Abstract
Background
Frequent blood loss induces progressive depletion of iron stores, leading to iron deficiency and, ultimately, to overt iron-deficient anaemia. The erythropoietin-mediated bone marrow response to anaemia is under the control of hypoxia-inducible factors (HIF), the master regulators of oxygen and iron homeostasis. Since the HIF-1αPro-582-Ser variant is associated with elevated trans-activation capacity of hypoxia responsive elements of target genes, we investigated whether the HIF-1αPro-582-Ser polymorphism might influence the response to repeated blood withdrawals.
Materials and methods
Using polymerase chain reaction analysis and DNA sequencing, we retrospectively investigated the presence of HIF-1αPro-582-Ser in a series of 163 blood donors. Haematological findings, serum ferritin levels and frequency of donations were compared according to the mutational status of the HIF-1α gene.
Results
We found that male carriers of the HIF-1αPro-582-Ser polymorphism had higher haemoglobin and ferritin levels than individuals homozygous for the wild-type allele. Moreover, the HIF-1αPro-582-Ser polymorphism protected regular blood donors from developing iron deficiency and anaemia and predicted uninterrupted donation activity.
Discussion
These findings show for the first time that the HIF-1αPro-582-Ser polymorphism significantly affects red blood cell and iron homeostasis after blood loss, conferring to male carriers a resistance to anaemia. Regarding the female gender, large series of individuals should be investigated to establish whether there is an effect of the HIF-1αPro-582-Ser polymorphism in this population. Although these data need to be confirmed in prospective studies, they could have important implications in blood donor selection and donation procedures.
Keywords: hypoxia-inducible factor, blood donors, iron deficiency
Introduction
Red blood cell homeostasis is tightly regulated by circulating erythropoietin, a glycoprotein hormone that binds to erythroid progenitors in bone marrow and stimulates their survival, proliferation and differentiation. Anaemia resulting from acute or chronic blood loss causes tissue hypoxia and induces, among several metabolic adjustments, the increase of erythropoietin production. The molecular pathway responsible for cell responses to hypoxia can be simplified into three major constituents: the prolyl hydroxylase domain-containing proteins (PHD1, PHD2, and PHD3), the hypoxia-inducible factors (HIF) and the protein product of the Von Hippel Lindau oncosuppressor gene (pVHL), which is the recognition component of an E3 ubiquitin ligase complex1,2. HIF proteins consist of heterodimeric complexes that are composed of an O2-labile α-subunit (HIF-1α and HIF-2α) and a stable 2-subunit (HIF-1β). Although significant overlap exists, array studies indicate differential induction by the two HIF-1α and HIF-2α isoforms, with apoptosis and gluconeogenesis preferentially induced by HIF-1 and angiogenesis preferentially induced by HIF-23. Under normal oxygen tension, PHD hydroxylate the α subunit of HIF on Pro402 and Pro564 (HIF1α) or Pro405 and Pro531 (HIF-2α), and target HIF-α for binding and degradation by pVHL1–2. In conditions of hypoxia, HIF-α hydroxylation by PHD is hampered: as a consequence, pVHL binding is reduced and HIF-α subunits accumulate, dimerise with HIF-b subunits, migrate into the nucleus, bind to hypoxia responsive elements (HRE) of HIF target genes (including EPO, the gene encoding erythropoietin) and activate their transcription1,2. Importantly, erythropoiesis involves large amounts of iron that are available for bone marrow for haemoglobin synthesis. Indeed, it is not surprising that in the liver, HIF-1α also stimulates iron uptake by repressing the gene encoding hepcidin (which inhibits ferroportin, the major protein responsible for intestinal iron uptake) and activates the synthesis of transferrin, responsible for transporting iron from the intestine to the bone marrow via the transferrin receptor4.
Several germline mutations of oxygen-sensing pathway genes impair HIF hydroxylation (i.e. HIF-2α and PHD mutations) or degradation (i.e. VHL mutations): all of them are associated with hereditary forms of erythrocytosis due to increased erythropoietin secretion4,5. In addition, a single nucleotide polymorphism located within exon 12 of the human HIF-1α gene (the HIF-1αPro-582-Ser mutation) was recently identified6. This polymorphism results from a C to T change at position 1772, and produces the substitution of proline at position 582 by serine6. Although HIF-1αPro-582-Ser shows higher in vitro transcriptional activity than the wild-type form6, it does not appear to be associated with increased or inappropriately normal erythropoietin levels7. Considering the pivotal role played by HIF-1α as a regulator of red cell and iron homeostasis4, we hypothesised that HIF-1αPro-582-Ser might confer carriers increased resistance to iron deprivation and anaemia as compared to wild-type subjects. In order to test this hypothesis, we investigated the effect of HIF-1αPro-582-Ser on haematological variables and iron stores in a series of blood donors.
Materials and methods
Study population
We evaluated a total of 169 blood donors (135 males and 34 females; median age 40.9 years; range 19.6–64.2) referring to three different blood transfusion centres (“Sacred Heart” Catholic University of Rome, United Hospitals of Jesolo/San Donà and Portogruaro and “Sapienza” University of Rome). Of these 169 donors, 88 were first- or second-time donors, while 81 were regular blood donors who had made more than two donations in the preceding year. Thirty-two subjects had been included in a previous study investigating possible causes of high haematocrit level (more than 48% in males and more than 46% in females) in regular donors. They were tested negative for all molecular abnormalities associated with primary erythrocytosis (including JAK2, VHL and HIF2a mutations) according to previously described methods8,9; in addition, haemoglobinopathies and possible causes of secondary erythrocytosis were ruled out. These donors were, therefore, considered eligible to continue donating and were included in the presently investigated series of donors. Haematological parameters and ferritin serum levels at the time of the first donation were recorded for each donor. For 94 subjects, serum iron data were also available. The study was approved by the institutional review boards.
HIF mutational analysis
HIF-1αPro-582-Ser mutation was investigated as previously reported8. Briefly, genomic DNA was isolated using standard procedures from peripheral blood9. The polymerase chain reaction (PCR) was carried out in a final volume of 25 μL containing 200 μmol/L of each dNTP, 1 unit of Taq polymerase (Promega, Madison, WI, USA), 1X reaction buffer (100 mmol/L Tris HCl [pH 9.0], 500 mmol/L KCl, 15 mmol/L MgCl2), 0.2 μmol/L of primers and 200 ng of DNA to be amplified. The primers used were: forward 5′-GTGTGGCCATTGTAAAAAC- 3′, reverse 3′ TTCCAGCAGACTCAAATACA- ′5′. Amplification was performed on an iCycler (BioRad, Hercules, CA, USA), with the following conditions: denaturation at 95 °C for 8 min, followed by 35 cycles of denaturation at 95 °C for 40 sec, annealing at 55 °C for 40 sec and extension at 72 °C for 40 sec, with final elongation at 72 °C for 5 min. The fragments were separated by electrophoresis on 2% agarose gels containing ethidium bromide, and visualized by UV illumination. The PCR product was then treated with EXOSap (UBS, Sial, Rome, Italy) and directly sequenced using a BigDye Terminator kit v3.1 (Applied Biosystem, Foster City, CA, USA) using the same primers as for the PCR amplification, in an ABI PRISM 3100 Genetic Analyser (Applied Biosystems).
Statistical analysis
Statistical analysis was performed with GraphPad software (GraphPad Software Inc., La Jolla, CA, USA) using the Mann-Whitney test for continuous variables, and Fisher’s exact test or the χ2 test for categorical variables, as appropriate. Survival analysis was performed with the Kaplan-Meier method using the log-rank test for the comparison of curves. P values <0.05 were considered statistically significant.
Results
Prevalence of HIF-1αPro-582-Ser polymorphism in the study population
Among the 169 investigated subjects, 48 individuals (28.4%) carried the HIF-1αPro-582-Ser mutation in a homozygous (2.4%) or heterozygous (26.0%) form. Of these 48 individuals, 36 were males and 12 females. The prevalence of the polymorphism was similar in both sexes (P =0.282) and was comparable to that previously reported in individuals of both Caucasian and East Asian ethnicity10.
Effect of the HIF-1αPro-582-Ser polymorphism on haematological parameters
Considering the low number of homozygous cases, for the purpose of our analyses we pooled heterozygous and homozygous cases together. However, since previous data indicated a possible link between gender and effect of the polymorphism10, we carried out the analysis separately in males and females. The haematological parameters of wild-type and mutated donors are shown in Table I and Table II for male and female subjects, respectively. We observed that there were significant differences in haematological parameters between HIF-1αPro-582-Ser male carriers and wild-type subjects with the former having higher haemoglobin (Hb) concentrations (P =0.004), haematocrit (Hct, P =0.013), mean corpuscular haemoglobin (MCH, P =0.006), mean corpuscular haemoglobin concentration (MCHC, P =0.048) and serum ferritin levels (P =0.002). No differences were found with respect to red blood cell (RBC) count, mean corpuscular volume (MCV), white blood cell (WBC), neutrophil and platelet counts and serum iron levels (Table I). In three of the male donors (two mutated and one wild-type) the ferritin levels visibly exceeded the upper limit of the normal range but also after excluding them from the analysis, ferritin levels differed significantly between HIF-1αPro-582-Ser carriers and wild-type individuals (mean values ± SEM 121±18 ng/mL versus 74±6 ng/mL, respectively; P =0.028). In contrast to the findings in the male population, among female donors no differences were detected in haematological parameters and ferritin concentrations between wild-type and HIF-1αPro-582-Ser mutated subjects (Table II).
Table I.
Laboratory findings in male donors grouped according to HIF-1α status.
| HIF-1α wild-type N =99 | HIF-1αPro-582-Ser N =36 | P value | |
|---|---|---|---|
| Age (years) | 44,2 | 38,6 | 0.029 |
| Median (range) | (20,5–62) | (19,6–64) | |
| Hb g/dLa | 15,6 | 16,3 | 0,004 |
| Median (range) | (13,5–18,1) | (13.8–18.9) | |
| MCV, fLb | 88 | 88,8 | 0.192 |
| Median (range) | (79,8–97) | (80,8–96) | |
| MCH pgc | 29,7 | 30,4 | 0,006 |
| Median (range) | (22,1–33,3) | (27,5–36,5) | |
| MCHC g/dLd | 33,8 | 34,3 | 0,048 |
| Median (range) | (30,6–36,6) | (32,6–35,9) | |
| Haematocrit %e | 45,5 | 48,4 | 0.013 |
| Median (range) | (39,0–53,8) | (38,4–54,4) | |
| RBC × 1012/Lf | 5.05 | 4.98 | 0.804 |
| Median (range) | (3.98–5.82) | (4.28–5.54) | |
| WBC × 109/Lg | 6.75 | 6.41 | 0.521 |
| Median (range) | (3.68–11.10) | (3.72–11.38) | |
| Platelets × 109/Lh | 206 | 220 | 0.273 |
| Median (range) | (129–316) | (118–343) | |
| Ferritin ng/mLi | 58 | 101 | 0.002 |
| Median (range) | (15–311) | (24–732) | |
| Iron serum gamma/dLj* | 88 | 85 | 0.903 |
| Median (range) | (38–193) | (59–134) |
Legend
normal Hb values: 13–17 g/dL for males;
normal MCV values: 81–99 fL;
normal MCH values 26–33 pg;
normal MCHC values 30–35 g/dL;
normal haematocrit values 42–52%;
normal RBC values 4.3–6.1×1012/L;
normal WBC values 4.1–9.8×109/L;
normal platelet values 140–450×109/L;
normal ferritin values 24–336 ng/mL;
normal serum iron levels 60–160 gamma/dL;
data obtained in 65 donors.
Table II.
Laboratory findings in female donors grouped according to HIF-1α status.
| HIF-1αwild type N =22 | HIF-1αPro-582-Ser N =12 | P value | |
|---|---|---|---|
| Age (years) | 37,9 | 34,5 | 0.379 |
| Median (range) | (21,1–55,6) | (20,4–63,2) | |
| Hb g/dLa | 13,1 | 13,3 | 0,971 |
| Median (range) | (12,4–14,9) | (12,5–15.9) | |
| MCV, fLb | 87,8 | 86,8 | 0.773 |
| Median (range) | (80–92,9) | (83,9–93,2) | |
| MCH pgc | 29,5 | 29,2 | 0,198 |
| Median (range) | (26,7–31,1) | (27,0–30,5) | |
| MCHC g/dLd | 33,5 | 33,5 | 0,402 |
| Median (range) | (29,8–36,2) | (31,6–34,3) | |
| Haematocrit %e | 39,0 | 40,0 | 0.880 |
| (Median (range) | (36,7–49,4) | (36,5–44,0) | |
| RBC × 1012/Lf | 4.57 | 4.61 | 0.561 |
| Median (range) | (3.93–5.28) | (4.29–5.10) | |
| WBC × 109/Lg | 7.11 | 6.48 | 0.397 |
| Median (range) | (4.60–10.24) | (4.93–11.95) | |
| Platelets × 109/Lh | 250 | 241 | 0.387 |
| Median (range) | (174–336) | (178–336) | |
| Ferritin ng/mLi | 23 | 27 | 1.000 |
| Median (range) | (23–161) | (17–66) | |
| Serum iron gamma/dLj* | 102 | 90 | 0.375 |
| Median (range) | (50–160) | (42–176 ) |
Legend
normal Hb values: 12–16 g/Dl;
normal MCV values: 81–99 fL;
normal MCH values 26–33 pg;
normal MCHC values 30–35 g/dL;
normal haematocrit values 37–47%;
normal RBC values 4–5.4×1012/L;
normal WBC values 4.1–9.8×109/L;
normal platelet values 140–450×109/L;
normal ferritin values 11–307 ng/mL;
normal iron serum levels 40–150 gamma/dL;
data obtained in 29 donors.
Effect of the HIF-1αPro-582-Ser polymorphism on donation referral
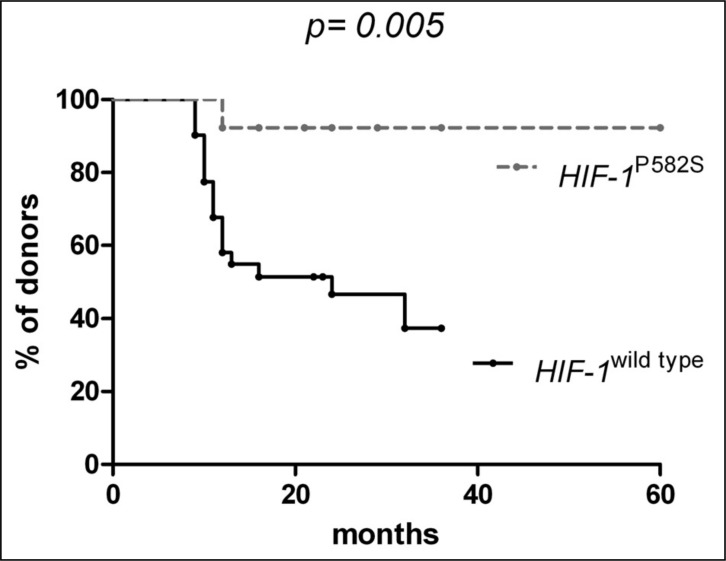
Since we found that the HIF-1αPro-582-Ser variant from exerts a more robust constitutive stimulating effect on erythropoiesis and on iron storage than the wild-type form, it could be expected that the HIF variant form might be also more efficacious in counteracting the effects of blood loss. In order to assess whether the HIF-1αPro-582-Ser polymorphism might protect donors from iron deprivation, we retrospectively investigated its effect on blood donation referral because of iron deficiency or anaemia. From the population of regular blood donors, we selected a subgroup of 45 subjects with a history of regular donations for at least 3 years; in this series we retrospectively evaluated the time elapsed between the first donation and the first donation postponement because of low ferritin levels (24 ng/mL in males and 11 ng/mL in females) or low Hb concentration (below than 13.5 g/dL in males and 12.5 g/dL in females). Thirty-two were HIF-1α wild-type and 13 had the HIF-1αPro-582-Ser mutation. We found that the median time for donation postponement in wild-type donors was 24 months, while it was undefined in mutated donors (Figure 1, P =0.005). This finding strongly suggests that HIF-1αPro-582-Ser carriers are less subjected to iron deficiency and anaemia than wild-type individuals.
Figure 1.
Time elapsed from the first donation to the first donation postponement for low ferritin levels or low Hb concentration in regular blood donors grouped according to HIF-1a mutational status.
Discussion
Iron deficiency is the most common cause of anaemia and one of the most frequent causes of blood donor deferral. Indeed, being able to identify subjects at low risk of iron deprivation may help to select them for specific donation procedures or more intensive donation programmes. The oxygen-sensing pathway includes a complex of genes able to increase red blood cell production in response to hypoxia. In particular, the HIF-1a gene modulates the synthesis of five gene products (erythropoietin, erythropoietin receptor, hepcidin, transferrin, and transferrin receptor) involving several different organs (kidney, liver, intestine, blood and bone marrow) to control erythropoiesis1,4. HIF-1α levels are regulated by the conditional interaction of HIF-1α with pVHL, which binds the N-terminal transactivation domain (N-TAD) within the oxygen-dependent degradation domain (ODD) of the HIF-1α molecule1,3. In the HIF-1αPro-582-Ser variant the amino acid change is located in the N-TAD6 and, whereas it does not directly affect the hydroxylation of proline at position 564 within the ODD, it has been associated with higher transcriptional activity than the wild-type form, suggesting that the mutation might affect HIF-1α transcriptional activity or HIF-1α stability7. In accordance with these observations, we found that HIF-1αPro-582-Ser donors had higher Hb concentration, Hct, MCH, MCHC values and serum ferritin levels than HIF-1a wild-type donors. Interestingly, in the female donor population, these haematological parameters were not affected by the presence of the HIF-1αPro-582-Ser polymorphism. Although this finding needs to be confirmed in prospective studies in larger series, it is not surprising that the effects of the polymorphism are influenced by the surrounding hormonal milieu. Recent data indicated that HIF-1a mediates the effects of testosterone in stimulating the proliferation of haematopoietic CD34+ cells11. In addition, it is well acknowledged that the HIF-1αPro-582-Ser polymorphism has a gender-specific effect in tumours: actually, among several cancers, it seems specifically associated with an increased risk of prostate and breast cancers10.
The relatively high prevalence of the HIF-1αPro-582-Ser polymorphism, reaching figures of 25–30% in this study and previous ones6,10,12–14, is rather surprising considering its unfavourable association with cancers10 and with poor prognosis histopathological features in the malignancies6. It is, therefore, reasonable to hypothesise that HIF-1aPro-582-Ser status may confer carriers some benefits. For example, a previous study carried out in the general population showed that HIF-1αPro582Ser carriers aged 60 years or older have significantly lower change in maximal oxygen consumption measured before and after aerobic exercise training13. Moreover a recent study performed in elite endurance athletes suggested that HIF-1αPro582Ser status is associated with higher responsiveness to endurance training14. In this study we show that the HIF-1αPro582Ser polymorphism exerts an evident protective effect against repeated blood loss: HIF-1αPro582Ser carriers are less prone to develop iron deficiency and anaemia than wild-type donors. These data parallel the recent observations of Miasnikova et al., demonstrating that identical mutations of the VHL gene in the homozygous state cause hereditary polycythaemia with a high mortality rate from vascular complications, while in the heterozygous state they protect carriers from anaemia15.
The main limit of this study undoubtedly lies in its cross-sectional retrospective design, which implies possible bias in donor selection. Indeed, our observations on the effect of HIF-1αPro582Ser polymorphism in both male and female donor populations require confirmation in prospective studies and through a deeper investigation of the iron status. A prospective investigation would enable the collection of data on parameters which are affected at extremely early phases of iron deficiency, such as reticulocyte sub-population counts, red cell distribution width and plasma total iron binding capacity. Finally, prospective studies might elucidate whether the HIF-1αPro582Ser variant has an effect on hepcidin and erythropoietin production. In conclusion, although our data are preliminary, they do indicate a novel approach for stratifying blood donors according to risk of iron deprivation and for selecting individuals suitable for more intensive blood donation programmes.
Footnotes
Authors’ contributions
Authors’ contributions: Lorenza Torti, Luciana Teofili, Sara Capodimonti, Agostino Tafuri, Francesco Fiorin, Giuseppina Massini, Gabriella Girelli, Luigi Maria Larocca and Gina Zini: conception and design of the study, contribution of samples, data collection and analysis, manuscript writing, final approval of the manuscript. Sara Capodimonti, Eugenia Rosa Nuzzolo and Maria Grazia Iachininoto performance of molecular analysis, manuscript writing, final approval of manuscript.
The Authors declare no conflicts of interest.
Funding
This work was supported by Fondi di Ateneo Progetti D1 2012 (Università Cattolica). Agostino Tafuri was supported by grants from Italian MIUR, PRIN 2010–2011, and from “Sapienza” University of Rome, 2009-2011.
References
- 1.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;14:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–47. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 3.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–9. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]
- 5.Gordeuk VR, Stockton DW, Prchal JT. Congenital polycythemias/erythrocytoses. Haematologica. 2005;90:109–16. [PubMed] [Google Scholar]
- 6.Tanimoto K, Yoshiga K, Eguchi H, et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis. 2003;24:1779–83. doi: 10.1093/carcin/bgg132. [DOI] [PubMed] [Google Scholar]
- 7.Percy MJ, Mooney SM, McMullin MF, et al. A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1alpha (HIF-1alpha) does not impair Pro-564 hydroxylation. Mol Cancer. 2003;2:31. doi: 10.1186/1476-4598-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giona F, Teofili L, Moleti ML, et al. Thrombocythemia and polycythemia in patients younger than 20 years at diagnosis: clinical and biologic features, treatment, and long-term outcome. Blood. 2012;119:2219–27. doi: 10.1182/blood-2011-08-371328. [DOI] [PubMed] [Google Scholar]
- 9.Teofili L, Giona F, Martini M, et al. Markers of myeloproliferative diseases in childhood polycythemia vera and essential thrombocythemia. J Clin Oncol. 2007;25:1048–53. doi: 10.1200/JCO.2006.08.6884. [DOI] [PubMed] [Google Scholar]
- 10.Zhao T, Lv J, Zhao J, Nzekebaloudou M. Hypoxia-inducible factor-1alpha gene polymorphisms and cancer risk: a meta-analysis. J Exp Clin Cancer Res. 2009;28:159. doi: 10.1186/1756-9966-28-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Fu L, Han Y, et al. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur J Pharmacol. 2012;684:116–24. doi: 10.1016/j.ejphar.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Clifford SC, Astuti D, Hooper L, et al. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx and HIF-1a in renal cell carcinoma. Oncogene. 2001;20:5067–71. doi: 10.1038/sj.onc.1204602. [DOI] [PubMed] [Google Scholar]
- 13.Prior SJ, Hagberg JM, Phares DA, et al. Sequence variation in hypoxia-inducible factor 1alpha (HIF1A): association with maximal oxygen consumption. Physiol Genomics. 2003;15:20–6. doi: 10.1152/physiolgenomics.00061.2003. [DOI] [PubMed] [Google Scholar]
- 14.Döring F, Onur S, Fischer A, et al. A common haplotype and the Pro582Ser polymorphism of the hypoxia-inducible factor-1alpha (HIF1A) gene in elite endurance athletes. J Appl Physiol. 2010;108:1497–500. doi: 10.1152/japplphysiol.01165.2009. [DOI] [PubMed] [Google Scholar]
- 15.Miasnikova GY, Sergueeva AI, Nouraie M, et al. The heterozygote advantage of the Chuvash polycythemia VHLR200W mutation may be protection against anemia. Haematologica. 2011;96:1371–4. doi: 10.3324/haematol.2011.045609. [DOI] [PMC free article] [PubMed] [Google Scholar]