Abstract
Background and Purpose
Although neuroendoscopy (NE) has been applied to many cerebral diseases, the effect of NE for intraventricular hemorrhage (IVH) secondary to spontaneous supratentorial hemorrhage remains controversial. The purpose of this study was to analyze the effect of NE compared with external ventricular drainage (EVD) alone or with intraventricular fibrinolysis (IVF) on the management of IVH secondary to spontaneous supratentorial hemorrhage.
Methodology/ Principal Findings
A systematic search of electronic databases (PubMed, EMBASE, OVID, Web of Science, The Cochrane Library, CBM, VIP, CNKI, and Wan Fang database) was performed to identify related studies published from 1970 to 2013. Randomized controlled trials (RCTs) or observational studies (OS) comparing NE with EVD alone or with IVF for the treatment of IVH were included. The quality of the included trials was assessed by Jaded scale and the Newcastle-Ottawa Scale (NOS). RevMan 5.1 software was used to conduct the meta-analysis.
Results
Eleven trials (5 RCTs and 6 ORs) involving 680 patients were included. The odds ratio (OR) showed a statistically significant difference between the NE + EVD and EVD + IVF groups in terms of mortality (OR, 0.31; 95% CI, 0.16-0.59; P=0.0004), effective hematoma evacuation rate (OR, 25.50, 95%CI; 14.30, 45.45; P<0.00001), good functional outcome (GFO) (OR, 4.51; (95%CI, 2.81-7.72; P<0.00001), and the ventriculo-peritoneal (VP) shunt dependence rate (OR, 0.16; 95%CI; 0.06, 0.40; P<0.0001).
Conclusion
Applying neuroendoscopic approach with EVD may be a better management for IVH secondary to spontaneous supratentorial hemorrhage than NE + IVF. However, there is still no concluive evidence regarding the preference of NE vs. EVD alone in the case of IVH, because insufficient data has been published thus far. This study suggests that the NE approach with EVD could become an alternative to EVD + IVF for IVH in the future.
Introduction
Intraventricular hemorrhage (IVH) is common disease in neurosurgery, and is mostly secondary to spontaneous intracerebral hemorrhage (ICH), traumatic brain injury (TBI) or aneurysmal and arteriovenous malformation rupture [1]. IVH is a proven risk factor for poor prognosis, and mortality estimates for IVH range from 50% to 80% [2,3]. For IVH secondary to spontaneous supratentorial hemorrhage, the mortality and poor prognosis rate are 72% and 86%, respectively [4]. The outcome is often worsened by development of acute hydrocephalus, mass effect of ventricular blood, the toxicity of intraventricular blood clots, and chronic hydrocephalus.
During the past two decades, the medical and surgical management of IVH has remained one of the most difficult challenges for most neurosurgeons. Early treatment of IVH focused on the control of intracranial pressure (ICP). However, it had limited effects on avoiding acute and delayed hydrocephalus. Although the best medical management had been applied, mortality continues to be as high as 50% and the first year survival rate of IVH is only 38% [5]. External ventricular drainage (EVD) is the choice for controlling acute obstructive hydrocephalus, and a systematic analysis confirmed that EVD could significantly decrease the mortality of IVH [6]. Meanwhile, no study has thus far proven that EVD alone could effectively improve the functional outcome of IVH patient and prevent the development of hydrocephalus, which may suggest that there are other risk factors affecting the long term prognosis. The noxious effects of IVH may cause impairment of cerebrospinal fluid circulation, intracranial hypertension, and the development of hydrocephalus [7-9]. Therefore, expeditious evacuation of the intraventricular blood appears to be the only way to reduce mortality and the incidence of hydrocephalus [10]. A multivariate analysis conducted by Steiner T 2006 [11] provided substantial support that faster removal of IVH was an excellent therapeutic target. The aim of intraventricular fibrinolysis (IVF) is aiming to maintain catheter patency and speed up the resolution and drainage of intraventricular blood by applying thrombolytic agents (e.g., rtPA and urokinase). Some case series [12-14] and a recent meta-analysis [15] presented improved survival and functional outcome in IVH patient treated by IVF as compared to EVD alone. However, due to the low quality of the studies, there were not sufficient data to support IVF prevents the development of chronic hydrocephalus in patients with IVH.
Currently, early evacuation of IVH could limit the negative effects of ventricular blood clots and prevent delayed hydrocephalus. Several studies [16-19] applied a minimally invasive technique, neuroendoscopy (NE), for fast and complete evacuation of IVH early on, and achieved a good functional outcome and low VP-dependence rate in patients with IVH secondary to hypertensive ICH.
The purpose of this present study was to evaluate the efficacy and safety of the NE approach compared with EVD alone or with IVF in the treatment of IVH secondary to spontaneous supratentorial hemorrhage. Therefore, we conducted this meta analysis and review the relative literature.
Materials and Methods
Literature Search and Study Selection
Relevant studies were identified by systematic searches of the published articles comparing NE versus EVD alone or with IVF for patients with IVH secondary to spontaneous supratentorial hemorrhage (YP.L and N.Z). The search was not restricted to articles in English and included articles published between January 1966 and April 2013. We searched for relevant studies in the English electronic databases (PubMed, EMBASE, and the Cochrane Library) and Chinese electronic database (CBM, VIP, CNKI, WanFang). The search strategy used both medical subject headings (MeSH) term and keywords searches for intraventricular hemorrhage, IVH, intracerebral hemorrhage, ICH, neuroendoscopy, endoscopy, external ventricular drainage, EVD, ventriculo-peritoneal (VP) shunt, intraventricular fibrinolysis, IVF, which were combined with the Boolean connectors. We looked through grey literature in China through the Chinese Academic Conference Pap (CACP), and we also search the unfinished clinical trials in the Cochrane central registry of controlled trials database to identify relevant journal and reference lists of retrieved articles.
Two independent reviewers (YP.L and N.Z) assessed the literature based on the titles and abstracts to identify potentially relevant articles. Disagreements were resolved through a discussion. Full versions of all relevant articles were obtained and inspected. Literature selection was present in the PRISMA flow chart (Figure 1) according to the PAISMA guidelines [20].
Figure 1. The PRISMA flow chart of the meta-analysis.
Inclusion Criteria
When the primary electronic search was completed, a well designed randomized controlled trial (RCT) had not been found. Therefore, we decided to include both small RCTs and prospective or retrospective observational studies (OSs) in this meta-analysis. The following inclusion criteria were used for selecting the potential studies: (1) the study reports results of comparing NE with EVD alone or NE +EVD with EVD + IVF for IVH; (2) the patients were adults; (3) the study reported the outcome measures of the meta-analysis (reported primary or secondary outcomes); and (4) at least 2 months follow-up.
Outcome Measures
The primary outcome was mortality at the end of the follow-up (2 months). Secondary outcomes included the following: (1) effective hematoma evacuation rate, defined as hematoma evacuation rate >60%; (2) good functional outcome (GFO), defined as a patient being able to care for him/herself, corresponding to a modified Rankin Scale (mRS) of 0, 1, 2, or 3, a Glasgow Outcome Scale (GOS) of 4 or 5, or a Activities of Daily Living (ADL) score [21] of 1, 2, or 3; (3) Ventriculo-peritoneal (VP) dependent rate.
Data Extraction and qualitative assessment
The relevant data from selected studies were independently extracted by 2 reviewers (YP.L and N.Z). The following pieces of information were extracted: author name, publication year, sample size, study group (mean age, number of patient), GCS on admission, the Graeb score, type of study, surgical procedure, information regarding study quality, follow-up, primary and secondary outcomes.
Methodological quality of the including studies was assessed by two observers. The Jaded scale 1996 [22] and the Newcastle-Ottawa Scale (NOS) [23] were introduced to evaluate methodological quality of RCTs and OSs.
Statistical Methods
Meta-analysis was performed using the RevMan software (version 5.1; The Cochrane Collaboration). The odds ratio (OR) with 95% confidence intervals (CIs) was used to assess outcomes of the studies, including mortality rate, effective hematoma evacuation rate, GFO, and VP-dependence rate. Statistical significance was accepted as P value less than 0.05. Because of the small number of studies included in this meta-analysis, I Square value statistics were performed to evaluate heterogeneity between NE and EVD group in each study. The OR was calculated by applying the fixed effect model of random effect model according to I2 values which defined 0-25% as low, 25-50% as moderate, 50-75% as high, and > 75% as extreme. Both Begg’s funnel plot and “fail-safe” numbers [24] were performed to assess the publication bias of the literature. The sensitivity analysis was performed in each study and the impact of different interventions was evaluated.
Results
Description of the Studies
Figure 1 shows a flow chart of the study selection and inclusion process. The primary search yielded 1285 potentially relevant articles (Figure 1). Of these, 1162 were excluded after reading the title and abstract. Then the full text of the remaining 21 articles was read by 2 independent reviewers (YP.L and HZ.Z). Nine studies were further excluded because of inadequate postoperative follow-up duration (<1 month, 3 articles) and insufficient clinical data (comparing NE with traditional craniotomy, 4 article, or cannot extract the data of primary or secondary outcomes, 2 articles).
Twelve articles were identified in this meta-analysis. The Basaldella’s study [17] was excluded because the reported cases of IVH had causes other than spontaneous supratentorial ICH, including ruptured aneurysms, pure IVHs, arteriovenous malformations (AVMs), or posterior fossa hemorrhages. Finally, we included 11 studies [16,18,19,25-32] with a total of 680 IVH patients (Table 1). The sample size of the trials ranged from 18 to 140. Three studies were published in English [16,18,19], and 8 in Chinese [25-32]. These articles were published between 2007 and 2013. Five studies were described as RCT [16,19,26,27,30], and other 6 articles [18,25,28,29,31,32], which lack an optimal randomization method, were included as OS. These 5 RCTs included 388 IVH patients with 191 patients treated through the NE approach (49.2%). The six observational studies included 292 IVH patients, of whom 147 underwent NE (50.3%). One study [29] was prospective, and 5 were retrospective [18,25,28,31,32]. In the included studies, two articles [18,29] applied the NE approach alone, and 9 studies used EVD followed by the NE procedure. We used patients treated with EVD alone or with IVF as the control group. The control group of three studies [18,19,29] was EVD alone, and that of the other 8 studies [16,25-28,30-32] was EVD + IVF. Seven studies clearly described the detail of the fibrinolysis agent and dose of IVF. In 3 studies [18,19,26], the results of GFO were presented in means ± the standard deviationists so that we could not extract the data according to definition of GFO. The other studies clearly presented the following outcome measures: three used GOS, 2 used mRS, and 5 used ADL at 6 months after the operation. The details of the surgical procedure and functional outcome measures are shown in Table 2. Because the studies available utilized different methodologies, we performed two comparisons in our meta-analysis, including NE versus EVD alone and NE + EVD versus EVD + IVF.
Table 1. Characteristics of included studies.
| Initial GCS |
Graeb Scale |
Age (y) |
Cases (M) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Inclusion criteria | NE | EVD | NE | EVD | NE | EVD | NE | EVD | Outcomes | Side-effect | Followup(m) |
| Zhang Z16 | 2007 | Any age, < 48 h, | 9 (8-12) | 6(8-12) | NR | NR | 58 | 58 | 22(13) | 20(12) | Mortality | Rebleeding rate | 2 |
| Diagnosed by CT | 13 (<8) | 14(<8) | GOS(2 m) | Cerebral infection | |||||||||
| ICH < 30 ml | |||||||||||||
| Fuminari K18 | 2010 | Any age, IVH | 5.4 | 7.5 | 8.9 | 7.8 | 58.9 | 64.3 | 10(7) | 8(6) | Mortality | Rebleeding rate | 6 |
| Acute hydrocephalus | mRS(12 m) | Cerebral infection | |||||||||||
| VP dependent | |||||||||||||
| EVD duration | |||||||||||||
| Chen CC19 | 2011 | Any age, < 48 h | 8.54 | 9.83 | 6.9 | 4.54 | 65.54 | 62.17 | 24(NR) | 24(NR) | Mortality | Not mentioned | 3 |
| Acute hydrocephalus | VP shunt rate | ||||||||||||
| IVH from ICH | GOS(3m) | ||||||||||||
| TM Song23 | 2010 | Age < 70, < 24 h | 9.21 | 9.27 | NR | NR | 62.5 | 61.9 | 28(15) | 25(13) | Evacuation rate | Not mentioned | 2 |
| Diagnosed by CT | GCS(2w and 2m) | ||||||||||||
| No trauma history | |||||||||||||
| M Lang24 | 2009 | 20-76 years, < 24 h | 22(12-14) | 22(12-14) | 22(3-5) | 22(3-5) | 56.3 | 54.2 | 80(56) | 60(36) | Mortality | Hydrocephalus | 6 |
| IVH caused by ICH | 35(10-12) | 29(10-12) | 35(5-7) | 29(5-7) | Evacuation rate | ||||||||
| 23(8-10) | 9(8-10) | 23(8-10) | 9(8-10) | ADL Scale | |||||||||
| VP shunt rate | |||||||||||||
| GCS(2w and 2m) | |||||||||||||
| HB Duan25 | 2007 | Any age, < 48 h | 12(12-14) | 23(12-14) | 12(3-5) | 23(3-5) | 53.1 | 55.8 | 33(21) | 61(40) | Evacuation rates | ||
| Diagnosed by CT | 15(10-12) | 29(10-12) | 15(5-7) | 29(5-7) | VP shunt rate | ||||||||
| ICH > 30 ml | 6(8-10) | 9(8-10) | 6(8-10) | 9(8-10) | GCS(2w and 2m) | ||||||||
| IVH from ICH | GOS(3m) | ||||||||||||
| HL Zhang26 | 2008 | Any age, < 6 h | 10.3 | 11.5 | 7.4 | 7.1 | 62.7 | 61.8 | 37(22) | 33(20) | Mortality | Not mentioned | 3 |
| Pupil mydriasis < 1h | Evacuation rate | ||||||||||||
| Hypertension history | GOS(3 m) | ||||||||||||
| IVH caused by ICH | |||||||||||||
| LL Yu27 | 2012 | Any age | All 11.25 | All 6.89 | 55.7 | 57.3 | 40(25) | 40(27) | Mortality | Not mentioned | 6 | ||
| Hypertension history | Evacuation rate | ||||||||||||
| IVH from ICH | ADL Scale (at 6 mo) | ||||||||||||
| ZW Lv28 | 2011 | Any age | NR | NR | NR | NR | NR | NR | 32(17) | 32(18) | Mortality | Cerebral infection | 6 |
| Hypertension history | ADL Scale (6 m) | Hydrocephalus | |||||||||||
| Diagnosed by CT | VP hunt rate | ||||||||||||
| ICH <30 ml | |||||||||||||
| LF Wang29 | 2011 | Any age, < 48 h | 4(9-12) | 6(9-12) | NR | NR | 57.8 | 59.2 | 17(11) | 22(14) | Mortality | Cerebral infection | 6 |
| Hypertension history | 11(7-9) | 12(7-9) | ADL Scale (6 m) | Hydrocephalus | |||||||||
| IVH diagnosed by CT | 2(5-6) | 12(5-6) | VP hunt rate | ||||||||||
| WJ Li30 | 2013 | 31-75 years, < 48 h | 6(8-14) | 7(8-14) | NR | NR | 57.5 | 55.2 | 21(12) | 24(14) | Mortality | Not mentioned | 6 |
| ICH <30 ml | 15(<8) | 17(<8) | ADL Scale (6 m) | ||||||||||
| IVH caused by ICH | GCS (2w) | ||||||||||||
NE: Neuroendoscopy; EVD: External ventricular drainage; IVF: intraventricular fibrinolysis; ICH: intracerebral hemorrhage; VP: Ventriculo-peritonea;l GOS: Glasgow Outcome Scale; mRS: modified Rankin Scale; ADL: Activities of Daily Living; NR: not report;
Table 2. Surgical procedure and functional outcome measure of included studies.
| Outcome | GFO(%) |
IVF |
Fibrinolytic agent |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Design | measures | NE | EVD | Approach | NE | EVD | NE | EVD | Surgical access | VCS | 4th ventricular |
| Zhang Z16 | RCT | GOS | 13(59) | 6(30) | NE + EVD | Y | Y | Urokinase | Urokinase | Frontal | Y | N |
| 25,000 IU | 25,000 IU | Occipital | ||||||||||
| Every 8 hours | Every 8 hours | |||||||||||
| Fuminari K18 | Retrospective OS | mRS | 3(30) | 0(0) | NE alone | N | N | -- | -- | Frontal | N | Y |
| Unilateral | ||||||||||||
| Chen CC19 | RCT | GOS | NR | NR | NE + EVD | N | N | -- | -- | Frontal | N | N |
| Occipital | ||||||||||||
| Unilateral | ||||||||||||
| TM Song23 | Retrospective OS | GCS | NR | NR | NE + EVD | Y | Y | Urokinase | Urokinase | Frontal | N | N |
| 20,000 IU | 20,000 IU | Unilateral | ||||||||||
| 1 time per day | 1 time per day | |||||||||||
| M Lang24 | RCT | ADL | 71(88) | 38(63) | NE + EVD | Y | Y | Urokinase | Urokinase | Frontal | N | Y |
| 20,000-40,000 IU | 20,000-40,000 IU | Unilateral | ||||||||||
| 3 times per day | 3 times per day | |||||||||||
| HB Duan25 | RCT | GCS | NR | NR | NE + EVD | Y | Y | Urokinase | Urokinase | Shortest | Y | N |
| 20,000 IU | ||||||||||||
| 2 times per day | ||||||||||||
| HL Zhang26 | Retrospective OS | GOS | 27(73) | 11(33) | NE + EVD | Y | Y | Urokinase | Urokinase | Frontal | N | Y |
| 50,000 IU | 50,000 IU | Unilateral | ||||||||||
| Every 4 to 8 hours | 4 to 8 hours | |||||||||||
| LL Yu27 | Prospective OS | ADL | 35(87) | 27(68) | NE alone | N | N | -- | -- | Frontal | Y | N |
| Unilateral | ||||||||||||
| ZW Lv28 | RCT | ADL | 27(84) | 16(50) | NE + EVD | N | Y | -- | Urokinase | Frontal | N | N |
| 20,000 IU | Occipital | |||||||||||
| Every 4 to 8 hours | Unilateral | |||||||||||
| LF Wang29 | Retrospective OS | ADL | 15(88) | 14(63) | NE + EVD | Y | Y | Urokinase | Urokinase | Frontal | N | N |
| 200,000 IU | 200,000 IU | Bilateral | ||||||||||
| 2 times per day | 2 times per day | |||||||||||
| WJ Li30 | Retrospective OS | ADL | 17(89) | 13(76) | NE + EVD | Y | Y | Urokinase | Urokinase | Frontal | NR | N |
| 200,000 IU | 200,000 IU | Unilateral | ||||||||||
| 2 times per day | 2 times per day | |||||||||||
NE: neuroendocopy; EVD: external ventricular drainage; IVF: intraventricular fibrinolysis; VCS: ventriculocisternostomy; OS: observational study; GOS: Glasgow Outcome Scale; mRS: modified Rankin Scale; ADL: Activities of Daily Living; Y: yes; N: no performed.
Primary Outcome
Mortality of IVH Patients
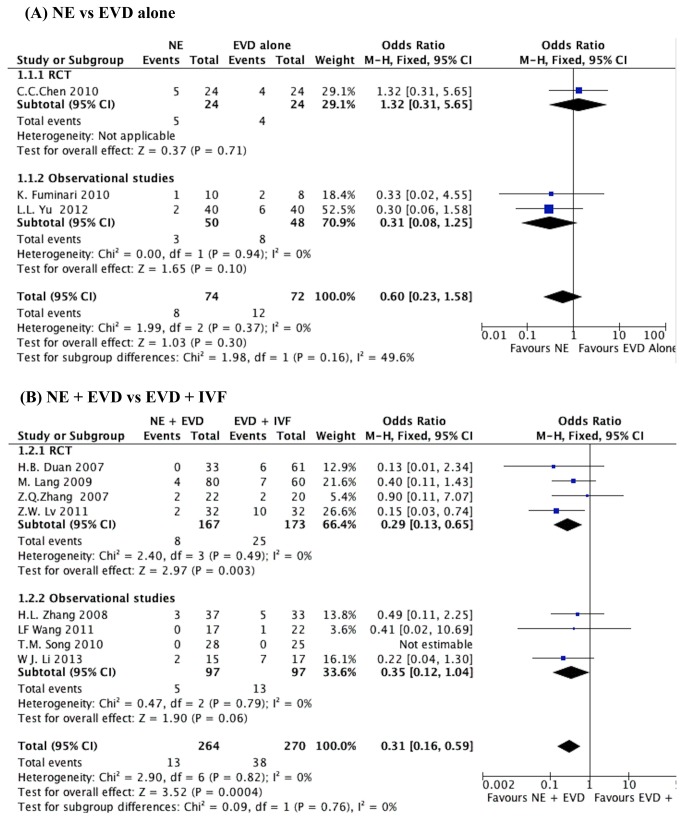
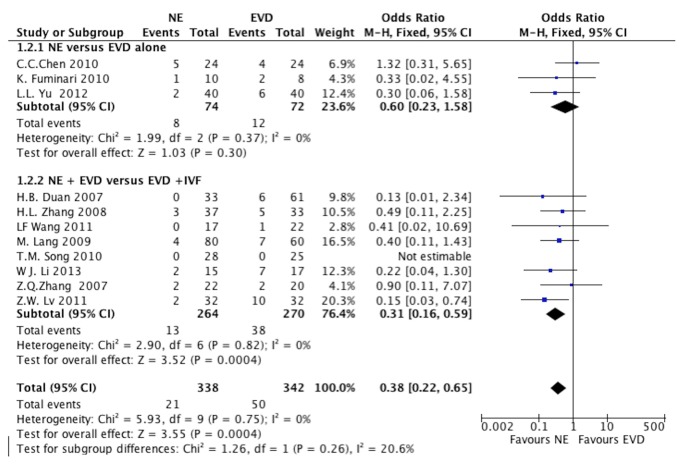
All included studies investigated the mortality with 5 RCTs. There were 3 studies [18,19,29] that presented mortality of NE versus EVD alone, including one RCT [19]. When the test of heterogeneity showed no significant differences in each study (I2=0), then fixed-effects model was used. A mortality of 14.8% was noted in NE group compared with 16.6% in the EVD alone group. The pooled OR was 0.60 (95% CI, 0.23-1.58; P=0.30), as shown in Figure 2 A.
Figure 2. The mortality of IVH patients at the end of the follow-up.
(A) NE group versus EVD alone group, (B) NE + EVD group versus EVD +IVF group. (IVH, intraventricular hemorrhage; NE, neuroendoscopy; EVD, external ventricular drainage; IVF, intraventricular fibrinolysis).
Eight studies [16,25-27,30-32] presented mortality of NE + EVD versus EVD + IVF, including 4 RCTs [16,26,27,30]. When the test of heterogeneity had no significant differences in each study (I2=0), the fixed-effects model was applied to analyze. A mortality of 4.9% was noted in the NE + EVD group compared with 14.1% in the EVD +IVF group. The overall pooled OR was 0.31 (95% CI, 0.16-0.59; P=0.0004), as shown in Figure 2 B. There was no difference between randomized and observational studies (P=0.99).
Secondary Outcome
Effective Hematoma Evacuation Rate
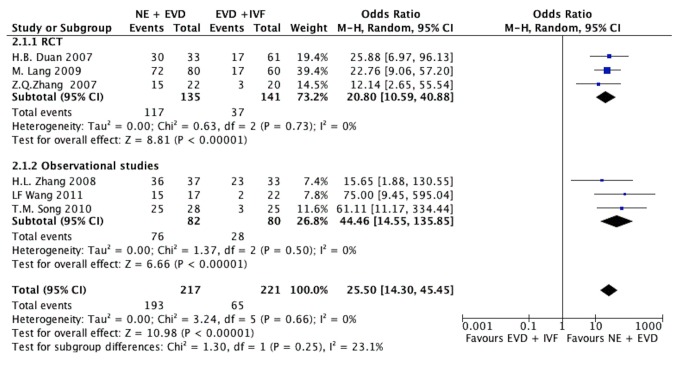
Seven studies [16,25-27,29,31] presented data of the effective hematoma evacuation, which was defined as removal of more than 60% of the IVH. One study [29] presented the effective hematoma evacuation rate was 67.5% in the NE group compared to 27.5% in the EVD alone group (P<0.05). The other 6 studies compared NE +EVD with EVD +IVF. When statistical heterogeneity among the studies was low (I2=0%), the fixed-effects model was adopted. The effective hematoma evacuation rate was 88.9% in the NE + EVD group compared to 29.4% in the EVD +IVF group (P< 0.05). The meta-analysis showed that the overall effective hematoma evacuation rate was statistically higher in the NE + EVD group (OR, 25.50, 95%CI; 14.30, 45.45; P<0.00001) (Figure 3).
Figure 3. The results of hematoma evacuation rate in IVH patients comparing NE + EVD group and EVD + IVF group.
(IVH, intraventricular hemorrhage; NE, neuroendoscopy; EVD, external ventricular drainage; IVF, intraventricular fibrinolysis.)
Good Functional Outcome (GFO)
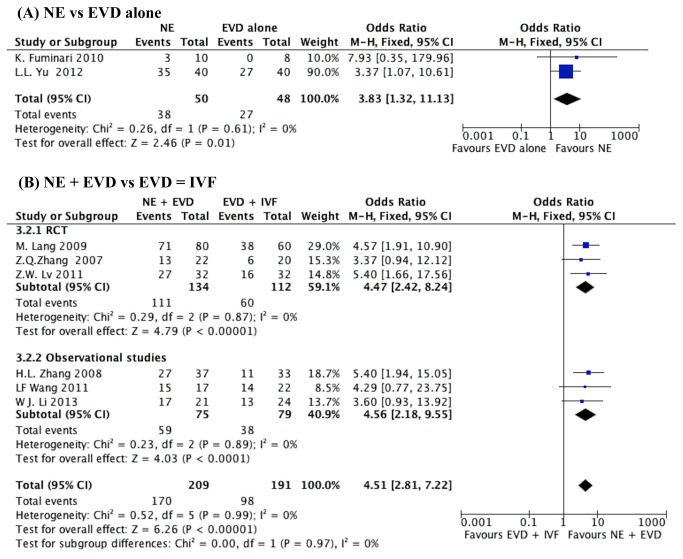
Eight studies [16,18,26,28-32] presented the data of GFO, including 3 RCTs [16,26,30]. Two studies [18,29] compared NE with EVD alone. No statistically significant heterogeneity was observed between studies (I2=0%); therefore, the fixed effect model was applied. Meta-analysis showed that the GFO in the NE group was 76%, which was higher than 56% in the EVD alone group (OR, 3.83, 95%CI, 1.32-11.13; P=0.01). (Figure 4 A)
Figure 4. The result of good functional outcome (GFO) in IVH patients.
(A) NE group versus EVD alone group, (B) NE + EVD versus EVD + IVF group. (IVH, intraventricular hemorrhage; NE, neuroendoscopy; EVD, external ventricular drainage; IVF, intraventricular fibrinolysis).
Six studies reported the GFO comparing NE + EVD with EVD + IVF, including 3 RCTs (Figure 4 B). The test of heterogeneity showed no significant differences in each study (I2=0%). The pooled OR was 4.51 (95%CI, 2.81-7.72) with an overall effect of 6.26 (P<0.00001).
The VP-Dependence Rate
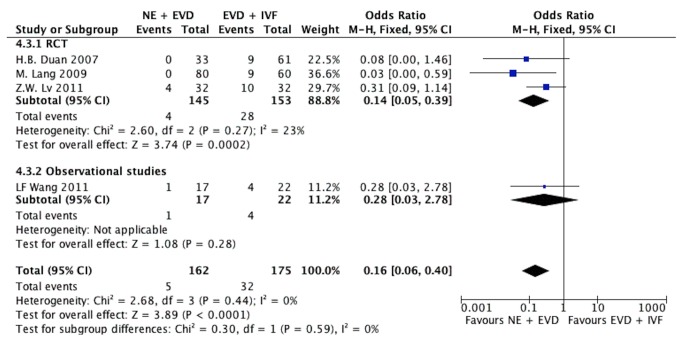
Six studies [18,19,26,27,30,31] presented data on the rate of ventriculo-peritoneal shunt (VP shunt) surgery. There were two studies, including one randomized study, comparing NE with EVD alone. In the study by Fuminari [18], none of the IVH patients needed to undergo VP shunt surgery. A study by Chen [19] presented a VP-dependence rate of 47.6% in the NE alone group compared with 90.5% in the EVD alone group (P<0.05).
Four studies [26,27,30,31] presented VP-dependence rates comparing NE + EVD with EVD + IVF, including 3 randomized studies (Figure 5). The test of heterogeneity had no significant differences in each study (I2=0%); therefore, we used the Peto fixed-effects model. The pooled OR was 0.16 (95%CI; 0.06, 0.40; P<0.0001). There was no difference between the randomized and observational studies (P=0.59).
Figure 5. The results of the dependent rate of ventriculo-peritoneal shunt surgery in IVH patients comparing NE + EVD group and EVD + IVF group.
(IVH, intraventricular hemorrhage; NE, neuroendoscopy; EVD, external ventricular drainage; IVF, intraventricular fibrinolysis).
Sensitivity Analysis
A sensitivity analysis was performed on the studies included in the meta-analysis by deleting each individual data set to assess the influence of the study on the pooled ORs. The results suggested that no individual study significantly affected the pooled ORs. Sensitivity analysis of two different comparisons between NE versus EVD alone and NE + EVD versus EVD + IVF was also performed to identify the effect of IVF on the pooled results, and it showed that the difference between these two comparisons was not significant (P=0.26, test for subgroup differences), thereby indicating that the results are statistically robust (Figure 6).
Figure 6. Sensitivity analysis of two intervention comparison (NE versus EVD alone; NE + EVD versus EVD + IVF) on mortality.
Ventriculocisternostomies (VCS) may impact the mortality and prognosis of IVH patients; therefore, the sensitivity analysis was applied determining the effect of VCS between the NE + EVD and EVD + IVF groups. Three studies reported performing VCS in the NE group and those also placed the EVD followed by NE. The results showed no significant difference in mortality, GFO, and VP-dependence rate with P values (test for subgroup differences) of 0.61, 0.98, and 0.95, respectively. The sensitivity analysis revealed no impact of the included studies that had performed VCS on our results (Figure S1-3).
Qualitative Assessment and Publication Bias
The quality of the studies included in this meta-analysis is shown in Table 2. It is can be seen from the funnel plot that the publication bias was low regarding mortality (Figure 7), VP-dependence rate and GFO, but moderate regarding hematoma evacuation rate (Figure S4-6 and Table S1). The results of “fail-safe” numbers of included studies on four outcome measures are shown in Table 3.
Figure 7. Funnel plot of included studies.
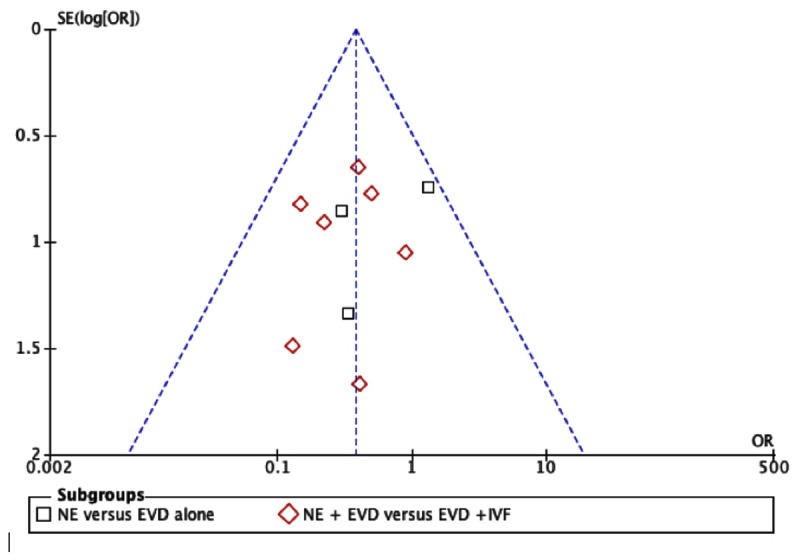
Table 3. Fail-safe numbers of primary and secondary outcome of included studies.
|
Fail-safe numbers
|
||||
|---|---|---|---|---|
| Variable | k(number of study) | Z | Np=0.01 | Np=0.05 |
| Mortality | 10 | 12.24743 | 17.62984 | 45.77024 |
| Hematoma evacuation rate | 7 | 34.13483 | 207.6266 | 426.2193 |
| GFO | 8 | 18.64928 | 56.06375 | 121.3113 |
| VP dependent rate | 5 | 12.22171 | 22.51391 | 50.53624 |
The fail-safe numbers are calculated as Np=0.05= (ΣZ/1.64)2-k; Np=0.01 = (ΣZ/2.33)2-k, where k is the number of studies.
The approach presented the potential for unpublished or missing studies to alter our conclusions; a low fail-safe number indicate more complicated publication bias methodologies.
Discussion
With a rising interest in minimally invasive techniques, the advancements in neuroimaging have promoted the establishment of modern neuroendoscopy [33]. Endoscopic surgery has many advantages, including minimal invasiveness, high evacuation rate, low incidence of complications, better protection of brain tissue, and less surgery-related injuries [34]. Although NE has been applied to many cerebral diseases, the effect of NE on IVH remains controversial, and it is still not clear whether it can improve the prognosis compared to EVD alone or with IVF. To our knowledge, this study is the first meta-analysis to evaluate the clinical effects of neuroendoscopy in the treatment of IVH. Eleven eligible trials (6 RCTs and 5 OSs) were identified and the pooled result comparing NE + EVD and EVD + IVF in the management of IVH secondary to spontaneous intracerebral hemorrhage showed the superiority of neuroendoscopy on mortality, effective hematoma evacuation rate, GFO and VP-dependence rate. The results indicated that neuroendoscopy with EVD may be superior to EVD + IVF for IVH. However, this study was limited by the small sample of clinical trials (just 5 small RCTs and 6 OSs), and this result still needs further clinical trials for confirmation.
The high mortality (range from 43% to 83%) and aggressive progression of IVH may relate to the volume of IVH, obstruction of cerebrospinal fluid (CSF) circulation and toxic effect of ventricular blood clots, which could lead to secondary brain damage and acute obstructive hydrocephalus, especially when the third and fourth ventricles are involved [35]. Blood clots in ventricles could obstruct the CSF circulation and cause mass effects. These may lead to obstructive hydrocephalus and secondary brain damage, which is the main reason for neurological deterioration after the first day [36]. An analysis by Hamada of the best available data suggests a 10%-15% absolute benefit might be achieved in IVH subjects, if blood clots are removed from the brain [37]. Therefore, clearing the ventricular hematoma has been shown to dramatically improve CSF circulation and symptoms.
The debate regarding which surgical intervention should be used to remove the hematoma continues. IVH secondary to spontaneous supratentorial hemorrhage can be treated with the following different surgical interventions: EVD, IVF, and neuroendoscopy. Placement of an EVD was performed to treat acute obstructive hydrocephalus. However, an EVD cannot effectively improve the prognosis of IVH because the catheter is often obstructed by blood clots. A study by Morgan revealed the circadian blood clot dissolution rate was only 10.8% [38]. Furthermore, EVDs also have a high postoperative infection rate, and several studies reported that EVD-related infections have occurred in approximately 10% of IVH patients [39].
In the past two decades, many studies have focused on testing new treatment modalities for IVH, which could result in faster evacuation and drainage of ventricular blood, including IVF and NE. In 2011, a meta-analysis conducted by Gaberel suggested that IVF was probably recommended in IVH secondary to small spontaneous ICH. Recently, an ongoing CLEAR-IVH RCT [15] including 500 patients evaluate the efficacy of the recombinant tissue plasminogen activator (rtPA) in IVF treatment for IVH, and the results will be available in 2015. Therefore, the definitive recommendation remains unanswered. In contrast, neuroendoscopy is an emerging minimal invasive technique, and more widely applied for faster removal of IVH, especially in China. Several small RCTs and observational studies compared these two approaches in treating IVH, and suggested that NE may be as efficient as IVF. Horvath demonstrated that an endoscopic removal of a hematoma offers a more adequate treatment option than EVD placement in patients with IVH [10]. There are other studies [40-42] demonstrating that NE result in high rate of hematoma evacuation (ranging from 83.4-99%). In our study, the mortality in IVH patient showed no statistically significant difference between the NE alone and EVD alone groups. However, the results also showed superiority of NE + EVD compared to EVD + IVF in terms of mortality. Mortality in four different intervention strategies was 4.9% for NE + EVD, 14.1% for EVD + IVF, 14.8% for NE, and 16.6% for EVD alone. Therefore, the placement of an EVD followed by NE maintains the patency of CSF pathways, allowing good control of ICP and faster resumption of normal CSF circulation. These factors may decrease the mortality and improve neurological function. Furthermore, NE could also evacuate most of ventricular blood earlier on, which effectively prevents acute hydrocephalus. In this study, the effective evacuation rate of NE group was 88.9%, which was significantly higher than 29.4% in EVD group (P<0.00001). Thus, the initial goal of IVH management should use the NE approach to evacuate intraventricular blood to reverse the ventricular dilation rapidly and restore normal ICP.
Chronic hydrocephalus was the most severe complication for IVH and was reported in nearly all recent studies. A study [43] revealed that endoscopic third ventriculostomy was a safe and effective treatment for hydrocephalus related to IVH, and the author reported that 34 IVH patients underwent endoscopic third ventriculostomy, and two of the 34 cases (5.9%) needed VP shunts. Follow-up clinical outcomes indicated that the NE approach could effectively improve the neurological function of patients (P=0.01). To explore proper management of IVH, the NE approach was preferred because of fewer complications and lower VP-dependence rate. Our study showed that VP-dependence rate of the NE + EVD group was significantly lower than the EVD + IVF group (P<0.00001). In the included studies the EVDs were all placed after performance of the NE procedure, 2 of the studies used IVF, and 2 performed VCS, indicating that placement of EVD with IVF followed by NE early on appears to prevent development of chronic hydrocephalus.
In terms of prognosis, the following four scales are usually used to evaluate the outcome: the GCS, the GOS, the mRS, and the ADL scale. Recent trials have demonstrated that IVH patients with an initial GCS score of more than 9 had a good recovery [44]. They suggested that the outcomes may depend on the initial GCS. We included two studies that compared GCS between these two interventions. Therefore, we could not analyze the GCS score because we only have two articles and could not extract data from them. In this study, the GFO was applied as a outcome measure to assess neurological functional recovery. We found significant differences in two comparisons. The GFO was 76% in the NE alone group, 56% in EVD alone group, 81.2% in NE + EVD group, and 51.3% in the EVD + IVF group. Thus, use of the NE approach with EVD could potentially improve the prognosis. The satisfactory prognosis through the NE approach may be determined by several benefits as follows: (1) Excellent visual quality in the deep and narrow cerebral ventricular system could enhance the hematoma evacuation rate. (2) Endoscopic surgery through a working channel can significantly reduce the surgery-related injury rate. (3) The incidence of complications (such as the dependence on VP shunts) was lower in the NE group than the EVD alone and EVD + IVF groups. (4) Relative short NICU stays and operative time.
Side effects are another problem that needed more consideration. Four of the included studies presented data on complications related to the NE approach. Zhang et al. [16] reported that rebleeding and cerebral infections did not occur in the NE group and that two patients had cerebral infections in the NE with EVD group. Wang reported that infections did not occur in the NE group, while three patients in the EVD + IVF group developed infections. Other included studies did not present any particular complications of the NE procedure. This issue will require further investigation of comparative clinical trials.
Limitations
There are several limitations to this study (1). Five RCTs and six OSs were selected in this study, and the sample size in some of them was rather small, which might generate bias of clinical results. In addition, small-volume, observational studies tended to produce more impressive effects than those with large-volume and randomized studies (2). Although the clinical outcomes have shown that neuroendoscopic approach with EVD placement can improve the quality of life for patients, the data on neurological function after 2 years have never been reported (3). There were insufficient data comparing NE versus EVD alone (just three included studies) (4). There is a possibility of publication bias, as suggest by the funnel plot (Figure S4-S6) (5). The random and double-blinded methods are difficult to conduct in the surgical field [45], which are also a limitation that is difficult to eliminate in reality.
Conclusions
In summary, this study revealed that applying a neuroendoscopic minimally invasive surgical approach with EVD placement may be a better management of IVH secondary to spontaneous supratentorial hemorrhage than NE + IVF. NE with EVD placement could be an alternative to EVD + IVF for IVH in the future. Although NE with EVD placement for treatment of IVH showed optimistic results in this analysis, further large multicenter randomized controlled trials are still needed to confirm this conclusion.
Supporting Information
PRISMA 2009 Checklist.
(DOC)
Qualitative Assessment of included studies.
(DOC)
sensitivity analysis of VCS influence the mortality.
(DOC)
sensitivity analysis of VCS influence the GFO.
(DOC)
sensitivity analysis of VCS influence the VP dependent rate.
(DOC)
Funnel plot of hematoma evacuation rate between NE group and EVD + IVF group.
(DOC)
Funnel plot of GFO between NE group and EVD + IVF group.
(DOC)
Funnel plot of VP dependent rate between NE group and EVD + IVF group.
(DOC)
Acknowledgments
The authors wish to express our gratitude to Dr.Guangyu Lu from Ruprecht-Karls Universitaet Heidelberg, for her editorial assistance.
Funding Statement
There is no current external funding source for this study.
References
- 1. Rosen DS, Macdonald RL, Huo D, Goldenberg FD, Novakovic RL et al. (2007) Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg 107: 261-265. doi: 10.3171/JNS-07/08/0261. PubMed: 17695378. [DOI] [PubMed] [Google Scholar]
- 2. Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, Investigators S (2006) Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl 96: 65-68 [DOI] [PubMed] [Google Scholar]
- 3. Huttner HB, Köhrmann M, Berger C, Georgiadis D, Schwab S (2006) Influence of intraventricular hemorrhage and occlusive hydrocephalus on the long-term outcome of treated patients with basal ganglia hemorrhage: a case-control study. J Neurosurg 105: 412-417. doi: 10.3171/jns.2006.105.3.412. PubMed: 16961136. [DOI] [PubMed] [Google Scholar]
- 4. Gaberel T, Magheru C, Emery E (2012) Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev 35: 485-494; discussion: 22732889. [DOI] [PubMed] [Google Scholar]
- 5. Nishikawa T, Ueba T, Kajiwara M, Miyamatsu N, Yamashita K (2009) A priority treatment of the intraventricular hemorrhage (IVH) should be performed in the patients suffering intracerebral hemorrhage with large IVH. Clin Neurol Neurosurg 111: 450-453. doi: 10.1016/j.clineuro.2009.01.005. PubMed: 19231066. [DOI] [PubMed] [Google Scholar]
- 6. Gaab MR (2011) Intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH): improvement of bad prognosis by minimally invasive neurosurgery. World Neurosurg 75: 206-208. doi: 10.1016/j.wneu.2010.10.003. PubMed: 21492714. [DOI] [PubMed] [Google Scholar]
- 7. Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB (1982) Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 143: 91-96. PubMed: 6977795. [DOI] [PubMed] [Google Scholar]
- 8. Staykov D, Huttner HB, Schwab S (2012) [New treatment strategies for intraventricular hemorrhage]. Med Klin Intensivmed Notfmed 107: 192-196. PubMed: 22526062. [DOI] [PubMed] [Google Scholar]
- 9. Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G et al. (2007) Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC Neurol 7: 1. doi: 10.1186/1471-2377-7-1. PubMed: 17204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horváth Z, Veto F, Balás I, Kövér F, Dóczi T (2000) Biportal endoscopic removal of a primary intraventricular hematoma: case report. Minim Invasive Neurosurg 43: 4-8. doi: 10.1055/s-2000-8410. PubMed: 10794560. [DOI] [PubMed] [Google Scholar]
- 11. Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K et al. (2006) Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 59: 767-773. doi: 10.1227/01.NEU.0000232837.34992.32. PubMed: 17038942. [DOI] [PubMed] [Google Scholar]
- 12. Staykov D, Bardutzky J, Huttner HB, Schwab S (2011) Intraventricular fibrinolysis for intracerebral hemorrhage with severe ventricular involvement. Neurocrit Care 15: 194-209. doi: 10.1007/s12028-010-9390-x. PubMed: 20524079. [DOI] [PubMed] [Google Scholar]
- 13. Nieuwkamp DJ, Gans K, Rinkel GJ, Algra A (2000) Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol 247: 117-121. doi: 10.1007/PL00007792. PubMed: 10751114. [DOI] [PubMed] [Google Scholar]
- 14. Gaberel T, Magheru C, Parienti JJ, Huttner HB, Vivien D et al. (2011) Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage: a meta-analysis. Stroke 42: 2776-2781. doi: 10.1161/STROKEAHA.111.615724. PubMed: 21817146. [DOI] [PubMed] [Google Scholar]
- 15. Webb AJ, Ullman NL, Mann S, Muschelli J, Awad IA et al. (2012) Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventricular thrombolytic: the clot lysis – Evaluating Accelerated Resolution of IVH (CLEAR IVH) Program. Stroke 43: 1666–1668. doi: 10.1161/STROKEAHA.112.650523. PubMed: 22474059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Z, Li X, Liu Y, Shao Y, Xu S et al. (2007) Application of neuroendoscopy in the treatment of intraventricular hemorrhage. Cerebrovasc Dis 24: 91-96. doi: 10.1159/000103122. PubMed: 17519550. [DOI] [PubMed] [Google Scholar]
- 17. Komatsu F, Komatsu M, Wakuta N, Oshiro S, Tsugu H et al. (2010) Comparison of clinical outcomes of intraventricular hematoma between neuroendoscopic removal and extraventricular drainage. Neurol Med Chir (Tokyo) 50: 972-976. doi: 10.2176/nmc.50.972. PubMed: 21123979. [DOI] [PubMed] [Google Scholar]
- 18. Chen CC, Liu CL, Tung YN, Lee HC, Chuang HC et al. (2011) Endoscopic surgery for intraventricular hemorrhage (IVH) caused by thalamic hemorrhage: comparisons of endoscopic surgery and external ventricular drainage (EVD) surgery. World Neurosurg 75: 264-268. doi: 10.1016/j.wneu.2010.07.041. PubMed: 21492728. [DOI] [PubMed] [Google Scholar]
- 19. Basaldella L, Marton E, Fiorindi A, Scarpa B, Badreddine H et al. (2012) External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: a comparative retrospective analysis on outcome and shunt dependency. Neurosurg Focus 32: E4. doi: 10.3171/2011.12.FOCUS11323. PubMed: 22463114. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC et al. (2009) The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLOS Med 6(7): e1000100. doi: 10.1371/journal.pmed.1000100. PubMed: 196315071962251219621070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185: 914–919. doi: 10.1001/jama.1963.03060120024016. PubMed: 14044222. [DOI] [PubMed] [Google Scholar]
- 22. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ et al. (1996) Assess the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trails 17: 1-12. doi: 10.1016/S0197-2456(96)90740-0. [DOI] [PubMed] [Google Scholar]
- 23. Wells G, Shea B, O’Connell D (2006) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa Health Research Institute (OHRI). [Google Scholar]
- 24. Rosenberg MS (2005) The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59: 464-468. doi: 10.1111/j.0014-3820.2005.tb01004.x. PubMed: 15807430. [DOI] [PubMed] [Google Scholar]
- 25. Song TM, Yan SW (2010) Analysis of clinical therapeutic effect of early applying minimally invasive surgery for intraventricular hemorrhage. Jilin Medical journal 31: 2205-2206. [Google Scholar]
- 26. Lang M, Tang SH, Liu JH (2009) Neuroendoscopic surgery for intraventricular hemorrhage: a clinical research. Minimally Invasive Medicine Journal 4: 362-364. [Google Scholar]
- 27. Duan HB, Kuai D, Hao CY, Liu YT, Liu H et al. (2007) Endoscopic surgery for hypertensive cerebral hemorrhage with intraventricular hematoma, a randomized controlled trial. China Journal of Emergency Resuscitation and Disaster Medicine 2: 75-77. [Google Scholar]
- 28. Zhang HL, Zhan RC, Qi ZL, Tian AM, Qi JG (2008) Early applied neuroendoscopy for intraventricular hematoma caused by hypertensive. Chinese Journal Of Practical Nervous Diseases 11: 18-20. [Google Scholar]
- 29. Yu LL, Guo YM, Zhan J, Wu YS (2012) Comparison of neuroendoscope and craniotomy for intraventricular hemorrhage: a controlled trails. Chinese Journal of Postgraduates of Medicine 35: 59-61. [Google Scholar]
- 30. Lu ZW, Zhang MR, Li MK (2011) Comparison of treatment effect on hypertension ventricular hemorrhage using endoscope-controlled operations, Burr-hole craniotomy and urokinase perfusion through ventricular puncture. Clinical Medicine of China 27: 1192-1195. [Google Scholar]
- 31. Wang LF, Wang XZ, Zhang WZ, Lin L, Wu PH (2011) Clinical analysis the neuroendoscopic surgery for intraventricular hemorrhage. China Prac Med 06: 64-66. [Google Scholar]
- 32. Li WJ, Liu QG, Liu MM (2013) Evacuation of intraventricular hemorrhage via neuroendoscopy. Chinese Journal of Postgraduates of Medicine 35: 47-48. [Google Scholar]
- 33. Ritschl E, Auer LM (1987) Endoscopic evacuation of an intracerebral and intraventricular haemorrhage. Arch Dis Child 62: 1163-1165. doi: 10.1136/adc.62.11.1163. PubMed: 3688921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou H, Zhang Y, Liu L, Huang Y, Tang Y et al. (2011) Minimally invasive stereotactic puncture and thrombolysis therapy improves long-term outcome after acute intracerebral hemorrhage. J Neurol 258: 661-669. doi: 10.1007/s00415-011-5902-7. PubMed: 21340523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garton HJ, Kestle JR, Cochrane DD, Steinbok P (2002) A cost-effectiveness analysis of endoscopic third ventriculostomy. Neurosurgery 51: 68-77; discussion: [DOI] [PubMed] [Google Scholar]
- 36. Singh D, Saxena A, Jagetia A, Singh H, Tandon MS et al. (2012) Endoscopic observations of blocked ventriculoperitoneal (VP) shunt: a step toward better understanding of shunt obstruction and its removal. Br J Neurosurg 26: 747-753. doi: 10.3109/02688697.2012.690908. PubMed: 22591406. [DOI] [PubMed] [Google Scholar]
- 37. Hamada H, Hayashi N, Kurimoto M, Umemura K, Nagai S et al. (2008) Neuroendoscopic removal of intraventricular hemorrhage combined with hydrocephalus. Minim Invasive Neurosurg 51: 345-349. doi: 10.1055/s-0028-1085449. PubMed: 19061146. [DOI] [PubMed] [Google Scholar]
- 38. Morgan T, Awad I, Keyl P, Lane K, Hanley D (2008) Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl 105: 217-220. doi: 10.1007/978-3-211-09469-3_41. PubMed: 19066112. [DOI] [PubMed] [Google Scholar]
- 39. Patil V, Lacson R, Vosburgh KG, Wong JM, Prevedello L et al. (2013) Factors associated with external ventricular drain placement accuracy: data from an electronic health record repository. Acta Neurochir (Wien).[Epub ahead of print]. PubMed: 23700258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsieh PC, Cho DY, Lee WY, Chen JT (2005) Endoscopic evacuation of putaminal hemorrhage: how to improve the efficiency of hematoma evacuation. Surg Neurol 64: 147-153; discussion: 10.1016/j.surneu.2004.11.028. PubMed: 16051009. [DOI] [PubMed] [Google Scholar]
- 41. Zhu H, Wang Z, Shi W (2012) Keyhole endoscopic hematoma evacuation in patients. Turk Neurosurg 22: 294-299. PubMed: 22664995. [DOI] [PubMed] [Google Scholar]
- 42. Kuo LT, Chen CM, Li CH, Tsai JC, Chiu HC et al. (2011) Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage: case selection, surgical technique, and long-term results. Neurosurg Focus 30: E9. doi: 10.3171/2011.2.FOCUS10313. PubMed: 21456936. [DOI] [PubMed] [Google Scholar]
- 43. Oertel JM, Mondorf Y, Gaab MR (2009) Endoscopic third ventriculostomy in obstructive hydrocephalus due to giant basilar artery aneurysm. J Neurosurg 110: 14-18. doi: 10.3171/2008.7.JNS0887. PubMed: 18991498. [DOI] [PubMed] [Google Scholar]
- 44. Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA et al. (2000) Treatment of intraventricular hemorrhage with urokinase : effects on 30-Day survival. Stroke 31: 841-847. doi: 10.1161/01.STR.31.4.841. PubMed: 10753985. [DOI] [PubMed] [Google Scholar]
- 45. Brand RA (2009) Standards of reporting: the CONSORT, QUORUM, and STROBE guidelines. Clin Orthop Relat Res 467: 1393-1394. doi: 10.1007/s11999-009-0786-x. PubMed: 19296187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.
(DOC)
Qualitative Assessment of included studies.
(DOC)
sensitivity analysis of VCS influence the mortality.
(DOC)
sensitivity analysis of VCS influence the GFO.
(DOC)
sensitivity analysis of VCS influence the VP dependent rate.
(DOC)
Funnel plot of hematoma evacuation rate between NE group and EVD + IVF group.
(DOC)
Funnel plot of GFO between NE group and EVD + IVF group.
(DOC)
Funnel plot of VP dependent rate between NE group and EVD + IVF group.
(DOC)