Abstract
Background
Antibodies against the human neutrophil alloantigen-3a (HNA-3a) are involved in severe cases of transfusion-related acute lung injury (TRALI), but the susceptibility of patients towards HNA-3a antibody differs largely. HNA-3a antibodies induce granulocyte aggregation. However, it is unresolved whether plasma proteins are required for granulocyte aggregation.
Materials and methods
We investigated whether HNA-3a-antibody-induced aggregation of polymorphonuclear cells is dependent on plasma factors by using and modifying the granulocyte agglutination test (GAT).
Results
Polymorphonuclear cells homozygous for HNA-3a did not aggregate when incubated with HNA-3a antibodies in a plasma-protein-free GAT setup. When the GAT was performed using polymorphonuclear cells re-suspended in phosphate-buffered saline containing proteins, HNA-3-mediated aggregation was observed. Moreover, using Tween®20 for blocking the plates, reconstituted the granulocyte aggregation in a protein-free medium. This indicates that granulocyte aggregation probably occurs by direct granulocyte-granulocyte interaction(s) or is mediated by substances released by neutrophils after activation.
Discussion
Granulocyte aggregation induced by HNA-3a antibodies does not require human plasma proteins. Interindividual variability in the response to HNA-3a antibodies does not depend on differences in patient’s plasma proteins.
Keywords: transfusion-related acute lung injury (TRALI), human neutrophil alloantigen-3a (HNA-3a), choline transporter-like protein 2 (CTL2), plasma factor, granulocyte agglutination test (GAT)
Introduction
Transfusion-related acute lung injury (TRALI) is a serious complication of blood transfusion. Immune-mediated TRALI is caused by antibodies against human leucocyte antigens (HLA) or human neutrophil antigens (HNA), which are transmitted to the patient by blood products obtained from donors who had been immunised against these antigens during pregnancy or transfusion. The antibodies bind to and activate the leucocytes of the patient, which results in acute lung injury1,2. Plasma-rich blood products are more often involved in TRALI cases than, for example, red blood cell concentrates3,4. However, there is considerable heterogeneity of the biological complications caused by TRALI-inducing antibodies. Some of this might be explained by different antibody titres in blood products obtained from different blood donors or by multiple transfusions which might result in administration of large volumes of HNA- or HLA-antibody-containing plasmas. The individual predisposition of patients might also be an important risk factor for developing TRALI. It is well documented that plasma obtained from the same immunised donor can cause TRALI of rather variable severity in different patients1,5,6. Among TRALI-inducing antibodies, HNA-3a antibodies are usually involved in severe TRALI7–10. In contrast to HLA, HNA-1 and HNA-2 antibodies, the potency of HNA-3a antibodies to induce TRALI even in healthy humans has already been described1. However, little is known about the mechanism of HNA-3a antibody-induced TRALI in vivo. Potentially, resolving the mechanisms of granulocyte aggregation induced by these antibodies in vitro might provide additional information on the pathogenesis of TRALI. In the view of the severity of TRALI induced by HNA-3a antibodies, it is possible that the pathogenesis differs from that of other TRALI antibodies. Similarly, it is conceivable that granulocyte aggregation, a very pronounced in vitro feature of HNA-3a antibodies in the granulocyte aggregation test (GAT), could play a key role in the pathogenesis of TRALI induced by these antibodies.
We hypothesised that granulocyte aggregation might be dependent on the presence of plasma components. These hypothesised plasma factors might stabilise the HNA-3a antigen on granulocytes, which is sensitive to conformational changes11,12, or they may mediate granulocyte aggregation by bridging granulocyte surface proteins as described for platelet aggregation13. In addition, specific compositions of these factors in a patient’s plasma might explain the heterogeneity of the clinical complications induced by HNA-3a antibodies.
We therefore assessed the role of plasma factors in the aggregation of polymorphonuclear cells (PMN) in vitro and found that HNA-3a-antibody-induced granulocyte aggregation occurs in a plasma-free environment.
Materials and methods
HNA-3 antibody plasmas and control plasmas
HNA-3a- and HNA-3b-antibody-containing plasma samples were obtained from alloimmunised blood donors (identified by serological screening and/or through their implication in TRALI cases) and characterised by flow cytometry, the GAT, granulocyte immunofluorescence test (GIFT) and lymphocyte immunofluorescence test (LIFT) using a panel of genotyped granulocytes and lymphocytes, as described elsewhere14. Control plasma (ABx) was pooled from ten healthy non-transfused male blood donors of blood group AB. Granulocyte reactive antibodies were excluded in these plasmas by serological investigations.
Immunoglobulin G purification
Immunoglobulin G (IgG) fractions were purified from filtered (0.45 μm, Sarstedt AG, Nümbrecht, Germany) plasma dilutions by affinity chromatography using Protein-G-Sepharose (GE Healthcare, Uppsala, Sweden). IgG was eluted using 100 mM glycine-HCl buffer (Carl Roth, Karlsruhe, Germany) and subsequent neutralised with 1 M Tris-HCl (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Eluates were dialysed against 0.9% sodium chloride. After sterile filtration (0.22 μm filter, Carl Roth), the protein concentration was estimated using a modified Bradford assay and adjusted to 7 mg/mL.
For some experiments, HNA-3a or HNA-3b antibodies were affinity purified from corresponding plasmas using HNA-3a or HNA-3b transfected human embryonic kidney (HEK 239T) cells. In detail, 5 mL of HNA-3a or HNA-3b-expressing cells (1×107 cells) were incubated with 1 mL of anti-HNA-3a or anti-HNA-3b plasma, respectively (for 45 minutes, at 37 °C, agitating every 10 minutes). After two washing steps (5 minutes, 1,000 × g), antibody elution, neutralisation, IgG purification and dialysis was performed as described above.
Genotyping of granulocyte donors
HNA-3 genotyping of granulocyte donors was performed by sequence specific primer-polymerase chain reaction as previously described13.
Polymorphonuclear cell preparation
PMN were isolated from ethylene-diamine-tetraacetic acid (EDTA)-anticoagulated whole blood of healthy, HNA-3a and HNA-3b homozygous donors by dextran sedimentation and subsequent gradient centrifugation of the leucocyte-rich supernatant (Biocoll; d =1.077 g/mL, Biochrom AG, Berlin, Germany). Residual red blood cells were lysed in ice-cold ammonium chloride for 5 minutes at 4 °C. Cells were washed twice (140 × g; 5 minutes at room temperature) with 10 mL phosphate-buffered saline (PBS without Ca2+ and Mg2+, Biochrom AG). Subsequently, cells were resuspended in either ABx plasma, PBS, or different 0.5% protein solutions [BSA I (Serva GmbH, Heidelberg, Germany, 100% purity); BSA II (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany); milk powder (Carl Roth, blotting grade) or blocking reagent® (Roche, Mannheim, Germany)]. Cell concentration was adjusted to 5×103 cells/μL.
Analyses of anti-HNA-3a-IgG binding
To analyse the binding of HNA-3a antibodies to neutrophils re-suspended in various protein solutions, cells, at a concentration of 5×106/50 μL, were incubated with 150 μL of anti-HNA-3a-IgG or control IgG for 30 minutes at 37 °C. Subsequently, the cells were washed twice (see above) and stained with polyclonal rabbit anti-human-IgG-fluoroscein isothiocyanate (1:100 in PBS w/o; Dako, Glostrup, Denmark) for 30 minutes at room temperature. Suspensions were washed twice (see above), pellets were re-suspended in 500 μL PBS and then analysed by flow cytometry.
The modified granulocyte agglutination test
To address the role of a plasma factor in granulocyte aggregation, the standard GAT13 was performed with important modifications. Aliquots of 2 μL of different cell suspensions (5×103 cells/μL in PBS or different protein solutions, as described above) were incubated with either 6 μL plasma (anti-HNA-3a plasma, anti-HNA-3b plasma, or control plasma) or 6 μL IgG fraction (7 mg/mL) of the respective plasma in oil-free Terasaki plates (Greiner Bio-One, Frickenhausen, Germany). In other experiments, blocked Terasaki plates (see below) were used to incubate 2 μL of neutrophils in protein-free cell suspension buffer (5×103 cells/μL in PBS) with 6 μL of IgG fractions from either HNA-3a antibody-containing plasma or control plasma. In additional experiments, the HNA-3a-IgG-fraction was diluted (1:4) in PBS or PBS containing different concentrations of BSA (0.1–10%) and incubated with PMN resuspended in PBS or PBS containing 0.5% BSA II. Furthermore, in some assays, isolated neutrophils (see above) were pretreated with EDTA (5%, Merck, Darmstadt, Germany) prior to incubation with the anti-HNA-3a IgG-fraction or control IgG in the GAT.
In all experiments, cavities were finally covered with oil (Bio-Rad Medical Diagnostics GmbH, Dreieich, Germany) and plates were incubated for at least 2 hours at 37 °C. Neutrophil aggregation was assessed by microscopy.
Blocking of Terasaki plates
Cavities of Terasaki plates were incubated with 10 μL solution containing either 0.5% BSA I, 0.5% BSA II, 0.5% milk powder, or blocking reagent® (following the manufacturer’s instructions) for 2 hours at room temperature or overnight at 4 °C. Blocked cavities were washed with 10 μL PBS (without Ca2+ and Mg2+). In additional experiments, the plates were coated “protein-free” by pre-incubation with 0.5% Tween®20 solution (Carl Roth). Additionally, HNA-3a antibodies which were affinity-purified on transfected HEK 293T cells were used in control experiments with blocked plates.
Furthermore, we tested whether blocking the plates would increase the sensitivity of the standard GAT by comparing granulocyte aggregation in non-blocked and blocked plates (see above) with PMN from two different donors and five serially diluted anti-HNA-3a plasmas.
Results
Effect of plasma on HNA-3a antibody-induced neutrophil aggregation
Induction of neutrophil aggregation is a very characteristic feature of HNA-3a antibodies, which can only be detected in the GAT14. According to the standard GAT, PMN are usually re-suspended in autologous plasma prior to incubation with HNA-3a antibody-containing plasma. To investigate whether neutrophil aggregation is dependent on plasma-specific factors, we modified the GAT using plasma-free conditions by using PBS as cell re-suspension buffer and IgG fractions of anti-HNA-3a or control plasmas.
In contrast to the plasma-containing setup (control plasma as the granulocyte re-suspension buffer and/or anti-HNA-3a plasma for treatment), PMN failed to aggregate when the GAT was performed in a plasma-free setup (Table I). In addition, aggregation occurred in an allele-specific manner, as HNA-3a antibodies (in plasma or as purified IgG fraction) were not able to aggregate HNA-3b homozygous cells (data not shown).
Table I.
Relevance of re-suspension buffer.
Legend
+: ≥10 % aggregation; −: no aggregation.
GAT reactivity of granulocytes homozygous for HNA-3a re-suspended in either plasma or PBS and incubated with HNA-3a plasma or its corresponding IgG fraction. Using this original GAT setting, granulocytes re-suspended in PBS aggregated in the presence of anti-HNA-3a plasma, but not in the presence of the respective IgG fraction. These results are representative of experiments with granulocytes from ten different donors.
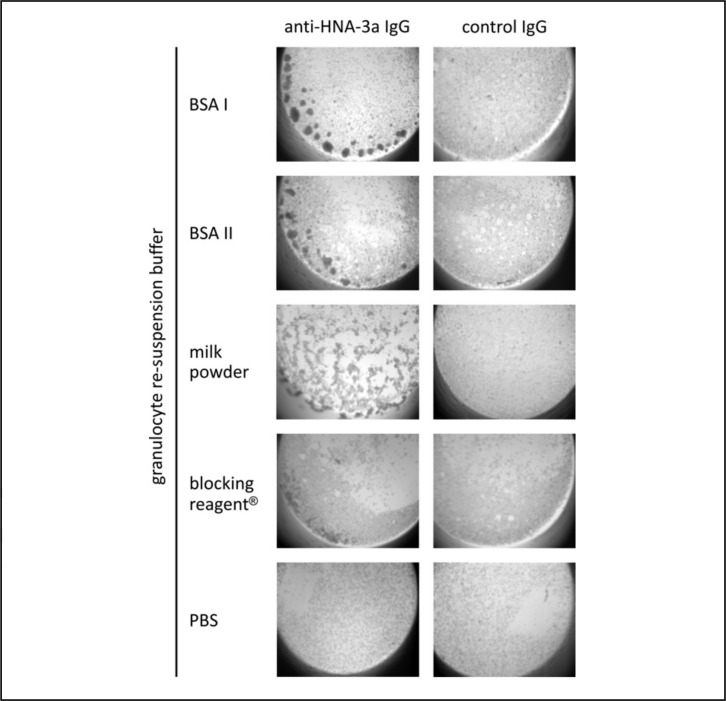
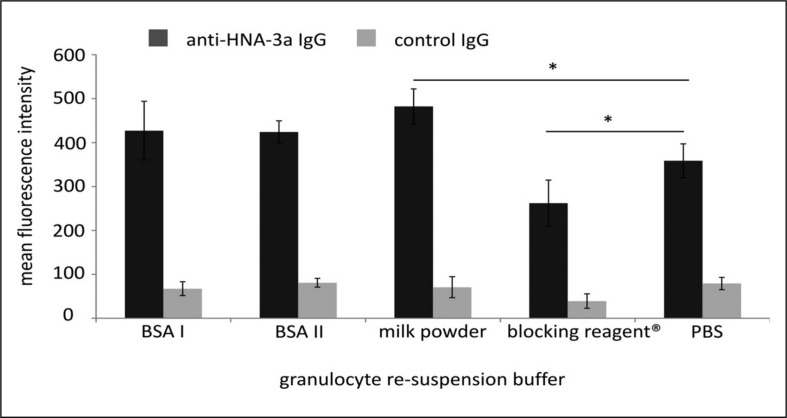
Interestingly, anti-HNA-3a-induced granulocyte aggregation was observed when neutrophils were suspended in PBS containing 0.5% BSA (from two different manufacturers) in the modified GAT assay. The same results were obtained with 0.5% milk powder and blocking reagent (Figure 1). Aggregation of HNA-3a homozygous PMN was HNA-3a antibody-specific since neither the IgG fractions of control plasma ABx nor of a HNA-3b antibody-containing plasma induced aggregation (data not shown). However, different patterns of neutrophil aggregates were observed with PMN suspended in different buffers (Figure 1) when compared to standard GAT. While re-suspension of cells in BSA caused normal, well-defined, three-dimensional aggregates, re-suspension in milk powder and blocking reagent® resulted in not delimited and partially spread HNA-3a-antibody-induced aggregates. To further investigate this observation, we analysed the binding ability of HNA-3a antibodies to PMN re-suspended in different solutions and found that re-suspension of PMN in blocking reagent® and milk powder did indeed alter the binding of anti-HNA-3a IgG to the cells (Figure 2).
Figure 1.
GAT with granulocytes homozygous for HNA-3a, re-suspended in PBS or PBS containing 0.5% of either BSA I, BSA II, milk powder, or blocking reagent® and incubated with the anti-HNA-3a IgG fraction or a control IgG fraction. The granulocytes aggregated after incubation with anti-HNA-3a IgG. Non-specific aggregation due to the protein additives could be excluded, since no aggregation was observed when cells were incubated with control IgG. These results are representative of experiments with granulocytes from six different donors.
Figure 2.
Binding of anti-HNA-3a IgG (dark grey) or control IgG (grey) to PMN after re-suspension in different solutions. No difference could be observed after re-suspension of the PMN in BSA compared to re-suspension in buffer. In contrast, re-suspension in milk powder or blocking reagent® resulted in stronger or weaker binding of anti-HNA-3a IgG to CTL2 compared to non-treated PMN. These results are representative of experiments with granulocytes from three different donors.
Interestingly, reconstitution of the aggregation of washed PMN by HNA-3a IgG fractions only occurred when the supplemental proteins were added to the cell suspension buffer, but not when supplements were present in the IgG fraction. Addition of BSA, for example, to the HNA-3a IgG fraction did not result in PMN aggregation, possibly indicating a non-specific interaction of PMN with the Terasaki plate (Table II).
Table II.
Impact of BSA in the IgG dilution buffer.
| Anti-HNA-3a IgG dilution | Granulocyte re-suspension buffer | |
|---|---|---|
|
|
||
| PBS | PBS with 0.5% BSA | |
| in PBS | − | + |
| in PBS with 0.5 % BSA | − | + |
Legend
+: ≥10 % aggregation; −: no aggregation.
GAT reactivity of HNA-3a homozygous granulocytes re-suspended in PBS or PBS containing 0.5% BSA, which were incubated with two HNA-3a IgG dilutions (1:4) in PBS or PBS containing 0.5% BSA. Without protective agents in the re-suspension buffer, granulocytes did not aggregate when incubated with anti-HNA-3a IgG. However, when granulocytes were re-suspended in 0.5% BSA prior to the GAT, aggregation occurred with both anti-HNA-3a IgG dilutions (n =3).
Recovery of neutrophil aggregation without plasma by blocking the Terasaki plates
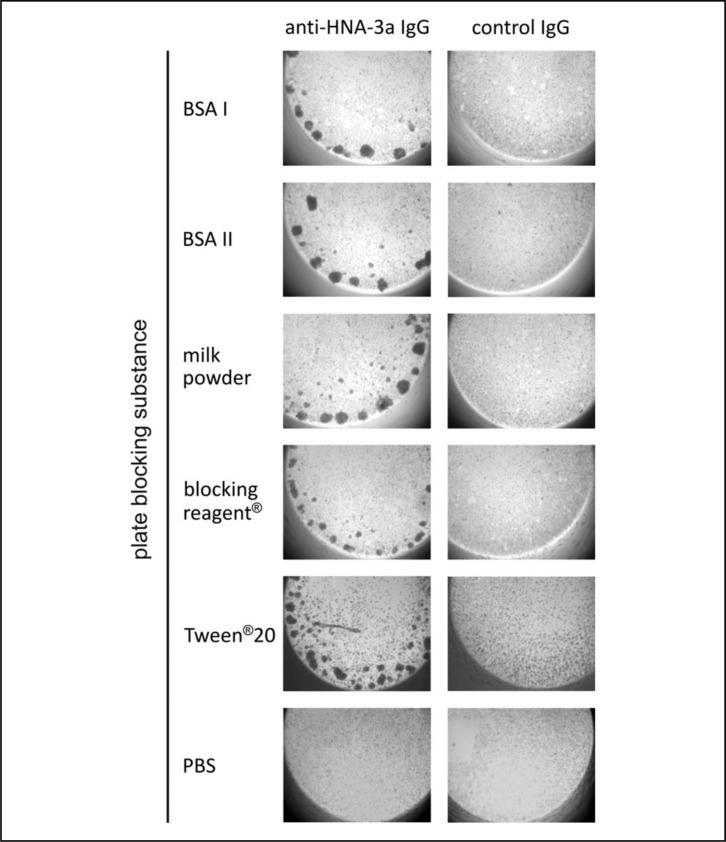
To test whether the failure of neutrophil aggregation in the plasma/protein-free GAT is the consequence of a change in the antigenic epitope or whether this is rather related to an interaction of PMN or IgG with the Terasaki plate, we blocked the plates with several 0.5% protein solutions prior to performing the plasma-free GAT. Under these conditions, anti-HNA-3a antibody-containing IgG fractions induced the characteristic aggregation pattern when PMN were re-suspended in PBS (Figure 2). Additionally, neutrophil aggregation was also recovered when plates were blocked with 0.5% Tween®20 (Figure 3). Similar aggregation patterns were found when HNA-3a antibodies were used, which were affinity-purified on CTL2-expressing HEK cells. Again, this aggregation occurred in an allele-specific manner and no aggregation was observed with control IgG (data not shown).
Figure 3.
GAT with HNA-3a homozygous granulocytes resuspended in PBS which were incubated with either anti-HNA-3a IgG or control IgG. Prior to the GAT, Terasaki plates were blocked with 0.5% of either BSA I, BSA II, milk powder, Tween®20 or blocking reagent®. Control plates were incubated with PBS only. Using blocked Terasaki plates, granulocytes showed typical aggregation patterns after incubation with anti-HNA-3a antibodies, while they failed to aggregate when unblocked plates were used. Non-specific aggregation due to plate blocking could be excluded, since no aggregation was observed when cells were incubated with control IgG. This figure shows a representative GAT result for granulocytes from six different donors.
To investigate the impact of plate blocking on the sensitivity of the standard GAT, we determined the maximum anti-HNA-3a plasma dilution causing neutrophil aggregation on blocked and unblocked plates. Pre-blocking the Terasaki plates did not change the sensitivity of the standard GAT (plasma as granulocyte re-suspension buffer) using five serially diluted HNA-3a plasmas (data not shown).
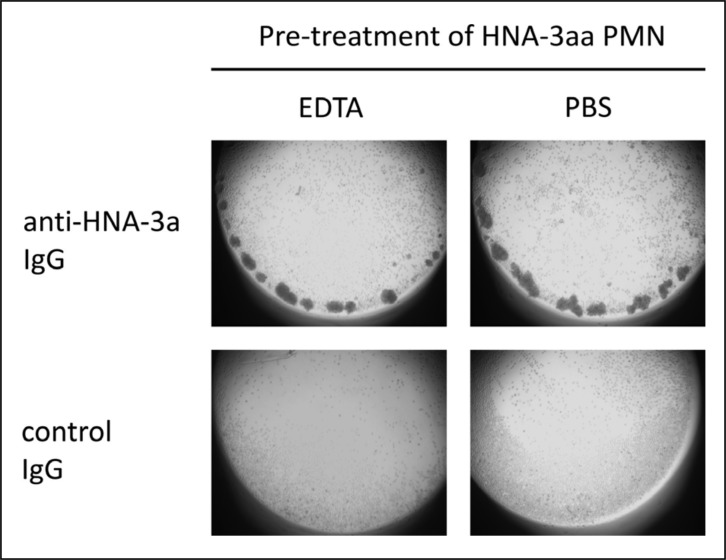
EDTA did not influence anti-HNA-3a-mediated neutrophil aggregation
To investigate the role of integrins in the aggregation mechanism, we performed the GAT in the presence of ETDA which captures free divalent ions. Treatment of PMN with EDTA prior to incubation with HNA-3a-IgG or control IgG did not alter the neutrophil aggregation induced by anti-HNA-3a (Figure 4).
Figure 4.
Granulocytes homozygous for HNA-3a were pretreated with EDTA or PBS and incubated with HNA-3a antibodies in the GAT. The aggregation ability of cells was not altered by using EDTA-treated cells instead of non-treated cells. No aggregation was observed with control IgG. These results are representative of experiments with granulocytes from three different donors.
Discussion
Aggregation of PMN is very probably an important step in the pathogenesis of TRALI caused by HNA- 3a antibodies1,15,16. Besides indications from many in vitro studies, Cherry and colleagues found neutrophil infiltrates and aggregates in the pulmonary vasculature of a patient who died of TRALI, suggesting that neutrophil aggregation is relevant in vivo17.
In this study, we investigated the contribution of plasma components to the neutrophil interaction, potentially influencing the susceptibility of patients to TRALI. Since HNA-3a antibody-mediated neutrophil aggregation is a characteristic and reliable feature, the GAT was the method of choice to address this issue. Aggregation of PMN re-suspended in plasma (the original GAT setup) consistently occurred when PMN were incubated with HNA-3a antibody-containing plasma or with the respective anti-HNA-3a IgG fractions. In contrast, when the GAT was performed in a plasma-free environment, PMN first failed to aggregate, suggesting the involvement of a potential aggregation-cofactor in the plasma, similar to the need of fibrinogen for platelet aggregation.
However, when granulocytes were re-suspended in PBS buffer containing different non-plasma proteins, anti-HNA-3a IgG induced neutrophil aggregation was reconstituted. This indicates that neutrophil aggregation induced by HNA-3a antibodies might occur independently of specific plasma proteins. It is therefore likely that the individual plasma composition of the patient/recipient homozygous for HNA-3a is not the cause of the variable susceptibility of patients to HNA-3a antibody-induced TRALI.
In the view of the conformation dependency of the HNA-3a antigen, it is conceivable that protein-free solutions might slightly alter the three-dimensional structure of CTL2 (choline transporter-like protein 2), which bears the epitope to which HNA-3a antibodies bind11,12, and might therefore also alter neutrophil aggregation. However, blocking of the Terasaki plates was at least as effective in reconstituting granulocyte aggregation as re-suspending PMN in BSA-containing buffer. It is therefore more likely that protein solutions are blocking non-specific binding of IgG or PMN to the plate rather than stabilising the antigenic structure of CTL2. Aggregation in a plasma-free environment could only be restored by adding the protein-containing solutions to the cell suspension or by pre-blocking the plate, but not by adding BSA to the HNA-3a IgG fraction. These results indicate that in our experimental setting PMN rather than the antibodies bind “non-specifically” to the plate in plasma/protein-free environments, preventing their aggregation by immobilisation of the PMN. Interestingly, the different protein solutions used as PMN re-suspension buffer resulted in the formation of aggregates of different shapes. Since there are differences between the blocking substances, it is likely that these solutions had varying capacity to inhibit non-specific interactions of granulocytes with the plate surface possibly caused by weak interactions, such as ionic or hydrophobic reciprocity, which may also affect the aggregation pattern. This phenomenon could also explain the different impacts of these solutions on HNA-3a antibody-binding to granulocytes (Figure 2), since reduced binding of anti-HNA-3a IgG to cells resuspended in blocking reagent corresponds with reduced aggregation observed in the GAT.
While the need to block plastic surfaces with proteins to prevent IgG/cell adherence is not a new concept, our experiments help the understanding of the mechanisms of granulocyte activation and aggregation induced by HNA-3a antibodies. Taken together, our data clearly show that granulocyte aggregation occurs even in the absence of plasma, indicating that aggregation of granulocytes is mediated by direct granulocyte-granulocyte interactions. While this is new and potentially important information on the interaction between granulocytes and HNA-3a antibodies, these findings prompt many new questions, which require further experiments to understand the mechanism of HNA-3a antibody-induced granulocyte activation and aggregation.
We have already shown that F(ab’)2 fragments of HNA-3a antibodies are able to induce aggregation of PMN. Thus, granulocyte aggregation occurs independently of the Fc-part of HNA-3a IgG antibodies and therefore independent of cross-linking7 of granulocytes by binding of the HNA-3a antibodies with their F(ab’)2 moiety to CTL2 on one granulocyte and by the Fc-part of the antibody to Fc-receptors on another granulocyte. However, it is possible that Fc-receptor-dependent activation occurs in addition to Fc-receptor-independent activation in vivo.
It is currently unknown how HNA-3a antibodies activate granulocytes and whether signals are transmitted by CTL2. An up-regulation of activation marker CD11b has been described for HNA-1 and -2, but proof of anti-HNA-3a-induced neutrophil activation is currently still missing. To assess the involvement of integrins, such as CD11b, which mediate homotypic neutrophil aggregation and require divalent ions18,19, we performed the GAT in the presence of ETDA to capture free divalent ions. However, we found no differences between EDTA-treated and non-treated (buffer) granulocytes, indicating that HNA-3a-mediated aggregation does not depend on integrins.
Although most in vitro studies are not directly comparable with the in vivo condition, the present study provides an instrumental setting to analyse granulocyte aggregation pathways by HNA-3a antibodies in a plasma-free environment e.g. by using proteomic tools and secretome analyses to show whether protein relevant to aggregation are released from neutrophils upon their activation. In summary, this experimental setup makes analyses of HNA-3a-antibody-dependent signalling feasible.
Despite the plasma-protein-free buffers used, we cannot exclude that granulocytes store plasma proteins in their granules, which may be released upon activation. Moreover, although we tried to use highly purified proteins in our experiments, contamination by other plasma proteins can not be definitively excluded, although this is extremely unlikely in Tween®20. We therefore do not have the ultimate proof that aggregation of PMN induced by HNA-3a antibodies occurs only via a direct cell-cell interaction.
In conclusion, neutrophil aggregation by HNA-3a antibodies can occur in a plasma-free medium. Blocking of surfaces is important for any experiments performed with HNA-3a antibodies and granulocytes in a plasma-free environment. Our results indicate that granulocyte aggregation induced by HNA-3a antibodies is potentially independent of specific plasma proteins mediating neutrophil activation and interaction.
Acknowledgements
This work was supported by an unrestricted grant from the Red Cross Blood Donation Service West, Hagen, Germany, by the Land Mecklenburg-Vorpommern, Exzellenzinitiative UG 07-064, and by the Germany’s Ministry of Education and Research (BMBF), Innovation Center - Humoral Immune Responses in Cardiovascular Disorders (ZIK-HIKE) FKZ 03Z2CN11 and FKZ 03Z2CN12.
Footnotes
Autorship contributions
Nicole Schubert performed the serological experiments and wrote the manuscript. Tom Berthold and Stefan Muschter designed the experiments, purified the IgG fractions and wrote the manuscript. Jan Wesche genotyped the HNA-3a from granulocyte donors and purified the IgG fractions via HEK cell-based adsorption and elution. Birgitt Fürll designed and supervised the experiments and evaluated the results. Angelika Reil and Jürgen Bux identified and provided the HNA-3a alloantibodies.
Andreas Greinacher designed the study, supervised the experiments, evaluated the results and wrote the manuscript.
All authors read and approved the final version of the manuscript.
Conflict of interest disclosure
The University Greifswald and the Red Cross Blood Donation Service West, Hagen have submitted a patent application for the HNA-3a antigen.
None of the authors has any conflict of interest to declare.
References
- 1.Bux J. Antibody-mediated (immune) transfusion-related acute lung injury. Vox Sang. 2011;100:122–8. doi: 10.1111/j.1423-0410.2010.01392.x. [DOI] [PubMed] [Google Scholar]
- 2.Keller-Stanislawski B, Reil A, Günay S, et al. Frequency and severity of transfusion related acute lung injury-German haemovigilance data (2006–2007) Vox Sang. 2010;98:70–7. doi: 10.1111/j.1423-0410.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 3.Silliman CC, Fung YL, Balla B, et al. Transfusion-related acute lung injury (TRALI): current concepts and misconceptions. Blood Reviews. 2009;23:245–55. doi: 10.1016/j.blre.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bux J, Sachs UJH. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136:788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: from bedside to bench and back. Blood. 2011;117:1463–71. doi: 10.1182/blood-2010-04-278135. [DOI] [PubMed] [Google Scholar]
- 6.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–67. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greinacher A, Wesche J, Hammer E, et al. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16:45–8. doi: 10.1038/nm.2070. [DOI] [PubMed] [Google Scholar]
- 8.Curtis BR, Cox NJ, Sullivan MJ, et al. The neutrophil alloantigen HNA-3a (5b) is located on choline transporter-like protein 2 and appears to be encoded by an R>Q154 amino acid substitution. Blood. 2010;115:2073–6. doi: 10.1182/blood-2009-11-248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davoren A, Curtis BR, Shulman IA, et al. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: a report of 2 fatalities. Transfusion. 2003;43:641–5. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 10.Reil A, Keller-Stanislawski B, Günay S, et al. Specificities of leukocyte alloantibodies in transfusion-related acute lung injury and results of leukocyte antibody screening of blood donors. Vox Sang. 2008;95:313–7. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 11.Berthold T, Wesche J, Kuhnert K, et al. Epitope mapping of antibodies directed against the human neutrophil alloantigen 3a. Transfusion. 2011;51:2160–7. doi: 10.1111/j.1537-2995.2011.03115.x. [DOI] [PubMed] [Google Scholar]
- 12.Curtis BR, Sullivan MJ, Holyst MT, et al. HNA-3a-specific antibodies recognize choline transporter-like protein-2 peptides containing arginine, but not glutamine at position 154. Transfusion. 2011;51:2168–74. doi: 10.1111/j.1537-2995.2011.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mody NA, King MR. Platelet adhesive dynamics. Part II: high shear-induced transient aggregation via GPIb alpha-vWF-GPIb alpha bridging. Biophys J. 2008;95:2556–74. doi: 10.1529/biophysj.107.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reil A, Wesche J, Greinacher A, et al. Geno- and phenotyping and immunogenicity of HNA-3. Transfusion. 2011;51:18–24. doi: 10.1111/j.1537-2995.2010.02751.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt AE, Adamski J. Pathology consultation on transfusion-related acute lung injury (TRALI) Am J Clin Pathol. 2012;138:498–503. doi: 10.1309/AJCPFF6JKXM7BYOI. [DOI] [PubMed] [Google Scholar]
- 16.Sachs UJ. Recent insights into the mechanism of transfusion-related acute lung injury. Curr Opin Hematol. 2011;18:436–42. doi: 10.1097/MOH.0b013e32834bab01. [DOI] [PubMed] [Google Scholar]
- 17.Cherry T, Steciuk M, Reddy VVB, Marques MB. Transfusion-related acute lung injury: past, present, and future. Am J Clin Pathol. 2008;129:287–97. doi: 10.1309/D3F7BXH466AE3G0P. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Chen JF. The regulation of integrin function by divalent cations. Cell Adh Migr. 2012;6:20–9. doi: 10.4161/cam.18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon SI, Rochon YP, Lynam EB, et al. Beta 2-integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood. 1993;82:1097–106. [PubMed] [Google Scholar]