Abstract
Background and purpose
Pre-existing diabetes worsens brain functionality in ischemic stroke. We have previously shown that type-2-diabetic rats exhibit enhanced dysfunctional cerebral neovascularization and when these rats are subjected to cerebral ischemic reperfusion injury develop hemorrhagic transformation (HT) and greater neurological deficits. However, our knowledge of vascular and functional plasticity during the recovery phase of diabetic stroke is limited. This study tested the hypothesis that vascular repair is impaired in the post-stroke period in diabetes, and this is associated with poor sensorimotor and cognitive function. We further hypothesized that glycemic control prevents impaired vascularization and improves functional outcome in diabetes.
Methods
Vascularization was assessed in the ipsilateral and contralateral hemispheres in control, diabetes and diabetes plus metformin groups 14 days after ischemic reperfusion injury as well as in respective sham controls. 3-dimensional reconstruction of the FITC stained vasculature was achieved by confocal microscopy and stereological parameters including vascular volume and surface area were measured. Astrogliosis was determined by GFAP staining. The relative rates of sensorimotor recovery, cognitive decline and spontaneous activity were assessed.
Results
Vascular density in the peri-infarct area was significantly reduced in diabetes whereas there was reparative neovascularization in control rats. Astroglial swelling and reactivity was more pronounced in diabetic stroke compared to control stroke. Diabetes blunted sensorimotor recovery and also exacerbated anxiety-like symptoms and cognitive deficits. Glycemic control started after stroke partially prevented these changes.
Conclusion
Diabetes impairs post-stroke reparative neovascularization and impedes the recovery. Glycemic control after stroke can improve neurovascular repair and improve functional outcome.
INTRODUCTION
Stroke or cerebral infarction affects 15 million people globally, with one third of the affected population having permanent disability, impacting quality of life 1, 2. Diabetes, hypertension, hypercholesterolemia and/or aging add to the complexity of stroke outcomes. It is estimated that more than 30% of the stroke patients have diabetes and these patients suffer from a greater risk of hemorrhagic transformation, increased mortality and slower recovery 3–6. Recent clinical studies suggest that diabetes also contributes to cognitive decline and dementia and these can occur after stroke further compromising functional outcomes 7–11. There is a great need for better understanding of stroke recovery in diabetes.
Harmonized regulation of angiogenesis and neurogenesis is very important for brain repair and improvement of functional outcome after cerebral injury 12–14. Indeed, stimulation of angiogenesis is being evaluated as a therapeutic modality in stroke 12, 15. We have shown that diabetes causes dysfunctional cerebral neovascularization that is characterized by increased immature microvasculature, augmented remodeling and permeability. These vessels also have insufficient pericyte coverage rendering them more susceptible to reperfusion injury 16–19. However, the impact of ischemia/reperfusion (I/R) on cerebrovascular repair in diabetes is unknown. A recent study reported that neuronal plasticity and functional recovery after stroke is blunted in type 1 diabetic rats 20. Understanding the changes associated with the vasculature will provide a better insight into the processes responsible for modulation of reparative angiogenesis and neurogenesis and ultimately improvement of functional outcomes. Building on these studies, the current study tested the hypotheses that 1) vascular repair is impaired in the post-stroke period in diabetes, 2) motor and cognitive recovery is blunted after diabetic stroke, and 3) glycemic control in the post-stroke period improves cerebrovascularization and this is associated with better functional outcome.
METHODS
Animal procedures and experimental design
All animal surgical and behavioral procedures were carried out in accordance with the NIH guidelines under protocols approved by Georgia Regents University and Charlie Norwood VA Medical Center. Wistar rats were purchased from Harlan, (Indianapolis, ID, USA) and the Goto-Kakizaki rats (GK) were purchased from Taconic (Hudson, NY, USA). We used five cohorts of male rats (250–300g, 10–12 weeks) were randomized to sham or stroke surgery: sham control and diabetes, stroked control and diabetes, and stroked diabetic rats treated with metformin (300mg/kg/day in drinking water) after stroke till sacrifice. Treatment was started when blood glucose reached above 140 mg/dL which occurred at Day 1 in majority of the animals (a small fraction of animal reached that level at Day 2). Blood glucose was measured during the light cycle every day and the levels ranged from 86–103 mg/dL, in control rats, 155–217 mg/dL, in the diabetic group and metformin treatment lowered the glucose levels to 81–115 mg/dL, respectively. The mean body weight was 318 ± 6 g in the control group, 271 ± 7 g in the diabetic group and 299 ± 12 g in the metformin treated group.
Method of ischemic stroke
I/R injury was induced by 90-min occlusion of the middle cerebral artery as previously published (Detailed methodology is given in supplementary files).
Measurement of post-stroke vascularization
Vascularization patterns were assessed in cortex and striatum around the infarct territories as well as in corresponding regions in the contralateral hemisphere using the space-filling model as reported earlier 17 (Detailed methodology is given in supplementary files).
Assessing astrogliosis and swelling
The FITC stained sections were co-stained with anti-GFAP antibody and imaged at 63X immediately around the infarcts and in the corresponding contralateral hemispheres of the same sections. Astrocytic swelling was evaluated by measuring the somatic volumes using Volocity software. Number of processes projecting from individual astrocytes was counted as the astrocytic reactivity using the Fiji software after skeletonizing the images.
Evaluation of neurological outcomes
All neurological outcomes were measured blindly by assessing sensorimotor functions, anxiety like symptoms using the elevated plus maze and T-maze, short-term cognitive test using the novel object recognition task (Detailed methodology is given in supplementary data).
Statistical Analysis
All data points are expressed in mean ± SEM. Assumptions of normality were tested and rank transformations were used as needed. Vascularization after stroke was compared using a 2 disease (control vs. diabetes) by 2 stroke hemisphere (sham vs. ischemic or sham vs. contralateral) ANOVA within area (cortex or striatum). An interaction was tested and if significant would indicate a differential effect of stroke on vascularity dependent on disease status. The effect of diabetes and of metformin treatment of diabetes on vascularization and astrogliosis after stroke was determined using a one-way ANOVA with three groups (control, diabetes, diabetes plus metformin) with separate analyses for each hemisphere (ischemic or contralateral) and each area (cortex or striatum). Changes in behavior and function were measured by determining the area under the curve (AUC) for responses across 14 days post-stroke. The effect of diabetes and of metformin treatment of diabetes on behavior and function after stoke was determined using a one-way ANOVA with three groups (control, diabetes, diabetes plus metformin) to analyze AUC as well as within-day measures of behavior and function. Tukey’s post-hoc tests were used to determine mean differences and to adjust for multiple comparisons for significant ANOVA effects. AUC was calculated using NCSS 2007 software (NCSS, LLC, and Kaysville, UT) and all statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Statistical significance was determined at alpha=0.05.
RESULTS
Vascularization in the ischemic and contralateral hemispheres
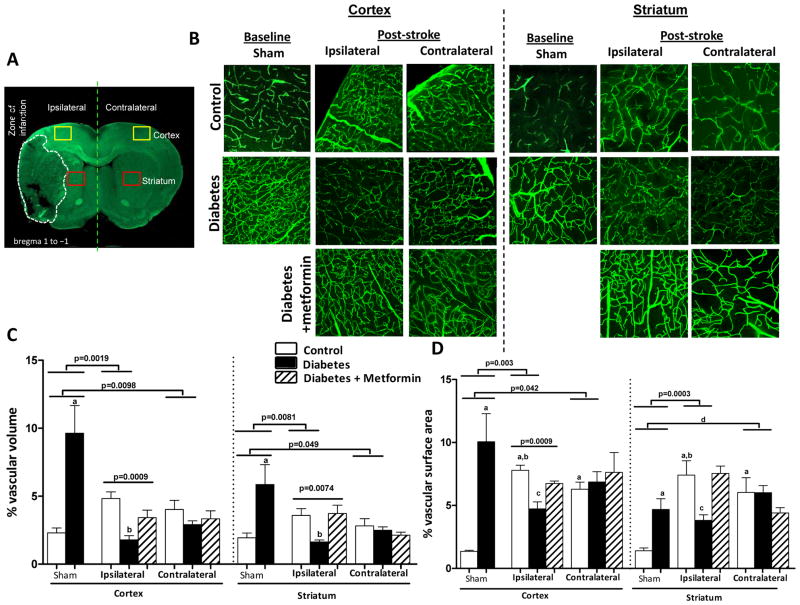
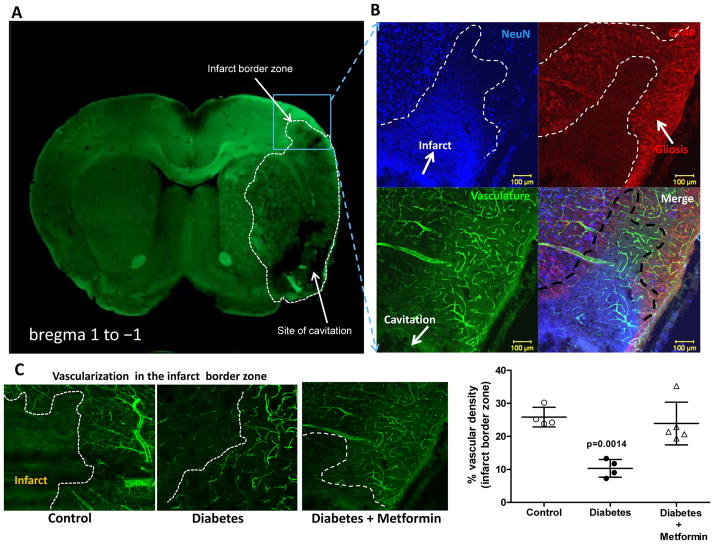
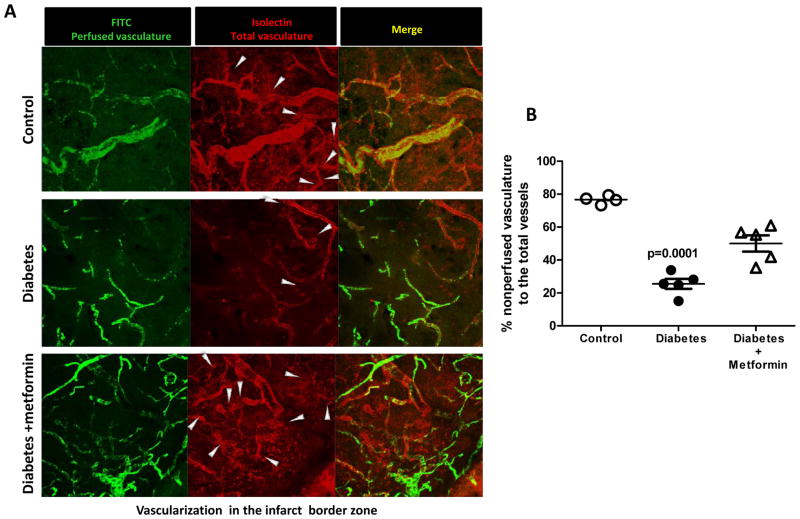
As reported earlier, the diabetic sham animals had greater vascularization compared to the control group 17. 14-days post-stroke vascular volume and surface area were dramatically enhanced in the ischemic hemisphere both in the cortex and striatum in the control group compared to the respective shams (Fig 1). The greater increase in surface area indicates that a significant portion of this increase comes from the enhancement of microvasculature. In diabetic animals, on the other hand, vascular volume was significantly decreased in the ischemic cortex and striatum indicating a strong disease (diabetes) and intervention (stroke) interaction. There was a decrease in the surface area only in the ischemic cortex. A comparison made between the ischemic and the contralateral hemisphere reveal that the distant sites display a similar response to that seen in the ischemic hemisphere but to a lesser extent. For example, contralateral region in the control groups had greater vascularization than in sham animals, while the contralateral site in diabetic animals displays attenuated vascularization. Metformin intervention initiated after stroke to achieve euglycemia corrected the decrease in vascular volume and surface area restoring it to control levels. When vascularization was assessed directly at the border of the infarct zone (Fig 2C), there were FITC-perfused vessels feeding into the infarcted area while diabetic rats show decreased vascular density. There was evident astrocyte activation as shown by enhanced GFAP staining around the infarct. Metformin treatment improved vascularization in the diabetic group (Fig 2B). Diabetes not only impaired the perfused vessels but also the non-perfused vasculature compared to control as indicated by white arrows (Fig 3). Metformin intervention improved the level of vascularity by increasing non-perfused vessel islands.
Figure 1.
Diabetes impairs post-stroke neovascularization in the ipsilateral and contralateral hemispheres. (A) Representative brain section depicting the region of interest in the ipsilateral and contralateral hemisphere shown in red and yellow squares. (B) Representative images contrasting ipsilateral and contralateral zones across the groups. (C) Plot depicting vascular volume across groups and treatment arm ap<0.05 vs sham control or ipsilateral control &diabetes, bp<0.05 vs control or diabetes+metformin. (D) Plot depicting vascular surface area across groups, ap<0.05 vs sham control, bp<0.05 vs ipsilateral diabetes, cp<0.05 vs control or diabetes+metformin, and dp=0.0018 vs sham. Data was analyzed with a 2×2 design for disease (control vs diabetes) and intervention (sham vs stroke) in the ipsilateral or contralateral hemispheres. There was significant interaction indicating important differences in vascularization at baseline and after stroke in the diabetes group. To determine the impact of glycemic control on post-stroke vascularization, one-way ANOVA was used (control, diabetes and diabetes+ metformin) and p values are shown on the graphs and post-hoc analyses are marked by letters. Mean ± SEM, n=6–9.
Figure 2.
Diabetes impairs post stroke cerebral neovascularization at the infarct border zone. (A) Representative image depicting the localization of the infarct border zone. (B) Representative images comparing vascular density at infarct border zone taken under 10X objective. (C) Graphical representation of the % vascular density around the area of infarction in all the groups. Mean ± SEM, n=4–6.
Figure 3.
Diabetes decreases isolectin staining (red, nonFITC-perfused vessels) after stroke indicating impairment of new vessel formation. (A) Representative images comparing non-perfused vessels at day-14 in the infarct border zone imaged using a 63X objective. White arrows represent the non-perfused vessel islands protruding from the perfused vessels (B) Graphical representation of the % nonperfused vasculature in the peri-infarct zone in all the groups. Mean ± SEM, n=4–5.
Diabetes aggravates astrogliosis post-stroke
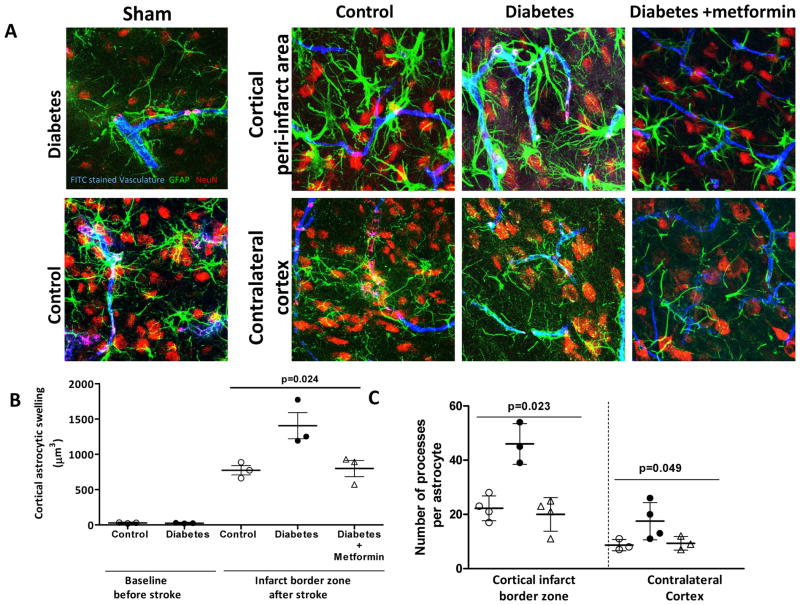
Astrocytic swelling was evaluated by measuring the somatic volumes. Diabetic animals showed increased swelling and number of astrocytic processes both around the zone of infarction and in the contralateral hemisphere. Metformin treatment prevented this response around the region of infarction (Fig 4A–C).
Figure 4.
Diabetes exacerbates astrogliosis post-stroke. (A) Representative images showing astrocytic morphology in the infarct border zone and non-lesional hemisphere as well as in the corresponding regions in sham animals (B) Diabetic stroke dramatically increases astrocytic swelling compared to control strokes and metformin treatment decreased swelling. (C) Number of astrocytic projections is significantly increased even after 14 days in diabetic stroke compared to control and glycemic intervention conserved astrocytic processes. Blue- FITC perfused vasculature, Green- Glial fibrillary acidic protein (GFAP), Red- NeuN. Mean ± SEM, n=3–4.
Glycemic intervention post stroke improves sensorimotor functions in diabetes
Neurological scores were similar at baseline and but decreased significantly in diabetic rats 24 hours post stroke. The control group had gradually improved sensorimotor function. The recovery in the diabetic group was significantly attenuated (Fig 5A). Metformin treatment reduced the sensorimotor deficits after day 5 in the diabetic group and the composite neurological scores were similar to that of the control group. Area under the curve (AUC, Day 1–14) was 112 ± 31, 67 ± 14a and 108 ± 21 for control, diabetes and diabetes + metformin groups, respectively (ap=0.012). Parallel results were obtained with the forelimb grip strength post stroke (Fig 5B). AUC (Day 0–14) was 11.4 ± 1.6, 8.2 ± 0.3a and 10.6 ± 0.3 for control, diabetes and diabetes + metformin groups, respectively (ap=0.0007).
Figure 5.
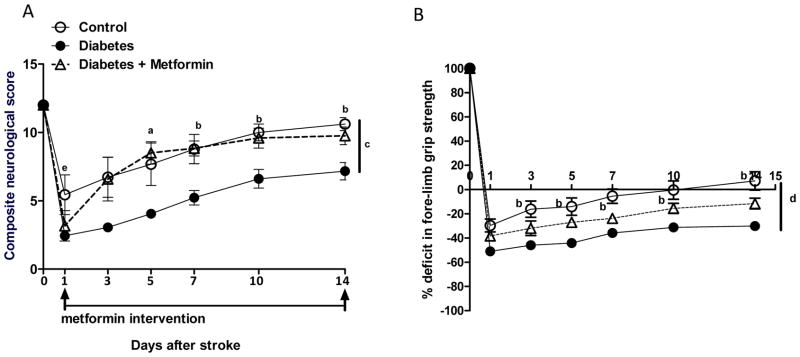
Glycemic intervention post stroke improves sensorimotor functions in diabetes. (A, B) Temporal profile of sensorimotor functions represented as composite neurological score. Diabetes significantly reduced the neurological scores after stroke. Improvement in the neurological scores was slower in the diabetic stroke group compared to control stroke. Glycemic intervention with metformin improved and restored the composite neurological scores comparable to the control group. (B) Temporal profile of forelimb grip strength shows a similar trend. The drop in the forelimb grip strength is not restored 14-days after stroke in the diabetic group compared to control. Glycemic intervention with metformin improved the forelimb grip strength. ap<0.05 vs diabetes, bp<0.01 vs diabetes, ep<0.001 vs diabetes, cAUC p=0.012 vs diabetes, dAUC p=0.0007 vs diabetes. Mean ± SEM, n=6–8.
Diabetes induces anxiety-like behaviors and cognitive deficits which are further exacerbated after stroke
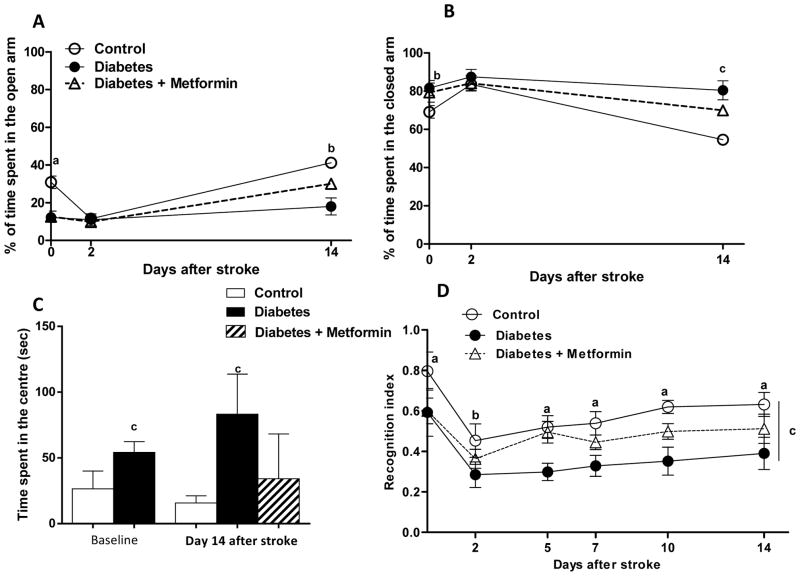
At baseline, diabetic rats demonstrated anxiety like behaviors as they spent longer time in the closed arm of the elevated plus maze. After stroke, both groups showed a similar pattern (Fig 6A). This response improved in subsequent days in the control group and at Day 14, control animals spent significantly less time than diabetics. Percent time spent in the open arm and freezing time or the time in the center of the maze was greater in diabetes (Fig 6B and C).
Figure 6.
Anxiety like symptoms are aggravated in diabetic stroke. (A, B) Graphical representation of the temporal changes in the percentage of time spent in the open and closed arm of the elevated plus maze. Diabetic groups tend to spend more time in the closed arm compared to the control groups and ischemic reperfusion injury worsened this outcome. (C) Freezing time was dramatically increased after stroke in the diabetic group, that already showed augmented time spent in the center of the maze before stroke. ap<0.001, bp<0.05, cp<0.01 vs control, Mean ± SEM, n=6–8. (D) Plot of the recognition index of all the groups at baseline and after stroke at various time points show that diabetes dampens the recognition index and stroke injury further worsens this index. Metformin intervention restored the recognition index in the diabetic stroke group. ap<0.001, bp<0.01, cAUC p<0.001 vs diabetes. Mean ± SEM, n=6–8.
Spontaneous T-maze alterations scores show a dramatic decrease in alteration scores tested at 48 hours post- stroke in both groups (supplementary figure). At baseline diabetes causes a reduction in the novel object recognition index indicating the negative impact of even short-term diabetes on cognition. All groups experienced a similar degree of deficits in the first few days following stroke but rats recovered moderately from cognitive dysfunction that is seen after stroke. Recovery in the diabetic stroked rats was impaired and metformin partially restored the cognitive function (Fig 6D).
DISCUSSION
Diabetes increases the risk and severity of stroke ultimately resulting in poor outcomes. Clinical evidence suggests that no history of diabetes is a predictor of recovery implicating that diabetes hampers recovery after stroke 7, 21, 22. However, preclinical evidence as to how diabetes influences stroke recovery is scarce. This study addressed this important gap in our knowledge and investigated the impact of diabetes a) on cerebrovascular remodeling and vascularization patterns, and b) motor and cognitive recovery following stroke.
Studies conducted on human and animal models reveal a critical place for therapeutic angiogenesis in recovery after stroke 23, 24. Angiogenesis around the infarct boundary in the ipsilateral ischemic hemisphere has been well characterized in animal models 23, 25. Increased angiogenesis has also been observed around the infarction in stroke patients. Morbidity, survival rates and neurological recovery are directly co-related to the degree of angiogenesis, microvascular density and restoration of blood flow after stroke 26, 27. Few studies have been conducted to assess brain repair in diabetic stroke and these reports suggest impaired vascular restoration after diabetic stroke 28, 29. Type-2 diabetic mice subjected to ischemia showed decreased number of microvessels after stroke in the ischemic hemisphere 28, 29. Another study, conducted on diabetic GK rats, similar to these studies, also show a reduced rate of angiogenesis 7-days after stroke 30. Most, if not all, of these studies employed conventional 2-dimensional strategies to assess microvascular density in the ischemic hemisphere, and the contralateral hemisphere was used as control tissue. We now provide a comprehensive report of 3-dimensional changes occurring in the brain vasculature in the infarcted zone as well as in peri-lesional cortical and subcortical regions in both ischemic and contralateral hemispheres 14-days after stroke as compared to sham-operated animals. This approach not only allowed us to compare the vascularization in control and diabetic animals but also assess the impact of stroke on cerebrovasculature architecture in the contralateral hemisphere. As recently reviewed 14, 31, most believe that angiogenesis only occurs in the ipsilateral hemisphere. Reports on remote functional plasticity and long-term hemodynamic changes occurring in both hemispheres substantiate our findings 32, 33. Our novel findings show that there is an increase in vascular density and remodeling in both ipsilateral and contralateral hemispheres in control animals. On the other hand, diabetic rats, which have increased yet dysfunctional angiogenesis at baseline, exhibit a dramatic decrease in vascular volume and surface area after stroke in both hemispheres. These results strongly suggest that the contralateral hemisphere is also affected by ischemic injury and responds to the damage and repair under normal conditions but presence of a confounding disease, such as diabetes, prevents this reparative response.
It is known that angiogenesis peaks one week after stroke followed by pruning and maturation of growing blood vessels 23, 24. Similar to these previous studies, stroke induced in normal rats stimulated an angiogenic response in the current study. While we did not directly study angiogenic markers, greater increase in surface area is indicative of increased microvasculature. In a given volume of tissue, an increase in vascular volume, which measures mainly the vascular lumen space, can be due to either remodeling of the vessel to get larger lumen and/or due to new vessel formation. In the latter, an increase in surface area, a measure of the area the vessel wall occupies, accompanies increased volume. As such when one looks at the relationship between volume and surface area, a linear association suggests that the increase in these two parameters increase in a parallel fashion and there is significant new microvessel formation. In this study, we detected increased vascular volume and even a greater increase in surface area. However, 14-days after stroke the diabetic GK rats had severely impaired vasculature around the infarct borders. Decreased vascularization was also observed in the contralateral hemisphere. Greater reduction in vascular volume suggests that there is significant vascular regression of existing vessels. Potential mechanisms underlying this response merit further investigation.
Glial cells, especially astrocytes, respond to vascular damage by cellular swelling and increased reactivity. Astrogliosis has been reported around the ischemic core and is increased progressively closer to the infarct border associated with glial scarring 34. This study provides evidence that stroke in association with diabetes heightens astrogliosis by increasing the swelling and number of astrocytic process densities in the peri-lesional zones. Marked increase in astrogliosis in the brain may alter neurovascular coupling to restore blood flow to the damage regions, causes inflammatory and trophic response 35, 36.
Clinical and preclinical studies report memory and cognition deficits associated with metabolic alterations and after ischemic reperfusion. Acutely after stroke, motor coordination is weakened that can propagate to permanent paralysis of the extremities. Studies report increased synaptic plasticity and neuronal reorganization with increased motor learning and activities 37. Animal behavior assessed after stroke also provides evidence of synaptic plasticity and progressive improvement in functional behavior associated with learning 38. A recent study showed that cortical plasticity in diabetic stroke is challenged compared to the stroke injury on animals without diabetes. Functional restoration was also compromised long-term after diabetic stroke 20. The current study evaluated temporal changes in sensorimotor functions, anxiety like symptoms, spontaneous activity and cognitive function after stroke with or without diabetes. Sensorimotor deficits occurred due to ischemic reperfusion injury improved and saturated after 10 days of stroke in the control rats. The extent of recovery was diminished in diabetic rats. Emerging evidence suggests that both type-1 and type-2 diabetic patients have poor neuropsychological functions affecting cognition 9, 11, 39–41. In our current study we elaborated on the temporal profile of cognitive functions after stroke in control and diabetic rats. Our results with novel object recognition and spontaneous alteration at T-maze experiments show that even at baseline memory and cognition-related tasks are impaired in diabetes. When stroke is overlayed on this pathology, recovery of cognitive function is severely affected.
We have earlier provided evidence of chronic glycemic control being an effective preventive strategy that confers vascular protection and reduces the risk of HT after ischemic reperfusion injury in diabetes 42. Although clinical trials focusing on the impact of glycemic control on cardiovascular outcomes resulted in debatable information, tight glycemic control has been an effective treatment for prevention of microvascular complications such as diabetic nephropathy and retinopathy 43–49. Metformin is a first line choice of drug commonly used to treat T2D patients. Metformin exerts its anti-hyperglycemic actions by reducing hepatic gluconeogenesis and increasing peripheral glucose uptake, insulin sensitivity and fatty acid oxidation. Metformin also has protective roles independent of its antihyperglycemic actions. Metformin improved anti-oxidant capacity in T2D patients who display excessive increased oxidation and glycation of albumin in the serum 50. Rosen and Wiernsperger showed improved anti-oxidant defense with the use of metformin in GK rats 51. We used metformin as an interventional strategy to target hyperglycemic status in diabetic rats in the post-stroke period. Clearly our findings provide compelling evidence that glycemic control by metformin in the post stroke period in rats that had not received any hypoglycemic therapy before effectively promotes vascular repair and improves sensorimotor and cognitive function after stroke.
In conclusion, these studies show that brain responds to repair ischemic injury by increasing vascularization even in remote sites but in the presence of diabetes this response is completely reversed and there is vascular regression. These changes are associated with delayed functional recovery after stroke. The mechanisms by which diabetes impairs vascular repair as well as mechanisms linking vascular regression to poor functional recovery are lines of research that we are pursuing further. Our results lend a strong support for glycemic control as an effective strategy in the prevention and/or improvement of cerebrovascular complications in diabetes.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by VA Merit Awards to S. C. F. (BX000891) and A. E. (BX000347), NIH grants to S. C. F. (NS063965), A. E. (NS054688), and AHA grants to A. E. (EIA 0740002N) and R.P. (10PRE4030037). Adviye Ergul is a research pharmacologist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
DISCLOSURES
None
References
- 1.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76:S85–90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–323. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- 3.Nannetti L, Paci M, Baccini M, Rinaldi LA, Taiti PG. Recovery from stroke in patients with diabetes mellitus. J Diabetes Comps. 2009;23:249–254. doi: 10.1016/j.jdiacomp.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen H, Nakayama H, Raaschou HO, Olsen TS. Stroke in patients with diabetes. The Copenhagen stroke study. Stroke. 1994;25:1977–1984. doi: 10.1161/01.str.25.10.1977. [DOI] [PubMed] [Google Scholar]
- 5.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 6.Mankovsky BN, Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004;20:268–287. doi: 10.1002/dmrr.490. [DOI] [PubMed] [Google Scholar]
- 7.Megherbi SE, Milan C, Minier D, Couvreur G, Osseby GV, Tilling K, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: Data from the European Biomed Stroke Project. Stroke. 2003;34:688–694. doi: 10.1161/01.STR.0000057975.15221.40. [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (accord mind): A randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saczynski JS, Jonsdottir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, et al. Cognitive impairment: An increasingly important complication of type 2 diabetes: The age, gene/environment susceptibility--reykjavik study. Am J Epidemiol. 2008;168:1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poels MM, Ikram MA, Vernooij MW, Krestin GP, Hofman A, Niessen WJ, et al. Total cerebral blood flow in relation to cognitive function: The rotterdam scan study. J Cereb Blood Flow Met. 2008;28:1652–1655. doi: 10.1038/jcbfm.2008.62. [DOI] [PubMed] [Google Scholar]
- 11.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: The framingham heart study. Neurobiol Aging. 2005;26 (Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ergul A, Alhusban A, Fagan SC. Angiogenesis: A harmonized target for recovery after stroke. Stroke. 2012 doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and mri indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 16.Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, et al. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: Role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Therap. 2012;342:407–415. doi: 10.1124/jpet.111.191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, et al. Enhanced cerebral but not peripheral angiogenesis in the goto-kakizaki model of type 2 diabetes involves vegf and peroxynitrite signaling. Diabetes. 2012;61:1533–1542. doi: 10.2337/db11-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: Relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ergul A, Li W, Elgebaly MM, Bruno A, Fagan SC. Hyperglycemia, diabetes and stroke: Focus on the cerebrovasculature. Vascul Pharmacol. 2009;51:44–49. doi: 10.1016/j.vph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, et al. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci. 2012;32:5132–5143. doi: 10.1523/JNEUROSCI.5075-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, et al. Functional recovery after ischemic stroke--a matter of age: Data from the austrian stroke unit registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 22.Liman TG, Heuschmann PU, Endres M, Floel A, Schwab S, Kolominsky-Rabas PL. Changes in cognitive function over 3 years after first-ever stroke and predictors of cognitive impairment and long-term cognitive stability: The erlangen stroke project. Dement Geriatr Cogn Disord. 2011;31:291–299. doi: 10.1159/000327358. [DOI] [PubMed] [Google Scholar]
- 23.Morris DC, Yeich T, Khalighi MM, Soltanian-Zadeh H, Zhang ZG, Chopp M. Microvascular structure after embolic focal cerebral ischemia in the rat. Brain Res. 2003;972:31–37. doi: 10.1016/s0006-8993(03)02433-8. [DOI] [PubMed] [Google Scholar]
- 24.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: Neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 27.Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci (Lond) 2006;111:171–183. doi: 10.1042/CS20060049. [DOI] [PubMed] [Google Scholar]
- 28.Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vascul Biol. 2005;25:1603–1609. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Bi X, Jia Q, Shangguan S. The possible mechanism for impaired angiogenesis after transient focal ischemia in type 2 diabetic gk rats: Different expressions of angiostatin and vascular endothelial growth factor. Biomed Pharm. 2010;64:208–213. doi: 10.1016/j.biopha.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Revs. 2010;6:238–244. doi: 10.2174/157340310791658802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayward NM, Yanev P, Haapasalo A, Miettinen R, Hiltunen M, Grohn O, et al. Chronic hyperperfusion and angiogenesis follow subacute hypoperfusion in the thalamus of rats with focal cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:1119–1132. doi: 10.1038/jcbfm.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin TN, Sun SW, Cheung WM, Li F, Chang C. Dynamic changes in cerebral blood flow and angiogenesis after transient focal cerebral ischemia in rats. Evaluation with serial magnetic resonance imaging. Stroke. 2002;33:2985–2991. doi: 10.1161/01.str.0000037675.97888.9d. [DOI] [PubMed] [Google Scholar]
- 34.Mogoanta L, Pirici D, Pop OT, Balseanu AT, Rolea E, Dahnovici RM. Study of vascular microdensity in areas of cerebral ischemia on experimental model. Romanian J Morphol Embryol. 2010;51:725–731. [PubMed] [Google Scholar]
- 35.Busch SA, Silver J. The role of extracellular matrix in cns regeneration. Curr Op Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, et al. Astroglial networks scale synaptic activity and plasticity. Proc Nat Acad Sci. 2011;108:8467–8472. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 38.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jefferson AL, Massaro JM, Beiser AS, Seshadri S, Larson MG, Wolf PA, et al. Inflammatory markers and neuropsychological functioning: The Framingham heart study. Neuroepidemiol. 2011;37:21–30. doi: 10.1159/000328864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 41.Elias PK, Elias MF, D’Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. Niddm and blood pressure as risk factors for poor cognitive performance. The framingham study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 42.Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, et al. Vascular protection in diabetic stroke: Role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30:1928–1938. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duckworth WC. Hyperglycemia and cardiovascular disease. Curr Atheroscler Rep. 2001;3:383–391. doi: 10.1007/s11883-001-0076-x. [DOI] [PubMed] [Google Scholar]
- 44.Lund SS, Tarnow L, Stehouwer CD, Schalkwijk CG, Teerlink T, Gram J, et al. Impact of metformin versus repaglinide on non-glycaemic cardiovascular risk markers related to inflammation and endothelial dysfunction in non-obese patients with type 2 diabetes. Eur J Endocrinol. 2008;158:631–641. doi: 10.1530/EJE-07-0815. [DOI] [PubMed] [Google Scholar]
- 45.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (ukpds 35): Prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (ukpds 34) UK Prospective Diabetes Study (UKPDS) group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 47.Schernthaner G. Diabetes and cardiovascular disease: Is intensive glucose control beneficial or deadly? Lessons from accord, advance, vadt, ukpds, proactive, and nice-sugar. Wien Med Wochenschr. 2010;160:8–19. doi: 10.1007/s10354-010-0748-7. [DOI] [PubMed] [Google Scholar]
- 48.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 49.Gaede P. Intensive glucose control and cardiovascular disease in type 2 diabetes--should we change the recommended target for glycated hemoglobin? Commentary to accord and advance trials. Polskie Archiwum Medycyny Wewnetrznej. 2008;118:619–621. [PubMed] [Google Scholar]
- 50.Faure P, Wiernsperger N, Polge C, Favier A, Halimi S. Impairment of the antioxidant properties of serum albumin in patients with diabetes: Protective effects of metformin. Clin Sci (Lond) 2008;114:251–256. doi: 10.1042/CS20070276. [DOI] [PubMed] [Google Scholar]
- 51.Rosen P, Wiernsperger NF. Metformin delays the manifestation of diabetes and vascular dysfunction in goto-kakizaki rats by reduction of mitochondrial oxidative stress. Diabetes Met Res Revs. 2006;22:323–330. doi: 10.1002/dmrr.623. [DOI] [PubMed] [Google Scholar]
- 52.Sachidanandam K, Hutchinson JR, Elgebaly MM, Mezzetti EM, Dorrance AM, Motamed K, et al. Glycemic control prevents microvascular remodeling and increased tone in type 2 diabetes: Link to endothelin-1. Am J Physiol. 2009;296:R952–959. doi: 10.1152/ajpregu.90537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.