West Nile virus: virology and clinical perspective
West Nile virus (WNV) infection is a vector-borne disease caused by a 50 nm, icosahedral, enveloped, ssRNA virus that is member of the genus Flavivirus and that belongs to the Japanese encephalitis virus (JEV) serogroup1. This serogroup also includes St Louis encephalitis virus (SLEV), Kunjin virus (KUNV) and Murray Valley encephalitis virus (MVEV). Sequencing and phylogenetic studies showed that, while WNV can be classified into as many as five distinct lineages2, it mainly circulates as two major genetic lineages, lineage 1 (L1) and lineage 2 (L2). L1 includes viral strains from North, Central and South America, Africa, the Middle East, Asia and Australia3,4. L2 includes strains historically isolated in Africa and traditionally associated with asymptomatic infections in human. Only recently, several virulent L2 isolates have been identified in Europe5 with human cases reported in Greece and Italy6,7.
The natural transmission cycle involves mosquitoes, particularly Culex species, acting as vectors, and wild birds, acting as amplifying hosts. Humans, horses and other mammals are considered incidental or dead-end hosts8,9. Vertical transmission and other transmission routes, i.e. breast-feeding, organ transplantation and blood transfusion, have also been documented10,11.
The majority of WNV infections in humans are asymptomatic while approximately 20% of infected individuals develop, after an incubation period of 3–14 days, a mild febrile illness for 3–6 days (West Nile fever, WNF) characterised by a variety of non-specific symptoms that do not allow WNF to be distinguished from other infectious illnesses on clinical examination. In less than 1% of infected individuals, particularly the elderly or immunocompromised subjects12, WNV infection can result in a severe disease with meningitis or encephalitis (West Nile neuro-invasive disease, WNND) and in long-term sequelae such as altered mental status, lethargy, cranial nerve palsies, acute flaccid paralysis and movement disorders13,14.
The diagnosis is based on clinical evaluation and on laboratory tests for specific IgM in serum (detectable one to several days after the onset of symptoms) either by enzyme-linked immunosorbent assays or by haemagglutination inhibition methods. As IgM cross-react with other flaviviruses, such as dengue virus, SLEV or USUTU virus (USUV)15, IgM-positive samples are subjected to a confirmatory test carried out using a plaque reduction neutralisation assay12,16. IgM can be persistent and these antibodies can sometimes be detected for months after infection17. Thus, in areas in which WNV has circulated for more than a season, the sole detection of IgM could lead to a misdiagnosis of WNV infection as the symptoms could be due to another febrile illness. In these cases, detection of IgG by an avidity assay can be useful to distinguish recent infections from past infections with persistent IgM. Although virus isolation is possible, cultures of blood, cerebral spinal fluid and tissues generally test negative in infected individuals because of the peculiar characteristics of WNV infection, i.e. brief duration of viraemia (1–11 days) and low viral loads (often <100 pfu/mL). As the detection of WNV RNA by nucleic acid amplification techniques (NAT) is quite sensitive in the early stage of infection, this is considered the method of choice to check for the possible presence of viraemia in blood and tissue/organ donors.
With respect to prevention, there is no human vaccine available to date. Only veterinary WNV vaccines are currently on the market: a formalin-inactivated WNV vaccine, a recombinant vaccine consisting of a canarypox virus vector with insertion and expression of WNV membrane and envelope proteins, and a recombinant vaccine with WNV membrane and envelope proteins expressed in a yellow fever vector18.
Currently, there are limited treatment options for patients infected with WNV. Only two classical antiviral compounds, interferon and ribavirin, showed promising results in vitro19 but it is unclear whether these compounds are effective in patients20. Passive transfer of anti-WNV immunoglobulin was shown to be effective in mouse and hamster models and may be helpful in patients as documented by some case reports of humans with WNND who improved after receiving immune γ-globulin20. The use of humanised or human monoclonal antibodies or antibody fragments with therapeutic activity against WNV infection appears promising20. Specific WNV intravenous immunoglobulin obtained from selected donors from WNV endemic regions, such as Israel, could help to control the active infection in treated patients in terms of higher chances of survival or diminished risks of immediate and/or long term sequelae21.
The emergence of West Nile virus as a blood-borne virus
Western hemisphere
WNV was first isolated in Uganda in 193722 and was associated with sporadic cases and outbreaks in Africa, the Middle East, and Asia23. Frequent outbreaks of severe WNND in humans and horses were subsequently reported in Europe and the Mediterranean Basin24,25. In 1999, the virus appeared for the first time in the USA, precisely in New York City26, and rapidly spread from the East coast to the West coast, reaching Canada in 2002. The first action undertaken by the two countries was the implementation in 2000 of a national veterinary surveillance plan for WNV27,28. In 2002, WNV was recognised in the USA as a blood-borne virus with 23 transfusion-transmitted WNV infections observed during the epidemic season10,29. The same year, virus transmission through solid organ transplantation was also reported11. Therefore, the Food and Drug Administration (FDA), strongly urged test kits manufacturers to develop WNV NAT assays for blood screening. Two manufacturers, world-wide leaders in this field, were each able to develop an assay and a test platform in a few months. Both kits were firstly implemented in the USA blood centres in summer 2003 under the FDA’s Investigational New Drug (IND) application: the Procleix WNV assay, based on transcription-mediated amplification technology, developed by Gen-Probe, and the TaqScreen WNV test, a real-time polymerase chain reaction method developed by Roche. The same year, Canadian blood centres also implemented NAT testing screening adopting the TaqScreen WNV test as a preventive measure. From July to November 2003, approximately 800 WNV presumptive viremic donors were detected in the USA30. When a sample tested positive the donor was temporarily deferred for 28 days (in Canada for 56 days). This 4-week deferral period was established based on the viraemic phase of a typical mosquito-borne WNV infection (1–11 days) thus providing reassurance that after this deferral period a specific humoural response can be detected and the virus is cleared from the blood. Because of automation constraints, the newly introduced WNV NAT tests were performed with minipools (MP) of either six (TaqScreen WNV test) or 16 (Procleix WNV assay) donations. Positive pools were resolved and tested again to identify the positive donation. Although this WNV NAT screening contributed to a decreased risk of blood-borne transmission in the USA, the MP strategy reduced the assays’ sensitivity and increased the possibility of missing donors with very low levels of viraemia. In fact, studies carried out during the 2003 WNV epidemic season identified at least six “MP-NAT breakthrough” infections which were attributed to blood units with levels of viraemia below the sensitivity of the MP-NAT assays31–33. In 2005, based on the data collected in the clinical trials showing that, in rare instances, the viraemic period may be as long as 104 days, a 120-day deferral for NAT-positive donors was recommended by the FDA34. With respect to donors’ symptoms, the initial FDA guidance for industry indicated deferral for donors in the case of suspected WNV infection when a combination of headache and fever was present35. After Orton et al.36 showed that this combination of symptoms made no detectable contribution to blood safety, the FDA guidance was revised to remove this deferral recommendation34. A subsequent independent study confirmed the low overall predictive value of combinations of specific symptoms (or individual symptoms) while pointing to a significant association between the total number of symptoms reported before donation and confirmed WNV infection37.
The spread of WNV up to 2012 caused more than 33,000 cases of human infection in the USA, including 1,506 deaths, and 5,099 human infections in Canada, including 44 deaths. This apparent stark difference between USA and Canada is appropriately placed in perspective if the respective population size is taken into consideration: with rates of human infection being 1.13/10,000 in the USA and 1.55/10,000 in Canada and corresponding mortality rates being 0.05/10,000 in the USA and 0.01/10,000 in Canada (Table I). In the European Union, a large outbreak of WNF occurred in Romania in 1996 with 352 cases of WNND and 17 deaths38. In subsequent years, the incidence of WNV in humans appeared to be quite low as only a few sporadic autochthonous cases were reported and only in defined geographical areas (Portugal, Spain, France, Italy, Czech Republic, Romania and Hungary)39. Meanwhile, sporadic imported cases were reported in Czech Republic, France, Germany, Denmark, the Netherlands, and Ireland40 caused by travelling to areas with ongoing transmission of WNV to humans, such as Canada, Israel and mainly the USA, where an increasing number of autochthonous infections had been described since 1999. The first WNV precautionary measure undertaken by the European Commission in 2004 was, therefore, a 28-day deferral period for blood donors leaving areas with ongoing transmission of WNV (Directive 2004/33/EC [Annex III]41 implementing Directive 2002/98/EC42). In 2007, WNV infection became a notifiable disease in compliance with Commission Decision 2007/875/EC43. Furthermore, Commission Decision 2008/426/EC44 established the criteria for case classification of human WNV infections (Table II) in order to allow comparability of the cases reported by member states to the Commission through the European Union Early Warning and Response System and to allow their compilation by the European Centre for Disease Prevention and Control (ECDC) in a disease risk assessment report to be issued annually. From 2008 to 2012, an increased number of WNV infections in humans were reported in the member states, especially in Greece, Italy, Hungary and Romania, as well as in neighbouring countries, namely Israel and Russia (Table III)39,45–57. The re-occurrence of WNV infections in the same places over the years could be a sign of the endemic nature of the disease rather than a new introduction of the virus58. In fact, mosquitoes may acquire the infection by vertical transmission between generations via the egg stage26,59. However, one cannot exclude that the implementation of a surveillance system could also have contributed to highlighting the above-mentioned re-occurrence.
Table I.
Human cases of WNV infections1 reported in the USA and Canada.
| Year | USA | Canada3 | ||
|---|---|---|---|---|
|
| ||||
| Human cases | Deaths | Human cases | Deaths | |
|
|
||||
| 1999 | 62 | 7 | - | - |
| 2000 | 21 | 2 | - | - |
| 2001 | 66 | 10 | - | - |
| 2002 | 4,156 | 284 | 414 | 14 |
| 2003 | 9,862 | 264 | 1,481 | 14 |
| 2004 | 2,539 | 100 | 25 | - |
| 2005 | 3,000 | 119 | 225 | 10 |
| 2006 | 4,269 | 177 | 151 | - |
| 2007 | 3,630 | 124 | 2,215 | - |
| 2008 | 1,356 | 44 | 36 | - |
| 2009 | 720 | 32 | 13 | - |
| 2010 | 1,021 | 57 | 4 | - |
| 2011 | 712 | 43 | 102 | - |
| 2012 | 5,387 | 243 | 450 | 6 |
|
| ||||
| Total | 33,804 | 1,506 | 5,099 | 44 |
| Ratio4 ×10,000 | 1.13 | 0.05 | 1.55 | 0.01 |
Including neuroinvasive disease (such as meningitis or encephalitis) and non-neuroinvasive disease (WN fever);
source: CDC, accessed on 11/12/2012 (http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detailed.htm);
source: Public Health Agency of Canada, accessed on 27/10/2012 (http://www.phac-aspc.gc.ca) and Community and Hospital Infection Control Association - Canada, accessed on 29/04/2011 at: http://www.chica.org/links_wnv.php;
mean values of the population in USA (297,013,100) and Canada (32,887,690) in the reported years. Source: http://www.indexmundi.com/.
Table II.
Criteria for case classification of human WNV infections (Commission Decision 2008/426/EC).
|
Clinical criteria Any person with fever or one of the following:
|
|
Laboratory criteria Laboratory test for case confirmation At least one of the following:
|
|
Epidemiological criteria At least one of the following two epidemiological criteria:
|
Table III.
Human cases of WNV infections1 reported in Europe and neighbouring countries.
| Country | Year | Reference | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| 2008 | 2009 | 2010 | 2011 | 20122 | ||
| Albania | - | - | - | 2 | - | 39 |
| Croatia | - | - | - | - | 5 | 56 |
| Greece | - | - | 262 | 69 | 161 | 39, 45, 46 |
| Hungary | 14 | 7 | 19 | - | 12 | 47 |
| Italy | 8 | 18 | 3 | 14 | 50 | 39, 68 |
| Israel | 104* | 33 | 59 | 45, 46* | 49 | |
| Kosovo | - | - | - | - | 4 | 40 |
| Macedonia | - | - | - | 4 | 6 | 48 |
| Montenegro | - | - | - | - | 1 | 40 |
| Romania | - | 2 | 49 | 10 | 14 | 39 |
| Russia Fed. | - | 10 | 206 | 136 | 447 | 50, 51 |
| Serbia | - | - | - | - | 70 | 57 |
| Spain | - | - | 2 | - | - | 52 |
| Turkey | - | 1 | 7 | 3 | - | 53, 54 |
| Tunisia | - | - | - | 3 | 63 | 55 |
| Ukraine | - | - | - | 8 | 12 | 40 |
Including neuroinvasive disease (such as meningitis or encephalitis) and non-neuroinvasive disease (WN fever).
From ecdc.europe.eu, accessed on 30/11/2012.
The first human cases of WNND prompted Italy in 2008 and Greece in 2010 to implement WNV NAT screening for blood donations collected from areas with ongoing transmission of WNV60,61.
Italy
In Italy, a national veterinary surveillance plan for WNV has been in place since 2002 following the first outbreak of WNV infection in 14 horses reported in 1998 in the region of Tuscany62. This plan is aimed at identifying risk areas, at monitoring WNV circulation (based on wild bird mortality and on entomological and sentinel animal surveillance), as well as at checking for WNV seroconversion in horses residing in risk areas. New equine cases of symptomatic WNV infection were reported in September 2008, with an outbreak of 794 cases in the north-eastern part of Italy (Veneto, Emilia-Romagna, Lombardy)63 while eight human cases of WNND were reported in September–October 2008, five in the region of Veneto and three in the Emilia-Romagna region64,65. After the first three of these human cases of WNND had been reported in the Emilia-Romagna region (one in the province of Bologna and two in the province of Ferrara), the National Blood Centre (NBC) asked regional health authorities to adopt specific preventive measures in the presence of a “grade 3 risk” (human morbidity) as defined in the guidelines on the procedures against the circulation of WNV issued by the French Ministry of Health66,67. These preventive measures consisted in the introduction of WNV NAT to test all blood, peripheral, bone marrow and cord blood stem cell donations collected in the provinces of Bologna and Ferrara and in a nationwide 28-day deferral for blood donors who had spent at least one night in these areas. Blood centres were asked to use the test kits available on the market for the detection of WNV RNA. With the subsequent identification of the five additional human cases of WNND in the Veneto region68, the donations collected in the concerned provinces (Venice, Vicenza and Rovigo) were also subjected to these preventive measures. Beginning in December 2008, the NBC asked all the concerned parties to discontinue the preventive actions. No NAT-positive donors were detected, most likely due to the fact that the probability of detecting asymptomatic, viraemic donors is significantly related to the peak of activity of the vector insects which in Italy occurs in midsummer. A serological study of about 9,000 healthy blood donors carried out in the province of Ferrara from October 2008 to April 2009 showed a seroprevalence of about 0.68%58, thus confirming the circulation of WNV in this area. The risk of having an asymptomatic, viraemic donor in 2008 in the province of Ferrara was estimated to be 2.2/10,000 donations by applying the formula developed by Biggerstaff and Petersen29 which takes into consideration a number of assumptions as well as different parameters such as the duration of the outbreak and the incidence, the latter being estimated at 0.84/100,000 donations (CI 95% 0.17–2.46) based on the three human cases of WNND that occurred in the province of Ferrara.
In 2009, the NBC implemented, as a preventive measure, WNV NAT testing of all blood, peripheral, bone marrow and cord blood stem cell donations from 1 August to 1 November 2009. Based on the animal and vector surveillance data for WNV gathered both at the national and at the regional level, the areas specified for the implementation of these measures were identified as the provinces of Ferrara, Rovigo and Mantua (the latter belonging to the region of Lombardy). Furthermore, the NBC adopted the definition of “affected area” as a sub-regional area, corresponding in terms of an administrative entity to a province, in which a human case of WNND and/or a WNV-NAT positive donor is confirmed. In the event that an area fell within this definition, the NBC would enforce a nationwide 28-day deferral for blood donors who had spent at least one night in these areas. Accordingly, after the first WNV-NAT positive asymptomatic blood donor was detected in the province of Mantua at the beginning of August 2009, this national measure was applied. The same summer, one human case of WNND was reported in the province of Mantua. Between August and September 2009, the 28-day deferral was also applied to donors who had spent at least one night in the provinces of Rovigo (one WNV NAT-positive donation), Ferrara, Modena, Bologna, Treviso and Venice (17 human cases of WNND). In addition, the last four provinces were requested to implement WNV NAT for screening of blood and stem cell donations. Overall, a total of 59,815 blood donations were tested in 2009 for WNV by NAT and two of them were found to be positive for this virus. An increase in WNND cases was observed in 2009 with respect to the previous year (18 versus 8)68 with an estimated risk of 1.22–1.45/10,000 donations when applying the above-mentioned formula29.
The circulation of WNV in a large area of the eastern part of the Po river’s plain for two consecutive years showed that this territory was becoming suitable to support WNV establishment and possible endemicity. Moreover, the geographical distribution of WNND had widened, with the virus spreading from the eastern to the western regions of Northern Italy. This prompted the Ministry of Health to centralise the standard surveillance measures for early detection of WNV activity and for the assessment of the risk for public health. In spring 2010, a National Plan for Surveillance of Human WNND was implemented reporting the activities to be carried out annually between 15 June and 15 November, i.e. when the risk for WNV infection is higher in Italy69. In this context, the NBC implemented as preventive measures, from 15 July to 15 November 2010, WNV screening by NAT of blood donations in the provinces of Mantua, Rovigo, Ferrara, Modena, Bologna, Reggio Emilia and Venice as well as the nationwide 28-day deferral. In addition, the NBC recommended blood centres in the affected areas to enhance pre- and post-donation information. The same summer, two human cases of WNND were reported in the province of Venice. In mid-autumn 2010, the same measures were extended to the province of Vicenza, belonging to the Veneto region, after one human case of WNND was reported in this area. Furthermore, three human cases of WNF were reported in the Veneto region70. Overall, 118,295 blood donations were tested in 2010 for WNV by NAT, with six of them testing positive. The final number of reported human cases of WNND decreased to three, with an incidence of 0.15/100,000, considering only the Veneto region, and an overall incidence of 0.06/100,000, considering all the affected areas68. The risk was estimated at 0.49–0.92/10,000 donations.
In 2011, the NBC confirmed the same preventive measures as in the previous year, the same period for their implementation (15 July–15 November 2011, later extended to 30 November 2011 due to the exceptional heat in the autumn months) and the same concerned areas, i.e. those in which human cases of WNND had been reported the previous year. As additional safety measures the NBC implemented storage of plasma/serum samples in the pre-NAT testing period and post-transfusion haemovigilance. In late summer 2011, WNV NAT testing was also extended to the provinces of Treviso, Belluno, Udine, Oristano and Olbia Tempio (in the regions of Veneto, Friuli-Venezia-Giulia, and Sardinia) after human cases of WNND were reported. Furthermore, the province of Ancona implemented WNV NAT testing on stem cell donations after the first human case of WNF was identified, according to the provisions set out by the National Transplant Centre (NTC) for organ and tissue donations. Overall, 119,345 blood donations were tested in 2011 for WNV by NAT, with four of them testing positive. The final number of reported human cases of WNND increased to 14 with an incidence of 0.18/100,000 considering all the affected areas. The risk was estimated at 0.19/10,000 donations. While no cases of transfusion-transmitted WNV infection had been observed up to 2011, four cases of WNV transmission occurred this year following a single multiorgan donation in north-eastern Italy71.
In 2012, the NBC, on the basis of the 2011 risk assessment of WNV transmission by transfusion of blood and blood components, and taking into consideration the Preparedness Plan in Europe prepared by Greece, Italy, Romania and France72, implemented the same preventive measures from 15 July to 30 November 2012 in the following areas concerned: the provinces of Udine (Friuli-Venezia-Giulia region), Treviso, Belluno and Venice (Veneto region) as well as all eight provinces in the Sardinia region.
As soon as NAT testing of blood donors was introduced, one donation was found positive for WNV in the province of Venice73. Sequence analysis of the viral RNA from the donor confirmed a 100% sequence identity with a lineage 1 WNV strain that was fully sequenced the year before from a blood donor resident in a nearby village, strongly suggesting overwintering of this strain in the area (the so-called WNV Livenza strain)73,74. This finding, in addition to underlining the importance of WNV NAT screening for the safety of blood, tissue, and organ donations, pre-announced 38 WNV clinical cases (21 WNND and 17 WNF) and 14 blood donations found positive for WNV by NAT in the Veneto region in summer 201274. Between August and September 2012, after four human cases of WNND were reported in the provinces of Udine, Gorizia and Pordenone, the same preventive measures were extended to all provinces of the Friuli-Venezia-Giulia region. The same actions were implemented at the end of September 2012 in the province of Matera (Basilicata region) when one WNND case was reported. Overall, 116,255 blood donations were tested in 2012 for WNV by NAT, with 14 of them testing positive. Considering also two human cases of WNND reported in Oristano (Sardinia region) between August and September 2012, the final number of human cases of WNND reported in 2012 amounted to 28. The risk was estimated at 0.26/10,000 donations.
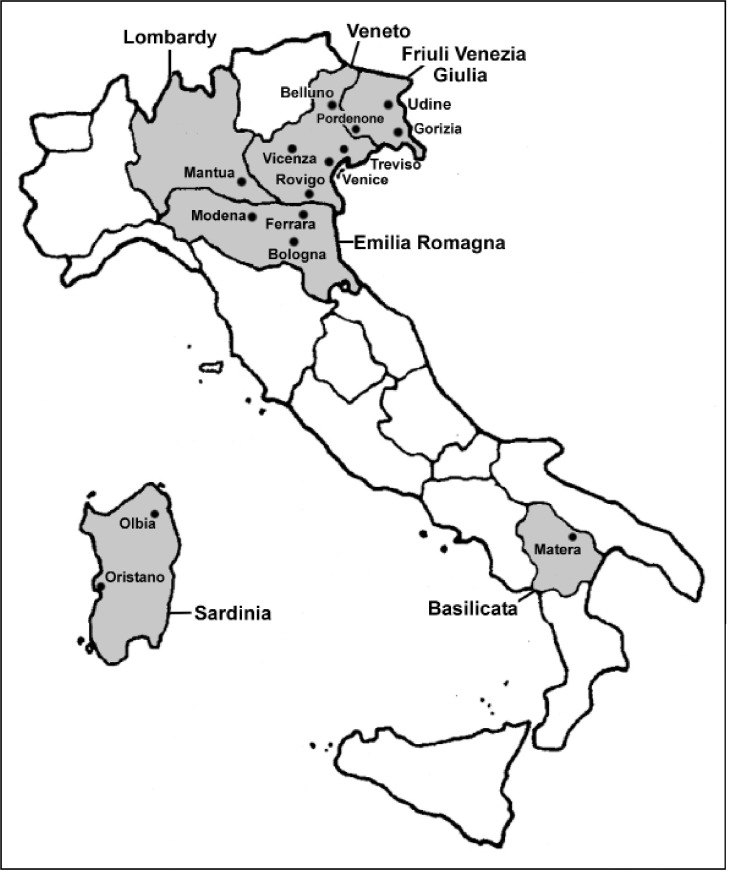
A map of the Italian regions and of the respective provinces concerned by WNV cases is provided in Figure 1. The WNND cases, blood donations tested and blood donations tested positive between 2009 and 2012 are reported in Table IVa and Table IVb along with their respective regional distribution. An overview of the safety measures for WNV infection adopted by the NBC between 2008–2012 is reported in Table V.
Figure 1.
Italian regions and respective provinces concerned by WNV cases.
Table IVa.
Overview of the the WNV situation in Italy between 2009–2010.
| Region | 2009 | 2010 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| N. of WNND cases | N. of blood donations tested | N. of positive donations | N. of WNND cases | N. of blood donations tested | N. of positive donations | |
| Basilicata | -- | -- | -- | 0 | 0 | 0 |
| Emilia-Romagna | 9 | 35,482 | 0 | 0 | 72,090 | 4 |
| Friuli | -- | -- | -- | 0 | 0 | 0 |
| Lombardy | 2 | 8,193 | 1 | 0 | 7,740 | 0 |
| Sardinia | -- | - | -- | 0 | 0 | 0 |
| Veneto | 7 | 16,140 | 1 | 3 | 38,465 | 2 |
|
| ||||||
| Total | 18 | 59,815 | 2 | 3 | 118,295 | 6 |
Table IVb.
Overview of the the WNV situation in Italy between 2011–2012.
| Region | 2011 | 2012 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| N. of WNND cases | N. of blood donations tested | N. of positive donations | N. of WNND cases | N. of blood donations tested | N. of positive donations | |
| Basilicata | 0 | 0 | 0 | 1 | 1,042 | 0 |
| Emilia-Romagna | 0 | 45,727 | 0 | 0 | 0 | 0 |
| Friuli | 2 | 61 | 0 | 4 | 30,455 | 0 |
| Lombardy | 0 | 0 | 0 | 0 | 0 | 0 |
| Sardinia | 4 | 1,076 | 0 | 2 | 30,476 | 0 |
| Veneto | 8 | 72,481 | 4 | 21 | 54,282 | 14 |
|
| ||||||
| Total | 14 | 119,345 | 4 | 28 | 116,255 | 14 |
Table V.
Safety measures for WNV infection adopted by the Italian National Blood Centre between 2008–2012.
| WNV safety measures | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|
| WNV NAT screening of donors (only in affected areas) | Yes | Yes | Yes | Yes | Yes |
| 28 days deferral period of donors who spent at least one night in affected areas | Yes | Yes | Yes | Yes | Yes |
| Enhancement of pre- and post-donation information | Yes | Yes | Yes | ||
| Stocks of plasma/serum samples to be retrospectively tested | Yes | Yes | |||
| Post-transfusion haemovigilance | Yes | Yes | |||
| Period of adoption | 10/10/2008 | 01/08/2009 | 15/07/2010 | 15/07/2011 | 15/07/2012 |
| 01/12/2008 | 31/10/2009 | 15/11/2010 | 30/11/2011 | 30/11/2012 |
Nucleic acid amplification technique assays for screening blood for West Nile virus
Two NAT assays are currently available on the market for screening blood for both WNV L1 and L2: the Roche cobas TaqScreen WNV Test platform (Roche Molecular Systems, Branchburg, USA) on a the cobas S 201 platform (Roche Instrument Centre, Rotkreuz, Switzerland) and the Procleix WNV Assay on the Procleix Tigris System (Gen-Probe and Novartis Diagnostics)75,76. Both NAT assays received FDA licensure for screening MP (6 and 16 donations, respectively) or in individual-donation (ID) format as well as for screening of organ and tissue donors (in the ID format).
With respect to WNV L1, both assays were validated for analytical sensitivity by the respective test kits’ manufacturers using dilution series of a WNV L1 secondary standard calibrated against the Health Canada reference preparation (HC-SC WNV Nat Ref 001/03)77. The analytical sensitivity, expressed as 95% limit of detection (LOD), proved to be about 40 copies/mL for cobas TaqScreen WNV and about 10 copies/mL for Procleix WNV (as reported in the respective packaging inserts).
Regarding WNV L2, both test kit manufacturers used the same WNV RNA Qualification Panel QWN701 (BBI Diagnostics) to validate the analytical sensitivity. A different 95% LOD was estimated: about 5 copies/mL for cobas TaqScreen WNV and about 20 copies/mL for Procleix WNV75,76.
A broad cross-reactivity to other flaviviruses such as JEV, MVEV, KUNV and SLEV was demonstrated for the cobas TaqScreen WNV72. With respect to Procleix WNV, false-positive results only to KUNV and not to other flaviviruses from the JEV serogroup were described76. However, Sambri et al. reported Procleix WNV to be cross-reactive to USUV in plasma samples while no false-positive results were observed testing the same plasma samples with cobas TaqScreen WNV78. Using plasma samples spiked with serial dilutions of USUV, it was possible to estimate that Procleix WNV detects USUV in plasma when the viral concentration is at least 1×106 TCID50. In a recent interlaboratory comparison, Pisani et al.79 showed that actually both NAT test kits can detect USUV (about 1,000,000 copies/mL) in a WNV-negative sample. Further investigation by the same authors confirmed that both NAT test kits can detect the USUV genome, though with a different sensitivity: up to the 1:32 dilution (approximately equal to 32,000 copies/mL) for Procleix WNV, between 1:16 and 1:32 (approximately between 62,500 and 32,000 copies/mL) for cobas TaqScreen WNV (G. Pisani, personal communication).
External quality assessment for laboratories using nucleic acid amplification technology to screen blood for West Nile virus
Participation in external quality assessment programmes (EQAP) is recognised as an important factor for quality assurance, as laid down in Directive 2005/62/EC80. Testing laboratories should, therefore, verify the quality of their techniques through ongoing participation in EQAP. Based on this consideration, since 2010 the NBC and the National Centre for Immunobiologicals Research and Evaluation (CRIVIB) of the Italian National Institute of Health, have organised an annual EQAP for Italian blood transfusion centres performing WNV NAT testing of blood donations79,81.
In the first NAT EQAP for WNV carried out in 201081, only WNV L1 was used to prepare the positive samples of the panels, while in the 2011 and 2012 EQAP reference materials representing both WNV L1 and L2 were used. The inclusion of L2 in the second EQAP79 proved to be an appropriate choice as there is now evidence that this lineage circulates also in Europe. In both the 2010 and 2011 EQAP, the 360 and 100 copies/mL-samples of WNV L1 were correctly detected as positive in 100% of the cases by both NAT assays. With respect to the samples containing less than 100 copies/mL, the results were as expected, i.e. in line with the 95% LOD stated by the test kits’ manufacturers for L1 (about 10 copies/mL for Procleix WNV and about 40 copies/mL for cobas TaqScreen WNV). Regarding WNV L2, the 16 copies/mL-samples were correctly detected as positive in 100% of the cases by both NAT assays and also in this case these results appear to fit with the respective 95% LOD of the test kits (approximately 20 copies/mL for Procleix WNV and about 5 copies/mL for cobas TaqScreen WNV). However, the cobas TaqScreen WNV appeared to be slightly more sensitive than the Procleix WNV toward this lineage, thus confirming the above-mentioned 95% LOD values of the two test kits. Finally, these findings with respect to both WNV lineages were confirmed in the 2012 EQAP, in which 100 copies/mL samples of WNV L1 and 100/50 copies/mL samples of WNV L2 were tested.
Overall, the 2010–2012 EQAP achieved their predefined objective, i.e. to provide participants with a valid tool to assess the quality of their analytical performance and the competence of their operators.
Individual-donation or minipool nucleic acid amplification technology?
There is no doubt that the introduction of WNV NAT testing of blood donations represents the most effective strategy to prevent WNV transmission via blood transfusion. However, there is still no consolidated opinion on whether WNV NAT should be applied to ID (ID-NAT) or MP of donations (MP-NAT).
WNV NAT screening was first implemented in the USA and Canada during the 2003 WNV epidemic season on either MP of six donations (TaqScreen WNV test) or MP of 16 donations (Procleix WNV assay) thus allowing the identification of 866 WNV NAT-positive donations in the USA and of 14 in Canada, with respective rates of 0.015–0.017% and 0.011%82,83. However, while no cases of transfusion-transmitted WNV infections were reported in Canada in 2003, six cases were reported in the USA that were associated with very low levels of RNA that had apparently escaped detection by MP-NAT. Retrospective studies using ID-NAT to test MP-NAT non-reactive specimens collected during that season identified additional reactive donations. These data prepared the ground for the introduction of a triggering strategy to switch from MP-NAT to ID-NAT during the 2004 WNV epidemic season both in the USA and Canada. In the USA, different switching thresholds were adopted by blood centres, all based on the number (1, 2 or 4) of MP-NAT presumed viraemic donations and/or a detection frequency of >1:1,000 rate in the geographic area of collection over a 7-day rolling period84,85. The de-triggering criterion, with a switch back to MP-NAT, was that no additional ID-NAT positive donations had to be detected over the course of 7 days of ID-NAT. Between 2004 and 2006 there were three additional transmissions of WNV by transfusion due to MP-NAT negative donations subsequently found to be positive by ID-NAT86,87. In 2007, two cases of probable transfusion-transmitted WNV from the same blood donor, whose donation was MP-NAT negative, were reported88. These cases could not be definitively proved as transfusion-transmitted WNV infections as blood samples or other components from the implicated donation were unavailable for testing. In 2008, the FDA proposed in a draft guideline, as a non-binding recommendation, to convert from MP-NAT to ID-NAT based on one MP-NAT presumed viraemic donor with no further criteria as triggering strategy while confirming the above-mentioned de-triggering approach89. Although not all blood centres adopted this recommendation, no new cases of WNV transfusion-transmitted cases have been reported since then.
In Canada, where MP-NAT of six donations was in use, a trigger for conversion from MP-NAT to ID-NAT was identified in an MP-NAT positive blood donor. ID-NAT could also be initiated by community cases (the community trigger) if the incidence was one case per 1,000 in rural areas or one case per 2,500 people in urban areas. Once WNV activity had decreased below the population trigger, or no additional positive donors were identified in the next 7 days for that area, testing could be switched back to MP-NAT90. As this strategy proved to be successful, it was re-adopted over the years with only minor modifications and it is still in use at present.
In Europe, WNV NAT screening for blood donations was introduced in Italy in 2008 and in Greece in 2010. In Italy this preventive measure was meant to be added to the routine NAT testing already in place for human immunodeficiency virus and hepatitis B and C viruses at blood centres (either on ID-NAT or on MP-NAT of six donations). Therefore, no triggering strategy was necessary for blood centres using ID-NAT. With respect to the blood centres using MP-NAT, the NBC recommended, since 2010, switching to ID-NAT when the area of collection falls into the definition of an “affected area”. No de-triggering strategy is currently in place, meaning that ID-NAT is continued until the end of the epidemic season.
WNV viral loads range from 50 to 690,000 copies/mL with a median of 3,500 copies/mL. Because of the lower analytic sensitivity of MP-NAT versus ID-NAT, approximately 30% of viraemic units can be identified only by ID-NAT due to the low viral load91. Most viraemic donations only detectable by ID-NAT have WNV-specific IgM antibodies92. These donations do not appear to be linked to WNV transfusion transmission cases unlike viraemic units negative for IgM antibodies86,93. Nevertheless, a residual transmission risk exists from newly infected donors who have not yet developed sufficient viraemia for their donations to be detected as positive by MP-NAT (like the 10 above-mentioned “breakthrough” transmissions reported from 2003 to 2007 in the USA). Although zero risk cannot be guaranteed in a transfusion setting, it appears that ID-NAT could represent the “gold-standard” approach to at least reduce residual risk. However, with the currently commercially available test kits, this has to be balanced against the burden that the introduction of ID-NAT would add, not just in financial terms but especially from a logistic point of view. When ID-NAT is not feasible, MP-NAT could be a highly effectively substitute for it if coupled with an appropriate strategy for triggering ID-NAT testing. This is supported not only by the experience gathered in the western hemisphere but also by methodological studies in which the effectiveness and efficiency of different triggering strategies were evaluated using simulating models84,85.
Conclusions
The importance of WNV as an emerging zoonotic pathogen is reflected by the increasing number of outbreaks reported worldwide every year. In Europe, particularly in the Mediterranean Basin, the mechanisms of WNV re-introduction and cycle of maintenance in infected areas remain to be elucidated, including the competence of potential mosquito vectors, the persistence of the virus in susceptible hosts and the genetic susceptibility of hosts to WNV infection. A valuable contribution to these unresolved issues is certainly provided by an integrated approach that includes veterinary and entomological surveillance in areas with favourable ecological conditions for the WNV cycle as well as by a renewed awareness among clinicians and veterinarians regarding the possibility of WNV causing cases of encephalitis and meningoencephalitis during periods of potential transmission.
With respect to blood donations, the WNV preventive measures adopted so far have certainly contributed to improving the safety of donations with regards to this virus. In the USA and Canada, 3342 and 240 presumptive NAT-positive donations were detected between 2003 and 2012, respectively (for reference, see notes 2 and 3 in Table I). Even if a risk of WNV transmission by transfusion remains, due to low viral load and the screening strategy adopted (MP versus ID), this risk can be considered very low as only a few documented cases of WNV infection have been reported in the USA after the introduction of NAT screening31–33. In Italy, the seasonal preventive measures for WNV implemented between 2008 and 2012 in affected areas appear to have effectively improved the safety of the blood supply as no cases of WNV transfusion transmission have been reported so far, either by the National Haemovigilance System or by the National Centre for Epidemiology, Surveillance and Health Promotion. We estimate that the implementation of blood screening for WNV by NAT in this 4-year period, in terms of assay kits and reagents, resulted in an investment of 4,551,000 Euros. However, it should be noted that in this period, out of a total of 413,710 blood donations tested for WNV, 26 viraemic donations were detected. Considering that most positive donations were whole blood donations and that each of these is systematically fractionated into three different blood components (red cells, buffy coat/platelets and plasma), a considerable number of blood recipients were spared exposure to these potentially infectious units. As a high proportion of blood recipients are immunocompromised and many of them are subjected to chronic or intensive transfusion treatments, they are at higher risk of contracting symptomatic WNV infection. Furthermore, once they become infected, they are more prone to develop WNND, thus becoming a heavy burden on the public health system. It should also be noted that the implementation of blood screening for WNV by NAT was based on an estimated risk of having up to one positive donation out of 10,000 donations in the peak period of WNV circulation in specific geographical areas. This estimated risk was at least about 10 times higher than the “as low as reasonably acceptable” (ALARA) risk estimated for hepatitis B virus in Italy. Considering that blood is routinely screened in Italy by NAT for hepatitis B virus (in addition to human immunodeficiency virus and hepatitis C virus), this WNV risk could not be overlooked and the appropriateness of this approach was demonstrated by the fact that at the end of the 4-year period the rate of detection of WNV-positive donations was 1 out of 15,912 donations.
Finally in terms of cost-effectiveness, a 2005 study by Custer et al.94 showed, by constructing a Markov model simulating patients receiving blood transfusions under seven different blood screening strategies, that the most cost-effective strategy is annual, national MP NAT. Conversely, in 2006 Korves et al. found, by analysing nine different blood screening strategies with the same Markov model, that universal screening for WNV is not the most appropriate strategy, even in high-infection-short duration transmission areas95,96. The most cost-effective strategy in high WNV prevalence areas is seasonal, targeted screening of donations designated for immunocompromised individuals by ID NAT. The authors suggest that this strategy should be taken into consideration by policy-makers as it appears to provide the right balance between financial resources and protection of public health. This would make more resources available to be invested in additional interventions focusing on vector mosquitoes, thus creating a virtuous circle in which affected areas are progressively reduced and the risk of WNV transmission decreased to a negligible level.
Acknowledgements
We are grateful to the Heads of the Regional Blood Centres of Basilicata, Emilia-Romagna, Friuli-Venezia-Giulia, Lombardy, Sardinia and Veneto regions for their helpful collaboration in providing data and advice.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Mackenzie JS, Barrett ADT, Deubel V. The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. In: Mackenzie JS, Barrett ADT, Deubel V, editors. Japanese Encephalitis and West Nile Viruses. New York: Springer-Verlag; 2002. pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 2.Bondre VP, Jadi RS, Mishra AC, et al. West Nile virus isolates from India: evidence for a distinct genetic lineage. J Gen Virol. 2007;88:875–84. doi: 10.1099/vir.0.82403-0. [DOI] [PubMed] [Google Scholar]
- 3.Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–46. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 5.Bakonyi T, Ivanics E, Erdelyi K, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12:618–23. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papa A, Bakonyi T, Xanthopoulou K, et al. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg Infect Dis. 2011;17:920–2. doi: 10.3201/eid1705.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnarelli P, Marinelli K, Trotta D, et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy. Euro Surveill. 2011;27 pii:20002. [PubMed] [Google Scholar]
- 8.Brault AC. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet Res. 2009;40:40–3. doi: 10.1051/vetres/2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulasekera VL, Kramer L, Nasci RS, et al. West Nile virus infection in mosquitoes, birds, horses, and humans, Staten Island, New York, 2000. Emerg Infect Dis. 2001;7:722–5. doi: 10.3201/eid0704.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pealer LN, Marfin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–45. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 12.Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis and prevention. Mayo Clin Proc. 2003;78:1137–44. doi: 10.4065/78.9.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617–24. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- 14.Loeb M, Hanna S, Nicolle L, et al. Prognosis after West Nile virus infection. Ann Intern Med. 2008;149:232–41. doi: 10.7326/0003-4819-149-4-200808190-00004. [DOI] [PubMed] [Google Scholar]
- 15.Vázquez A, Jiménez-Clavero MA, Franco L, et al. Usutu virus - potential risk of human disease in Europe. Euro Surveill. 2011;16 pii:19935. [PubMed] [Google Scholar]
- 16.Niedrig M, Sonnenberg K, Steinhagen K, et al. Comparison of ELISA and immunoassays for measurement of IgG and IgM antibody to West Nile virus in human sera against virus neutralization. J Virol Methods. 2007;139:103–5. doi: 10.1016/j.jviromet.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Prince HE, Tobler LH, Yeh C, et al. Persistence of West Nile virus-specific antibodies in viremic blood donors. Clin Vaccine Immunol. 2007;14:1228–30. doi: 10.1128/CVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seino KK, Long MT, Gibbs ET, et al. Comparative efficacies of three commercially available vaccines against West Nile Virus (WNV) in a short-duration challenge trial involving an equine WNV encephalitis model. Clin Vaccine Immunol. 2007;14:1465–71. doi: 10.1128/CVI.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson J, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107–8. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond MS. Progress on the development of therapeutics against West Nile virus. Antiviral Res. 2009;83:214–27. doi: 10.1016/j.antiviral.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Nathan D, Gershoni-Yahalom O, Samina I, et al. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. BMC Infect Dis. 2009;17:9–18. doi: 10.1186/1471-2334-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smithburn KC, Hughes TP, Burke AW, et al. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;20:471–92. [Google Scholar]
- 23.Murgue B, Zeller H, Duebel V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. In: Mackenzie JS, Barrett AD, Deubel V, editors. Japanese Encephalitis and West Nile Viruses. New York: Springer-Verlag; 2002. pp. 195–220. [DOI] [PubMed] [Google Scholar]
- 24.Blitvich BI. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- 25.Calistri P, Giovannini A, Hubalek Z, et al. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol J. 2010;4:29–37. doi: 10.2174/1874357901004010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–7. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Guidelines for surveillance, prevention, and control of West Nile virus infection - United States. MMWR Morb Mortal Wkly Rep. 2000;49:25–8. [PubMed] [Google Scholar]
- 28.Drebot MA, Lindsay R, Barker IK, et al. West Nile virus surveillance and diagnostics: a Canadian perspective. Can J Infect Dis. 2003;14:105–14. doi: 10.1155/2003/575341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion. 2002;42:1019–26. doi: 10.1046/j.1537-2995.2002.00167.x. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) West Nile virus screening of blood donations and transfusion-associated transmission United States, 2003. Update. MMWR Morb Mortal Wkly Rep. 2004;53:281–4. [PubMed] [Google Scholar]
- 31.Busch MP, Wright DJ, Custer B, et al. West Nile virus infections projected from blood donor screening data, United States, 2003. Emerg Infect Dis. 2006;12:395–402. doi: 10.3201/eid1203.051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Oliveira AM, Beecham BD, Montgomery SP, et al. West Nile virus blood transfusion-related infection despite nucleic acid testing. Transfusion. 2004;44:1695–9. doi: 10.1111/j.0041-1132.2004.04130.x. [DOI] [PubMed] [Google Scholar]
- 33.Busch MP, Tobler LH, Saldanha J, et al. Analytical and clinical sensitivity of West Nile virus RNA screening and supplemental assays available in 2003. Transfusion. 2005;45:492–9. doi: 10.1111/j.0041-1132.2005.04382.x. [DOI] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. Guidance for Industry: assessing donor suitability and blood and blood product safety in cases of known or suspected West Nile virus infection. Rockville, MD: Center for Biologics Evaluation and Research; 2005. [Accessed on 17/09/2013]. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm074111.htm. [Google Scholar]
- 35.Guidance for industry: revised recommendations for the assessment of donor suitability and blood and blood product safety in cases of known or suspected West Nile Virus infection. Rockville (MD): Center for Biologics Evaluation and Research; 2003. [Accessed on 17/09/2013]. [Cited May 6, 2003]. Available at: http://www.bpro.or.jp/publication/pdf_jptrans/us/us200305en.pdf. [Google Scholar]
- 36.Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46:272–7. doi: 10.1111/j.1537-2995.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 37.Custer B, Kamel H, Kiely NE, et al. Associations between West Nile virus infection and symptoms reported by blood donors identified through nucleic acid test screening. Transfusion. 2009;49:278–88. doi: 10.1111/j.1537-2995.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsai TF, Popovici F, Cernercu C, et al. West Nile encephalitis epidemic in south-eastern Romania. Lancet. 1998;352:767–71. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 39.European Centre for Disease Prevention and Control. Update 19 September 2011. Stockholm: 2011. Review of the epidemiological situation of West Nile virus infection in the European Union. [Google Scholar]
- 40.Berger SA. West Nile fever: global status. Gideon e-books; 2012. [Google Scholar]
- 41.Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components.
- 42.Commission Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003. Setting Standards of Quality and Safety for the Collection, Testing, Processing, Storage and Distribution of Human Blood and Blood Components and Amending Directive 2001/83/EC.
- 43.Commission Decision 2007/875/EC amending Decision No 2119/98/EC of the European Parliament and of the Council and Decision 2000/96/EC as regards communicable diseases listed in those decisions.
- 44.Commission Decision 2008/426/EC amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision N° 2119/98/EC of the European Parliament and of the Council.
- 45.Danis K. Outbreak of West Nile Virus infection in Greece, 2010. Expert Consultation on West Nile Virus infection; Europe Thessaloniki. 25-26-13 January 2011; Hellenic Centre of Disease Control and Prevention; [Accessed on 17/09/2013]. [cited 2011 Mar]. Available at: http://www.ecodev.gr/userfiles/file/pdf/Danis_WNV_Greece2011.pdf. [Google Scholar]
- 46.ECDC Threat Assessment. Outbreak of West Nile virus infection in Greece, July–August 2010. [Accessed on 17/09/2013]. Available at: http://www.ecdc.europa.eu/en/healthtopics/Documents/1009_Threat%20assessment_West_Nile_Virus.pdf.
- 47.Krisztalovics K, Ferenczi E, Molnár Z, et al. West Nile virus infections in Hungary, August–September 2008. Euro Surveill. 2008;13 pii:19030. [PubMed] [Google Scholar]
- 48.West Nile virus Eurasia (16): European Union. Archive Number: 20111128.3477. [Accessed on 17/09/2013]. Published Date: 28/11/2011. Available at: http://www.promedmail.org/direct.php?id=20111128.3477.
- 49.Kopel E, Amitai Z, Bin H, et al. Surveillance of West Nile Virus Disease, Tel Aviv District, Israel, 2005 to 2010. Euro Surveill. 2011;16 pii:19894. [PubMed] [Google Scholar]
- 50.West Nile virus Eurasia update: Russia, Greece. Archive Number: 20100902.3141. [Accessed on 17/09/2013]. Published Date: 02/09/2010. Available at: http://www.promedmail.org/direct.php?id=20100902.3141.
- 51.West Nile virus Eurasia (14): Russia (Volgograd) Archive Number: 20111122.3422. [Accessed on 17/09/2013]. Published Date: 22/11/2011. Available at: http://www.promedmail.org/direct.php?id=20111122.3422.
- 52.García-Bocanegra I, Jaén-Téllez JA, Napp S, et al. West Nile Fever outbreak in horses and humans, Spain, 2010. Emerg Infect Dis. 2011;17:2397–9. doi: 10.3201/eid1712.110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ergünay K, Özkul A. Confirmation of West Nile virus seroreactivity in central nervous system infections of unknown etiology from Ankara Province, Central Anatolia, Turkey. Mikrobiyol Bul. 2011;45:381–3. [PubMed] [Google Scholar]
- 54.Tapisiz A, Emiralioğlu N, Vural O, et al. The first report of West Nile virus infection in a child from Turkey. Turk J Pediatr. 2011;53:317–9. [PubMed] [Google Scholar]
- 55.West Nile virus North Africa (05): Tunisia (BZ) Archive Number: 20121126.14255190. [Accessed on 17/09/2013]. Published Date: 26/11/2012. Available at: http://www.promedmail.org/direct.php?id=20121126.14255190.
- 56.West Nile virus Eurasia (10): Croatia. Archive Number: 20120920.1303163. [Accessed on 17/09/2013]. Published Date: 20/09/2012. Available at: http://www.promedmail.org/direct.php?id=20120920.1303163.
- 57.West Nile virus Eurasia (12): Balkans. Archive Number: 20120921.1304610. [Accessed on 17/09/2013]. Published Date: 21/09/2012. Available at: http://www.promedmail.org/direct.php?id=20120921.1304610.
- 58.Angelini P, Tamba M, Finarelli AC, et al. West Nile virus circulation in Emilia-Romagna, Italy: the integrated surveillance system 2009. Euro Surveill. 2010;15 pii:19547. [PubMed] [Google Scholar]
- 59.Dohm DJ, Sardelis MR, Turell MJ. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera:Culicidae) J Med Entomol. 2002;39:640–4. doi: 10.1603/0022-2585-39.4.640. [DOI] [PubMed] [Google Scholar]
- 60.Papa A, Politis C, Tsoukaia A, et al. West Nile Virus Lineage 2 from blood donor, Greece. Emerg Infec Dis. 2012;18:688–9. doi: 10.3201/eid1804.110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danis K, Papa A, Papanikolaou E, et al. Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Eurosurveill. 16 2011. pii: 19951. [PubMed] [Google Scholar]
- 62.Autorino GL, Battisti A, Deubel V, et al. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 2002;8:1372–8. doi: 10.3201/eid0812.020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calistri P, Bruno R, Lelli R. West Nile disease in Italy. Arbo-Zoonet News. 2009;3:12–9. [Google Scholar]
- 64.Rossini G, Cavrini F, Pierro A, et al. First human case of West Nile virus neuroinvasive infection in Italy, September 2008 – case report. Euro Surveill. 2008;13 doi: 10.2807/ese.13.41.19002-en. pii:19002. [DOI] [PubMed] [Google Scholar]
- 65.Barzon L, Squarzon L, Cattai M, et al. West Nile virus infection in Veneto region, Italy, 2008–2009. Euro Surveill. 2009;14 doi: 10.2807/ese.14.31.19289-en. pii:19289. [DOI] [PubMed] [Google Scholar]
- 66.Grazzini G, Liumbruno GM, Pupella S, et al. West Nile virus in Italy: a further threat to blood safety, a further challenge to the blood system. Blood Transfus. 2008;6:235–7. doi: 10.2450/2008.0065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ministère du travail, des relations sociales, de la famille et de la solidarité, Ministère de la santé, de la jeunesse, des sports et de la vie associative. Guide de procédures de lutte contre la circulation du virus West Nile en France métropolitaine. [Accessed on 17/09/2013]. Available at: http://www.sante.gouv.fr/fichiers/bo/2005/05-08/a0080028a.pdf.
- 68.Rizzo C, Salcuni P, Nicoletti L, et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012;17 pii:20172. [PubMed] [Google Scholar]
- 69.Sorveglianza della malattia di West Nile in Italia, 2010. Ministero della Salute Italiana, Roma, 21 luglio 2010, Prot. n. 0033197-P-21/07/2010.
- 70.Barzon L, Pacenti M, Cusinato R, et al. Human cases of West Nile Virus Infection in north-eastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011;16 pii:19949. [PubMed] [Google Scholar]
- 71.Nanni Costa A, Capobianchi MR, Ippolito G, et al. West Nile virus: the Italian national transplant network reaction to an alert in the north-eastern region, Italy 2011. Euro Surveill. 2011;16 pii:19991. [PubMed] [Google Scholar]
- 72.West Nile Virus And Blood Safety: Introduction to a Preparedness Plan in Europe. Final Working Document 2012 v.2.1. [Accessed on 17/09/2013]. Prepared by: Greece, Italy, Romania and France. Available at: http://ec.europa.eu/health/blood_tissues_organs/docs/wnv_preparedness_plan_2012.pdf.
- 73.Barzon L, Pacenti M, Franchin E, et al. New endemic West Nile virus lineage 1a in northern Italy, July 2012. Euro Surveill. 2012;17 doi: 10.2807/ese.17.31.20231-en. pii:20231. [DOI] [PubMed] [Google Scholar]
- 74.Barzon L, Pacenti M, Franchin E, et al. Clinical and virological findings in the ongoing outbreak of West Nile virus Livenza strain in northern Italy, July to September 2012. Euro Surveill. 2012;17 pii:20260. [PubMed] [Google Scholar]
- 75.Pai A, Kleinman S, Malhotra K, et al. Performance characteristics of the Food and Drug Administration licensed Roche Cobas TaqScreen West Nile virus assay. Transfusion. 2008;48:2184–89. doi: 10.1111/j.1537-2995.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 76.Linnen JM, Deras ML, Cline J, et al. Performance Evaluation of the Procleix West Nile Virus Assay on Semi-Automated and Automated Systems. J Med Virol. 2007;79:1422–30. doi: 10.1002/jmv.20930. [DOI] [PubMed] [Google Scholar]
- 77.Saldanha J, Shead S, Heath A, et al. Collaborative study to evaluate a working reagent for West Nile virus RNA detection by nucleic acid testing. Transfusion. 2005;45:97–102. doi: 10.1111/j.1537-2995.2005.04151.x. [DOI] [PubMed] [Google Scholar]
- 78.Gaibani P, Pierro AM, Cavrini F, et al. False-positive transcription-mediated amplification assay detection of West Nile virus in blood from a patient with viremia caused by an USUV infection. J Clin Microbiol. 2010;48:3338–9. doi: 10.1128/JCM.02501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pisani G, Pupella S, Cristiano K, et al. Detection of West Nile virus RNA (lineages 1 and 2) in an External Quality Assessment Programme for laboratories screening blood and blood components for WNV by NAT. Blood Transfus. 2012;10:515–20. doi: 10.2450/2012.0036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.European Directive 2005/62/EC “implementing Directive 2002/98/EC of the European Parliament and of the Council as regards Community standards and specifications relating to a quality system for blood establishments”. Official Journal of the European Union L. 2005 Sep 30;256:41–8. [Google Scholar]
- 81.Pisani G, Pupella S, Marino F, et al. Interlaboratory study to evaluate the performance of laboratories involved in West Nile virus RNA screening of blood and blood components by nucleic acid amplification testing in Italy. Blood Transfus. 2011;9:425–9. doi: 10.2450/2011.0025-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fearon M. West Nile story: the transfusion medicine chapter. Future Virol. 2011;6:1423–34. [Google Scholar]
- 83.Cameron C, Reeves J, Antonishyn N, et al. West Nile virus in Canadian blood donors. Transfusion. 2005;45:487–91. doi: 10.1111/j.0041-1132.2005.04384.x. [DOI] [PubMed] [Google Scholar]
- 84.Custer B, Tomasula PA, Murphy EL, et al. Triggers for switching from minipool testing by nucleic acid technology to individual donation nucleic acid testing for West Nile Virus: analysis of 2003 data to inform 2004 decision making. Transfusion. 2004;44:1547–54. doi: 10.1111/j.0041-1132.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- 85.Biggerstaff BJ, Petersen LR, et al. A modeling framework for evaluation and comparison of trigger strategies for switching from minipool to individual-donation testing for West Nile virus. Transfusion. 2009;49:1151–9. doi: 10.1111/j.1537-2995.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 86.Macedo de Oliveira A, Beecham BD, Montgomery SP, et al. West Nile virus blood transfusion-related infection despite nucleic acid testing. Transfusion. 2004;44:1695–9. doi: 10.1111/j.0041-1132.2004.04130.x. [DOI] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention (CDC) West Nile virus transmission through blood transfusion – South Dakota, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:76–9. [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention (CDC) West Nile virus transmission through blood transfusion-South Dakota, 2006. MMWR Morb Mortal Wkly Rep Editorial Note. 2007;56:76–9. [PubMed] [Google Scholar]
- 89.Food and Drug Administration. Guidance for industry. Use of nucleic acid tests to reduce the risk of transmission of West Nile virus from donors of whole blood and blood components intended for transfusion and donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps). Draft guidance. Rockville (MD): Food and Drug Administration; 2008. [Google Scholar]
- 90.O’Brien SF, Scalia V, Zuber E, et al. West Nile Virus in 2006 and 2007: the Canadian Blood Services’ experience. Transfusion. 2010;50:1118–25. doi: 10.1111/j.1537-2995.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 91.Busch MP, Caglioti S, Robertson EF, et al. Screening the blood supply for West Nile Virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353:460–7. doi: 10.1056/NEJMoa044029. [DOI] [PubMed] [Google Scholar]
- 92.Stramer SL, Fang CT, Foster GA, et al. West Nile Virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–9. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention (CDC) Transfusion-associated transmission of West Nile Virus - Arizona, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:842–4. [PubMed] [Google Scholar]
- 94.Custer B, Busch MP, Marfin AA, et al. The cost-effectiveness of screening the U.S. blood supply for West Nile virus. Ann Intern Med. 2005;143:486–92. doi: 10.7326/0003-4819-143-7-200510040-00007. [DOI] [PubMed] [Google Scholar]
- 95.Korves CT, Goldie SJ, Murray MB. Cost-effectiveness of alternative blood-screening strategies for West Nile virus in the United States. PLoS Medicine. 2006;3:211–21. doi: 10.1371/journal.pmed.0030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korves CT, Goldie SJ, Murray MB. Blood screening for West Nile virus: the cost-effectiveness of a real-time, trigger-based strategy. Clin Infect Dis. 2006;43:490–3. doi: 10.1086/506570. [DOI] [PubMed] [Google Scholar]