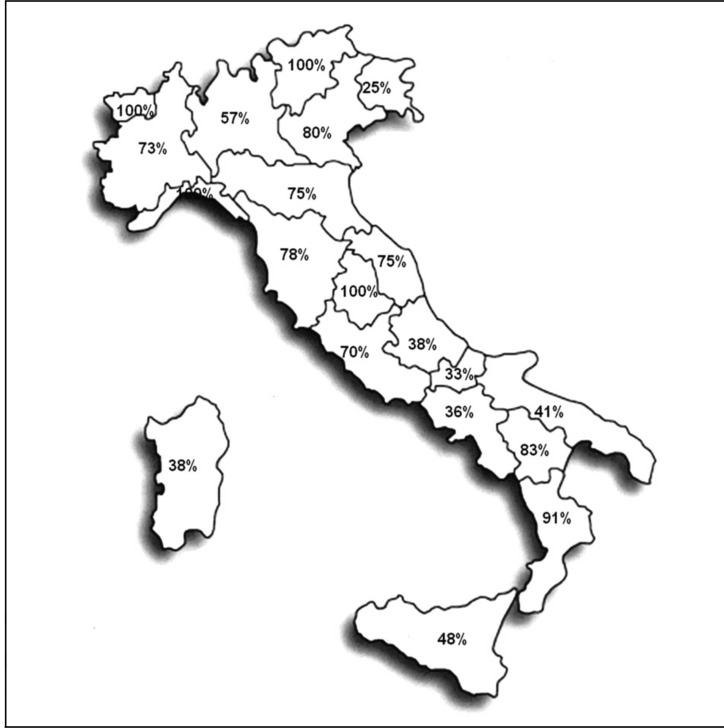
Of the 280 TS sent the survey, 176 (63%) responded. The percentage distribution of the TS participating in the various Italian regions is shown in Figure 1. It can be seen that the participation differed depending on geographical area, with higher response rates from TS in the Centre (80%) and North (79%) of Italy and lower rates from the South (61%) and the Islands (52%).
Figure 1.
Percentage response rate to the survey among the individual regions of Italy.
Of the 176 respondents, 12 TS only answered the first question, declaring that they did not carry out services aimed at preventing HDN. Thus, the number of TS that participated actively in the survey was 164.
Not all the questions were answered: the mean percentage of questions answered was 76% (min 27%–max 100%). The lowest percentage of answers (55%) was for the section concerning data on the incidence of HDN.
Results
Section 1. Types of services provided and legally required registers(Table I)
Table I.
Types of services provided and legally required registers.
| Only immuno-haematological tests on mother and newborns (A) | 46% |
| (A) + indication for giving IP (B) | 25% |
| (A) + (B) + record of IP having been given | 29% |
| Existence of a register of Rh(D) negative women who have undergone IP | 57%* |
| Paper records | 58% |
| Electronic records | 42% |
|
| |
| *Typology of records | |
| Record of IP having been given | 74% |
| Evaluation of FMH | 27% |
| Record of partner’s blood group | 24% |
| Evaluation of efficacy of IP | 22% |
With regards to the types of services provided with the purpose of preventing HDN, 46% of the TS declared that they performed only immunohaematological tests on the mother and newborn, 25% also provided indications concerning the IP to give and 29% recorded that the prophylaxis had been given. A HDN register concerning Rh(D) negative women to be given immunoprophylaxis was available in 55.5% of the TS (n =91); 42.1% (n =69) of the structures responded that they did not have a register, while 2.4% (n =4) did not answer the question. As far as concerns the information recorded, only 68 recorded that the IP had been given (74% of those stating that they had a register), 25 (27%) also recorded the outcome of an evaluation of FMH, 22 (24%) recorded the partner’s blood group and 20 (22%) recorded the efficacy of the IP. In 58% of the TS the register was in paper form, whereas in the other 42% electronic records were kept. When asked “Do you have specific software for the management of HDN?” only 13 TS (8%) responded positively.
Section 2. Immunoprophylaxis
This section of the questionnaire was designed to collect information on IP, such as the method of distributing the immunoglobulins, the timing and protocols for administering the prophylaxis and determination of its efficacy. Thirty-one percent of the TS did not reply to the questions in this section.
IP was regularly administered to Rh(D) negative or Rh(D) variant puerpera who gave birth to Rh(D) positive newborns. In 76% of cases the dose of anti-D immunoglobulins given was 1,500 IU (300 μg), in 17% the dose was 1250 IU (250 μg), while in the remaining 7% the dose was between 500 and 1,000 IU (100–200 μg) (Table II).
Table II.
Post-partum immunoprophylaxis and dose of anti Rh(D) immunoglobulins.
| Percentage of TS that perform post-partum IP | IP 1,500 IU | IP 1,250 IU | IP 500–1,000 IU |
|---|---|---|---|
| 100% | 76% | 17% | 7% |
In the case of dystocic, twin or Caesarean deliveries, a dose of at least 250 μg (1,250 IU) anti-D immunoglobulins was always given by 73% of the TS. In 80% of cases (132 TS) post-partum IP was given to Rh(D) negative or Rh(D) variant women even when it had not been possible to determine the Rh(D) type of the newborn. Eight TS (5%) stated that IP was not given in this situation, while 24 (15%) did not respond to the question.
In the case in which it was not possible to carry out IP within 72 hours of delivery, 15 TS (9%) declared that they would not administer it after this period, 35 TS (21%) did not respond to the question and 114 (70%) stated that they would give IP even though more than 72 hours had passed since delivery (Table III).
Table III.
Immunoprophylaxis in particular circumstances.
| Particular circumstances | Percentage of TS |
|---|---|
| Dystocic or twin deliveries | 73% yes (at least 250 μg) |
| 27% no | |
|
| |
| Post-partum IP given when it is not possible to determine the Rh(D) type of the newborn | 80% yes |
| 5% no | |
| 15% no response | |
|
| |
| IP given more than 72 h after delivery (when it was not possible to give it earlier) | 70% yes |
| 9% no | |
| 21% no response | |
With regards to antenatal IP, the questionnaire included some questions on prophylaxis in situations at risk of immunisation and others concerning prophylaxis to carry out between the 25th and 28th week of pregnancy.
When asked “In what ‘at risk’ situations do you give antenatal IP?”, a substantial percentage (36%) of the TS did not answer the question and gave no indication on the prophylaxis used. Antenatal IP was performed by 64% of the TS surveyed: as far as concerns the doses used, more than half (52%) did not indicate any dose, while 31% of the TS indicated that they used a maximum dose of 1,500 IU (Table IV).
Table IV.
Situations at risk of foetal-maternal haemorrhage in which antenatal prophylaxis is given and the doses used.
| “At risk” situations in which antenatal IP is given | No response | Prophylaxis given | 500 IU 100 μg | 1,000 IU 200 μg | 1,250 IU 250 μg | 1,500 IU 300 μg | Dose not indicated |
|---|---|---|---|---|---|---|---|
| Elective termination of pregnancy | 22% | 78% | 2% | 4% | 8% | 35% | 52% |
| Threatened abortion | 51% | 49% | 1% | 6% | 10% | 30% | 53% |
| Ectopic pregnancy | 37% | 63% | 1% | 6% | 11% | 29% | 54% |
| Invasive manoeuvres | 21% | 79% | 2% | 8% | 5% | 31% | 54% |
| Abdominal trauma | 54% | 46% | 0% | 8% | 12% | 31% | 49% |
| Haemorrhage during pregnancy | 41% | 59 % | 2% | 6% | 8% | 30% | 54% |
| Suspected foetal-maternal haemorrhage | 39% | 61% | 2% | 6% | 9% | 32% | 51% |
| Intrauterine death | 27% | 73% | 2% | 5% | 8% | 35% | 50% |
| Mean | 36% | 64% | 1% | 6% | 10% | 31% | 52% |
Forty-two (26%) of the TS routinely gave antenatal IP between the 25th and 28th week to all Rh(D) negative or Rh(D) variant women; 102 (62%) stated that they did not give antenatal IP, while 20 TS (12%) did not answer the question. With regards to the dose of anti-D immunoglobulins used, 30 TS (71.4%) used a maximum dose of 300 μg (1,500 IU), 6 TS (14,3%) gave a dose of 250 μg (1,250 IU), 5 TS (11,9%) used a dose between 100 μg (500 IU) and 200 μg (1,000 IU) (Table V), while one TS (2.4%) did not indicate the dose used.
Table V.
Antenatal prophylaxis in the third trimester.
| Antenatal IP between the 25th and 28th weeks | 26% yes | |
| 62% no | ||
| 12% no response | ||
|
| ||
| Dose used | 71.4% | 300 μg |
| 14.3% | 250 μg | |
| 11.9% | 100 μg – 200 μg | |
| 2.4% | no response | |
Section 3. Red cell typing and searches for irregular antibodies (Table VI)
Table VI.
Red cell typing and search for irregular antibodies.
| TS | |
|---|---|
| Search for weak D with the antiglobulin test in Rh(D) negative pregnant and puerperal women | 82% |
| Search for variant Rh(D) in Rh(D) negative pregnant and puerperal women | 59% |
| Foetal DNA typing in maternal blood | TS of Pavia |
| IAT at start of pregnancy* | 86.8% on all women |
| 13.2% only on Rh(D) negative women | |
| Sample used for determining the newborn’s blood group | 85% cord blood |
|
| |
| *Method | |
| 78% card | |
| 15% liquid phase | |
| 4% microplate | |
| 3% no response | |
The purpose of the questions concerning immunohaematological investigations was to determine the techniques and protocols adopted by each TS for typing the Rh(D) antigen and its variants and for searching for irregular antibodies. One question was on the type of request form used for immunohaematological investigations. More than 96% of the TS answered the questions in this section, apart from the question on the time of carrying out the indirect antiglobulin test in pregnancy, to which only 37% of the TS responded.
A search for weak D with the antiglobulin test in pregnant and puerperal Rh(D) negative women was carried out by 135 TS (82%), while 97 TS (59%) searched for variants of the Rh(D) antigen.
Only one centre in Italy (the TS in Pavia) reported typing foetal DNA in maternal blood. This test is carried out in the case of anti-D alloantibodies in the mother to determine whether the blood group of the foetus is compatible and in all pregnant Rh(D) negative women before they are given antenatal IP.
As far as concerns an indirect antiglobulin test at the start of pregnancy, 132 TS (86.8%) stated that they carried out this on all women, whether Rh(D) positive or Rh(D) negative, while 20 TS (13.2%) only performed it on Rh(D) negative women. Most of the TS (n =149, 78%) used cards to perform the indirect antiglobulin test, while 29 (15%) used a test-tube method (liquid phase), 7 (4%) used microplates and 5 (3%) did not give a response.
The newborn’s blood group was determined in a sample of cord blood in 139 TS (85%); the other TS did not reply (3 TS, 2%) or used samples of the baby’s blood (22 TS, 13%).
With regards to request forms for immunohaematological investigations, it was asked whether this form envisaged additional information for the immunohaematology laboratory carrying out the tests (for example, reason for the request, diagnostic outcome, relevant information such as ongoing pregnancy, a previous positive indirect Coombs’s test, previous IP): 67% (109 TS) replied positively, 27% (45 TS) answered that the request form did not envisage any historical information and 10 TS (6%) did not respond to the question.
Section 4. Evaluation of foetal-maternal haemorrhage
Two questions were asked about the evaluation of FMH. One concerned the type of method used, the other was on the clinical situations in which such an evaluation was performed.
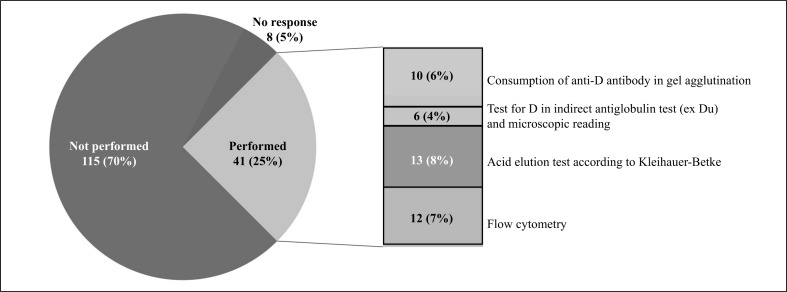
Only 41 TS (25%) carried out this type of evaluation, using the methods described in Figure 2, while 115 TS (70 %) did not make any assessment of the degree of the FMH. Eight (5%) of the TS did not answer this question.
Figure 2.
Evaluation of foetal-maternal haemorrhage and the methods used.
The entity of a FMH was evaluated after delivery by 31 TS (70% of those that carried out this investigation); 10 TS (23%) also evaluated haemorrhage during pregnancy in the case of events associated with a risk of bleeding (invasive obstetric manoeuvres, abdominal trauma, threatened abortion, etc.) occurring after the 20th week of pregnancy, whereas in 3 TS (7%) FMH was evaluated after delivery and only in the case of ‘at risk’ events occurring after the 28th week of pregnancy.
Section 5. Incidence of haemolytic disease of the newborn in 2010
In the part concerning the incidence of HDN in 2010 the TS were asked to state both the total number of cases of HDN caused by ABO, Rh(D) or other irregular antibodies, and the number of cases that required intrauterine transfusion or exchange transfusion.
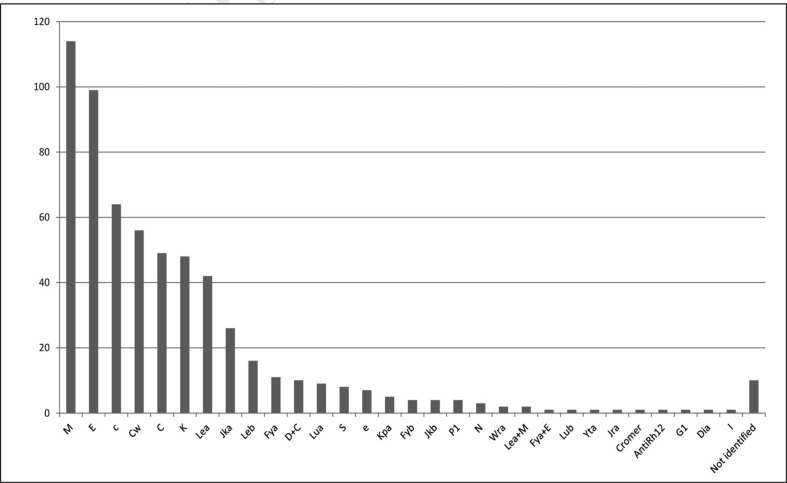
The number of deliveries recorded in 2010 was 203,384. Only 45 TS (27%) were able to indicate not only the total number of deliveries, but also the number of deliveries in Rh(D) negative women, which were 100,192 and 13,569 (13.5%), respectively. The overall data concerning the presence of irregular antibodies found in pregnancy are reported in Table VII. Anti-D antibodies were found in 245 pregnant women. Twenty-two of these cases had become sensitised despite IP having been administered regularly, on the occasion of a preceding birth of a Rh(D) incompatible baby. Only two TS were able to indicate the reason for the lack of protection from the IP: in one case the dose had been insufficient and in the other the IP had been performed late. The other 20 TS stated that they had not been able to determine the cause of the failed IP. Other irregular antibodies, not all pregnancy-related, were found in 602 pregnant women: the distribution of the antibodies identified is shown in Figure 3, in order of frequency.
Table VII.
Number of irregular antibodies found during pregnancy.
| Total n. of deliveries | 203,384 |
| N. of deliveries in TS that indicated the number of deliveries in Rh(D) negative women | 100,192 |
| N. of deliveries in Rh(D) negative women | 13,569 |
| N. of pregnancies in Rh(D) negative women in whom anti-D was found (excluding passive immunisation secondary to antenatal IP) | 245 |
| N. of pregnancies in Rh(D) negative women in whom active immunisation was recorded despite administration of IP | 22 |
| N. of pregnancies in women in whom other irregular antibodies were found | 602 |
Figure 3.
Pregnancies in which irregular antibodies (excluding anti-D) were found.
Overall, 1,661 cases of HDN were reported (Table VIII). Of these, 111 were due to anti Rh(D) incompatibility, but in only 40 cases was it necessary to perform intrauterine transfusion (8 cases) or exchange transfusion (32 cases). There were 94 cases of HDN due to other irregular antibodies. In 11 cases intrauterine transfusion (4 cases) or exchange transfusion (7 cases) was necessary. Finally, there were 1,456 cases of HDN due to ABO incompatibility, defined as the presence of a high titre of anti-A and/or anti-B IgG in maternal serum and/or a positive direct antiglobulin test (from 1+ up to 2–3+) on neonatal red blood cells and/or a positive eluate. Exchange transfusion was performed in 13 of these cases.
Table VIII.
Cases di HDN recorded in 2010 and the transfusion treatments used.
| Antibody identified | Number of cases | Intrauterine transfusion | Exchange transfusion |
|---|---|---|---|
| ABO system | 1,456 | 13 (0,9%) | |
| D | 111 | 8 (7,2%) | 32 (28,8%) |
| C | 26 | 3 (11,5%) | |
| C | 14 | 1 + 1 (C+D) (14,3%) | 1 (+D) (7,1%) |
| E | 17 | 3 (17,6%) | |
| K | 8 | 1 (12,5%) | |
| Fya | 7 | ||
| Jka | 7 | 1 (14,3%) | |
| E | 4 | ||
| M | 4 | ||
| Cw | 2 | ||
| Fyb | 1 | ||
| Jkb | 1 | ||
| yTA | 1 | ||
| Jra | 1 | ||
| Kpa | 1 | ||
| Total | 1,661 | 12 | 52 |
Discussion
Almost two-thirds (n =176; 63%) of the TS in Italy participated in this survey and the data collected can be considered important for providing a realistic picture of the management and prevention of HDN in this country. Compared to a previous survey published by SIMTI in 200715, to which only 69 TS had responded, this is certainly a gratifying result. Considering that the number of deliveries reported for 2010 by the TS participating in the survey was 203,384, and comparing this figure with the number of deliveries in Italy in the same period (n =549,794)17, we have data for about 37% of all pregnancies in Italy in 2010. The difference between the total number of pregnancies and the pregnancies surveyed is due, in part, to the fact that only two-thirds of the Italian TS participated in the survey, in part because some women deliver outside of hospital facilities (in private clinics or at home) and are not easily included in the survey and, above all, because many TS were not able to report the number of deliveries within their own hospital or private clinics within their catchment area.
The analysis of the data collected by this survey raise numerous issues worthy of discussion.
The rate of responses to the individual questions varied, being on average 76% and in no case reaching 100%; the lowest rate of response (55%) was to section 5 of the questionnaire (incidence of HDN and irregular antibodies during pregnancy). Only 27% of the TS were able to communicate the data on pregnancies of Rh(D) negative women. Considering only the data from the TS which communicated both the total number of deliveries and the number of deliveries by Rh(D) negative women, the percentage of the latter was about 13.5%, which is slightly lower than that expected for Rh(D) negative subjects in the European population18,19. The difficulty in obtaining the total number of deliveries and the number of those specifically in Rh(D) negative women derives from the fact that many of the TS do not have records specifically dedicated to HDN and most of the software systems used for the management and registration of transfusion activities do not allow easy extraction of the data relative to pregnant Rh(D) negative women.
Although a register of women to be given IP is required by law, such a register was kept in little more than half of the TS (n =91; 57%). In 58% of the cases the register was in paper form, while in the remaining 42% an electronic format was used. Very often the TS were not able to manage a register for organisational reasons, given the frequent lack of collaboration with maternity clinics (private or public), which are actually the structures in which the prophylaxis is performed and which should communicate to its reference TS that the prophylaxis has been given. The lack of a register of women given IP causes significant difficulties in guaranteeing full traceability of services delivered. It would, therefore, be desirable that the information technology systems that manage the various activities of TS have the possibility of recording and organising all the information useful for ensuring the best management and prevention of HDN. Finally, it would also be desirable that the information about women at risk of HDN could be consulted and updated on-line both by the staff of the TS, and by hospital and community gynaecologists/obstetricians in order that the immunohaematological history of patients at risk of HDN is always kept under control during a pregnancy.
With regards to services aimed at preventing HDN, just over a half of the TS (54%) provided information on the IP to perform, while 46% only carried out the immunohaematological tests requested, leaving the obstetrician to manage the prophylaxis. Analysing the data it seems almost as if these TS are not interested in the management of the prophylaxis, considering this the exclusive responsibility of the specialist obstetrician. The lack of interest is evident from the absence of responses to the part of the questionnaire concerning methods of administering IP in the antenatal period in particular risk situations: more than one-third of the TS did not answer or did not know how to answer these questions since they were not directly involved in the management of the prophylaxis. We, however, believe that the immunohaematologist should take a leading role in the governance of the whole process and not be consulted only when transfusion support is requested for a newborn already with HDN. We, therefore, hope that the indication for IP, when necessary, and the dose of the anti Rh(D) immunoglobulin, are always included in the immunohaematological report of newborn and mother’s blood groups.
One very reassuring finding was that post-natal IP is performed by almost all (97%) the hospitals involved in the survey and that in more than 75% of the cases the dose was more than 1,500 IU. On the other hand, antenatal IP for events that could potentially cause immunisation (invasive obstetric manoeuvres, abortions or threatened abortions, trauma to the pregnant abdomen or other similar situations) was performed by 63% of the TS surveyed, although it should be noted the percentage of structures not replying (37%) to this question was very high. Prophylaxis in the 25th and 28th week was administered by 42 TS (25%). This is certainly an improvement on the situation documented by the survey in 2007, when only 4 of the 69 TS which responded to the survey systematically gave antenatal IP in the 28th week15. Considering that one of the causes of failed prevention of HDN by anti-D is the passage of neonatal red blood cells into the maternal circulation during pregnancy5,11,20–22, antenatal prophylaxis should always be given to reduce the risk of immunisation23–28.
Not all the TS checked the efficacy of the IP administered and, among those that did, there were notable differences in the times that the test was performed. Although it is often difficult to convince women to undergo this evaluation of efficacy, greater involvement of obstetricians and better standardisation of practices would be desirable. In this case investigations for irregular antibodies, with the indirect immunoglobulin test, should be carried out at least 6 months after the administration of the last dose of anti-D immunoglobulins. Given the high sensitivity of currently used methods to search for irregular antibodies (card agglutination, microplate tests, etc.), tests carried out before 6 months could indicate the presence of traces of anti-D immunoglobulins in the circulation and the test would, therefore, inevitably have to be repeated in the following weeks.
The indirect antiglobulin test to search for weak D in pregnant and puerperal Rh(D) negative women was carried out by 82% of the TS, while more than a half (n =97; 59%) were able to investigate Rh(D) variants. Exact typing of the Rh(D) antigen in pregnant women and newborns is the key to correct IP. If investigations for weak D are performed and found to be positive, studies for variant Rh(D) should also be carried out, since it is not uncommon that subjects with weak D antigens (that is, with a low number of D antigen sites and low expression) also lack some epitopes and should, therefore, be classified as having a weak D variant. While most subjects with weak D are to all effects considered Rh(D) positive, and therefore in the case of pregnant women do not require IP, in contrast, the rare weak D variant subjects, such as those with weak D types 4.2, 7 and 11, who lack one or more components of the D antigen complex, resemble Rh(D) negative individuals and, therefore, in the case of pregnancy must be given IP11,29–32. Only one centre in Italy types cell-free foetal DNA (cffDNA) directly from a sample of maternal plasma. When a pregnant woman has a clinically significant antibody for HDN and the father is a heterozygote for the antigen in question, it may be important to genotype the foetus. Until recently the foetal DNA used for molecular typing of the foetus’s blood group was obtained by amniocentesis or chorionic villus biopsy. These invasive techniques do, however, carry a small risk of spontaneous abortion and can increase the levels of maternal antibodies. These risks can be avoided by precise determination of the foetal Rh(D) genotype from samples of plasma from the peripheral blood of the mother. Numerous studies have been published33–36 on the accuracy of determining foetal Rh factor from cffDNA and a meta-analysis of 37 studies published between 1993 and 2009 revealed that the overall diagnostic accuracy is greater than 96.5%37. By determining the foetal Rh type before the 28th week of gestation, prophylaxis with anti-D immunoglobulins can be reserved only for those women with a Rh(D) positive foetus, avoiding giving immunoglobulins in the case of Rh(D) negative foetuses. Further feasibility studies are necessary to establish whether the test can be applied on a large scale in the near future and whether it can also be used for the detection of other red cell antigens involved in HDN38–40.
The majority of the TS (86.4%) use a sample of cord blood to determine a newborn’s blood group, although a substantial proportion of the structures (n =22, 13.6%) still use blood samples from the new born baby. One of the issues under discussion is whether Rh typing should be carried out on all newborns, or whether it can be limited to only the newborns born of Rh(D) negative mothers. Rh(D) typing of Rh(D) positive mothers does not give any sort of information and could, therefore, be avoided.
There was considerable variability in the time of carrying out the indirect antiglobulin test during pregnancy and greater standardisation of this aspect would be welcome. The test was carried out by 86.8% of the TS on all women at the beginning of their pregnancies, while 20 TS stated that they only performed it on Rh(D) negative women. The request forms for immunohaematological tests provided for historical information in only two-thirds of the TS; thus in 45 TS the immunohaematology laboratory did not have all the information useful for the optimal interpretation of the results of the tests performed, for example, in the case of positivity for anti-D, the distinction between active or passive immunisation from previous IP. Only a request form with information on the patient’s history can help to resolve some of the immunohaematologists’ doubts.
Only 41 TS evaluated FMH. The remaining 115 TS that participated in the survey (70.1%) did not carry out any assessment of bleeding and the amount of anti-D immunoglobulins administered is not related to the extent of the FMH. This finding contrasts starkly with the results of the previous study in which almost all the participating TS declared that they evaluated FMH (n =67; 97% of the participating structures). In most cases FMH was evaluated after the delivery, whereas in some centres it was also carried out during pregnancies in cases at risk of haemorrhage. Also in this case, greater standardisation of the doses of anti-D immunoglobulins to use for prophylaxis would be desirable.
The number of Rh(D) negative women with anti-D antibodies during pregnancy was 245. However, not all TS that notified the presence of anti D indicated the total number of Rh(D) negative women and it was not, therefore, possible to calculate the real incidence of anti D. Taking into consideration only the data from the TS which communicated both the number of deliveries by Rh(D) negative women and the presence of anti D (n =146), the incidence was 1.08%. This percentage of immunised women was essentially the same as that in the survey conducted in 2004, in which the incidence was 0.96%. These data were, however, supplied by only 45 TS of 164 (27%), once again confirming the difficulty in obtaining this sort of information.
There were 111 cases of HDN due to anti-D immunisation; in 22 cases the sensitisation occurred despite the women having received correct IP on the occasion of a preceding Rh(D) incompatible delivery. The incidence of HDN due to anti Rh(D), calculated taking into consideration only the data reported by the TS that communicated the total number of deliveries in 2010, was 0.32 cases every 1,000 pregnancies; it is not possible to relate this to the total number of Rh(D) negative women because the denominator is not available. Most of the cases of HDN due to anti Rh(D) were of only modest clinical significance: among the 111 cases of HDN notified, intrauterine transfusion was carried out in only 8 (7.2%) and exchange transfusion was required in 32 cases (28.8%). In the remaining 71 cases (64%) no major transfusion treatment was performed.
Irregular antibodies to blood group antigens other than Rh(D) were found in 602 pregnancies. In most cases the antibodies were of little clinical relevance for either the pregnancy or effects on the foetus/newborn. Ninety-four foetuses/newborns developed a haemolytic disease that led to the performance of TIU in 4 (4,2%) and ET in 7 (7.4%) (Table VII).
There were 1,456 cases of HDN due to ABO incompatibility, defined as the presence of a high titre of anti-A and/or anti-B IgG and/or a positive direct antiglobulin test (from 1+ up to 2–3+) on neonatal red blood cells and/or a positive eluate. There were only 13 (0.9%) clinically challenging cases that necessitated exchange transfusion. In the other cases either no treatment was necessary or phototherapy was applied to correct hyperbilirubinaemia or small volumes of red cell concentrates were transfused to correct anaemia. The use of intravenous immunoglobulins in these cases contributed to a significant reduction in the number of exchange transfusions compared with the data collected in the 2007 survey, in which exchange transfusion was carried out in a total of 37 (2.4%) of 1,535 cases of HDN due to ABO incompatibility.
Overall, 64 (3.8%) of the total 1,661 cases of clinically relevant HDN required transfusion treatments such as intrauterine transfusion and exchange transfusion. This percentage is undoubtedly an overestimate, because although the reported number of cases of HDN requiring transfusion therapy is realistic, since the TS were always involved and could easily extract the information on intrauterine transfusion and exchange transfusion performed, the same cannot be said for the total number of pregnancies.
In conclusion, despite all the limitations that partial collection of data can give, particularly with regards to the real incidence of the pathology, the data obtained in this survey definitely provide a representative picture of HDN in Italy and the way immunoprophylaxis of HDN due to anti Rh(D) is managed. The information that the survey has made available could provide the basis for updating the SIMTI recommendations on the management and prevention of HDN.
Acknowledgements
We thank all the Transfusion Structures that participated in the survey: Alba, Alessandria, Aosta, Arezzo, Ascoli Piceno, Asti, Avezzano, Avola, Bagno A Ripoli, Bari Carbonara, Barletta, Bassano Del Grappa, Battipaglia, Belluno, Biella, Bologna Maggiore, Bologna S. Orsola Malpighi, Bolzano, Brescia, Bussolengo, Busto Arsizio, Cagliari, Caltagirone, Caltanissetta, Campobasso, Camposampiero, Casale Monferrato, Caserta, Castelfranco Veneto, Castellammare Di Stabia, Castrovillari, Catania Cannizzaro, Catania Vittorio Emanuele, Catanzaro, Chiari, Chieti, Chioggia, Città Di Castello, Civitanova Marche Alta, Conegliano Veneto, Cosenza, Crema, Cremona, Domodossola, Eboli, Enna, Esine, Este, Feltre, Fermo, Ferrara, Firenze San Giovanni Di Dio, Firenze Careggi, Foligno, Forlì, Frosinone, Gallarate, Gallipoli, Garbagnate Milanese, Genova Villa Scassi, Genova Galliera, Genova San Martino, Genova Gaslini, Grosseto, Imperia, Ivrea, Jesi, La Spezia Sant’Andrea, Lagonegro, Lamezia Terme, Lanusei, L’Aquila, Latina, Lavagna, Lecce, Legnago, Lentini, Lido Di Camaiore, Lido Di Ostia, Livorno, Locri, Lodi, Lucca, Macerata, Massa, Matera, Melfi, Messina Papardo-Piemonte, Mestre, Milano Policlinico, Milano San Carlo Borromeo, Modena, Modica, Mondovì, Monopoli, Napoli San Giovanni Bosco, Napoli Policlinico Federico II, Novara, Nuoro, Oristano, Padova, Palermo Policlinico, Paola, Parma, Paternò, Pavia, Perugia, Pesaro, Piacenza, Pietra Ligure, Pinerolo, Pisa, Pistoia, Polla, Pontedera, Potenza, Ragusa, Ravenna, Reggio Calabria, Rimini, Roma Torvergata, Roma Ospedale San Pietro, Roma Ospedale Sant’Eugenio, Roma Umberto I, Roma Bambino Gesù, Roma San Giovanni Calibita-Fatebenefratelli, Roma Pertini, Roma San Filippo Neri, Roma San Camillo, Roma Santo Spirito, Roma Gemelli, Rossano Calabro, San Benedetto Del Tronto, San Donà Di Piave, San Gavino Monreale, Savigliano, Savona, Sciacca, Senigallia, Seriate, Sesto San Giovanni, Siena, Sondrio, Taormina, Terni, Tivoli, Torino Molinette, Torino San Giovanni Bosco, Torino Oirm Sant’Anna, Torrette Di Ancona, Tortona, Trapani - Erice, Trento, Treviglio, Udine, Vallo Della Lucania, Varese, Verona, Vibo Valentia, Vicenza, Vigevano, Vimercate, Viterbo.
Footnotes
The Authors declare no conflicts of interest.
End note
The Study Group on the “Incidence and Prophylaxis of HDN in Italy”, which carried out and analysed the survey, was appointed by the Board of Directors of SIMTI and consisted of the Authors of this report, Francesco Bennardello and Giuseppe Curciarello, as well as Daniela Inverardi, Sisto Vecchio and Stefania Villa.
References
- 1.Mollison PL, Walker W. Controlled trials of the treatment of haemolytic disease of the newborn. Lancet. 1952;i:429–33. doi: 10.1016/s0140-6736(52)91949-1. [DOI] [PubMed] [Google Scholar]
- 2.Woodrow JC, Donohoe WT. Rh-immunization by pregnancy: results of a survey and their relevance to prophylactic therapy. Br Med J. 1968;4:139–44. doi: 10.1136/bmj.4.5624.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sansone G. La malattia emolitica neonatale, ieri e oggi. In: Sansone G, Dambrosio F, editors. Atti del Convegno sui Problemi Attuali della MEN, 1971. Genoa: Stringa Editore; 1971. pp. 17–26. [Google Scholar]
- 4.Tovey LA. Towards the conquest of Rh haemolytic disease: Britain’s contribution and the role of serendipity. Transfus Med. 1992;2:99–109. doi: 10.1111/j.1365-3148.1992.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 5.Huchet J, Dallemagne S, Huchet, et al. Ante-partum administration of preventive treatment of Rh-D immunisation in rhesus-negative women. Parallel evaluation of transplacental passage of fetal blood cells. Results of a multicentre study carried out in the Paris region. J Gynecol Obstet Biol Reprod. 1987;16:101–11. [PubMed] [Google Scholar]
- 6.MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynaecol. 1999;106:492–7. doi: 10.1111/j.1471-0528.1999.tb08304.x. [DOI] [PubMed] [Google Scholar]
- 7.Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. Br Med J. 1997;315:1588. doi: 10.1136/bmj.315.7122.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tovey LA, Townley A, Stevenson BJ, Taverner J. The Yorkshire antenatal anti-D trial in primigravidae. Lancet. 1983;2:244–6. doi: 10.1016/s0140-6736(83)90232-5. [DOI] [PubMed] [Google Scholar]
- 9.Chilcott J, Lloyd Jones M, Wight J, et al. A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are Rhesus-negative. Health Technol Assess. 2003;7:iii-62. doi: 10.3310/hta7040. [DOI] [PubMed] [Google Scholar]
- 10.Turner RM, Lloyd-Jones M, Anumba DOC, et al. Routine antenatal anti-D prophylaxis in women who are Rh(D) negative: meta-analyses adjusted for differences in study design and quality. PLoS ONE. 2012;7:e30711. doi: 10.1371/journal.pone.0030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reali G. Forty years of anti-D immunoprophylaxis. Blood Transfus. 2007;5:3–6. doi: 10.2450/2007.0b18-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowman JM. Alloimmune Hemolytic Disease of the Fetus and Newborn (Erythroblastosis Fetalis): Diagnosis, Management, and Prevention. GLOWM. 2008 doi: 10.3843/GLOWM.10201. [DOI] [Google Scholar]
- 13.Fung Kee Fung K, Eason E SOGC Maternal-Fetal Medicine Committee; SOGC Genetics Committee. Prevention of Rh alloimmunization. SOGC Clinical Practice Guidelines, No. 133, September 2003. J Obstet Gynaecol Can. 2003;25:765–73. doi: 10.1016/s1701-2163(16)31006-4. [DOI] [PubMed] [Google Scholar]
- 14.Legge 21 ottobre 2005, n. 219. Nuova disciplina of the attività trasfusionali e della produzione nazionale di emoderivati. Gazzetta Ufficiale della Repubblica Italiana, n. 251, 27/10/2005.
- 15.Velati C. A survey of the current use of anti-D immunoprophylaxis and the incidence of haemolytic disease of the newborn in Italy. Blood Transfus. 2007;5:7–14. doi: 10.2450/2007.0018-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biffoni F, D’Angiolino A, Massaro AL, et al. Recommendations for the management of haemolytic disease of the newborn. Blood Transfus. 2006;4:237–50. [Google Scholar]
- 17.ISTAT: Demografia in cifre. Nati per sesso e provincia - Anno di iscrizione. 2010. [Accessed on: 06/04/2012]. Available at: http://demo.istat.it/altridati/IscrittiNascita/2010/T1.13.pdf.
- 18.Wagner FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2:10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrone AO, Mazzei C. The Rhesus system: new insights with particular reference to weak D phenotypes. Blood Transfus. 2003;4:299–313. [Google Scholar]
- 20.Sebring ES, Polensky HF. Detection of fetal-maternal hemorrhage in Rh immune globulin candidates. A rosetting technique using enzyme-treated Rh2Rh2 indicator erythrocytes. Transfusion. 1982;22:468–71. doi: 10.1046/j.1537-2995.1982.22683068604.x. [DOI] [PubMed] [Google Scholar]
- 21.Moise KJ. Red blood cell alloimmunisation in pregnancy. Semin Hematolol. 2005;42:169–78. doi: 10.1053/j.seminhematol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 22.British Committee for Standards in Haematology (BCSH - a) Guidelines for the estimation of fetomaternal haemorrhage. Transfus Med. 1999;9:87–92. [PubMed] [Google Scholar]
- 23.Pilgrim H, Lloyd-Jones M, Rees A. Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation. Health Technol Assess. 2009;13:iii, ix–xi, 1–103. doi: 10.3310/hta13100. [DOI] [PubMed] [Google Scholar]
- 24.Royal College of Physicians of Edinburgh and Royal College of Obstetricians and Gynaecologist of UK, London. Statement from the Consensus Conference on anti-D prophilaxis. Vox Sang. 1998;74:127–8. doi: 10.1046/j.1423-0410.1998.7420127.x. [DOI] [PubMed] [Google Scholar]
- 25.Liumbruno GM, D’Alessandro A, Rea F, et al. The role of antenatal immunoprophylaxis in the prevention of maternal-foetal anti-Rh(D) alloimmunisation. Blood Transfus. 2010;8:8–16. doi: 10.2450/2009.0108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies J, Chant R, Simpson S, Powell R. Routine antenatal anti-D prophylaxis - is the protection adequate? Transfus Med. 2011;21:421–6. doi: 10.1111/j.1365-3148.2011.01106.x. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Clinical Excellence (NICE) Routine antenatal anti-D prophylaxis for women who are Rhesus D negative. [Accessed on 20/04/2013]. Available at: http://www.nice.org.uk/nicemedia/pdf/TA156Guidance.pdf.
- 28.Royal College of Obstetricians and Gynaecologists (revised in May 2002) Clinical green top guidelines: - Use of anti-D immunoglobulin for Rh prophylaxis. [Accessed on 20/04/2013]. Available at: http://www.neonatalformulary.com/pdfs/uk_guidelines/RHESUS(D)IMMUNOGLOBULIN-RCOG_guideline.pdf.
- 29.Flegel WA, Wagner FF. Molecular biology of partial D and weak D: implications for blood bank practice. J Clin Lab. 2002;48:53–9. [PubMed] [Google Scholar]
- 30.British Committee for Standards in Haematology (BCSH) Guidelines for blood grouping and red cell antibody testing during pregnancy. Transfus Med. 1996;6:71–4. [PubMed] [Google Scholar]
- 31.Royal College of Obstetricians and Gynaecologists. Clinical green top guidelines: the Use of anti-D immunoglobulin for Rh prophylaxis (revised March 2011) [Accessed on 20/04/2013]. Available at: http://www.rcog.org.uk/files/rcog-corp/GTG22AntiD.pdf.
- 32.Flegel WA. Molecular genetics and clinical applications for RH. Transfus Apher Sci. 2011;44:81–91. doi: 10.1016/j.transci.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo YM. Fetal DNA in maternal plasma. Ann N Y Acad Sci. 2000;906:141–7. doi: 10.1111/j.1749-6632.2000.tb06604.x. [DOI] [PubMed] [Google Scholar]
- 34.Lo YMD, Hjelm NM, Fidler C, et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med. 1998;339:1734–8. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- 35.Wright C. Report of the UK expert working group. Cambridge PHG Foundation; 2009. [Accessed on 20/04/2012]. Cell-free fetal nucleic acids for non-invasive prenatal diagnosis. Available at: http://www.phgfoundation.org/reports/4985/ [Google Scholar]
- 36.Finning K, Martin P, Daniels G. The use of maternal plasma for prenatal RhD blood group genotyping. Methods Mol Biol. 2009;496:143–57. doi: 10.1007/978-1-59745-553-4_11. [DOI] [PubMed] [Google Scholar]
- 37.Geifman-Holtzman O, Grotegut CA, Gaughan JP. Diagnostic accuracy of noninvasive fetal Rh genotyping from maternal blood - a meta-analysis. Am J Obstet Gynecol. 2006;195:1163–73. doi: 10.1016/j.ajog.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Daniels G, Finning K, Martin P, Summers J. Fetal RhD genotyping: a more efficient use of anti-D immunoglobulin. Transfus Clin Biol. 2007;14:568–71. doi: 10.1016/j.tracli.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Benachi A, Delahaye S, Leticee N, et al. Impact of non-invasive fetal RhD genotyping on management costs of rhesus-D negative patients: results of a French pilot study. Eur J Obstet Gynecol Reprod Biol. 2012;162:28–32. doi: 10.1016/j.ejogrb.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Finning K, Martin P, Summers J, Daniels G. Fetal genotyping for the K (Kell) and Rh C, c, and E blood groups on cell-free fetal DNA in maternal plasma. Transfusion. 2007;47:2126–33. doi: 10.1111/j.1537-2995.2007.01437.x. [DOI] [PubMed] [Google Scholar]