Introduction
Cobalamin metabolism is complex and involves a series of processes any of which, if absent, may lead to cobalamin deficiency. The main causes of cobalamin deficiency are food-cobalamin malabsorption (53%), pernicious anaemia (33%), insufficient nutritional vitamin B12 intake (2%) and post-surgical malabsorption (1%)1,2. Dietary causes of the deficiency are limited to elderly people who are already malnourished or to strict vegetarians while malabsorption occurs in patients suffering from several gastrointestinal conditions3.
Vitamin B12 is an important co-enzyme required for tetra-hydrofolate production which itself is necessary for normal DNA synthesis. B12 deficiency, therefore, results in impaired DNA formation. The consequent slowing down of cell division leads to the formation of megaloblastic cells especially of those cells with a rapid turnover such as haematopoietic cells and intestinal epithelial cells1.
Although measurement of vitamin B12 levels is the gold standard for the diagnosis of B12 deficiency some reports do exist concerning difficulties in its assay4–8. During the 1970s and 1980s vitamin B12 was measured using a 57Co-based radioisotope; since the 1990s, however, with the introduction of automated analysers, most methods are based on solid-phase competitive chemiluminescence enzyme immunoassays9,10. The principal problems with these more recent assays are caused by the presence of intrinsic factor antibodies and heterophilic antibodies in the test sample. Obviously, this is a significant limitation to B12 assays in pernicious anaemia and raises the need to find more sensitive and specific tests to confirm vitamin B12 deficiency11,12. Despite these specificity restrictions and controversy about sensitivity, measurement of plasma cobalamin is still the gold standard for diagnosing vitamin B12 deficiency and its determination can provide intriguing, but misleading information in clinical practice13.
Case report
A 59-year old woman with severe anaemia (haemoglobin 58 g/L) who had been suffering from progressively increasing palpitations, profound fatigue and exertional dyspnoea for about one month was admitted to our hospital in May 2011. She had a good, well-balanced diet and was not taking any medication apart from thyroxine.
On clinical examination the only significant findings were mild glossitis and pallor. The woman’s full blood count showed macrocytic anaemia (haemoglobin 58 g/L, mean corpuscular volume 106.0 fL), thrombocytopenia (42×109/L), and low counts of white blood cells (2.280×109/L), neutrophils (0.930×109/L) and reticulocytes (18×109/L). The peripheral blood smear showed severe anisopoikilocytosis, large polychromatophilic erythrocytes and hypersegmented neutrophils.
Vitamin B12 and folate deficiency were considered immediately, but on analysis their values were in the normal range (NR), >1,000 pg/mL (NR 193–982 pg/mL) and 4.5 ng/mL (NR 3.0–17,0 ng/mL), respectively, as was iron concentration (ferritin 70 ng/mL, transferrin saturation 30%), while the concentration of lactate dehydrogenase was very high (15,000 U/L, NR 250–400 U/L) and bilirubin was elevated 32.49 μmol/L (NR 5.1–17.0 μmol/L).
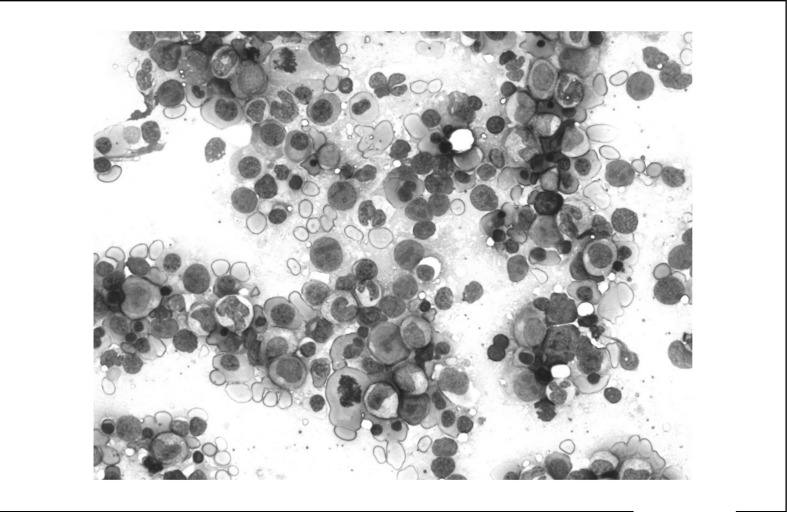
The bone marrow (Figure 1) showed megaloblastic erythroblasts, megaloblastic metamyelocytes with large bone-shaped nuclei and neutrophils with hypersegmentation. Myelodyspastic syndrome/acute leukaemia was excluded on the basis of this morphological picture as well as the patient’s normal karyotype.
Figure 1.
Bone marrow is represented almost completely by a picture of dysplastic erythropoiesis with several megaloblastic erythroblasts typical of vitamin B12 deficiency.
Repeated analyses showed the vitamin B12 concentration close to the upper limit at 910 pg/mL and folate at 10.3 ng/mL; intrinsic factor and parietal cell antibodies were strongly positive. In the interim the patient received two red cell units and was discharged from hospital but, 2 weeks later, was admitted again with anaemia (Hb 67 g/L) and the same symptoms as previously.
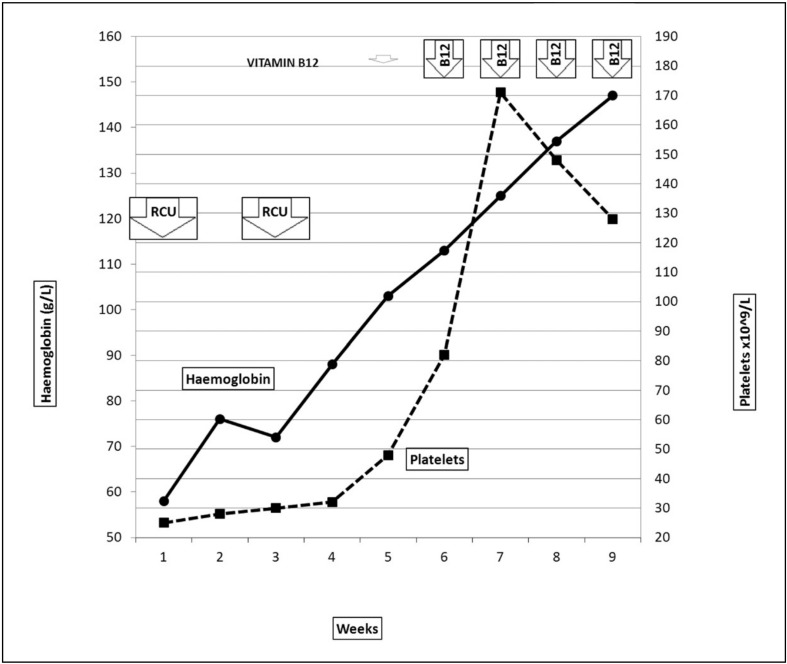
A diagnosis of vitamin B12 deficiency was still suspected despite the initial normal B12 values and with four repeated measurements being 331, 357, 380, and 297 pg/mL it was decided to treat this patient with a pharmacological dose of subcutaneous vitamin B12 (1,000 mg/day for 1 week followed by once a week 4 times). After 2 weeks the patient’s condition improved and complete clinical and haematological recovery occurred within the next month (Figure 2).
Figure 2.
Time-dependent curves of haemoglobin concentration and platelet counts.
B12 vitamin was administered daily from days 1 to 7 and once a week for the following 21 days; complete recovery was achieved within 1 month of starting treatment with B12. RCU = two red cell units.
Discussion
Over the last 10 years several definitions of cobalamin deficiency have been published, depending mainly on population studies and the assay used1–3. The availability of vitamin B12 at the cellular level depends on its absorption from the ileum and its transport in blood to the liver and bone marrow via a carrier protein (transcobalamin II). In the circulation vitamin B12 is bound to two proteins, transcobalamin and haptocorrin.
Until the 1990s serum vitamin B12 levels were measured using radioisotope assays, which were replaced more recently by competitive-binding luminescence assays. Few studies have compared the different methods4,7,12.
Salomon reported normal plasma cobalamin levels in patients with clinical signs of vitamin B12 deficiency who later improved after treatment with the vitamin2. Others have documented assays being repeated using kits from different manufacturers the results of which substantiated the diagnosis of vitamin B12 deficiency making evident the false normal results originally obtained4–8.
The assay carried out in our laboratory (Siemens Centaur, Beckman Access, Siemens Immulite 2000/2500, San Diego, CA, USA) was equally open to error and it is possible that a false normal vitamin B12 level may have been caused by interference from a high-titre of intrinsic factor antibody4. However, abnormal haemoglobin and erythrocyte mean cell volume values support suspected pernicious anaemia, but normal levels do not rule out the presence of vitamin B12 deficiency, leading to a high risk of clinical error.
In conclusion, our experience further supports the fact that when the diagnosis of B12 deficiency is suspected on the basis of clinical findings and additional tests, supplementation treatment should be administered even if the assayed level of the vitamin is not low and, if possible, an assay from another manufacturer is recommended.
Acknowledgements
This work was supported in part by the Associazione Italiana Leucemie Treviso (AIL Treviso), Laura Candiotto is a fellow of AIL Treviso.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Carmel R. Current concepts in cobalamin deficiency. Annual Rev Med. 2000;51:357–75. doi: 10.1146/annurev.med.51.1.357. [DOI] [PubMed] [Google Scholar]
- 2.Salomon LR. Cobalamin responsive disorders in the ambulatory care setting: unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood. 2005;105:978–85. doi: 10.1182/blood-2004-04-1641. [DOI] [PubMed] [Google Scholar]
- 3.Andrès E, Affenberger S, Vinzio S, et al. Food cobalamin malabsorption in elderly patients: clinical manifestation and treatment. Am J Med. 2005;118:1154–9. doi: 10.1016/j.amjmed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Carmel R, Agrawal YP. Failures of cobalamin assays in pernicious anemia. N Engl J Med. 2012;367:385–6. doi: 10.1056/NEJMc1204070. [DOI] [PubMed] [Google Scholar]
- 5.Yang DT, Cook RJ. Spurious elevations of vitamin B12 with pernicious anemia. N Engl J Med. 2012;366:1742–3. doi: 10.1056/NEJMc1201655. [DOI] [PubMed] [Google Scholar]
- 6.Boven LA, van Wijnen M. False normal vitamin B12 levels caused by assay error. Blood. 2011;118:492. doi: 10.1182/blood-2010-11-315564. [DOI] [PubMed] [Google Scholar]
- 7.Emancipator K, Mansbach L, Robert T, Waskiewicz D. Failure of assay to identify low cobalamin concentrations: representatives of Bayer Diagnostics respond. Clin Chem. 2000;46:2018–9. [PubMed] [Google Scholar]
- 8.Hamilton MS, Blackmore S, Lee A. Possible cause of false normal B-12 assays. Br Med J. 2006;333:654–5. doi: 10.1136/bmj.333.7569.654-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christenson RH, Dent GA, Tuszynski A. Two radioassays for serum vitamin B12 and folate determination compared in a reference interval study. Clin Chem. 1985;31:1358–60. [PubMed] [Google Scholar]
- 10.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Inter Med. 1999;159:1289–98. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 11.Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clin Chem. 2000;46:1277–83. [PubMed] [Google Scholar]
- 12.Goringe A, Ellis R, McDowell I, et al. The limited value of methylmalonic acid, homocystein and holotranscobalamin in the diagnosis of early B12 deficiency. Haematologica. 2006;91:231–4. [PubMed] [Google Scholar]
- 13.Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency, an update. Haematologica. 2006;91:1506–11. [PubMed] [Google Scholar]