Abstract
The purpose of the study was to determine the effects of exercise-associated menstrual disorders and hormonal contraceptives (HC) on systemic inflammatory markers and endothelial function in female athletes. Thirty-nine active women (≥5 h of aerobic exercise per wk), aged 18-33 y, participated in this cross-sectional study comparing women with menstrual disorders (MD, n = 10; 0-9 cycles·y-1), eumenorrheic women (E, n = 13; 10-13 cycles·y-1), and HC users (HC, n = 16; 12 cycles·y-1). Fasting serum samples were collected during the early follicular phase (d2-5) for the menstruating women. Tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), C-reactive protein (CRP), soluble vascular adhesion molecule-1 (sVCAM-1), total cholesterol (TC), high- and low density lipoprotein-cholesterol (HDL-C, LDL-C), triglycerides (TG), reproductive hormones, and cortisol were measured in serum. Estradiol, progesterone, and cortisol were not statistically different between MD and E groups; cortisol was significantly greater in the HC versus E group (p = 0.002). TC (p = 0.005), LDL-C (p = 0.03), and CRP (p = 0.05) were increased in the HC versus MD and E groups. TNF-α was significantly higher in the HC (p=0.001) compared with the E group. There were no significant group differences in the concentrations of sVCAM-1 or IL-6. TNF-α and cortisol were positively correlated (r=0.31, p = 0. 058), as were sVCAM-1 and estradiol (r = 0.41, p = 0.010). In conclusion, HC use, but not exercise- associated menstrual disorders, is associated with increased TNFα and LDL-C.
Key Points.
Serum lipids and markers of inflammation were not altered by exercise-associated oligomenorrhea or amenorrhea.
Hormonal contraceptive users had elevated total and LDL cholesterol compared with regularly menstruating non-HC users.
C-reactive protein and tumor necrosis factor-α, but not soluble vascular adhesion molecule-1, were increased in hormonal contraceptive users.
The long-term effect of these changes on cardiovascular disease is unknown.
Key words: Cytokines, soluble vascular adhesion molecule, female reproductive disorders
Introduction
Female athletes experience menstrual disorders due to suppression of the hypothalamic-pituitary-ovarian axis by low energy availability, which results from an imbalance between energy intake and expenditure. Hypoestrogenemia causes loss of bone mass, increasing current bone fragility and risk for future osteoporosis. In addition, risk factors for cardiovascular disease (CVD) are more common in these apparently healthy, premenopausal women than in regularly menstruating athletes (O’Donnell and De Souza, 2004). Amenorrheic athletes have greater total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and triglycerides (TG) than their regularly menstruating counterparts (Friday et al., 1993). Recently, endothelial dysfunction, manifest as reduced flow-mediated dilation has been identified as another consequence of athletic amenorrhea (Rickenlund et al., 2005a; Zeni Hoch et al., 2003). It is not known if these risk factors that predict CVD in the general adult population, also are predictive in young women with exercise-associated menstrual disorders.
Postmenopausal women and women with anorexia nervosa also exhibit adverse changes in serum lipids and impaired endothelial function, suggesting that low circulating estradiol plays a causal role in these negative events (O’Donnell and De Souza, 2004). Estrogens protect against CVD by: decreasing the concentration of LDL-C in blood; reducing oxidation of LDL and other lipids; and stimulating vasodilation by increasing the production of nitric oxide and prostacyclin, thereby improving blood flow (Taddei et al., 1996) (Kublickiene et al., 2005). Estrogen replacement therapy (ERT) with estradiol or conjugated equine estrogens improves the lipid profile in postmenopausal women. Addition of progestins to the hormonal replacement therapy (HRT) may abrogate the benefits of exogenous estrogens, but the results are equivocal (Dubey et al., 2004; Silvestri et al., 2003).
Monocyte adhesion to vascular endothelial cells is an early critical step in the atherogenic process; attached monocytes differentiate to macrophages, which actively sequester cholesterol, forming fatty streaks in blood vessels. Vascular adhesion molecule-1 (VCAM-1), which is expressed by vascular endothelial and smooth muscle cells, mediates adhesion via binding of leukocytes to integrins (Cybulsky and Gimbrone, 1991), and inflammatory cytokines are known to upregulate VCAM-1 expression (Mukherjee et al., 2003). Estrogen suppresses VCAM-1 in vivo (Nathan et al., 1999) and in vitro (Mukherjee et al., 2003), apparently via an indirect mechanism, as no estrogen response element has been identified in the VCAM-1 promoter. Soluble VCAM-1 (sVCAM-1) arises via proteolytic cleavage from the cell surface; the concentration of sVCAM-1 in serum is proportional to its expression on endothelial cells (Pigott et al., 1992).
Although the deleterious effects of exercise-associated amenorrhea on blood lipids has been appreciated for sometime, impaired endothelial function was only recently described by Zeni-Hoch et al. (2003). Rickenlund et al. (2005b) subsequently investigated the mechanistic links between estrogen, inflammatory cytokines, vascular adhesion molecules, and endothelial function. In a cross-sectional study and a treatment trial with hormonal contraceptives, impaired flow-mediated dilation was associated with elevated sVCAM-1, but not with TNF-α, CRP or IL-6 in female athletes (Rickenlund et al., 2005a; 2005b). Because the beneficial effect of estrogen on endothelial function is thought to be mediated through cytokine-stimulated expression of adhesion molecules, further investigation of this causative pathway is warranted.
Thus, the purpose of this study was to determine the effects of exercise-associated menstrual disorders and hormonal contraceptive use on TNF-α, CRP, IL-6 and sVCAM-1 and on serum lipids in non-eating disordered female athletes. We hypothesized: 1) menstrual disorders secondary to low energy availability would be associated with increased TC, TG, TNF-α , IL-6, CRP, cortisol, and sVCAM-1; 2) oral contraceptive users would have increased TC, LDL-C, TNF-α , IL-6, CRP, cortisol, and decreased sVCAM-1 compared to regularly menstruating women.
Methods
Study participants
A total of 39 recreationally-trained (at least 5 hours of aerobic, weight-bearing exercise per week) female athletes, aged 18-33, were recruited from local multi-sport clubs and the University of Missouri in Columbia. Subjects were classified as: having an exercise-associated menstrual disorder (MD, n = 10; 0-9 cycles·year-1 during the previous 12 months); eumenorrheic women (E, n = 13; 10-13 cycles·year-1); using hormonal contraceptives for at least 12 months (HC, n = 16; 12 cycles·year-1) without a history of menstrual disorders. Four of the women in the MD group were amenorrheic (0 cycles·year-1) and six were oligomenorrheic (average 6 cycles·year-1). Nine subjects currently were taking monophasic oral contraceptives (combinations of norgestimate, or levonorgestrel, or norethindrone, or drospirenone and ethinyl estradiol) and 7 subjects currently were taking triphasic oral contraceptives (norgestimate and ethinyl estradiol). Prior to initial screening, all subjects were informed of any risks associated with this study, read a consent form, and gave oral consent as had been approved by the University of Missouri Health Sciences Institutional,
Review Board. Subjects with a current or previous disease, or use of medications, affecting bone including eating disorders, or who were pregnant or lactating, were excluded from the study. Subjects were paid $25 upon completion of the study. All procedures involving human subjects were in accordance with the ethical standards of the University of Missouri Institutional Review Board and with the Helsinki Declaration of 1975 as revised in 1983.
Anthropometric data
Subject weight was determined to the nearest 0.05 kg and height was determined to the nearest 0.5 cm, and used to calculate body mass index (BMI; kg·m-2). Body composition was determined from three skinfold measurements and percent body fat was calculated by the Jackson-Pollock method (Siri, 1961).
Serum lipids, hormones, and inflammatory markers
Blood (20 ml) was drawn between 0800 and 1000 hours after an overnight fast and prior to exercise from an antecubital vein. Serum was separated by centrifugation at 2000 g for 15 minutes and stored at -80oC. Sample collection occurred during the early follicular phase of the menstrual cycle, defined as days 2-5 of menstruation, for regularly menstruating subjects, i.e., eumenorrheic, and hormonal contraceptive users, and on an arbitrary day for amenorrheic subjects. All of the participants had concentrations of LH and FSH that were less than 15 IU/liter and 11 IU/liter, respectively, consistent with the early follicular phase or anovulatory amenorrhea.
TC, HDL-C and TG were measured using enzymatic methods (Sigma Dianostics, St. Louis, MO). LDL-C was estimated using the Friedwald equation (Friedewald et al., 1972). TNF-α, IL-6, sVCAM-1, and CRP were measured using ELISA (ALPCO Diagnostics, Windham, NH; intra-assay CVs were 5.5%, 10.2%, 5.7%, 9.1% respectively). LH, FSH, cortisol, progesterone, and parathyroid hormone (PTH) were measured using ELISA (ALPCO Diagnostics, Windham, NH; intra-assay CVs were 8.6%, 6.6%, 10.4%, 9.1%, 2.7%, respectively). Estradiol, leptin, and prolactin (PRL) were measured using ELISA (Diagnostic Systems Laboratories, Inc, Webster, Texas; intra-assay CVs were 6.5%, 7.9%, 6.8%, respectively).
Questionnaires
All subjects completed a medical, menstrual, and sports history questionnaire. Physical activity was quantified using a 7-day written training log of activity type, duration, and frequency. The Compendium of Physical Activities was used to estimate daily energy expenditure (Ainsworth et al., 2000). Menstrual history was assessed using a written questionnaire that consisted of a timeline on which participants indicated menarche, missed menstrual periods, initiation and cessation of hormonal contraceptive use.
Statistical analyses
CRP, TC, and TG were not normally distributed, so analyses were performed using a logarithmic transformation. One-way ANOVA with the least significant difference technique was used to determine differences among groups. Pearson correlations between inflammatory markers and hormones (cortisol, estradiol, progesterone) were performed. Statistical analyses were conducted using SAS version 8.2 (Cary, 1999) and statistical significance was set at p < 0.05.
Results
Participant characteristics
The MD group had significantly lower body weight, BMI, and percent body fat than the E and HC groups (Table 1). The number of missed menstrual cycles since menarche was greater in the MD group than in the E and HC groups, but did not differ between E and HC groups. Finally, the HC group had been using hormonal contraceptives for a significantly greater number of months than the MD and E groups. There was no significant difference between MD and E groups in the number of months of hormonal contraceptive use. No significant differences existed among the menstrual status groups in hours of physical activity participation per week or in energy expended in exercise per day. The HC group had significantly greater concentrations of cortisol and lower concentrations of progesterone in serum than the E and MD groups (Table 2).
Table 1.
Characteristics of female athletes with menstrual disorders (MD), regular menstrual cycles (E), or hormonal contraceptive (HC) users. Data are means (±SD) or median and range.
| MD a (n = 10) |
E b (n = 13) |
HC c (n = 16) |
|
|---|---|---|---|
| Age (yrs) | 22.7 (3.2) | 21.1 (3.8) | 23.2 (3.54) |
| Menarchal age (yrs) | 13.4 (1.5) | 13.8 (1.7) | 13.0 (1.3) |
| Weight (kg) | 54.2 (6.1) b | 62.2 (7.8) a | 61.8 (8.1) a |
| Height (m) | 1.66 (.05) | 1.68 (.05) | 1.67 (.06) |
| BMI (kg·m-2) | 19.6 (1.7)b | 22.0 (2.2) a | 22.2 (2.4) a |
| Body fat (%) | 18.2 (3.7) b | 20.4 (5.2) a,c | 23.2 (4.4) a |
| Lean body mass (kg) | 41.3 (5.5) | 46.4 (5.8) | 45.1 (5.8) |
| Menstrual cycles per year | 4 (3) b | 12 (1) a | 12 (1) a |
| Missed menstrual cycles (since menarche) | 47 (16-57) b | 4 (0-21) a | 3.5 (0-36) a |
| Months on hormonal contraceptives | 12 (0-84) c | 0 (0-120) c | 78 (36-192) a,b |
| Physical activity (hrs·wk-1) | 11.2 (3.9) | 9.2 (3.0) | 11.7 (6.0) |
| Physical activity energy expenditure (kcal·d-1) | 622 (237) | 604 (213) | 596 (220) |
Superscripts denote significantly (p < 0.05) differences between the groups.
Table 2.
Hormones, markers of inflammation and endothelial function in serum of female athletes with menstrual disorders (MD), regular menstrual cycles (E), or hormonal contraceptive (HC) users. Data are means (± SD) or median and range.
| Hormone | MD a (n = 10) |
E b (n = 13) |
HC c (n = 16) |
Normal Range |
|---|---|---|---|---|
| Estradiol (pmol·L-1) | 378 (117) | 330 (143) | 323 (198) | 110–1470 |
| Progesterone (nmol·L-1) | 3.56 (1.23) c | 3.15 (.62) c | 2.60 (1.03) a,b | < 3.2 |
| LH (IU·L-1) | 6.4 (1.5-17.1) | 6.8 (4.0-14.1) | 2.5 (1.1-17.1) | 1.7–15 |
| FSH (IU·L-1) | 4.7 (2.7-6.1) | 4.2 (2.7-6.4) | 3.5 (1.6-15.0) | 1.8–11.2 |
| Leptin (ng·mL-1) | 6.5 (1.7-16.1) | 6.8 (2.1-22.8) | 12.3 (4.3-27.9) | 2.5–9.0 |
| Cortisol (nmol·L-1) | 1137 ± 448 c | 1068 ± 379 c | 1653 ± 276 a,b | 170–860 |
| PTH (ng·L-1) | 47 (12) | 42 (19) | 43 (24) | 10–65 |
| Prolactin(pmol·L-1) | 503 (261-1043) | 565 (274-1710) | 636 (270-1913) | < 4000 |
| TNF-a (pg·mL-1) | 19.1 (17.9-33.2)c | 12.4 (6.7-33.1) c | 34.9 (18.6-43.1) a,b | <2 |
| CRP (mg·L-1) | .50 (0-5.76) c | 2.40 (0-13.41) c | 4.42 (0-13.87) a,b | <10 |
| IL-6 (pg·mL-1) | 7.1 (2.9-12.4) | 9.1 (2.2-36.8) | 11.5 (3.5-88.7) | <150 |
| sVCAM-1 (ng·mL-1) | 955 (572-1245) | 1072 (753-1726) | 970 (790-1194) | 400-800 |
Superscripts denote significantly (p < 0.05) differences between the groups.
We expected that the concentration of estradiol in serum would be low in all groups. The women with exercise-associated menstrual disorders were presumed to have suppressed ovarian hormone production due to decreased LH secretion. We expected the menstruating women to have low circulating estradiol because we measured reproductive hormones during the early follicular phase of the menstrual cycle when estradiol secretion is lowest. Thus, we did not expect to detect statistically significant differences in estradiol concentrations among groups.
Serum lipids
TC and LDL-C were significantly higher in the HC group compared to the E and MD groups (p < 0.01, Table 3). HDL-C and TG did not differ among groups (p = 0.1, Table 3). There were no differences in serum lipids between amenorrheic and oligomenorrheic women in the MD group (data not shown).
Table 3.
Cholesterol and triglyceride concentrations in serum of female athletes with menstrual disorders (MD), regular menstrual cycles (E), or hormonal contraceptive (HC) users. Data are means (± SD).
| Hormone | MD a (n = 10) |
E b (n = 13) |
HC c (n = 16) |
Desirable Range |
|---|---|---|---|---|
| TG (mmol·L-1) | 1.06 (.36) | 1.03 (.23) | 1.28 (.43) | <1.695 |
| TC (mmol·L-1) | 3.83 (.47) c | 3.94 (.67) c | 4.74 (.96) a,b | <5.18 |
| LDL-C (mmol·L-1) | 2.02 (.72) c | 2.02 (.60)c | 2.61 (.76)a,b | <2.59 |
| HDL-C (mmol·L-1) | 1.33 (.31) | 1.45 (.33) | 1.53 (.26) | >1.036 |
Superscripts denote significantly (p < 0.05) differences between the groups.
Inflammatory markers
TNF-α and CRP were significantly elevated in the HC compared to the E group (Table 2, Figure 1). The MD group tended to have elevated TNF-α compared to the E group, but this difference was not statistically significant (p = 0.062). sVCAM-1 and IL-6 did not differ among groups (p > 0.05). There was a positive correlation between TNF-α and cortisol (r = 0.31, p = 0.058, n = 39; data not shown). This association remained significant when HC users were excluded from the analysis (r = 0.44, p = 0.03, n = 23). sVCAM-1 was positively correlated with estradiol (r = 0.41, p = 0.010; data not shown), but was not significantly correlated with TNF-α or IL-6. There were no differences in serum markers of inflammation between amenorrheic and oligomenorrheic women in the MD group (data not shown).
Figure 1.
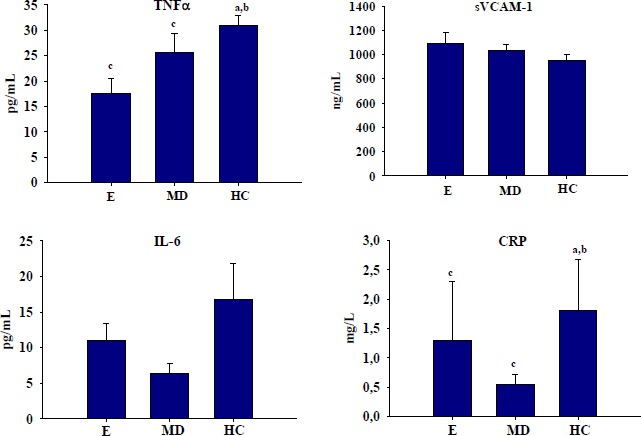
Serum markers of inflammation and endothelial function in female athletes with regular menstrual cycles (Ea), menstrual disorders (MDb), or hormonal contraceptive users (HCc). Data are means (± SD). Superscripts denote significantly (p < 0.05) differences between the groups.
Discussion
Contrary to our hypothesis, female athletes with exercise-associated menstrual disorders did not have significant alterations in serum inflammatory markers or blood lipids. We expected women with menstrual disorders to have significantly greater serum TNF-α , IL-6, TC, LDL-C, and TG than eumenorrheic women. Estrogen suppresses TNF-α and IL-6 gene expression (Salem, 2004) and elevations in TNF-α and IL-6 have been observed in postmenopausal women and in individuals with anorexia nervosa (Gianni et al., 2004; Kahl et al., 2004; O’Donnell and De Souza, 2004). We did not expect to observe group differences in serum estradiol because blood was collected from regularly menstruating subjects during the early follicular phase of the menstrual cycle. Assuming that women with menstrual disorders have lower cumulative estradiol exposure over the course of the menstrual cycle than eumenorrheic women, the lack of group differences in blood lipids was unexpected.
Hormonal contraceptive users had elevated cortisol, CRP, TNF-α, TC, and LDL-C in serum compared to eumenorrheic athletes. Oral estrogens and progestins in hormonal contraceptives previously have been shown to increase TC, LDL-C, TG (Guazzelli et al., 2005), as well as, TNF-α and CRP in premenopausal women (Rickenlund et al., 2005b). There is evidence that the increase in CRP associated with HC use, results from hepatic metabolism of the steroid hormones rather than induction of an inflammatory response (Silvestri et al., 2003). Transdermal administration of hormonal contraceptives does not result in altered serum lipoproteins or CRP (Dreon et al., 2003).
In the only other published observations of serum and endothelial inflammatory markers in women with exercise-associated menstrual disorders, Rickenlund et al., 2005a reported that oligomenorrheic and amenorrheic athletes had similar sVCAM-1, TNF-α , IL-6, and CRP compared with eumenorrheic athletes, despite impaired flow-mediated dilation. The results of the present study are consistent with these findings, strengthening the evidence that altered systemic cytokines may not be the link between estrogen inadequacy and impaired endothelial function.
In the current study, sVCAM-1 was not correlated with TNF-α or IL-6, consistent with the findings of Rickenlund et al., 2005a in female athletes. Souter et al., 2005 reported a significant positive relationship between TNF-α and sVCAM-1 throughout the menstrual cycle in sedentary women. Although TNF-α and IL-6 induce expression of cellular adhesion molecules, circulating concentrations of these inflammatory markers do not necessarily reflect their bioactivity, which is modulated by soluble receptors, nor are they indicators of localized cytokine autocrine and paracrine activity (Fruhbeck et al., 2001). Exogenous estrogens also cause a divergent response of the cytokines and sVCAM-1 in vivo (Rickenlund et al., 2005b; Souter et al., 2005). Oral contraceptives decreased sVCAM-1, but increased TNF-α and CRP, in amenorrheic and eumenorrheic female athletes (Rickenlund et al., 2005b). In regularly menstruating, sedentary premenopausal women, hormonal contraceptive use decreased sVCAM-1, but had no effect on serum TNF-α (Souter et al., 2005).
The effect of synthetic estrogens (e.g., ethinyl estradiol), commonly found in hormonal contraceptives, on serum sVCAM-1 in vivo is surprising based on regulation of VCAM-1 expression by estrogens in cultured endothelial cells (Mukherjee et al., 2003). In vitro experiments demonstrate that suppression of TNF-α-induced VCAM-1 expression by estrogen is mediated by binding of estrogen receptor β (ERβ) but not ERα (Mukherjee et al., 2003). Although 17-β estradiol binds ERα and ERβ, ethinyl estradiol only binds ER and has no effect on VCAM-1 expression in vitro. Our findings are consistent with these in vitro experiments.
The positive correlation between estradiol and sVCAM-1 observed in this study was unexpected and may be an artifact of the study design. Although, hormonal contraceptives suppress sVCAM-1 (Rickenlund et al., 2005b; Souter et al., 2005) in premenopausal women, the amount of this adhesion molecule in circulation does not vary across the menstrual cycle (Souter et al., 2005). Because we measured reproductive hormones during the early follicular phase of the menstrual cycle when estradiol secretion is lowest, we were unable to determine group differences in cumulative estrogen exposure throughout the course of the menstrual cycle. Thus, our ability to examine the relationship between estradiol and sVCAM-1 was impaired. When the number of menstrual cycles per year was used as a proxy variable for cumulative estrogen exposure, the relationship between cycle number and sVCAM-1 was not significant.
A limitation of this study is that the MD groups included women who were amenorrheic and oligomenorrheic. Rickenlund et al., 2005a reported that flow mediated dilation was more impaired in women with exericse-associated amenorrhea than in those with oligomenorrhea; however, TNF-α , IL-6 and sVCAM-1 did not differ between groups. Similarly, TNF-α , IL-6, CRP, and sVCAM-1 were not different between amenorrheic and oligomenorrheic subjects in the current study in post hoc analyses.
The relatively small sample size is another limitation of this study, reducing the power to detect group differences. Retrospective power analyses demonstrate that a sample size of 32 subjects is required to detect a significant difference in IL-6 and TNF-α between the eumenorrheic and menstrual disturbance groups at power = 0.80 and p = 0.05. However, the required sample sizes to detect group differences in CRP and sVCAM-1 are 137 and 274, respectively, at power = 0.80 and p = 0.05. Thus, there may be differences in the inflammatory cytokines associated with menstrual disorders. However, it is unlikely that true differences in CRP and sVCAM-1 exist between the E and MD groups.
Conclusions
In conclusion, we did not observe altered serum markers of inflammation and endothelial function in women with exercise-associated menstrual disorders. Regular use of hormonal contraceptives, however, significantly increased total and LDL cholesterol, TNF-α , and CRP in physically active women, as previously reported (Souter et al., 2005). Future studies are needed to determine if the increased proinflammatory cytokines in serum initiate the atherosclerotic process and impair vascular endothelial function. Moreover, the absence of consistent relationships between the inflammatory cytokines, adhesion molecules, and estradiol, indicates that further studies are needed to elucidate the mechanisms by which estrogen regulates endothelial function.
Acknowledgements
This project was funded by the Department of Nutritional Sciences, Marget Mangel Research Catalyst Fund, Elizabeth Hegarty Foundation, Food for the 21st Century Summer Research Intern Program.
Biographies
Pamela S. HINTON
Employment
Ass. Prof., Department of Nutritional Sciences, University of Missouri-Columbia, USA.
Degree
PhD
Research interests
Iron deficiency and training adaptations, bone health and menstrual dysfunction.
E-mail: hintonp@missouri.edu
R. Scott RECTOR
Employment
Graduate Research Assistant, Department of Nutritional Sciences, University of Missouri-Columbia, USA.
Degree
MS
Research interests
Effects of exercise during weight regain on markers of inflammation and the metabolic syndrome
E-mail: RSRector@mizzou.edu
James E. PEPPERS
Employment
Medical Student, Department of Nutritional Sciences, University of Missouri-Columbia, USA.
Degree
BS
Rebecca D. IMHOFF
Employment
Department of Nutritional Sciences, University of Missouri-Columbia, USA.
Degree
MS
Research interests
Exercise-associated menstrual disorders and bone health.
Laura S. HILLMAN
Employment
Interim Chair, Department of Nutritional Sciences, University of Missouri-Columbia, USA .
Degree
MD
Research interests
Bone metabolism in childhood diseases.
References
- Ainsworth B.E., Haskell W.L., Whitt M.C., Irwin M.L., Swartz A.M., Strath S.J., O’Brien W.L., Bassett D.R., Jr., Schmitz K.H., Emplaincourt P.O., Jacobs D.R., Jr., Leon A.S. (2000) Compendium of physical activities: an update of activity codes and MET intensities. Medicine and Science in Sports and Exercise 32, 498-504 [DOI] [PubMed] [Google Scholar]
- Cary N.S.I.I. (1999) Statistical analyses system. Version 8.2. Cary, NC: SAS Institute Inc. [Google Scholar]
- Cybulsky M.I., Gimbrone M.A., Jr. (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251, 788-791 [DOI] [PubMed] [Google Scholar]
- Dreon D.M., Slavin J.L., Phinney S.D. (2003) Oral contraceptive use and increased plasma concentration of C-reactive protein. Life Science 73, 1245-1252 [DOI] [PubMed] [Google Scholar]
- Dubey R.K., Imthurn B., Zacharia L.C., Jackson E.K. (2004) Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension 44, 789-795 [DOI] [PubMed] [Google Scholar]
- Friday K.E., Drinkwater B.L., Bruemmer B., Chesnut C.3rd., Chait A. (1993) Elevated plasma low-density lipoprotein and high-density lipoprotein cholesterol levels in amenorrheic athletes: effects of endogenous hormone status and nutrient intake. Journal of Clinical Endocrinology and Metabolism 77, 1605-1609 [DOI] [PubMed] [Google Scholar]
- Friedwald W.T., Levy R.I., Fredrickson D.S. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry 18, 499-502 [PubMed] [Google Scholar]
- Fruhbeck G., Gomez-Ambrosi J., Muruzabal F.J., Burrell M.A. (2001) The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. American Journal of Physiology Endocrinology and Metabolism 280, E827-847 [DOI] [PubMed] [Google Scholar]
- Gianni W., Ricci A., Gazzaniga P., Brama M., Pietropaolo M., Votano S., Patane F., Agliano A.M., Spera G., Marigliano V., Ammendola S., Agnusdei D., Migliaccio S., Scandurra R. (2004) Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study. Journal of Clinical Endocrinology and Metabolism 89, 6097-6099 [DOI] [PubMed] [Google Scholar]
- Guazzelli C.A., Lindsey P.C., de Araujo F.F., Barbieri M., Petta C.A., Aldrighi J.M. (2005) Evaluation of lipid profile in adolescents during long-term use of combined oral hormonal contraceptives. Contraception 71, 118-121 [DOI] [PubMed] [Google Scholar]
- Kahl K.G., Kruse N., Rieckmann P., Schmidt M.H. (2004) Cytokine mRNA expression patterns in the disease course of female adolescents with anorexia nervosa. Psychoneuroendocrinology 29, 13-20 [DOI] [PubMed] [Google Scholar]
- Kublickiene K., Svedas E., Landgren B.M., Crisby M., Nahar N., Nisell H., Poston L. (2005) Small artery endothelial dysfunction in post-menopausal women: in vitro function, morphology and modification by estrogen and SERMs. Journal of Clinical Endocrinology and Metabolism 90, 6113-6122 [DOI] [PubMed] [Google Scholar]
- Mukherjee T.K., Nathan L., Dinh H., Reddy S.T., Chaudhuri G. (2003) 17-epiestriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (VCAM-1) mRNA expression. Journal of Biological Chemistry 278, 11746-11752 [DOI] [PubMed] [Google Scholar]
- Nathan L., Pervin S., Singh R., Rosenfeld M., Chaudhuri G. (1999) Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo: possible mechanisms for gender differences in atherosclerosis. Circulation Research 85, 377-385 [DOI] [PubMed] [Google Scholar]
- O’Donnell E., De Souza M.J. (2004) The cardiovascular effects of chronic hypoestrogenism in amenorrhoeic athletes: a critical review. Sports Medicine 34, 601-627 [DOI] [PubMed] [Google Scholar]
- Pigott R., Dillon L.P., Hemingway I.H., Gearing A.J. (1992) Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochemistry Biophysiology Researcg Communication 187, 584-589 [DOI] [PubMed] [Google Scholar]
- Rickenlund A., Eriksson M.J., Schenck-Gustafsson K., Hirschberg A.L. (2005a) Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. Journal of Clinical Endocrinology and Metabolism 90, 1354-1359 [DOI] [PubMed] [Google Scholar]
- Rickenlund A., Eriksson M.J., Schenck-Gustafsson K., Hirschberg A.L. (2005b) Oral contraceptives improve endothelial function in amenorrheic athletes. Journal of Clinical Endocrinology and Metabolism 90, 3162-3167 [DOI] [PubMed] [Google Scholar]
- Salem M.L. (2004) Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Current Drug Targets of Inflammation and Allergy 3, 97-104 [DOI] [PubMed] [Google Scholar]
- Silvestri A., Gebara O., Vitale C., Wajngarten M., Leonardo F., Ramires J.A., Fini M., Mercuro G., Rosano G.M.(2003) Increased levels of C-reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation 107, 3165-3169 [DOI] [PubMed] [Google Scholar]
- Siri W.E. (1961) Body composition from fluid spaces and density: analysis of methods. In: Techniques for measuring body composition. : Brozek J., Henschel A.Washington, DC: National Academy of Sciences, National Research Council; 223-224 [Google Scholar]
- Souter I., Janzen C., Martinez-Maza O., Breen E.C., Stanczyk F., Chaudhuri G., Nathan L. (2005) Serum levels of soluble vascular cell adhesion molecule-1 are decreased in women receiving oral contraceptives compared with normally menstruating women: implications in atherosclerosis. Fertility and Sterility 83, 1480-1488 [DOI] [PubMed] [Google Scholar]
- Taddei S., Virdis A., Ghiadoni L., Mattei P., Sudano I., Bernini G., Pinto S., Salvetti A. (1996) Menopause is associated with endothelial dysfunction in women. Hypertension 28, 576-582 [DOI] [PubMed] [Google Scholar]
- Zeni Hoch A., Dempsey R.L., Carrera G.F., Wilson C.R., Chen E.H., Barnabei V.M., Sandford P.R., Ryan T.A., Gutterman D.D. (2003) Is there an association between athletic amenorrhea and endothelial cell dysfunction? Medicine and Science in Sports and Exercise 35, 377-383 [DOI] [PubMed] [Google Scholar]


