Abstract
Background
Surgical intervention may pose significant risk of life-threatening bleeding in patients with von Willebrand's disease; prophylactic treatment with von Willebrand factor/factor VIII concentrate is generally indicated for von Willebrand's disease characterized by moderate to severe qualitative and quantitative deficiencies of Willebrand factor to raise and maintain both Willebrand factor and FVIII at haemostatic levels for surgical prophylaxis.
Materials and methods
Since prospective clinical data in such situations were lacking, two recent, prospective, multicentre studies evaluated the prophylactic perioperative use of the on Willebrand factor/ factor VIII concentrates, Humate-P® and Haemate P. Despite some differences in the two studies, one conducted in the USA (n =35) and one in the European Union (n =27), the designs were similar enough to allow for a limited pooled analysis of data. In both studies, preoperative loading doses and subsequent maintenance doses were calculated using individual subject-derived incremental in vivo recovery values, although von Willebrand factor:ristocetin cofactor and FVIII:coagulation activity target levels differed between the protocols. Efficacy was rated daily by the investigator as excellent, good, moderate, or poor.
Results
Overall haemostatic efficacy (rating of excellent/good), assessed 24 hours after the last infusion (USA) or taken as the worst rating between surgery and day 14 (EU), was achieved in 95% of the pooled population of 62 adults and children. Efficacy did not appear to be affected by dosing variations. The rate of possibly related adverse events was low (8 subjects; 13%); one of these events was considered serious (pulmonary embolism).
Discussion
This pooled analysis of a relatively large number of patients for a rare disease confirms the feasibility of pharmacokinetically guided dosing of von Willebrand factor/factor VIII concentrate and highlights its efficacy and safety in the prevention of excessive perioperative bleeding.
Keywords: von Willebrand's disease, factor VIII, prophylaxis, elective surgery
Introduction
von Willebrand's disease (VWD), the most common inherited bleeding disorder, is markedly heterogeneous with regard to type and severity1,2. The bleeding tendency in VWD results from deficient production or function of von Willebrand factor (VWF) and a secondary defect of factor VIII (FVIII). Surgery, even relatively minor procedures, in those patients with moderate to severe qualitative and quantitative deficiencies of VWD, can be associated with a life-threatening risk of excessive bleeding. The treatment of choice in such situations is prophylactic administration of a VWF/FVIII concentrate to replace the missing or impaired coagulation factors.
Humate-P® and Haemate P (CSL Behring, Marburg, Germany) are VWF/FVIII concentrate products marketed for more than 20 years in the United States of America (USA) and European Union (EU), respectively. The differences between the two products are minor, with the primary ones being: (i) the plasma used for Haemate P is not solely sourced from USA Food and Drug Administration (FDA)-licensed plasma collection centres, and (ii) the excipient albumin for Haemate P originates from the USA and EU (Germany, Austria), while that for Humate-P is exclusively from the USA. In comparison to other VWF/FVIII products, these products are noteworthy for their higher percentage of high molecular weight multimers, thought to be critically important in correcting the haemostatic defects in patients with VWD3. Haemate P was introduced in Europe in 1981 for the management of haemophilia A and VWD. Humate-P was first approved by the FDA in April 1986, with an indication for use in patients with haemophilia A (treatment and prevention of bleeding), and subsequently approved in 1999 for the treatment of spontaneous and trauma-induced bleeding in patients with severe VWD. The efficacy and safety of this VWF/FVIII concentrate in patients with VWD have been well documented4–11 and the concentrate continues to be regarded as a standard for therapy for moderate and severe bleeds as well as for prevention of bleeding in surgical procedures12.
The use of VWF/FVIII concentrates had not been systematically and prospectively studied in clinical situations involving surgical prophylaxis, although a number of retrospective studies, surveys and case reports have described the successful use of Humate-P/Haemate P in such situations10,11,13–19. To further address the need for evidence-based clinical recommendations for perioperative administration, two prospective clinical studies were conducted with Humate-P and Haemate P in patients with VWD undergoing elective surgery in the USA and EU, respectively. Despite some differences in protocol specifics, particularly with regard to dosing guidelines, it was felt that the similarities afforded an opportunity to analyse the safety and efficacy of Humate-P/Haemate P on a larger scale than might otherwise be feasible for this rare disease. While the pooled analysis provides a more robust dataset for evaluation of the overall efficacy of this VWF/FVIII concentrate, a comparison of individual outcomes from the two trials is also presented as a means of assessing the relative merits of particular dosing strategies. Detailed findings from the individual studies have been published elsewhere8,9,20.
Materials and methods
Both studies were prospective, multicentre, open-label trials. The American study was carried out primarily in the USA (15 centres), along with two centres in Europe, and the EU study was conducted at 12 European centres.
Selection of patients
Both studies included adults and children (>5 years of age in the EU study; any age in the USA study) with a clinical and laboratory diagnosis of VWD who were not sufficiently responsive to desmopressin for management of surgery and who were scheduled to undergo elective surgery.
Surgery classification
Before surgery, each anticipated procedure was categorised by the investigator into one of the following categories: (i) oral surgery (simple tooth extraction); (ii) minor surgery (simple operations not considered a risk to life which could be performed in an outpatient setting with or without sedation); (iii) major surgery (operations involving considerable hazard or risk to life or limb, frequently involving general anaesthesia). Extractions of more than two teeth and removal of more than one impacted wisdom tooth were considered major surgery in the USA study, while extractions of four or more teeth were considered major surgery in the EU.
Pharmacokinetic determinations
Participants in both studies received an initial single infusion of 60 IU VWF:ristocetin cofactor (RCo)/kg (USA) or ~80 IU VWF:RCo/kg (EU) of VWF/FVIII concentrate for the purpose of pharmacokinetic (PK) assessments within 1 week (USA) or 2 to 4 weeks (EU) prior to surgery. Half-life and incremental in vivo recovery (IVR) were determined for VWF:RCo and coagulation FVIII activity (FVIII:C) and used for perioperative dosing calculations.
Laboratory methods
In the USA study, central laboratory support and testing were provided by Mayo Clinical Trials Services (Rochester, MN, USA), whereas in the EU study, the plasma samples were assayed at the laboratories of the local study centres using standard methods and also at a central coagulation laboratory (Ulrich Budde, Hamburg, Germany). The details of the laboratory methods have been published previously8,9,20.
Perioperative dosing of Humate-P/Haemate P
Dosing regimens for the surgical phases of both the USA and EU studies were tailored for each patient using the calculated IVR from the PK phase. In the USA study, individually determined terminal half-lives of VWF:RCo were used to establish dosing frequency. There were notable differences in loading and maintenance dosing calculations between the two protocols (Table I). Unless stated otherwise, all dosing values reported in this paper reflect VWF:RCo units.
Table I.
Dosing guidelines followed in the USA and EU VWF/FVIII concentrate studies.
| USA study | EU study | ||
|---|---|---|---|
|
|
|||
| PK infusion | 60 IU VWF:RCo/kg | 80 IU VWF:RCo/kg | |
| Loading dose | Before amendment | After amendment | |
| Target | 1.5 × full dosea | Oral and minor surgery: | VWF:RCo >100% |
| VWF:RCo ≥100% | VWF:RCo 50%–60% | FVIII:C >80% | |
| FVIII:C ≥100% | FVIII:C >40% | ||
| Major surgery: | |||
| VWF:RCo 80%–100% | |||
| FVIII:C 80%–100% | |||
|
| |||
| Maintenance dose | Before amendment | After amendment | All: VWF:RCo >50% for ≥6 daysb |
| Target trough levels | VWF:RCo ≥50% | Oral and minor surgery: | Type 1, minor surgery: |
| FVIII:C ≥80% (>50% after day 3) | After day 3: FVIII:C >30% | FVIII:C >50% for ≥4 days | |
| Major surgery: | Types 2 and 3, minor surgery: | ||
| VWF:RCo and FVIII:C >50% (>30% after day 3) | FVIII:C >50% for ≥7 days | ||
| All major surgery: | |||
| FVIII:C 80%–100% for 7–14 days | |||
|
|
|||
| Minimum duration | Oral surgery: | Oral surgery: | At least one repeat dose in 24 hours |
| At least one dose post-surgery | At least one dose post-surgery | ||
| Minor surgery: | Minor surgery: | ||
| At least 48 hours post-surgery | At least 48 hours post-surgery | ||
| Major surgery: | Major surgery: | ||
| Anticipated for at least 72 hours | Anticipated for at least 72 hours | ||
Legend
Full dose: PK-derived dose that would achieve a VWF:RCo level of 100%; in initial protocol, the loading dose was taken as 1.5 times the full dose to compensate for possible extra consumption during surgery.
Excluding oral surgery.
FVIII: factor VIII; PK: pharmacokinetic; RCo: ristocetin cofactor; VWF: von Willebrand factor; FVIII:C: coagulation factor VIII activity.
In the USA study, loading doses were initially calculated similarly for all types of surgery. The pre-surgical loading dose was given as 1.5 times the "full dose", defined as the PK-derived dose that would achieve a VWF:RCo level of 100% and a predicted FVIII:C level of at least 100%. The rationale for the use of an initial dose of 1.5 times the full dose was to compensate for predicted clotting factor consumption prior to surgery; however, because of a lack of evidence of increased factor consumption, the protocol was amended after 15 subjects had been treated to adjust loading doses to achieve target plasma levels of 50% to 60% VWF:RCo for oral or minor surgery and levels of 80% to 100% VWF:RCo for major surgery. Doses were calculated as loading dose = (Δ×b.w.)/IVR, where Δ is the target VWF:RCo increase (IU/dL) to achieve the desired plasma level and b.w. is body weight in kilograms. For subjects with an IVR ≥2, a denominator of 2 was used. Doses were rounded to the nearest full vial of VWF/FVIII concentrate, with a maximum deviation of 10%. Similarly, maintenance dosage recommendations were initially the same for all subjects, but changed to reflect the nature of surgery following the protocol amendment (see Table I). In the USA study, maintenance infusions were spaced according to individual VWF:RCo half-life values; VWF/FVIII concentrate was given every 12 hours for those with a half-life of >10 hours and every 8 hours for those with a half-life of 6 to 10 hours. In subjects with a VWF:RCo half-life shorter than 6 hours, a dosage recommendation was made by the Data Safety Monitoring Board (DSMB). Maintenance regimens could be modified by the investigator depending on observed VWF:RCo and FVIII:C levels following surgery.
The EU study stipulated loading doses targeting a VWF:RCo of >100% and FVIII:C of >80% using the same formula: loading dose = (Δ×b.w.)/IVR. Maintenance dosing for all subjects was targeted to achieve a VWF:RCo trough level of >50% for at least 6 days. Target trough FVIII:C levels varied according to the types of VWD and surgery (Table I). Postoperative doses in the EU study were repeated at the discretion of the investigator, but were recommended at least once every 24 hours.
Efficacy assessments
Haemostatic efficacy was assessed in both studies at various time points by the investigators using a four-point ordinal scale: excellent (normal haemostasis), good (mildly abnormal haemostasis; slight oozing), moderate/poor (moderate, controllable bleeding), and none (severe haemorrhage that is difficult to control). In the EU study, this assessment was made on the day of surgery and daily for 14 days thereafter; in the USA study, haemostatic efficacy was rated directly after the end of the surgical procedure, 24 hours after the last VWF/FVIII concentrate infusion, and 14 days post-surgery.
The main efficacy parameter for this combined analysis was overall haemostatic efficacy, as specified by the USA protocol, as either: (i) the assessment 24 hours after the last infusion of Humate-P or (ii) the day 14 assessment, whichever came earlier. Overall haemostatic efficacy was not a defined outcome in the EU study, and no particular time point was selected to represent overall efficacy. Furthermore, the EU study did not mandate an assessment 24 hours after the last infusion of VWF/FVIII concentrate. Therefore, for the purposes of this combined analysis, overall haemostatic efficacy for EU subjects was defined as the worst rating recorded by investigators between surgery and day 14.
Results
Subjects
In the USA study, efficacy was assessed using the surgical-phase Full Analysis Set (FAS) population, characterized by subjects who met all of the following criteria: (i) achieved a peak VWF:RCo level after the PK infusion of ≥50 IU/dL; (ii) received a pre-surgery loading infusion of Humate-P; and (iii) were assessed for efficacy at some post-surgery time point or dropped out of the study due to lack of efficacy. Thirty-five subjects met these criteria. For this combined analysis, subjects from the EU study were included based upon post hoc FAS criteria consistent with the USA study. Based on these criteria, 27 EU subjects were eligible for the combined analysis, forming a total combined cohort of 62 subjects. The only EU subject who was ineligible for the pooled analysis received a loading dose of VWF/FVIII concentrate, but did not subsequently undergo surgery. This subject was, however, included in the safety analysis.
The great majority of subjects in both studies were adults between the ages of 16 and 64 years, with a similar male:female ratio in the two studies8,9. In both populations, the samples of patients were characterized by similar proportions of VWD types 1, 2, and 3. Within the category of type 2 disease, there were, however, greater proportions of subjects with types 2B and 2M in the USA study (9% vs 0% and 14% vs 4%, respectively), and a greater proportion of type 2A disease in the EU study (33% vs 6%).
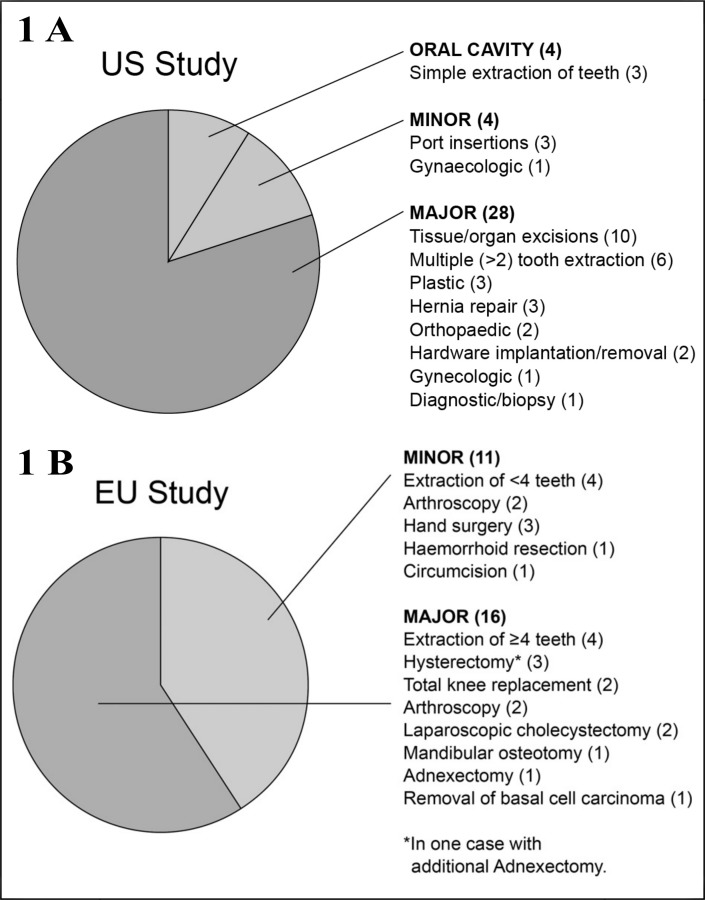
Figure 1 provides a breakdown of the types of surgery performed in both studies. The proportion of surgical procedures classified as major was somewhat higher in the USA study than in the EU one (80% vs 59%), although it should be noted that the definitions for major and minor surgery were not identical between the protocols.
Figure 1.
1A Distribution of surgery types in USA and EU study of Humate-P; 1B Distribution of surgery types in EU study of Haemate P.
Pharmacokinetic parameters
The main PK analysis in both studies focused on the population without major protocol deviations during the PK phase, which included 41 subjects in the USA study and 28 subjects in the EU study. While a detailed description of all PK findings is beyond the scope of this manuscript, Table II outlines the parameter means that were of importance with regard to dosing in both studies (half-life and incremental IVR for VWF:RCo and FVIII:C). Despite differences in PK dosing, half-life and IVR values were quite similar between the USA and EU studies.
Table II.
Pharmacokinetic parameters used for VWF/FVIII concentrate dose determinations in the USA and EU studies.
| Parameter | USA study (n =41) | EU study (n =28) |
|---|---|---|
| Effective half-life | ||
| h (range) | 6.8 (0.2–74.9) | 6.3 (1.1–14.1) |
| Terminal half-life | ||
| h (range) | 11.7 (3.5–74.9) | 10.0 (2.8–28.3) |
| VWF:RCo IVR (incremental), IU/dL per IU/kg | ||
| Median (range) | 2.4 (1.1–4.2) | 1.9 (0.6–4.5) |
| FVIII:C IVR (incremental), IU/dL per IU/kg | ||
| Median (range) | 2.7 (1.4–5.7) | 2.8 (1.5–4.2) |
Legend FVIII: factor VIII; IVR: in vitro recovery; RCo: ristocetin cofactor; VWF: von Willebrand factor.
In the USA study, 19 of the 41 subjects demonstrated an initial distribution phase with a median VWF:RCo half-life of 1.2 hours (range, 0.2–3.2 hours). Terminal half-life in all USA subjects was 11.7 hours (range, 3.5–74.9 hours), and a median volume of distribution at steady state (Vss) of 52.7 mL/kg was observed, suggesting a moderate distribution of VWF:RCo beyond plasma. In the EU study, the median VWF:RCo terminal half-life was 15.6 hours (range, 9.0–28.4 hours), with a median Vss of 68.8 mL/kg (range, 46.2–89.5 mL/kg).
Efficacy
Thirty-five subjects in the USA study and 27 subjects in the EU study were eligible for the combined efficacy analysis. Overall haemostatic efficacy was rated as effective for all subjects, except for a single EU subject in whom efficacy was rated by the investigator as moderate at the end of minor surgery (arthroscopic synovectomy); all further assessments in this subject were rated as excellent. In the USA study, the overall efficacy was rated as excellent in 33 of the 35 patients. Overall haemostatic efficacy for the pooled sample was, therefore, 95%. Because of the high overall efficacy rating, no differences in efficacy could be detected between the types of surgery.
In the USA study, a DSMB reviewed all assessments of overall haemostatic efficacy and provided an independent adjudication. The DSMB agreed with all investigator assessments of overall haemostatic effectiveness except for two subjects for whom the effectiveness was down-graded from "excellent" to "moderate/poor". One type 2A subject was enrolled in the study for a planned hysteroscopy for fibroid removal and dilation and curettage, but experienced severe vaginal bleeding requiring a follow-up hysterectomy the following day. She received one unplanned transfusion of packed red blood cells and two transfusions of fresh-frozen plasma. FVIII:C levels ranged from 68% to 98%, and VWF: RCo values ranged from 88 to 132 IU/dL throughout this 2-day period. Although the investigator had graded immediate haemostatic efficacy as "excellent" in this subject, the DSMB downgraded the overall evaluation to "moderate/poor" after a thorough review of all contributory findings. Another subject with type 2A VWD experienced prolonged gastrointestinal bleeding following bariatric surgery and liver biopsy. The investigators rated overall haemostatic efficacy as "excellent", which the DSMB ultimately downgraded to "moderate/poor." Factor VIII:C and VWF:RCo levels were in the therapeutic range throughout the course of the bleeding event, which the investigator considered a surgical complication.
For the USA study, the median and mean actual estimated blood loss during surgery did not exceed the expected volume (22 [±25] mL for oral surgery, 18 [±19] mL for minor surgery, 81[±93] mL for major surgery), regardless of the type of surgery. Four subjects received red blood cell transfusions, with individual transfusion volumes ranging from 300 to 4,279 mL. Three of these subjects experienced serious haemorrhagic adverse events. In two of these subjects, the DSMB considered haemostasis ineffective, despite FVIII and VWF:RCo levels within the therapeutic ranges, as mentioned previously. In the EU study, only one subject who underwent a bilateral total knee replacement required a transfusion after exhibiting low pre-operative haematocrit; no obvious postoperative bleeding was observed and overall haemostatic efficacy was still rated as excellent in this subject.
Safety
Table III summarises adverse event frequencies for the surgical phases of the individual studies and the combined analysis. The pooled safety data did not identify any notable variations in the trends observed from the individual study findings. The most commonly reported events were nausea, constipation, dizziness, pain, and haemorrhage. While a majority of subjects in each study reported at least one adverse event (USA, 86%; EU, 75%), few events were considered related to treatment. The rate of adverse events considered possibly related to VWF/FVIII concentrate treatment was 13% for the combined population. In the USA study, there were three possibly related events in three subjects during the surgical phase: headache, nausea, and dizziness, which were generally mild, brief, and self-limiting; the nausea was of moderate intensity and lasted 30 days. There were five possibly related adverse events during the surgical phase of the EU study: pulmonary embolism (classified as serious), thrombophlebitis of the leg, vomiting, rash, and increased levels of alanine aminotransferase. All events except the pulmonary embolism (moderate) were of mild intensity. The pulmonary embolism occurred in an 81-year-old subject 10 days (and 10 VWF/FVIII concentrate infusions) after undergoing major surgery for bilateral knee prostheses. Her baseline FVIII:C and VWF:RCo levels were 70% and 8%, respectively. She had several risk factors for thrombosis (old age, major orthopaedic surgery, high postoperative FVIII:C levels, and thrombocytosis) and had not received any standard antithrombotic prophylaxis. The thrombophlebitis of the leg occurred in a 51-year-old subject 7 days (and 4 VWF/FVIII concentrate infusions) after undergoing major surgery for a hysterectomy. Her baseline FVIII:C and VWF:RCo levels were 24% and <10%, respectively.
Table III.
Summary of total and most frequent adverse events (≥10% in either study) experienced during surgical-phase treatment with VWF/FVIII concentrate.
| Number (%) of subjects | |||
|---|---|---|---|
|
|
|||
| Adverse Event | USA (n =35) | EU (n =28) | Pooled (n =63) |
| Any AE | 30 (86%) | 21 (75%) | 51 (81%) |
| Possibly related AE | 3 (9%) | 5 (18%) | 8 (13%) |
| Nausea | 13 (37%) | 2 (7.1%) | 15 (24%) |
| Constipation | 5 (14%) | 2 (7.1%) | 7 (11%) |
| Dizziness | 5 (14%) | 0 | 5 (8%) |
| Pain | 5 (14%) | 6 (21.4%) | 11 (17%) |
| Fever | 4 (11%) | 0 | 4 (6%) |
| Haemorrhage* | 4 (11%) | 6 (21.4%) | 10 (16%) |
| Epistaxis | 2 (6%) | 3 (10.7%) | 5 (8%) |
| Headache | 1 (3%) | 3 (10.7%) | 4 (6%) |
Legend AE: adverse event; FVIII: factor VIII; VWF: von Willebrand factor;
Only comprises events coded as "haemorrhage".
Bleeding events
Among all surgical-phase subjects in the combined safety analysis (n =63), 19 (30%) subjects experienced 35 bleeding events. Five subjects experienced bleeding from at least two sites. Twelve subjects experienced 19 episodes of wound bleeding or injection-site bleeding, five subjects experienced epistaxis, one patient experienced two episodes of cerebral haemorrhage/subdural haematoma postoperatively (days 2 and 3) after placement of a subdural grid for localisation of the focus of seizures, and one patient had two episodes of gastrointestinal bleeding postoperatively (days 10 and 15) after bariatric surgery. Other bleeding events, each reported in a single subject, included menorrhagia, groin bleed, ear bleed, haemoptysis, haematuria, and shoulder bleed. Fourteen of the 35 bleeding events occurred after completion of VWF/ FVIII treatment. None of these events were considered possibly related to VWF:FVIII administration.
Dosing
Table IV describes the median daily loading and maintenance doses for the USA and EU populations, as well as total doses received throughout treatment. The median loading doses were lower for oral surgery than for minor and major surgeries, but there was a notable overlap in the ranges across types of surgery. The median loading dose for minor surgery was higher than for major surgery, but this was skewed by high median loading doses for minor surgery in the small paediatric cohort. Paediatric loading doses for major surgery were lower than those used in adult subjects. In general, median loading doses became progressively higher with more severe types of VWD (56.5, 62.2, 70.9, 40.7, and 74.0 IU/kg for types 1, 2A, 2B, 2M, and 3, respectively).
Table IV.
Median daily loading and maintenance doses and total doses received by surgery type for the USA, EU, and pooled populations.
| Median doses of VWF:RCo, IU/kg (range) | ||
|---|---|---|
|
|
||
| USA | EU | |
| Oral surgery | n =3 | Not assessed separately |
| Loading | 42.6 (38.6–121.1) | – |
| Daily maintenance | 24.7 (21.3–40.5) | – |
| Total therapy | 64 (63–202) | – |
| Minor surgery | n =4 | n =11 |
| Loading | 114.3 (48.9–135.3) | 62.2 (44.4–155.6) |
| Daily maintenance | 36.3 (35.4–93.8) | 39.2 *(30.7–59.6) |
| Total therapy | 292 (226–859) | 238.5 (143.6–849.3) |
| Major surgery | n =28 | n =16 |
| Loading | 55.5 (17.4–113.9) | 66.5 (39.7–151.4) |
| Daily maintenance | 39.7 (21.7–100.0) | 49.7 (28.8–123.8) |
| Total therapy | 241 (79–1,699) | 448.5 (167.1–1,297.4) |
There was a general trend toward increasing daily maintenance doses going from oral procedures via minor to major types of surgery, although there was some overlap. Subjects with type 2A VWD had the highest median daily maintenance dose (45.6 IU/kg; range, 29.6–123.8 IU/kg), followed by subjects with type 3 VWD (40.5 IU/kg; range, 21.3–78.8 IU/kg), and those with type 1 VWD (39.7 IU/kg; range, 25.6–100.0 IU/kg).
The median total dose of VWF/FVIII used in subjects undergoing minor surgery was similar in the USA (292 IU/kg) and EU studies (238 IU/kg). For subjects undergoing major surgery, the median total dose was higher in EU subjects (448 IU/kg; range, 167–1,297 IU/kg) than in USA subjects (241 IU/kg; range, 79–1,699 IU/kg), although the range was narrower in the EU population.
For the pooled sample, the median duration of post-surgery treatment was shortest for oral surgery (1 day; range, 1–2 days), followed by minor surgery (4 days; range, 1–17 days) and major surgery (7 days; range, 1–26 days). Duration of therapy for major surgery was shorter for paediatric subjects than for adult subjects (4 days vs 7 days), but the small number of paediatric subjects (n =8) makes these results difficult to interpret. Median treatment duration was longest in subjects with type 3 VWD (7 days; range, 1–16 days), followed by subjects with type 2M VWD (6.5 days; range, 6–10 days).
Discussion
These data from the individual studies and the pooled analysis of findings support the efficacy of Humate-P and Haemate P as prophylaxis against excessive bleeding in subjects with all types of VWD undergoing elective surgery. The combined population of 62 subjects represents the largest prospective surgical cohort with VWD studied to date. In the pooled assessment, using efficacy criteria defined by the USA protocol, effective haemostasis was achieved in 95% of subjects, including a high percentage of subjects undergoing major surgery. This finding is of particular interest given the differences in dosing between the two source studies. Efficacy was apparently not affected either by dosing differences between the two protocols or by the reduced loading doses enacted by a protocol amendment midway through the USA trial.
For minor surgery, the median loading dose was almost twice as high in the USA study than in the EU study (114 vs 62 IU/kg); however, the total dose of VWF/ FVIII concentrate used for these procedures was similar between studies (292 vs 238 IU/kg, respectively). For major surgeries, median loading doses were comparable between studies (56 vs 67 IU/kg), while total doses used were notably higher in the EU study than in the USA study (448 vs 241 IU/kg). In essence, Humate-P/ Haemate P was highly effective in preventing excessive bleeding in essentially all treated subjects, within the range of doses administered.
These findings corroborate previously reported experience with Humate-P and Haemate P in the prophylaxis of surgical bleeding2,17,21. Thompson et al.2 described the use of Humate-P in the management of 42 urgent surgical procedures in 39 subjects with congenital VWD in a prospective study. Overall efficacy was rated as "excellent/good" in 100% of subjects. Gill et al.21 described another prospective study evaluating the use of Humate-P to manage urgent bleeding or for non-elective surgical bleeding prophylaxis in 33 subjects with VWD. Overall haemostatic efficacy was rated as "excellent/good" in 52 of 53 evaluable bleeding events. In one subject, efficacy was rated as "none" on the first day because the bleeding was not resolved, but daily efficacy ratings for the next 6 days of treatment were reported as "excellent/good." Franchini et al.17 reported retrospectively on the use of Haemate P for the prophylaxis of excessive bleeding in 26 subjects with VWD who underwent a total of 43 surgical or invasive procedures. Red blood cell transfusions were required in five major surgical procedures, although the transfused volumes were similar to those for subjects without VWD undergoing similar procedures. Haemostasis was rated as excellent in all but one procedure; a subject who received a single preoperative infusion of Haemate-P experienced haemorrhage 3 days following a dental procedure. Additional administration of Haemate-P for 2 days was successful in controlling the bleeding.
Based upon the combined findings of the USA and EU surgery studies with Humate-P/Haemate P, the FDA has formulated dosing recommendations for prophylaxis of excessive surgical bleeding. A dosing calculator is available on the Humate-P web site22. Whenever possible, the patient's incremental IVR should be measured, along with baseline VWF:RCo and FVIII:C values. However, if an individualised IVR cannot be obtained, the IVR can be assumed to be 2 IU/dL per IU/kg. Loading doses should be administered 1 to 2 hours prior to surgery. Loading doses should be designed to achieve plasma levels of approximately 100 IU/dL VWF:RCo in the case of major surgery and 50 to 60 IU/dL in the case of minor or oral surgery and should also be based upon baseline plasma levels of VWF:RCo and a target plasma FVIII:C of 80 to 100 IU on day 1 for major surgery and 40 to 50 IU for minor/oral surgery. Dosing recommendations are the same for all types of VWD. Maintenance doses of half the loading dose should be administered postoperatively every 8 hours, changing to a 12-hour regimen on the second day of treatment. Treatment for 2 to 4 days generally suffices for oral and minor surgery, while it may be greater than 4 days for major surgery. Monitoring of trough VWF:RCo and FVIII:C is advisable at least daily, in order to adjust dosing if necessary and monitor for any pronounced increase of coagulation factors.
As an example, the loading dose of Humate-P/ Haemate P required (assuming a target VWF:RCo level of 100 IU/dL, baseline VWF:RCo level of 20 IU/dL, an IVR of 2.0, Δ of 80 IU/dL, and a body weight of 70 kg) would be calculated as follows: (80 IU/dL×70 kg)/2 = 2,800 IU VWF:RCo required. Attaining a target peak FVIII:C plasma level of 80 to 100 IU FVIII:C/dL (major surgery) or 40 to 50 IU FVIII: C/dL (minor or oral surgery) might require additional dosing. Because the ratio of VWF:RCo to FVIII:C activity in Humate-P/Haemate P is approximately 2.4 to 1, any additional dosing will increase VWF:RCo proportionately more than FVIII:C. Assuming an incremental IVR of 2.0 IU VWF:RCo/dL per IU/kg infused, additional dosing to increase FVIII:C in plasma will also increase plasma VWF:RCo by approximately 5 IU/dL for each IU/kg of FVIII administered.
Many years of clinical experience have shown that Humate-P and Haemate P are safe and well tolerated. While the adverse event rate appeared to be high in the current studies and in the combined analysis (81%), this was most likely affected by the surgical setting of the trials. The rate of events considered possibly related to treatment was substantially lower (13%). In a similar study involving 42 urgent surgical procedures in 39 subjects, the overall adverse event rate was also high (57.1%) compared to the frequency of events that were considered possibly related to VWF/FVIII concentrate administration (16.7%)2. In the current study, most events were mild and there were no discontinuations related to tolerability issues. The one serious event, a case of pulmonary thromboembolism, is not an unknown risk of VWF/FVIII replacement in patients with VWD, especially in a setting such as major orthopaedic surgery, with its own inherent risks for thrombosis. In fact, the individual who experienced this event had a number of risk factors for thromboembolism.
Overall, this pooled and comparative analysis of findings from two similar, prospective trials of VWF/FVIII concentrate in subjects with VWD undergoing elective surgeries highlights its effectiveness in such settings. Furthermore, these data support the use of factor level monitoring and individualised dosing strategies, and have helped to foster the development of standardised dosing recommendations to assist clinicians in providing optimal perioperative care for patients with VWD.
Acknowledgements
Medical writing support was provided by Complete Publication Solutions, LLC; this support was funded by CSL Behring LLC, King of Prussia, PA, USA.
Footnotes
Financial Disclosure
The Authors declare that this study was funded by CSL Behring.
References
- 1.Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand's disease. Blood. 1987;69:454–9. [PubMed] [Google Scholar]
- 2.Thompson AR, Gill JC, Ewenstein BM, et al. Successful treatment for patients with von Willebrand disease undergoing urgent surgery using factor VIII/VWF concentrate (Humate-P) Haemophilia. 2004;10:42–51. doi: 10.1046/j.1351-8216.2003.00809.x. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman TS, Ruggeri ZM. von Willebrand's disease. Clin Haematol. 1983;12:175–200. [PubMed] [Google Scholar]
- 4.Blanchette V, Israels SJ, Akabutu J, Bergman GE. Report of the efficacy and safety of Haemete P in Canadian von Willebrand disease (vWD) patients. Thromb Haemost. 1997;77:513. [Google Scholar]
- 5.Ewenstein BM, Gill JC, Thompson A. Treatment of von Willebrand disease (vWD) in patients with urgent life-or-limb-threatening bleeding or emergent surgery with Humate-P (factor VII/vWF concentrate) Haemophilia. 2000;6:277. [Google Scholar]
- 6.Federici AB, Castaman G, Franchini M, et al. Clinical use of Haemate P in inherited von Willebrand's disease: a cohort study on 100 Italian patients. Haematologica. 2007;92:944–51. doi: 10.3324/haematol.11124. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M. Surgical prophylaxis in von Willebrand's disease: a difficult balance to manage. Blood Transfus. 2008;6(Suppl 2):s33–8. doi: 10.2450/2008.0035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill JC, Shapiro A, Valentino LA, et al. von Willebrand factor/ factor VIII concentrate (Humate-P) for management of elective surgery in adults and children with von Willebrand disease. Haemophilia. 2011;17:895–905. doi: 10.1111/j.1365-2516.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 9.Lethagen S, Kyrle PA, Castaman G, et al. von Willebrand factor/factor VIII concentrate (Haemate P) dosing based on pharmacokinetics: a prospective multicenter trial in elective surgery. J Thromb Haemost. 2007;5:1420–30. doi: 10.1111/j.1538-7836.2007.02588.x. [DOI] [PubMed] [Google Scholar]
- 10.Lillicrap D, Poon MC, Walker I, et al. Efficacy and safety of the factor VIII/von Willebrand factor concentrate, Haemate-P/ Humate-P: ristocetin cofactor unit dosing in patients with von Willebrand disease. Thromb Haemost. 2002;87:224–30. [PubMed] [Google Scholar]
- 11.Lubetsky A, Schulman S, Varon D, et al. Safety and efficacy of continuous infusion of a combined factor VIII-von Willebrand factor (vWF) concentrate (Haemate-P) in patients with von Willebrand disease. Thromb Haemost. 1999;81:229–33. [PubMed] [Google Scholar]
- 12.Federici AB. Management of von Willebrand disease with factor VIII/von Willebrand factor concentrates: results from current studies and surveys. Blood Coagul Fibrinolysis. 2005;16(Suppl 1):S17–21. doi: 10.1097/01.mbc.0000167658.85143.49. [DOI] [PubMed] [Google Scholar]
- 13.Alusi GH, Grant WE, Lee CA, et al. Bleeding after tonsillectomy in severe von Willebrand's disease. J Laryngol Otol. 1995;109:437–9. doi: 10.1017/s0022215100130373. [DOI] [PubMed] [Google Scholar]
- 14.Dobrkovska A, Krzensk U, Chediak JR. Pharmacokinetics, efficacy and safety of Humate-P in von Willebrand disease. Haemophilia. 1998;4(Suppl 3):33–9. doi: 10.1046/j.1365-2516.1998.0040s3033.x. [DOI] [PubMed] [Google Scholar]
- 15.Lethagen S. Haemostatic treatment in connection with surgery in patients with von Willebrand disease. Haemophilia. 1999;5:64–7. doi: 10.1046/j.1365-2516.1999.0050s2064.x. [DOI] [PubMed] [Google Scholar]
- 16.Nitu-Whalley IC, Griffioen A, Harrington C, Lee CA. Retrospective review of the management of elective surgery with desmopressin and clotting factor concentrates in patients with von Willebrand disease. Am J Hematol. 2001;66:280–4. doi: 10.1002/ajh.1058. [DOI] [PubMed] [Google Scholar]
- 17.Franchini M, Rossetti G, Tagliaferri A, et al. Efficacy and safety of factor VIII/von Willebrand's factor concentrate (Haemate-P) in preventing bleeding during surgery or invasive procedures in patients with von Willebrand disease. Haematologica. 2003;88:1279–83. [PubMed] [Google Scholar]
- 18.Michiels JJ, Berneman ZN, van der Planken M, et al. Bleeding prophylaxis for major surgery in patients with type 2 von Willebrand disease with an intermediate purity factor VIII-von Willebrand factor concentrate (Haemate-P) Blood Coagul Fibrinolysis. 2004;15:323–30. doi: 10.1097/00001721-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Naqvi A, Endres-Brooks J, Montgomery RR. Successful cardiac surgery in a young girl with type 2a von Willebrand disease using continous infusions of Haemete-P. Thromb Haemost. 1997;77:514. [Google Scholar]
- 20.Di Paola J, Lethagen S, Gill J, et al. Presurgical pharmacokinetic analysis of a von Willebrand factor/factor VIII (VWF/FVIII) concentrate in patients with von Willebrand's disease (VWD) has limited value in dosing for surgery. Haemophilia. 2011;17:752–8. doi: 10.1111/j.1365-2516.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 21.Gill JC, Ewenstein BM, Thompson AR, et al. Successful treatment of urgent bleeding in von Willebrand disease with factor VIII/VWF concentrate (Humate-P): use of the ristocetin cofactor assay (VWF:RCo) to measure potency and to guide therapy. Haemophilia. 2003;9:688–95. doi: 10.1046/j.1351-8216.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- 22.CSL Behring LLC. Humate-P dosing calculator. [Accessed on 26 October 2012]. Available at: http://www.humate-p.com/Professional/Resources-and-Tools/Dosage-Calculator.aspx.