Abstract
Background
Once-daily dosing of abacavir and lamivudine has been approved for adults, but paediatric data are insufficient. We conducted a pharmacokinetic study of once-daily and twice-daily abacavir and lamivudine in children aged 3–<36 months.
Methods
Children with stable HIV type-1 (HIV-1) RNA levels after 12 weeks treatment with twice-daily abacavir (8 mg/kg) with or without lamivudine (4 mg/kg) underwent plasma pharmacokinetic sampling. Children then switched to once-daily abacavir (16 mg/kg) with or without lamivudine (8 mg/kg), and sampling was repeated 4 weeks later. The area under the plasma concentration–time curve over 24 h (AUC0–24) and the maximum concentration (Cmax) were compared using geometric mean ratios (GMRs); 90% confidence intervals (CIs) within the range of 0.80–1.25 were considered bioequivalent.
Results
A total of 18 children (4, 6 and 8 in the 3–<12, 12–<24 and 24–<36 month age ranges, respectively) provided pharmacokinetic data for abacavir (17 for lamivudine). The GMR of AUC0–24, once-daily versus twice-daily, was 1.07 (90% CI 0.92–1.23) for abacavir and 0.91 (90% CI 0.79–1.06) for lamivudine. Cmax almost doubled on once-daily versus twice-daily dosing: abacavir and lamivudine GMRs were 2.04 (90% CI 1.73–2.42) and 1.78 (90% CI 1.52–2.09), respectively. At baseline, 12, 24 and 48 weeks, 89%, 94%, 100% and 89% of children had HIV-1 RNA<400 copies/ml, respectively.
Conclusions
Bioequivalence was demonstrated on AUC0–24 between twice-daily and once-daily abacavir; very similar AUC0–24 values were seen for twice-daily and once-daily lamivudine. Given that viral load suppression rates were maintained, these data suggest that once-daily abacavir and lamivudine might be an option for children aged 3–<36 months.
Introduction
AIDS-related mortality and morbidity has decreased substantially since the introduction of combination antiretroviral therapy (ART) in HIV-infected children [1,2]; however, long-term therapeutic success depends on good adherence [3–6]. Adherence is related to several factors, including volume and palatability of medication, the complexity of medication schedules and interference with daily activities [6–9]. In adults, once-daily medication has been shown to enhance adherence and patient satisfaction with ART [10–13]. Good adherence and acceptability to once-daily ART has also been reported in children [14].
Abacavir and lamivudine have been approved for once-daily dosing for adults [15,16]. PENTA 13, a pharmacokinetic (PK) study performed in HIV type-1 (HIV-1)-infected children aged 2–13 years, showed that abacavir and lamivudine taken once-daily were well tolerated and non-inferior in terms of PK profiles and continued HIV-1 RNA suppression, compared with corresponding twice-daily regimens [17]. However, only three children under 3 years of age were included. Very young children have a fluctuating gastrointestinal absorption rate, a larger volume of distribution for water-soluble drugs and faster renal clearance per kg body weight (the latter being the principal route of elimination for both lamivudine and abacavir metabolites); therefore, it might not be appropriate to extrapolate the findings of PENTA 13 to infants and younger children [16,18–20].
PENTA 15 (trial number ISRCTN38147516) was a multicentre, open-label, within-child, crossover PK study in HIV-1-infected children aged 3–<36 months. The aim of the study was to compare plasma PK parameters of once-daily versus twice-daily dosing of abacavir and lamivudine. Secondary objectives were to describe age-related differences in once-daily versus twice-daily PK parameters in three age groups (3–<12, 12–<24 and 24–<36 months), and to describe child and family acceptability of (and adherence to) once-daily compared with twice-daily dosage regimens.
Methods
Study population
HIV-1-infected children aged 3–<36 months taking twice-daily abacavir (Ziagen®; GlaxoSmithKline, Research Triangle Park, NC, USA) with or without twice-daily lamivudine (Epivir®; GlaxoSmithKline), both as oral solutions for at least 12 weeks as part of combination ART, were included. HIV-1 RNA levels were required to be either <400 copies/ml or if ≥400 copies/ml to be stable or decreasing and <20,000 copies/ml, with no plans to change medication in the next 12 weeks. Likewise, the percentage of CD4+ T-cells was required to be stable or rising prior to entry. Children were excluded if they had an intercurrent illness, were receiving concomitant therapy except prophylactic antibiotics or had abnormal renal or liver function (grade ≥3). The protocol was approved by the ethics committee for each participating centre. All parents/guardians provided written informed consent.
Study design
At week 0, children on twice-daily abacavir 8 mg/kg (with or without twice-daily lamivudine 4 mg/kg) had blood samples taken at 0 (pre-dose), 1, 2, 3, 4, 6, 8 and 12 h after observed intake of medication. Children then switched to once-daily abacavir 16 mg/kg (and once-daily lamivudine 8 mg/kg if applicable). The same total daily dose of both drugs was given. Children took once-daily medication in the morning until the week 4 visit when a second PK sampling (at 0 [pre-dose], 1, 2, 3, 4, 6, 8, 12 and 24 h) was performed. Children were subsequently followed until week 48, with the intention for all to remain on once-daily abacavir (with or without once-daily lamivudine) at least until week 12. Switch back to twice-daily dosing was at the discretion of the physician and family. After week 4, once-daily medication dosing could be changed from the morning to a different, but regular, time of day. Carers were asked to complete questionnaires on adherence to ART (week 0, 4 and 12) and on acceptability of once-daily dosing (week 0 and 12).
Pharmacokinetic analyses
Plasma concentrations of abacavir and lamivudine were determined by a validated HPLC assay with ultraviolet detection at a wavelength of 260 nm; measurements were performed at the Department of Pediatric Pharmacology and Pharmacogenetics (Clinical Investigation Center CIC Inserm 9202, Hôpital Robert Debré, Paris, France). Methods were cross-validated with the Department of Clinical Pharmacy of the Radboud University Nijmegen Medical Center (Nijmegen, the Netherlands), where the assay was developed [21]. The lower limit of quantification (LLQ) was 0.015 mg/l for both abacavir and lamivudine. PK parameters (area under the plasma concentration–time curve [AUC0–T, h•mg/l], apparent oral clearance relative to body weight [CL/F/kg, l/(h•kg)], maximum plasma concentration [Cmax, mg/l]; minimum plasma concentration [Cmin, mg/l] and elimination half-life [t1/2, h]) were calculated using non-compartmental methods and the linear up/log down trapezoidal procedure within WinNonlin version 5.2 (Pharsight Corporation; Mountain View, CA, USA). The options ‘uniform weighting’ and ‘best-fit’ were used and concentrations below LLQ were set to ‘0’ (when it appeared in the absorption phase) or ‘missing’ (when it appeared in the elimination phase). For AUC0–T, T was 12 h for twice-daily and 24 h for once-daily dosing. Daily AUC (AUC0–24) was estimated for the twice-daily regimen by calculating AUC0–24=2×AUC0–T.
Sample size
Target enrolment was 18 children, 6 in each age group: 3–<12, 12–<24 and 24–<36 months. If a child did not complete both PK evaluation days on twice-daily and once-daily regimens, their data was not evaluable for the purposes of the study and an additional child in the relevant age group was recruited as a replacement. On the basis of plasma abacavir PK data from the PENTA 13 study [17], the standard deviation of change in log10(AUC0–24) between twice-daily and once-daily dosing was approximately 0.13 log10 (h•mg/l). Eighteen children providing plasma abacavir PK data on both twice-daily and once-daily regimens was expected to provide at least 80% power for the width of the 90% confidence interval (CI) for the mean log10(AUC0–24) difference between twice-daily and once-daily to be <0.113. For example, the 90% CI for a ratio of geometric mean AUC0–24 of 1.00 (no difference between twice-daily and once-daily AUC0–24 observed) would be 0.88–1.14.
Statistical analyses
Statistical calculations were performed using Stata version 10 (StataCorp LP, College Station, TX, USA). For each child, ratios of AUC0–24, CL/F/kg and Cmax for once-daily versus twice-daily were calculated. Overall geometric mean ratios (GMRs) were calculated after log10 transformation of the within-patient ratios. For AUC0–24 and CL/F/kg, a GMR with 90% CI including 1.00 and falling entirely within the range 0.80–1.25 was considered bioequivalent. In each age group (3–<12, 12–<24 and 24–<36 months), geometric means (GMs) were calculated separately for twice-daily and once-daily; GMRs were calculated for once-daily versus twice-daily. Data were compared with published results from steady-state PK studies in children using 8 mg/kg twice-daily or 16 mg/kg once-daily abacavir and 4 mg/kg twice-daily or 8 mg/kg once-daily lamivudine (tablets and oral solution; PENTA 13 [17]); comparisons were also made with adults using 300 mg twice-daily or 600 mg once-daily abacavir tablets (CAL102120 [22]) and 150 mg twice-daily or 300 mg once-daily lamivudine tablets (EPV1001 [23]). Major resistance mutations were defined according to the 2008 International AIDS Society–USA guidelines [24].
Results
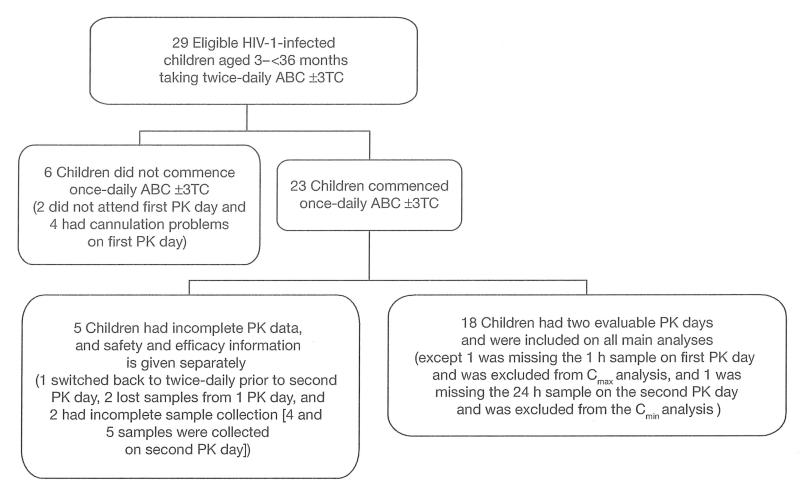
A total of 18 children, enrolled between July 2006 and May 2008, had two evaluable PK days and were included in all main analyses (Figure 1). Safety and efficacy information on five children who commenced once-daily therapy, but who had incomplete PK data, is given separately (one switched back to twice-daily prior to the second PK day, two had lost samples from one PK day, and two had incomplete sample collection [only 4 and 5 samples were collected on the second PK day]). Six children were recruited but did not commence once-daily therapy and were not followed: two did not attend their first PK day and four had cannulation problems on their first PK day.
Figure 1.
PENTA 15 participant flow
ABC, abacavir; Cmax, maximum plasma concentration; Cmin, minimum plasma concentration; HIV-1, HIV type-1; PK, pharmacokinetic; 3TC, lamivudine; ±, with or without.
Baseline characteristics of the 18 vertically HIV-1-infected children (4, 6 and 8 children in age groups 3–<12, 12–<24 and 24–<36 months, respectively) with two evaluable PK days are given in Table 1. One child in the 24–<36 months age group was evaluated for abacavir only (Table 1). Prior to the study, many children had been exposed to two ART classes (7 [39%] to nucleoside reverse transcriptase inhibitors [NRTIs] and protease inhibitors, and 9 [50%] to NRTIs and non-nucleoside reverse transcriptase inhibitors). Two (11%) children had been exposed to all three classes. Sixteen (89%) children were taking all their ART drugs twice-daily and the remaining two were taking a mixture of twice-daily and once-daily medication (one taking emtricitabine once-daily and one taking nevirapine once-daily).
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Evaluable children, n(%) | 18 (100) |
| Median age, months (IQR) | 23.4 (12.1–27.8) |
| Age range | |
| 3–<12 months, n (%) | 4 (22) |
| 12–<24 months, n (%) | 6(33) |
| 24–<36 months, n (%) | 8 (44) |
| Gender | |
| Male, n (%) | 10(56) |
| Female, n (%) | 8 (44) |
| Median body weight, kg (IQR) | 11.3 (10.3–13.0) |
| Ethnic origin | |
| White, n (%) | 3 (17) |
| Black, n (%) | 14(78) |
| Mixed, n (%) | 1 (6) |
| CDC stage | |
| N, n (%) | 7 (39) |
| A, n (%) | 3(17) |
| B, n (%) | 1 (6) |
| C, n (%) | 7 (39) |
| Median cumulative ART exposure, months (IQR) | 11.3 (7.4–21.6) |
| Antiretroviral therapy combinationa | |
| 3TC+ABC+NVP (±AZT or ±d4T), n (%) | 10 (56) |
| 3TC+ABC+LPV/r (±AZT), n (%) | 7 (39) |
| FTC+ABC+LPV/r, n (%) | 1 (6) |
All children were taking all antiretroviral therapy (ART) drugs twice daily, with two exceptions: one child, who was on lamivudine (3TC) and abacavir (ABC), was taking nevirapine (NVP) once daily; and one child on abacavir (ABC) and lopinavir/ritonavir (LPV/r) was taking emtricitabine (FTC) once daily. The child evaluated for ABC only was 24–<36 months of age, female, 15.0 kg, White, CDC stage C and taking FTC, ABC plus LPV/r. AZT, zidovudine; d4T, stavudine; IQR, interquartile range.
Pharmacokinetic analyses
One child, who had a missing 1 h sample on their first PK day, was excluded from the Cmax analysis (Figure 1). One child missed their 24 h sample on the second PK day and was excluded from Cmin analysis. Three children vomited post-dose on their second PK day. One child (AUC0–24 abacavir 21.54, AUC0–24 lamivudine 7.35, CL/F/kg abacavir 0.72 and CL/F/kg lamivudine 1.06) vomited approximately 50–100 ml at 20 min post-dose; all drugs were given again and the PK analysis restarted. One child (AUC0–24 abacavir 11.20, lamivudine 6.94; CL/F/kg abacavir 1.45 and lamivudine 1.10) had a very small vomit 20 min post-dose and drugs were not re-administered. One child (AUC0–24 abacavir 13.06, lamivudine 5.76; CL/F/kg abacavir 1.23 and lamivudine 1.17) vomited approximately 10 ml 1 h post-dose and drugs were not readministered. All three children were included in all PK analyses.
Abacavir pharmacokinetics
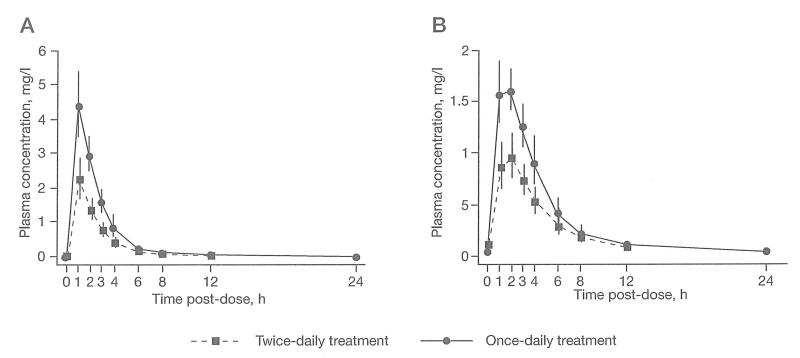
For the 18 children with evaluable abacavir PK data, median (interquartile range [IQR]) doses were 8.04 mg/kg (7.74–8.25) for twice-daily and 16.02 mg/kg (15.53–16.26) for once-daily. Bioequivalence between twice-daily and once-daily dosing was demonstrated on AUC0–24 (GMR once-daily versus twice-daily, 1.07 [90% CI 0.92–1.23]) and also for CL/F/kg (Table 2 and Figure 2A). GMRs for AUC0–24 and CL/F/kg showed no linear trend with age (P=0.6 for both). The GM of Cmax was approximately double on once-daily compared with twice-daily dosing; there was no difference between age groups (P=0.3).
Table 2.
Abacavir pharmacokinetic parameters
| PK parameter | Study (subdivided by age group) |
Evaluable patients, n |
GM (95% CI) twice-daily |
GM (95% CI) once-daily |
GMR (90% CI) once-daily versus twice-daily |
Reference |
|---|---|---|---|---|---|---|
| AUC0–24, h•mg/l | PENTA 15 | 18 | 10.85 (8.89–13.24) | 11.57 (9.89–13.53) | 1.07 (0.92–1.23) | – |
| 3–<12 months | 4 | 12.68 (6.52–24.64) | 15.90 (8.86–28.52) | 1.25(1.12–1.41) | – | |
| 12–<24 months | 6 | 13.89 (10.40–18.54) | 10.60 (7.68–14.63) | 0.76 (0.56–1.05) | – | |
| 24–<36 months | 8 | 8.34 (6.27–11.08) | 10.53 (8.87–12.50) | 1.26 (1.07–1.48) | – | |
| PENTA 13 | 14 | 9.91 (8.26–11.89)a | 13.37 (11.80–15.16)a | 1.35 (1.19–1.54) | [17] | |
| 2–6 years | 9 | 9.27 (7.06–12.18)a | 13.55 (11.19–16.42)a | – | – | |
| >6–13 years | 5 | 11.17 (8.76–14.24)a | 13.06 (10.91–15.63)a | – | – | |
| CAL102120b | 27 | 7.90 (6.66–9.39) | 8.52 (7.23–10.04) | 1.08(1.02–1.15) | [22] | |
| CL/F/kg, l/h•kg | PENTA 15 | 18 | 1.47 (1.21–1.79) | 1.38 (1.17–1.62) | 0.94 (0.81–1.08) | – |
| 3–<12 months | 4 | 1.23 (0.61–2.48) | 0.97 (0.52–1.81) | 0.79 (0.71–0.88) | – | |
| 12–<24 months | 6 | 1.17 (0.94–1.46) | 1.52 (1.11–2.09) | 1.30 (0.97–1.76) | – | |
| 24–<36 months | 8 | 1.91 (1.44–2.53) | 1.52 (1.29–1.78) | 0.79 (0.66–0.95) | – | |
| PENTA 13 | 14 | 1.58 (1.30–1.93)a | 1.16(1.01–1.34)a | 0.73 (0.64–0.84) | [17] | |
| 2–6 years | 9 | 1.80(1.37–2.36)a | 1.21 (1.00–1.47)a | – | – | |
| >6–13 years | 5 | 1.26 (0.96–1.64)a | 1.08 (0.81–1.44)a | – | – | |
| CAL102120b | 27 | 1.09 (0.91–1.29) | 1.01 (0.86–1.19) | 0.93 (0.86–1.00) | [22] | |
| Cmax, mg/l | PENTA 15 | 1.38 (1.17–1.62) | 4.68 (3.86–5.67) | 2.04(1.73–2.42) | – | |
| 3–<12 months | 4 | 2.43 (1.37–4.31) | 5.89 (2.83–12.26) | 2.42 (1.64–3.59) | – | |
| 12–<24 months | 6 | 3.18 (2.24–4.52) | 5.29 (3.70–7.56) | 1.66 (1.12–2.46) | – | |
| 24–<36 months | 7c | 1.67 (1.10–2.53) | 3.69 (2.95–4.61) | 2.21 (1.74–2.80) | – | |
| PENTA 13 | 14 | 2.14(1.79–2.56)a | 4.80 (4.04–5.71)a | 2.25 (1.83–2.77) | [17] | |
| 2–6 years | 9 | 1.94(1.50–2.51)a | 5.07 (3.92–6.56)a | – | – | |
| >6–13 years | 5 | 2.54 (2.00–3.22)a | 4.36 (3.39–5.60)a | – | – | |
| CAL102120b | 27 | 1.84(1.58–2.15) | 3.85 (3.34–4.42) | 2.09 (1.88–2.32) | [22] | |
| Cmin, mg/l | PENTA 15 | 17d | 0.03 (<0.015–0.08)e | <0.015(<0.015–0.07)e | N/A | – |
| T1/2, h | PENTA 15 | 18 | 1.66 (0.99–2.95)e | 2.15(l.40–4.98)e | N/A | – |
90% confidence interval (CI).
Adult study.
One child excluded because of a missing 1 h plasma concentration on the first pharmacokinetic (PK) sampling day.
One child excluded because of a missing 24 h plasma concentration on the second PK sampling day.
eMedian (range). AUC0–24, daily area under the plasma concentration–time curve; CL/F/kg, apparent oral clearance relative to body weight; Cmax, maximum plasma concentration; Cmin, minimum plasma concentration; GM, geometric mean; GMR, geometric mean ratio; N/A, not applicable; T1/2, elimination half-life.
Figure 2.
Geometric mean plasma concentrations with 95% confidence intervals
Geometric means (95% confidence interval) of plasma concentrations of (A) abacavir and (B) lamivudine.
The GMs of AUC0–24, CL/F/kg and Cmax on twice-daily and once-daily dosing were comparable with data obtained in children aged 2–13 years (PENTA 13 [17]; Table 2). Slightly higher AUC0–24 and CL/F/kg were observed in children in both the PENTA 13 and 15 studies compared with adults [22].
Lamivudine pharmacokinetics
For the 17 children with evaluable lamivudine PK data, median (IQR) doses were 4.04 mg/kg (3.87–4.13) for twice-daily and 8.02 mg/kg (7.77–8.13) for once-daily. Very similar AUC0–24 values were seen for twice-daily and once-daily dosing of lamivudine (GMR once-daily versus twice-daily, 0.91 [90% CI 0.79–1.06]; Table 3 and Figure 2B), but higher than expected variability resulted in the 90% CI falling slightly outside the bioequivalence criteria. Similar results were observed for CL/F/kg. As for abacavir, the GM of Cmax was approximately double on once-daily compared with twice-daily dosing. There were no statistically significant differences among age groups for all PK parameters (P=0.2, P=0.3 and P=0.1 for AUC0–24, CL/F/kg and Cmax, respectively).
Table 3.
Lamivudine pharmacokinetic parameters
| PK parameter | Study (subdivided by age group) |
Evaluable patients, n |
GM (95% CI) twice-daily |
GM (95% CI) once-daily |
GMR (90% CI) once-daily versus twice-daily |
Reference |
|---|---|---|---|---|---|---|
| AUC0–24, h•mg/l | PENTA 15 | 17 | 9.48 (7.89–11.40) | 8.66 (7.46–10.06) | 0.91 (0.79–1.06) | – |
| 3–<12 months | 4 | 9.24 (4.66–18.32) | 10.31 (6.26–16.96) | 1.11 (0.95–1.31) | – | |
| 12–<24 months | 6 | 9.54 (6.71–13.57) | 7.13 (5.84–8.71) | 0.75 (0.58–0.96) | – | |
| 24–<36 months | 7 | 9.58 (6.65–13.78) | 9.27 (7.11–12.09) | 0.97 (0.71–1.32) | – | |
| PENTA 13 | 19 | 8.88 (7.67–10.28)a | 9.80 (8.64–11.12)a | 1.12(1.03–1.21) | [17] | |
| 2–6 years | 10 | 7.60 (6.12–9.45)a | 8.80 (7.43–10.43)a | – | – | |
| >6–13 years | 9 | 10.55 (8.82–12.63)a | 11.04 (9.06–3.45)a | – | – | |
| EPV1001b | 60 | 9.21 (8.81–9.63) | 8.70 (8.28–9.14) | 0.94 (0.92–0.97) | [23] | |
| CL/F/kg.l/h•kg | PENTA 15 | 17 | 0.79 (0.65–0.96) | 0.86 (0.74–1.01) | 1.10(0.95–1.28) | – |
| 3–<12 months | 4 | 0.79 (0.36–1.73) | 0.72 (0.42–1.26) | 0.92 (0.73–1.16) | – | |
| 12–<24 months | 6 | 0.80 (0.55–1.18) | 1.05 (0.88–1.25) | 1.31 (1.00–1.71) | – | |
| 24–<36 months | 7 | 0.77 (0.53–1.12) | 0.81 (0.61–1.07) | 1.05 (0.77–1.43) | – | |
| PENTA 13 | 19 | 0.90 (0.78–1.04)a | 0.80 (0.70–0.92)a | 0.89 (0.82–0.96) | [17] | |
| 2–6 years | 10 | 1.09 (0.89–1.34)a | 0.92 (0.78–1.08)a | – | – | |
| >6–13 years | 9 | 0.73 (0.63–0.85)a | 0.69 (0.55–0.87)a | – | – | |
| EPV1001b | 60 | 0.44 (0.42–0.47) | 0.47 (0.44–0.50) | 1.06 (1.02– 1.10) | [23] | |
| Cmax, mg/l | PENTA 15 | 16c | 1.05 (0.88–1.26) | 1.87 (1.65–2.13) | 1.78 (1.52–2.09) | – |
| 3–<12 months | 4 | 1.04 (0.76–1.41) | 2.37 (1.57–3.57) | 2.29 (1.73–3.01) | – | |
| 12–<24 months | 6 | 1.22 (0.82–1.80) | 1.71 (1.46–2.02) | 1.41 (1.14–1.74) | – | |
| 24–<36 months | 6c | 0.92 (0.62–1.35) | 1.75 (1.39–2.20) | 1.91 (1.35–2.69) | – | |
| PENTA 13 | 19 | 1.11 (0.96–1.29)a | 2.09 (1.80–2.42)a | 1.90 (1.67–2.16) | [17] | |
| 2–6 years | 10 | 0.94 (0.78–1.13)a | 1.72 (1.48–1.99)a | – | – | |
| >6–13 years | 9 | 1.34(1.08–1.67)a | 2.59 (2.04–3.28)a | – | – | |
| EPV1001b | 60 | 1.19 (1.12–1.26) | 1.97 (1.84–2.11) | 1.66 (1.57–1.74) | [23] | |
| Cmin, mg/l | PENTA 15 | 16d | 0.08 (0.03–0.27)e | 0.05 (<0.015–0.21)e | N/A | – |
| T1/2, h | PENTA 15 | 17 | 2.99 (2.38– 4.54)e | 5.35 (2.83–13.23)e | N/A | – |
90% confidence interval (CI).
Adult study.
One child excluded because of a missing 1 h plasma concentration on the first pharmacokinetic (PK) sampling day.
One child excluded because of a missing 24 h plasma concentration on the second PK day.
Median (range). AUC0–24, daily area under the plasma concentration–time curve; CL/F/kg, apparent oral clearance relative to body weight; Cmax, maximum plasma concentration; Cmin, minimum plasma concentration; GM, geometric mean; GMR, geometric mean ratio; N/A, not applicable; T1/2, elimination half-life.
The GMs of AUC0–24, CL/F/kg and Cmax on twice-daily and once-daily dosing were comparable with data obtained in children aged 2–13 years (PENTA 13 [17]; Table 3). Additionally, AUC0–94 and Cmax were similar to adult data [23], although the CL/F/kg was slightly higher in children.
Safety and efficacy
Eighteen children with two evaluable pharmacokinetic days
During the study, no child changed ART regimen and no child discontinued once-daily abacavir (or once-daily lamivudine if applicable) prior to week 12. One child switched back to twice-daily abacavir and lamivudine at week 16, owing to adherence concerns and fluctuating HIV-1 RNA levels (although at time of switching HIV-1 RNA was <50 copies/ml and subsequent results were >50 copies/ml). Three children, all on zidovudine, abacavir, lamivudine and nevirapine at baseline, discontinued zidovudine during the trial. Three children switched from twice-daily to once-daily nevirapine (one subsequently switched back to twice-daily after therapeutic monitoring) and two children switched to once-daily lopinavir/ritonavir. Overall, 5 (28%) children experienced a period of taking all their ART medication once-daily during the study.
Virological control was maintained throughout the study. At baseline, 4, 8, 12, 24 and 48 weeks, 89% (16/18), 93% (14/15), 93% (14/15), 94% (16/17), 100% (18/18) and 89% (16/18) of children, respectively, had HIV-1 RNA<400 copies/ml. Moreover, the proportion of children with HIV-1 RNA<50 copies/ml remained stable over time. The percentage of CD4+ T-cells also remained stable. Of five children with HIV-1 RNA≥100 copies/ml at screening, three had centralized resistance tests and no major mutations were detected (for the other two children, one sample could not be amplified and one had no stored sample). Six children had HIV-1 RNA≥100 copies/ml during the first 12 weeks of follow-up and centralized resistance tests were performed on two (three could not be amplified and one had no stored sample), detecting no major mutations.
Four hospitalizations were reported as serious adverse events (one croup, one scalp laceration, one gastroenteritis, one diarrhoea and persistent vomiting [grade 2]); none were judged to be related to ART. Two children had adverse events (one diarrhoea and vomiting of medication [grade 2] and one febrile convulsion). No new CDC B and C events occurred during the study and no child discontinued treatment because of adverse events.
Five children with incomplete pharmacokinetic data
Among five children with incomplete PK data (Figure 1), three were followed until 48 weeks. All three remained on once-daily abacavir and lamivudine and had HIV-1 RNA<400 copies/ml throughout. One child was hospitalized to investigate vomiting and respiratory symptoms; tuberculosis was excluded and vomiting judged probably related to nevirapine. The other two children had limited follow-up data, but in both, vomiting was reported on once-daily abacavir and lamivudine. The first child had severe nausea after commencing once-daily therapy and switched back to twice-daily after 6 days. The second child was hospitalized at week 3 for vomiting with increase of transaminases (aspartate aminotransferase 59 U/l and alanine aminotransferase 46 U/l). This child remained on once-daily abacavir and lamivudine until their second PK assessment (PK data incomplete because of lost samples from first PK day), but then immediately switched back to twice-daily.
Adherence and acceptability
At baseline, 4 and 12 weeks, 18/18, 18/18 and 16/18 carers, respectively, completed questionnaires. Reported adherence since the last clinical visit on a visual analogue scale ranged between 70% and 100%: 71% (week 0), 80% (week 4) and 69% (week 12) recorded 100% adherence. At week 0, one child missed a lamivudine dose the day before the PK assessment day and another child missed one abacavir, lamivudine and lopinavir/ritonavir dose 2 days before the PK assessment day. At weeks 4 and 12, no carers reported missed doses over the previous 3 days.
At baseline, all carers thought switching from twice-daily to once-daily would make things easier for them (15/18 [83%] a lot and 3/18 [17%] a little). This was supported after the switch to once-daily abacavir/lamivudine, with all carers reporting that the switch had made things easier for them (11/16 [69%] a lot and 5/16 [31%] a little). Overall, all carers (apart from one who were unsure) preferred once-daily dosing and 14/16 (88%) preferred to give the once-daily dose in the morning.
Discussion
In HIV-1-infected children aged 3–<36 months, bioequivalence was demonstrated on AUC0–24 between twice-daily and once-daily dosing for abacavir; very similar AUC0–24 values were observed for lamivudine, although the 90% CI for the GMR fell slightly outside the pre-set bioequivalence criteria. As expected, the observed Cmax was approximately 2× higher on once-daily compared with twice-daily therapy for both drugs. Analysis of age-related differences was limited by the small sample size per age group; however, no consistent trend with age for AUC0–24, CL/F/kg or Cmax max was seen for either drug.
For abacavir, PK data in children aged 2–13 years (PENTA 13 [17]) were comparable with data in this study. CL/F/kg was higher in children from both studies than in adults (CAL102120 [22]). This might be explained by a difference in the relationship between CL/F and weight, as CL/F has been reported to increase linearly with weight in children [25] but exponentially (scaling 0.8) in adults [26]. This could lead to an apparent higher weight-normalized CL/F in children compared with adults. Studies covering a larger age range are required to further evaluate the effect of age and weight on clearance.
For lamivudine, age-dependent CL/F has been widely studied in children and a cutoff for change at approximately 6 years has been reported [27]; lamivudine AUC was reported to be similar in children aged >6 years receiving 8 mg/kg/day to adults receiving approximately 4 mg/kg/day (300 mg/day), but higher doses (>8 mg/kg/day) were required for children <6 years to achieve a comparable AUC. In our study, CL/F was comparable to that previously reported (0.66 l/[h•kg]) in 59 children with a similar age range: 29 days to 3 years [28]. Further work on a larger dataset is needed to confirm these results because of high variability; however, average AUC0–24 values for lamivudine in PENTA 15 were very similar to those observed in the adult data [23].
We did not measure concentrations of the active intracellular triphosphates of abacavir and lamivudine, as analysis is technically challenging and requires large volumes of blood. Although the correlation between plasma concentration of an NRTI and its intracellular triphosphate is imperfect, it is reasonable to assume that if plasma levels of the parent compound are similar on once-daily and twice-daily dosing then, as in adults [22,23], intracellular levels will also be similar.
PENTA 15 was designed primarily as a single-arm crossover PK study and so strong conclusions cannot be drawn about efficacy and safety of once-daily use of abacavir and lamivudine. However, we observed no evidence of loss of efficacy over 48 weeks and there were no major safety concerns. Of note, two children with incomplete PK data experienced vomiting shortly after commencing once-daily abacavir and lamivudine, which caused them to switch back to twice-daily. Large volumes of liquids might not be appropriate for all infants.
Virological efficacy results in this study were similar to those observed in children aged 2–13 years (PENTA 13 [17]); in PENTA 13, during 24 weeks of follow-up no child (n=20) discontinued once-daily dosing and similar proportions had undetectable HIV-1 RNA at baseline (16/20 [80%]) and 24 weeks (17/19 [89%]). In adults, a trial of 770 participants [15] demonstrated that abacavir once-daily in combination with once-daily lamivudine and efavirenz was non-inferior to abacavir twice-daily: at 48 weeks, 66% in the once-daily arm compared with 68% in the twice-daily arm had undetectable HIV-1 RNA. For lamivudine, a trial of 554 adults [16] demonstrated that lamivudine once-daily in combination with zidovudine and efavirenz was equivalent to twice-daily dosing: at 48 weeks, 61% in the once-daily arm compared with 59% in the twice-daily arm had undetectable HIV-1 RNA. This has led to once-daily abacavir and lamivudine being a recommended dosing option for these drugs in adults. Data from a substudy of the ARROW trial [29] on 24 Ugandan children aged 3–12 years have recently reported similar AUC0–24 values for twice-daily and once-daily abacavir and lamivudine. Further efficacy and safety data will be available from a planned randomization of several hundred children to receive twice-daily or once-daily dosing of abacavir and lamivudine within the ARROW trial.
Following the results from the CHER trial [30], guidelines recommend [31,32] infants start ART as soon as possible after diagnosis; therefore, children infected vertically with HIV-1 are likely to face lifelong treatment. In this study, good adherence to ART was reported throughout and all carers reported that daily dosing made life easier. Comments included, ‘it makes it a lot easier if you’re leaving a child with someone else, for example, a childminder’, ‘will not affect his sleeping any more’, ‘I will manage to work in the morning’, ‘tranquillity in the evening’ and ‘easier to remember’. In PENTA 13 [14], 71% of carers also reported that once-daily dosing of lamivudine/abacavir made things easier. Although once-daily dosing might be ‘less forgiving’, if doses are missed this might be offset by better long-term adherence and durability of ART with once-daily dosing, as is now the goal for all therapy in adults.
In conclusion, PENTA 15 showed no major concerns with respect to the pharmacokinetics, efficacy or safety of once-daily dosing of abacavir and lamivudine in children aged 3–<36 months. Data supports findings from the PENTA 13 trial and suggests that carers and children, as well as adults, could benefit from the option of safe, effective and more manageable daily ART regimens. However, at present, individual monitoring of children on once-daily therapy is essential and once-daily versus twice-daily regimens of abacavir and lamivudine should be further explored in large comparative efficacy and safety studies.
Acknowledgements
We thank all the children, families and staff from the centres participating in the PENTA 15 study.
Footnotes
Writing committee Evelyne Jacqz-Aigrain, Linda Harrison, Wei Zhao, Alexandra Compagnucci, Hannah Castro (née Green), Laura Farrelly, Yacine Saidi, Djamel Hamadache, Steven Welch, Uwe Wintergerst, Silvia Forcat, Ghenima Hadjou, Ghislaine Firtion, Wendy Snowden, Carlo Giaquinto, Diana Gibb and David Burger.
Disclosure statement WS declares a financial conflict of interest as a result of affiliation with GlaxoSmithKline. All other members of the writing committee declare no competing interests.
Additional file Additional file 1: A full list of all PENTA-associated comittees can be found at http://www.intmedpress.com/uploads/documents/AVT-09-OA-1392_Harrison_(PENTA_Group)_Add_file1.pdf
References
- 1.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 2.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 3.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Chi BH, Cantrell RA, Zulu I, et al. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol. 2009;38:746–756. doi: 10.1093/ije/dyp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaner JS, Reiss P, Cooper D, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. JAMA. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 6.Gibb DM, Goodall RL, Giacomet V, McGee L, Compagnucci A, Lyall H. Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. Pediatr Infect Dis J. 2003;22:56–62. doi: 10.1097/00006454-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Pontali E. Facilitating adherence to highly active antiretroviral therapy in children with HIV infection: what are the issues and what can be done? Paediatr Drugs. 2005;7:137–149. doi: 10.2165/00148581-200507030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Reddington C, Cohen J, Baldillo A, et al. Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis J. 2000;19:1148–1153. doi: 10.1097/00006454-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Stone VE, Jordan J, Tolson J, Miller R, Pilon T. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36:808–816. doi: 10.1097/00126334-200407010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Maitland D, Jackson A, Osorio J, Mandalia S, Gazzard BG, Moyle GJ. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med. 2008;9:667–672. doi: 10.1111/j.1468-1293.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 11.Molina JM. Efficacy and safety of once-daily regimens in the treatment of HIV infection. Drugs. 2008;68:567–578. doi: 10.2165/00003495-200868050-00001. [DOI] [PubMed] [Google Scholar]
- 12.Maggiolo F, Ripamonti D, Arici C, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3:371–378. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- 13.Portsmouth SD, Osorio J, McCormick K, Gazzard BG, Moyle GJ. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: a 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med. 2005;6:185–190. doi: 10.1111/j.1468-1293.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 14.LePrevost M, Green H, Flynn J, et al. Adherence and acceptability of once daily lmivudine and abacavir in human immunodeficiency virus type-1 infected children. Pediatr Infect Dis J. 2006;25:533–537. doi: 10.1097/01.inf.0000222415.40563.d4. [DOI] [PubMed] [Google Scholar]
- 15.Moyle GJ, Dejesus E, Cahn P, et al. Abacavir once or twice daily combined with once-daily lamivudine and efavirenz for the treatment of antiretroviral-naive HIV-infected adults: results of the Ziagen Once Daily in Antiretroviral Combination Study. J Acquir Immune Defic Syndr. 2005;38:417–425. doi: 10.1097/01.qai.0000147521.34369.c9. [DOI] [PubMed] [Google Scholar]
- 16.Dejesus E, McCarty D, Farthing CF, et al. Once-daily versus twice-daily lamivudine, in combination with zidovudine and efavirenz, for the treatment of antiretroviral-naive adults with HIV infection: a randomized equivalence trial. Clin Infect Dis. 2004;39:411–418. doi: 10.1086/422143. [DOI] [PubMed] [Google Scholar]
- 17.Bergshoeff A, Burger D, Verweij C, et al. Plasma pharmacokinetics of once- versus twice-daily lamivudine and abacavir: simplification of combination treatment in HIV-1-infected children (PENTA-13) Antivir Ther. 2005;10:239–246. [PubMed] [Google Scholar]
- 18.King JR, Kimberlin DW, Aldrovandi GM, Acosta EP. Antiretroviral pharmacokinetics in the paediatric population: a review. Clin rharmacokinet. 2002;41:1115–1133. doi: 10.2165/00003088-200241140-00001. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MA, Moore KH, Yuen GT, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66. doi: 10.2165/00003088-199936010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Yuen GJ, Weller S, Pakes GE. A review of the pharmacokinetics of abacavir. Clin Pharmacokinet. 2008;47:351–371. doi: 10.2165/00003088-200847060-00001. [DOI] [PubMed] [Google Scholar]
- 21.Verweij-van Wissen CP, Aarnoutse RE, Burger DM. Simultaneous determination of the HIV nucleoside analogue reverse transcriptase inhibitors lamivudine, didanosine, stavudine, zidovudine and abacavir in human plasma by reversed phase high performance liquid chromatography. J Chromatogr B Analyt Tecbnol Biomed Life Sci. 2005;816:121–129. doi: 10.1016/j.jchromb.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Moyle G, Boffito M, Fletcher C, et al. Steadv-state pharmacokinetics of abacavir in plasma and intracellular carbovir triphosphate following administration of abacavir at 600 milligrams once daily and 300 milligrams twice daily in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2009;53:1532–1538. doi: 10.1128/AAC.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuen GJ, Lou Y, Bumgarner NF, et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48:176–182. doi: 10.1128/AAC.48.1.176-182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: spring 2008. Top HIV Med. 2008;16:62–68. doi: 10.1007/s11750-007-0034-z. [DOI] [PubMed] [Google Scholar]
- 25.Jullien V, Urien S, Chappuy H, et al. Abacavir pharmacokinetics in human immunodeficiency virus-infected children ranging in age from 1 month to 16 years: a population analysis. J Clin Pharmacol. 2005;45:257–264. doi: 10.1177/0091270004272215. [DOI] [PubMed] [Google Scholar]
- 26.Jullien V, Treluyer JM, Chappuy H, et al. Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients. Br J Clin Pharmacol. 2005;59:183–188. doi: 10.1111/j.1365-2125.2004.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger DM, Verweel G, Rakhmanina N, et al. Age-dependent pharmacokinetics of lamivudine in HIV-infected children. Clin Pharmacol Ther. 2007;81:517–520. doi: 10.1038/sj.clpt.6100118. [DOI] [PubMed] [Google Scholar]
- 28.Tremoulet AH, Capparelli EV, Patel P, et al. Population pharmacokinetics of lamivudine in human immunodeficiency virus-exposed and -infected infants. Antimicrob Agents Chemother. 2007;51:4297–4302. doi: 10.1128/AAC.00332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musiime V, Ferrier A, Kitaka SB, et al. Pharmacokinetics of once versus twice daily lamivudine and abacavir in HIV-1 infected Ugandan children in the ARROW trial. 5th IAS Conference on HIV Pathogenesis; Cape Town, South Africa. 19–22 July 2009; Abstract WEPEB271. [Google Scholar]
- 30.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PENTA Steering Committee PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 32.Working Group on Antiretroviral Therapy and Medical Management of HIV-infected Children . Guidelines for the use of antiretroviral agents in pediatric HIV infection. US Department of Health and Human Services; [Accessed 17 June 2009]. Updated 23 February 2009. Available from http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf. [Google Scholar]