Abstract
Purpose
Previous studies suggest that the concentration of 25-hydroxyvitamin D [25(OH)D] in cord blood may show an inverse association with respiratory tract infections (RTI) during childhood. The aim of the present study was to examine the influence of 25(OH)D concentrations in cord blood on infant RTI in a Korean birth cohort.
Methods
The levels of 25(OH)D in cord blood obtained from 525 Korean newborns in the prospective COhort for Childhood Origin of Asthma and allergic diseases were examined. The primary outcome variable of interest was the prevalence of RTI at 6-month follow-up, as diagnosed by pediatricians and pediatric allergy and pulmonology specialists. RTI included acute nasopharyngitis, rhinosinusitis, otitis media, croup, tracheobronchitis, bronchiolitis, and pneumonia.
Results
The median concentration of 25(OH)D in cord blood was 32.0 nmol/L (interquartile range, 21.4 to 53.2). One hundred and eighty neonates (34.3%) showed 25(OH)D concentrations less than 25.0 nmol/L, 292 (55.6%) showed 25(OH)D concentrations of 25.0-74.9 nmol/L, and 53 (10.1%) showed concentrations of ≥75.0 nmol/L. Adjusting for the season of birth, multivitamin intake during pregnancy, and exposure to passive smoking during pregnancy, 25(OH)D concentrations showed an inverse association with the risk of acquiring acute nasopharyngitis by 6 months of age (P for trend=0.0004).
Conclusion
The results show that 89.9% of healthy newborns in Korea are born with vitamin D insufficiency or deficiency (55.6% and 34.3%, respectively). Cord blood vitamin D insufficiency or deficiency in healthy neonates is associated with an increased risk of acute nasopharyngitis by 6 months of age. More time spent outdoors and more intensified vitamin D supplementation for pregnant women may be needed to prevent the onset of acute nasopharyngitis in infants.
Keywords: Cohort studies, Infant, Respiratory tract infections, Umbilical cord blood, Vitamin D
Introduction
Vitamin D plays an important role in bone and mineral metabolism1). Recent evidence suggests that vitamin D deficiency is associated with obesity2), insulin resistance2), cardiovascular diseases3), autoimmune diseases3), asthma4), recurrent wheezing5), and infection5,6). It is estimated that 40% of pregnant women and 50% of newborns and infants in Western countries suffer from vitamin D insufficiency or deficiency7-9). A recent study based on the Fourth Korea National Health and Nutrition Examination Surveys, which involved 3,047 males and 3,878 females aged 10 years and older, showed that vitamin D insufficiency or deficiency is very common in Korea, particularly in the younger generation10).
The results of epidemiologic studies implementing cross-sectional designs suggest that vitamin D may protect against respiratory tract infections (RTI)11-13). However, few birth cohort studies have examined the role of cord blood vitamin D concentrations in the susceptibility of infants to RTI5,6). These associations have not been adequately examined in Asian populations. Such research is important, since Koreans, particularly the younger generation, have low vitamin D levels10,14).
The present healthy birth cohort study aimed to examine the association between cord blood 25(OH)D concentrations and the subsequent risk of RTI in infants during the first 6 months of life. The primary hypothesis was that cord blood 25(OH)D concentrations are inversely associated with the risk of RTI in infants.
Materials and methods
1. Study design, study population, data sources, and management
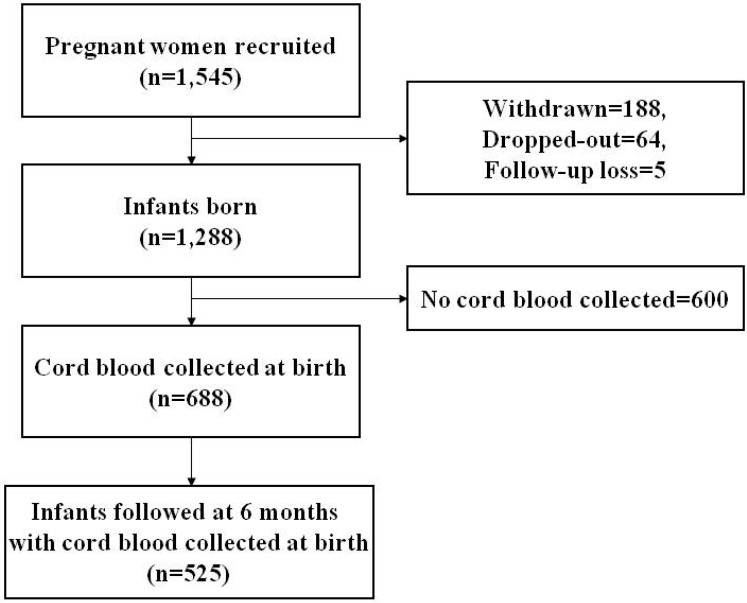
The COhort for Childhood Origin of Asthma and allergic diseases (COCOA) is an ongoing prospective hospital-based birth cohort study. Its aim is to examine the relationship between maternal lifestyle and the subsequent development of allergic diseases in Korean children. The present study investigated 1,545 unselected pregnant women from this cohort. The women received antenatal care at four different hospitals and seven different Public Health Centers between December 2007 and November 2011. Subjects with diabetes, preeclampsia, anemia, and severe infections during pregnancy, which might have an effect on the development of allergic disease, were excluded. The study was approved by the Institutional Review Boards of the Asan Medical Center (IRB No. 2008-0616), the Samsung Medical Center (IRB No. 2009-02-021), the Severance Hospital (IRB No. 4-2008-0588), and the Gangnam CHA Medical Center (IRB No. 2010-010). Written informed consent was obtained from the parents of each infant and confirmed by each IRB. Of the 1,545 pregnant women initially enrolled, 188 withdrew from the study, 64 failed to fulfill the inclusion criteria, and 5 were lost to follow-up at delivery. Thus, 1,288 subjects were included in the analysis. Cord blood was obtained from 688 infants. A total of 882 children were followed-up at 6 months of age. The primary outcome variable of interest was the prevalence of RTI at 6-month follow-up, as diagnosed by pediatricians and pediatric allergy and pulmonology specialists. RTI was classified as acute nasopharyngitis, rhinosinusitis, otitis media, croup, tracheobronchitis, bronchiolitis, or pneumonia. Finally, the association between cord blood vitamin D concentrations and the occurrence of RTI during the first 6 months of age was analyzed in 525 infants (Fig. 1).
Fig. 1.
Flow chart showing the study population.
2. Measurement of cord blood 25(OH) vitamin D levels
Cord blood was collected immediately after delivery and anticoagulated with sodium heparin. Plasma was prepared by centrifugation (10 minutes at 500×g) and stored at -80℃. Plasma 25(OH)D concentrations [nmol/L (converted to ng/mL by dividing by 2.496)] were measured in a chemiluminescence immunoassay (Roche, Indianapolis, IN, USA). The interassay variability for the pooled plasma analyses was 19% at 33 nmol/L 25(OH)D, 12% at 62 nmol/L, and 10% at 99 nmol/L. The plasma concentrations of 25(OH)D were analyzed both as a continuous variable and by division into tertiles5,15). The following definition was used to classify cord blood 25(OH)D concentrations: deficiency, <25.0 nmol/L; insufficiency, 25.0-74.9 nmol/L; and sufficiency, ≥75.0 nmol/L5,15).
3. Statistical analysis
Cord blood plasma 25(OH)D concentrations showed a normal distribution. Values were expressed as the mean or as a percentage according to the tertile of cord blood 25(OH)D concentrations. P values for trends were determined using the general linear model for continuous variables. The chi-square test was used to assess the association between categorical variables. Logistic regression analysis was performed to determine the effect of cord blood 25(OH)D concentrations on the risk of subsequent RTI, and was adjusted for potential confounders. Multivitamin use during pregnancy, season of birth (spring, summer, autumn, or winter), and passive smoking during pregnancy showed the highest association with cord blood 25(OH)D concentrations in the single variable analyses, and were therefore included in the regression models. Analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). A two-tailed P value of <0.05 was considered statistically significant.
Results
1. Population characteristics
Among the 525 newborns in whom cord blood 25(OH)D concentrations were measured and were followed-up at 6 months of age, 171 (32.6%) were recruited at Asan Medical Center, 246 (46.9%) at Samsung Medical Center, 77 (14.7%) at Severance Medical Center, and 31 (5.9%) at Gangnam CHA Medical Center. The median gestational age was 39+2 weeks (interquartile range [IQR], 38+3-40+0), and the mean birth weight, height, and head circumference were 3.2±0.4 kg, 49.5±1.8 cm, and 34.4±1.3 cm, respectively. The mean maternal age at birth was 32.2±3.4 years. Approximately half of the newborns were male (n=284, 54.1%). There was no difference in the baseline characteristics of the participating and non-participating subjects.
2. Cumulative incidence of respiratory tract infections during the first 6 months of life
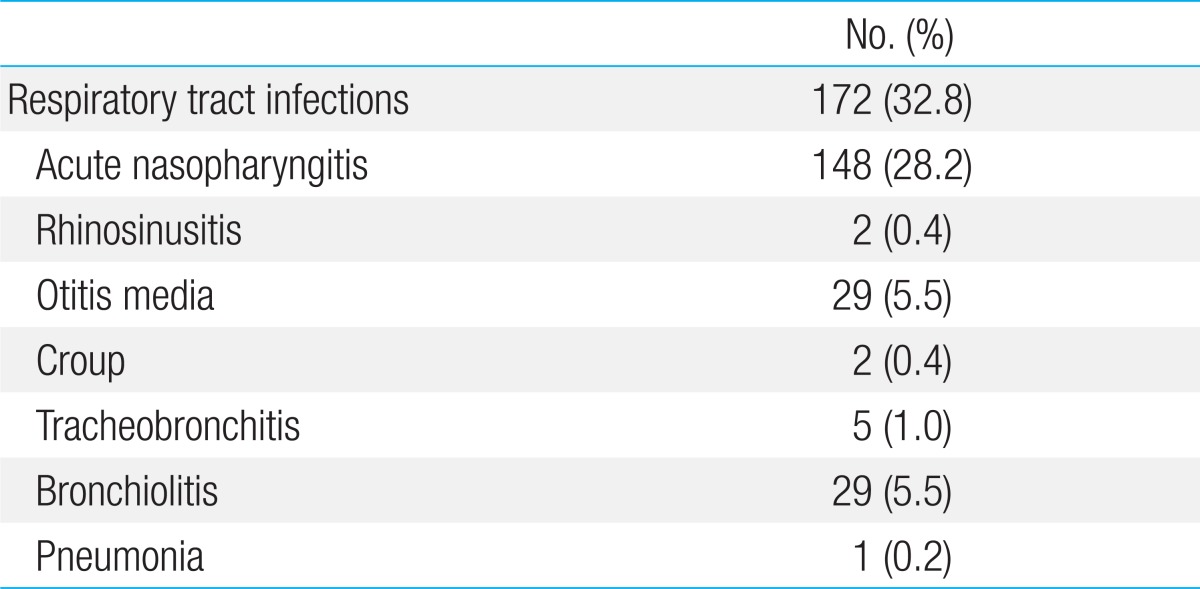
Table 1 shows the cumulative incidence of various RTI within the first 6 months of life. Among the 525 infants analyzed, the incidence of acute nasopharyngitis, rhinosinusitis, otitis media, croup, tracheobronchitis, bronchiolitis, and pneumonia was 148 (28.2%), 2 (0.4%), 29 (5.5%), 2 (0.4%), 5 (1.0%), 29 (5.5%), and 1 (0.2%), respectively. The total incidence of RTI was 172 (32.8%). Cases of rhinosinusitis (n=2), croup (n=2), tracheobronchitis (n=5), and pneumonia (n=1) were excluded from the analysis due to low incidence.
Table 1.
Cumulative incidence of respiratory tract infections during the first 6 months of life
3. High prevalence of vitamin D insufficiency or deficiency in healthy newborns
The mean cord blood plasma 25(OH)D concentration among healthy newborns was 32.0 nmol/L (IQR, 21.4 to 53.2). As shown in Table 2, low vitamin D concentrations were more common among neonates born in spring and winter, whereas high vitamin D concentrations were more common among those born in autumn (P for trend<0.0001). Higher concentrations of 25(OH)D were found among mothers who had taken multivitamins during pregnancy (P for trend=0.0008). Overall, 180 neonates (34.3 %) showed 25(OH)D concentrations of less than 25.0 nmol/L,
Table 2.
Potential confounders according to cord blood levels of 25(OH)D
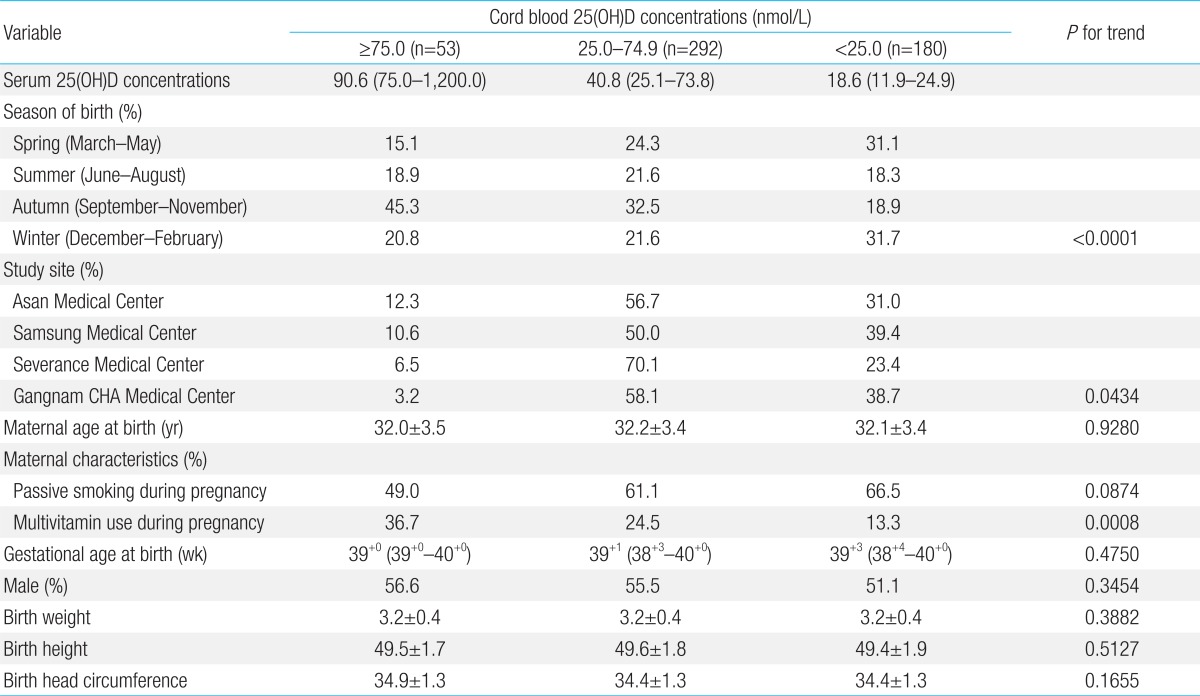
Values are presented as median (range), number (%), or mean±standard deviation. P-values for trends were determined using the Chi-square test (for passive smoking and multivitamin use) and by linear regression analysis (season of birth) using dummy variables. 25(OH)D, 25-hydroxyvitamin D.
4. Prevalence of respiratory tract infections in infants within the first 6 months of life according to cord blood concentrations of 25(OH)D
Table 3 shows the prevalence of RTI during the first 6 months of life according to cord blood concentrations of 25(OH)D. Infants with 25(OH)D concentrations less than 25.0 nmol/L were more likely to develop acute nasopharyngitis (P for trend=0.0004). There was no difference in the prevalence of otitis media or bronchiolitis according to cord blood 25(OH)D concentrations (P for trend=0.4554 and P for trend=0.3718, respectively). Extending the outcome to include the risk of any RTI, infants born with a 25(OH)D concentration of less than 25.0 nmol/L were more likely to develop RTI (P for trend=0.0021).
Table 3.
Prevalence of respiratory tract infections within the first 6 months of age according to cord blood concentrations of 25(OH)D
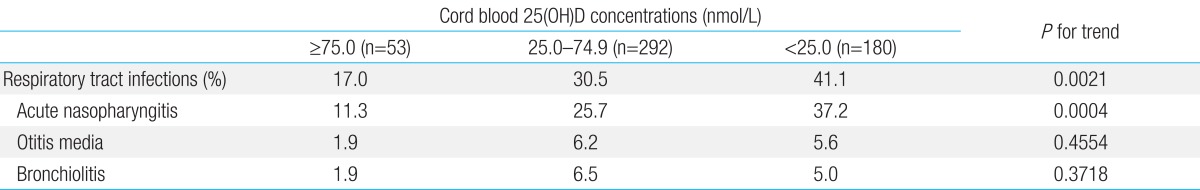
25(OH)D, 25-hydroxyvitamin D.
5. Cord blood 25(OH) vitamin D concentrations were associated with acute nasopharyngitis during the first 6 months of life
Binary logistic regression analysis showed that newborns showing 25(OH)D concentrations less than 25.0 nmol/L were 4.64 times more likely to develop acute nasopharyngitis than those showing concentrations of 75.0 nmol/L or higher (unadjusted odds ratio [OR], 4.64) (Table 4), and that newborns showing 25(OH)D concentrations of 25.0-74.9 nmol/L were 2.71 times more likely to develop acute nasopharyngitis than those showing concentrations of 75.0 nmol/L or higher (unadjusted OR, 2.71) (Table 4). Cord blood 25(OH)D concentrations had no effect on the development of otitis media or bronchiolitis (P for trend=0.3625 and P for trend=0.4819, respectively) (Table 4). The multivariate logistic model, adjusted for multivitamin use during pregnancy and the season of birth, showed that neonates showing cord blood 25(OH)D concentrations less than 25.0 nmol/L were 5.34 times more likely to develop acute nasopharyngitis within the first 6 months of life, and that newborns showing 25(OH)D concentrations of 25.0-74.9 nmol/L were 3.17 times more likely to develop acute nasopharyngitis than those showing concentrations of 75.0 nmol/L or higher (P for trend=0.0003) (Table 5). Cord blood 25(OH)D concentrations had no effect on the development of otitis media or bronchiolitis (P for trend=0.5031 and P for trend=0.4590, respectively) (Table 5). The multivariate logistic model adjusted for multivitamin use during pregnancy, season of birth, and exposure to passive smoking during pregnancy also demonstrated that infants showing cord blood 25(OH)D concentrations less than 25.0 nmol/L were 5.21 times more likely to develop acute nasopharyngitis within the first 6 months of life, and that newborns showing 25(OH)D concentrations of 25.0-74.9 nmol/L were 2.99 times more likely to develop acute nasopharyngitis than those with concentrations of 75.0 nmol/L or higher (P for trend=0.0004) (Table 5). Cord blood 25(OH)D concentrations had no effect on the development of otitis media or bronchiolitis (P for trend=0.3745 and P for trend=0.4485, respectively) (Table 5). When acute nasopharyngitis, otitis media, and bronchiolitis were analyzed together as RTI, the multivariate logistic model adjusted for multivitamin use during pregnancy, season of birth, and exposure to passive smoking during pregnancy showed that infants showing cord blood 25(OH)D concentrations less than 25.0 nmol/L were 3.56 times more likely to develop any RTI within the first 6 months of life, and that newborns showing 25(OH)D concentrations of 25.0-74.9 nmol/L were 2.06 times more likely to develop any RTI than those with concentrations of 75.0 nmol/L or higher (P for trend=0.0015) (Table 5).
Table 4.
Association between respiratory tract infections within the first 6 months of age and cord blood concentrations of 25(OH)D according to the binary logistic regression model
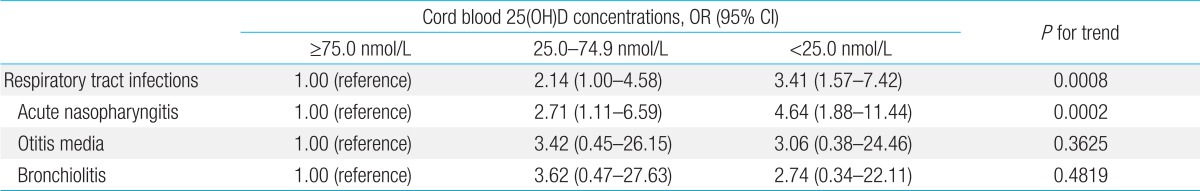
25(OH)D, 25-hydroxyvitamin D; OR, odds ratio; CI, confidence interval.
Table 5.
Association between respiratory tract infections within the first 6 months of age and cord blood concentrations of 25(OH)D according to the multivariate logistic regression analysis models
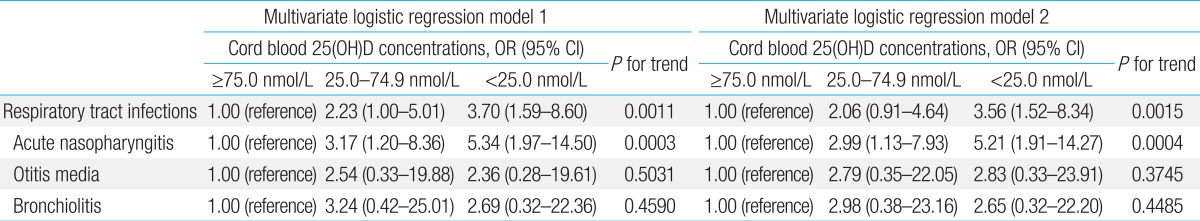
Multivariate logistic model 1 was adjusted for multivitamin use during pregnancy (yes/no) and season of birth (spring, summer, autumn, or winter); multivariate logistic model 2 was adjusted for multivitamin use during pregnancy (yes/no), season of birth (spring, summer, autumn, or winter), and exposure to passive smoking during pregnancy (yes/no). 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio; CI, confidence interval.
6. There was no association between respiratory tract infections during the first 6 months of life and season of birth, birth weight, vitamin supplementation after birth, feeding methods, and monthly household income
Binary logistic regression analyses showed that there was no association between RTI during the first 6 months of life and clinical factors such as the season of birth, birth weight, vitamin supplementation after birth, feeding methods such as breast feeding, bottle feeding, and mixed feeding, and monthly household income (data not shown).
Discussion
The results of this prospective birth cohort study of 525 apparently healthy Korean children show that 89.9% of healthy newborns in Korea are born with vitamin D insufficiency or deficiency (55.6% and 34.3%, respectively). Overall, 34.3% of the children showed vitamin D concentrations less than 25.0 nmol/L and 55.6% showed vitamin D concentrations between 25.0 and 74.9 nmol/L. Only 10.1% of children showed optimal concentrations of 25(OH)D. Low cord blood vitamin D concentrations were associated with a higher risk of developing acute nasopharyngitis during the first 6 months of life. By contrast, vitamin D status at birth was not associated with otitis media and bronchiolitis. This association seems to be robust, and shows a clinically and statistically significant association when controlling for potential confounders such as multivitamin use during pregnancy, season of birth, and exposure to passive smoking during pregnancy. To the best of our knowledge, this is the first longitudinal study to identify a relationship between cord blood 25(OH)D concentrations and the subsequent risk of RTI in an Asian population.
The prevalence of vitamin D insufficiency or deficiency in this birth cohort was much higher than that previously reported for Western countries5,6,9,16-18). This finding may be due to ethnic differences19), greater skin pigmentation1), and a different geographical latitude1). A recent study showed that vitamin D insufficiency or deficiency was highly prevalent in Korean adolescents, particularly girls14). This may be due to reduced consumption of dairy products and less time spent doing outdoor activities14). Although we did not measure maternal vitamin D concentrations, we can speculate that the low vitamin D status of adolescent girls may persist into adulthood, ultimately affecting the vitamin D status of their offspring.
The present study is the first to identify an inverse association between cord blood 25(OH)D concentrations and RTI, particularly acute nasopharyngitis, during infancy in an Asian population. There is no clear explanation as to how a single cord blood concentration can predict the risk of developing acute nasopharyngitis during early infancy; however, there are two theories to rationalize this finding. Firstly, vitamin D is important for maintaining immune homeostasis, and there might be certain time windows during pregnancy in which exposure to adequate levels of vitamin D is critical. Therefore, the vitamin D status of the pregnant woman may influence the development of the fetal and neonatal immune system20) to a much greater extent than that of older children. Furthermore, a recent birth cohort study showed that cord blood 25(OH)D concentrations protected against the development of RTI and wheezing, even in children at 5 years of age5). Secondly, human studies show that 1,25-dihydroxyvitamin D promotes surfactant production21), and downstream effectors of vitamin D have been identified in fetal lungs as early as at 14 weeks of gestation22); thus, it can be inferred that vitamin D may expedite fetal lung development23-26), thereby protecting against RTI.
A growing body of research into the role of vitamin D in innate immunity suggests that it plays an important role in regulating innate immune responses in the upper respiratory tract11-13). For example, vitamin D induces the lung epithelial cells to produce cathelicidin and β-defensin-2, peptides that have antimicrobial activity11,27). Indeed, the vitamin D receptor genes are located adjacent to the genes that encode these two antimicrobial peptides27). Recently, a study that measured serum 25(OH)D levels in 84 children with recurrent tonsillitis and 71 controls between the ages of 2 and 10 years found that serum 25(OH)D levels in the recurrent tonsillitis group were significantly lower than those in the controls12). This suggests that high serum 25(OH)D concentrations may protect children against recurrent tonsillitis12). In addition, previous studies incorporating a cross-sectional design show an association between low serum vitamin D concentrations and increased severity of RTI during childhood28-31). This finding is supported by in vitro studies reporting that vitamin D induces epithelial cells, macrophages, monocytes, and granulocytes to produce antimicrobial peptides such as cathelicidin and defensin 11), and also decreases chemokine and interferon release by virus-infected epithelial cells, thereby inhibiting the inflammatory response32,33). Other studies suggest that vitamin D concentrations of up to 100 nmol/L (40 ng/mL)34), or supplementation with vitamin D35), may expedite cathelicidin production.
The present study has some limitations. First, we did not ask the women how much sunlight they were exposed to. We included the season of birth in the analysis as a surrogate marker of sun exposure, which indicated that cord blood 25(OH)D levels are an independent predictor for the occurrence of acute nasopharyngitis in neonates. Second, we did not obtain any data regarding behavioral factors that may affect the cutaneous synthesis of vitamin D, such as the degree of skin pigmentation, sunscreen use, or coverage of the skin by clothing. Third, no detailed information about dietary habits was available. Finally, we did not measure maternal vitamin D concentrations, which are known to have a significant impact on cord blood vitamin D concentrations in the offspring.
In conclusion, we found that vitamin D insufficiency or deficiency is highly prevalent in healthy Korean newborns, and that low cord blood 25(OH)D concentrations are associated with an increased risk of developing acute nasopharyngitis during early infancy. The results suggest that more time spent outdoors and increased vitamin D supplementation may be necessary to increase vitamin D concentrations in pregnant women in order to protect their offspring from acute nasopharyngitis during early infancy. Randomized trials are warranted to address this question further.
Acknowledgments
This research was supported by a grant (Seokchun Award) from the Korean Pediatric Society in 2011 and funds (2008-E33030-00, 2009-E33033-00, and 2011-E33021-00) from the Research of Korea Centers for Disease Control and Prevention.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97:774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 4.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr. 2011;158:437–441. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 5.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 6.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 8.Merewood A, Mehta SD, Grossman X, Chen TC, Mathieu JS, Holick MF, et al. Widespread vitamin D deficiency in urban Massachusetts newborns and their mothers. Pediatrics. 2010;125:640–647. doi: 10.1542/peds.2009-2158. [DOI] [PubMed] [Google Scholar]
- 9.Camargo CA, Jr, Ingham T, Wickens K, Thadhani RI, Silvers KM, Epton MJ, et al. Vitamin D status of newborns in New Zealand. Br J Nutr. 2010;104:1051–1057. doi: 10.1017/S0007114510001674. [DOI] [PubMed] [Google Scholar]
- 10.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96:643–651. doi: 10.1210/jc.2010-2133. [DOI] [PubMed] [Google Scholar]
- 11.Bartley J. Vitamin D, innate immunity and upper respiratory tract infection. J Laryngol Otol. 2010;124:465–469. doi: 10.1017/S0022215109992684. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz I, Unuvar E, Zeybek U, Toptas B, Cacina C, Toprak S, et al. The role of vitamin D in children with recurrent tonsillopharyngitis. Ital J Pediatr. 2012;38:25. doi: 10.1186/1824-7288-38-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin YH, Kim KE, Lee C, Shin HJ, Kang MS, Lee HR, et al. High prevalence of vitamin D insufficiency or deficiency in young adolescents in Korea. Eur J Pediatr. 2012;171:1475–1480. doi: 10.1007/s00431-012-1746-0. [DOI] [PubMed] [Google Scholar]
- 15.Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–598. [PMC free article] [PubMed] [Google Scholar]
- 16.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202:436.e1–436.e8. doi: 10.1016/j.ajog.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Meer IM, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, et al. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84:350–353. doi: 10.1093/ajcn/84.1.350. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–1111. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erkkola M, Nwaru BI, Viljakainen HT. Maternal vitamin D during pregnancy and its relation to immune-mediated diseases in the offspring. Vitam Horm. 2011;86:239–260. doi: 10.1016/B978-0-12-386960-9.00010-1. [DOI] [PubMed] [Google Scholar]
- 21.Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, et al. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab. 2002;76:46–56. doi: 10.1016/s1096-7192(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 22.Brun P, Dupret JM, Perret C, Thomasset M, Mathieu H. Vitamin D-dependent calcium-binding proteins (CaBPs) in human fetuses: comparative distribution of 9K CaBP mRNA and 28K CaBP during development. Pediatr Res. 1987;21:362–367. doi: 10.1203/00006450-198704000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. Am J Physiol Lung Cell Mol Physiol. 2005;289:L617–L626. doi: 10.1152/ajplung.00129.2004. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besancon F, Cayre YE, et al. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89-90:93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 25.Marin L, Dufour ME, Nguyen TM, Tordet C, Garabedian M. Maturational changes induced by 1 alpha,25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. Am J Physiol. 1993;265(1 Pt 1):L45–L52. doi: 10.1152/ajplung.1993.265.1.L45. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, et al. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L496–L505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 28.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 29.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 30.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–393. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 31.Inamo Y, Hasegawa M, Saito K, Hayashi R, Ishikawa T, Yoshino Y, et al. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatr Int. 2011;53:199–201. doi: 10.1111/j.1442-200x.2010.03224.x. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 35.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]