Abstract
F-box proteins are the substrate recognition subunits of SCF (Skp1, Cul1, F-box protein) ubiquitin ligase complexes. Skp2 is a nuclear F-box protein that targets the CDK inhibitor p27 for ubiquitin- and proteasome-dependent degradation. In G0 and during the G1 phase of the cell cycle, Skp2 is degraded via the APC/CCdh1 ubiquitin ligase to allow stabilization of p27 and inhibition of CDKs, facilitating the maintenance of the G0/G1 state. APC/CCdh1 binds Skp2 through an N-terminal domain (amino acids 46–94 in human Skp2). It has been shown that phosphorylation of Ser64 and Ser72 in this domain dissociates Skp2 from APC/C. More recently, it has instead been proposed that phosphorylation of Skp2 on Ser72 by Akt/ PKB allows Skp2 binding to Skp1, promoting the assembly of an active SCFSkp2 ubiquitin ligase, and Skp2 relocalization/ retention into the cytoplasm, promoting cell migration via an unknown mechanism. According to these reports, a Skp2 mutant in which Ser72 is substituted with Ala is unable to promote cell proliferation and loses its oncogenic potential. Given the contrasting reports, we revisited these results and conclude that phosphorylation of Skp2 on Ser72 does not control Skp2 binding to Skp1 and Cul1, has no influence on SCFSkp2 ubiquitin ligase activity, and does not affect the subcellular localization of Skp2.
Keywords: Skp2, Akt, SCF, ubiquitin
Introduction
F-box proteins (a family of approximately 70 members in mammals) provide the substrate specificity of SCF (Skp1, Cul1, F-box protein) ubiquitin ligase complexes.1 Skp2 is the substrate targeting subunit of the SCF ubiquitin ligase that targets the CDK inhibitor p27 and other negative regulators of cell proliferation for proteasomal degradation.1 In late G1, after the cell has committed to the next round of cell division, p27 is eliminated via SCFSkp2 to allow activation of Cdk1 and Cdk2 and, consequently, promote progression through the subsequent phases of the cell cycle. To prevent premature degradation of p27, Skp2 levels are kept low during early- and mid-G1due to the APC/CCdh1 ubiquitin ligase, which mediates the ubiquitylation of Skp2.2,3
Skp2 contains two degradation motifs (degrons): the first degron (amino acids 3–6 in human) is a classical APC/C recognition motif (D box), while the second (amino acids 46–94) mediates the stable interaction between Skp2 and the APC/C activator protein Cdh1.2,3 The second degron contains a conserved CDK phosphorylation consensus site (Ser64 in human Skp2), which we found to be phosphorylated in endogenous Skp2 (unpublished results), and at least two other potential phosphorylation sites (Ser72 and Ser75). We mutated these three residues to alanine (individually or in combination) to determine their role in the APC/CCdh1-mediated degradation of Skp2. While these experiments were in progress, another group showed that phosphorylation of Ser64 and, to a lesser extent, Ser72 of Skp2 contributes to the stabilization of Skp2 by preventing its association with APC/CCdh1.4 Similar conclusions were reached by Gao et al.5 Furthermore, recently published reports assert that Ser72 phosphorylation by Akt/protein kinase B (PKB) plays an important role in SCFSkp2 assembly and activation6 and controls the subcellular localization of Skp2.5,6 Here, we investigated whether the phosphorylation status of Skp2 affects SCFSkp2 assembly, the binding of Skp2 with known interactors, and Skp2 subcellular localization.
Results and Discussion
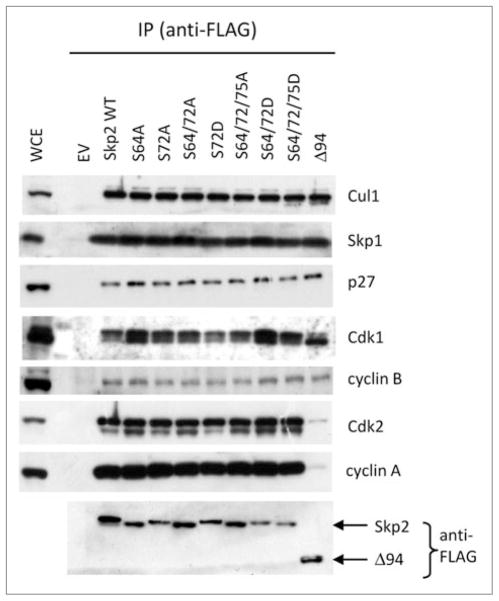
We used a panel of Skp2 phosphorylation site mutants and a deletion mutant lacking the first 94 amino acids [Skp2(Δ94)]. FLAG-tagged, wild type Skp2 and different Skp2 mutants were transiently expressed in HEK293T cells, and after lysing the cells, Skp2 was immunoprecipitated using an anti-FLAG antibody. With the exception of Skp2(Δ94), which, as previously reported,7 did not bind to the cyclin A-Cdk2 complex, the different Skp2 constructs displayed no differences in binding to endogenous Skp1, Cul1, or other interacting proteins (Fig. 1). Identical results were obtained using HeLa cells transiently transfected (to achieve high expression, as in Fig. 1 and in ref. 6) or retrovirally infected (to achieve low expression) with wild type and mutant Skp2 constructs (data not shown). Moreover, treatment of HEK293T cells with LY294002, an inhibitor of the PI(3)K-Akt pathway, did not affect the binding between endogenous Skp2 and either Skp1 or Cul1 (Fig. 2).
Figure 1.
Mutation of Ser72 does not affect the interaction of Skp2 with Skp1 or Cul1. HEK293T cells were transfected with the indicated FLAG-tagged Skp2 constructs or an empty vector (EV). Twenty-four hours post-transfection, whole cell extracts (WCE) were subjected to immunoprecipitation (IP) with anti-FLAG resin and immunoblotting was performed for the indicated proteins.
Figure 2.
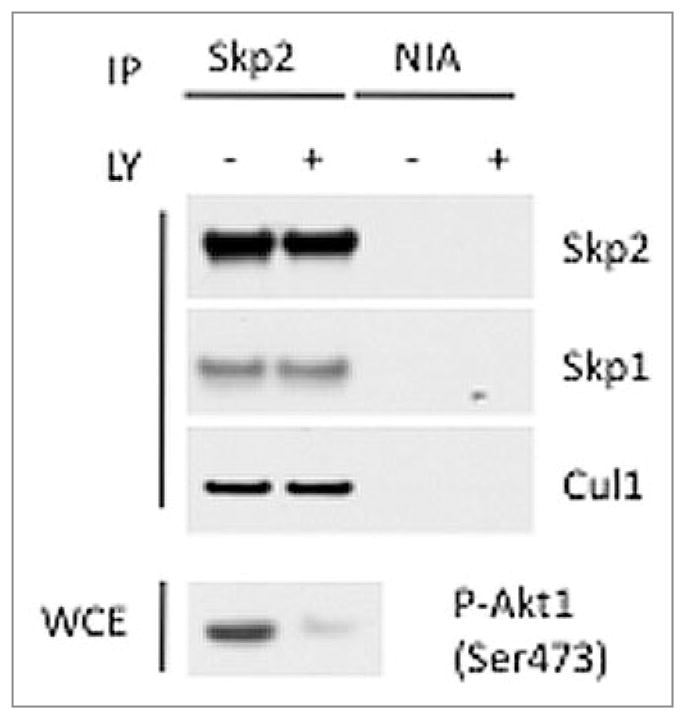
Pharmacologic inhibition of the PI(3)K-Akt pathway does not affect the binding of Skp2 to Skp1 or Cul1. HEK293T cells were stimulated with serum for 6 hours and treated with vehicle or 20 mM LY294002 for the final 5 hours. Endogenous Skp2 was immunoprecipitated, and SCF complex formation was monitored by immunoblotting with the indicated antibodies. The activation status of Akt1 was monitored by immunoblotting with anti-phospho-Akt1(Ser473) antibody. NIA, non-immunoprecipitating antibody.
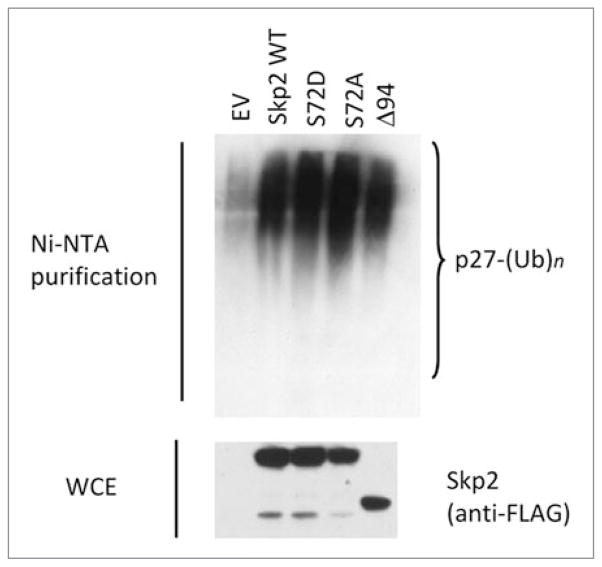
We also measured the in vitro ubiquitylation activity of the Skp2 panel and found that all Skp2 mutants were able to ubiquitylate p27 to an extent indistinguishable from that of wild type Skp2 (Fig. 3). Accordingly, expression of Skp2 mutants in HEK293T cells promoted the in vivo ubiquitylation of p27 as efficiently as wild type Skp2 (Fig. 4).
Figure 3.
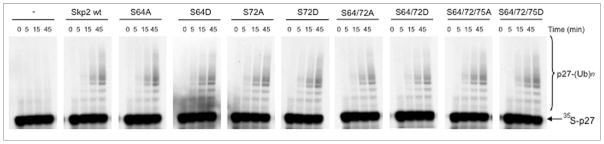
Mutation of Ser72 does not influence the in vitro ubiquitylation activity of Skp2. One microgram of in vitro transcribed/translated [35S]-labeled p27 produced in rabbit reticulocyte extract (which contains Skp1, Cul1 and Rbx1) was incubated in the presence of 0.1 μl purified, recombinant Cdk2-cyclin E complex and 0.1 μl purified, recombinant Cks1. In addition, the reaction was supplemented with 1 μl of in vitro transcribed/translated cold Skp2 (wild type and mutants), as indicated. The right bracket indicates a ladder of bands of relative molecular mass >27,000 Da, corresponding to polyubiquitylated p27; the arrow indicates unconjugated [35S]p27.
Figure 4.
Mutation of Ser72 does not influence the in vivo ubiquitylation activity of Skp2. HEK293T cells were transfected with His-tagged ubiquitin and either one of the indicated FLAG-tagged Skp2 constructs or an empty vector (EV). Twenty-four hours post-transfection, whole cell extracts (WCE) were treated with the proteasome inhibitor MG-132 for four hours prior to collection. After purification of ubiquitylated proteins with Ni-NTA resin, proteins were analyzed by immunoblotting with an antibody to p27. The bracket on the right side of the panels marks a ladder of bands >27,000 Da, corresponding to polyubiquitylated p27. The bottom panel shows the level of exogenous Skp2 as detected by immunoblotting.
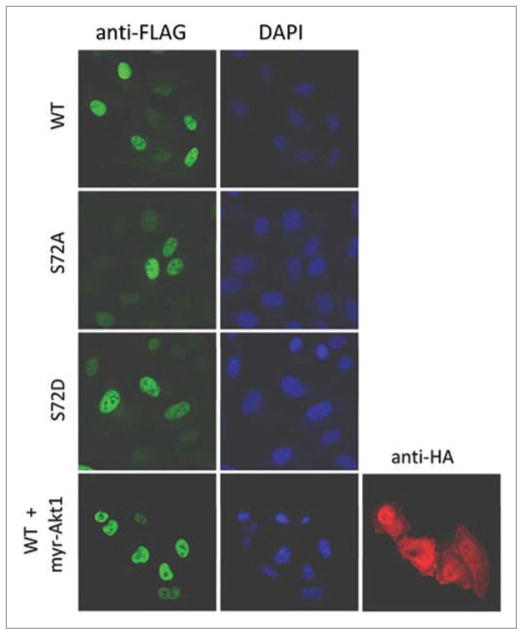
Finally, we examined the subcellular localization of FLAG-tagged Skp2(Ser72Ala) and Skp2(Ser72Asp) mutants in HeLa and U-2OS cells using indirect immunofluorescence. Like wild type Skp2, the two mutants were found to localize to the nucleus in 100% of the cells examined (Fig. 5 and data not shown). Moreover, co-expression of a constitutively active Akt1 (myr-Akt1) did not change the nuclear localization of Skp2 (Fig. 5).
Figure 5.
Mutation of Ser72 in Skp2 does not influence the cellular localization of Skp2. U-2 OS cells were transfected with the indicated FLAG-tagged Skp2 constructs in the absence or presence of HA-tagged myr-Akt1, and 48 hours post-transfection, cells were collected and analyzed by immunofluorescence with an anti-FLAG antibody, DAPI and anti-HA antibody, as indicated.
Thus, although Ser72 appears to regulate Skp2 stability in conjunction with Ser64,4,5 its phosphorylation status has no influence on SCFSkp2 complex formation, ubiquitin ligase activity, or Skp2 localization.
Our results contrast starkly with the study of Lin et al.6 which asserts that Skp2 is phosphorylated on Ser72, allowing Skp2 to bind Skp1 and Cul1, thus triggering p27 ubiquitylation. Instead, our data are in agreement with a large body of literature showing that the in vitro ubiquitylation activity of wild type Skp2 does not require the addition of Akt to the reaction (reviewed in refs. 8–10). This result also holds true for studies utilizing pre-assembly of the entire wild type SCFSkp2 complex in bacteria,11,12 where Skp2 is not phosphorylated. Similarly, Rodier et al. did not observe impaired SCF assembly or decreased activity for Skp2(Ser64/72Ala).4
Gao et al.5 and Lin et al.6 also suggest that phosphorylation of Skp2 on Ser72 (or mutation of Ser72 to Asp, which mimics phosphorylation) induces the relocalization—or retention—of Skp2 in the cytoplasm, an event that, in turn, promotes cell migration via an unknown mechanism. It is unclear whether Skp2 or the entire SCFSkp2 complex is relocalized to the cytoplasm. However, we have never observed differential localization of either Skp2(Ser72Ala) or Skp2(Ser72Asp) compared to wild type Skp2 (Fig. 5 and data not shown), nor have we observed cytoplasmic Skp2 in cells expressing myr-Akt1 (Fig. 5).
Despite a plethora of published studies on F-box proteins in eukaryotes, there is no evidence to suggest a role for the N-terminus of F-box proteins in regulating SCF assembly. On the contrary, the literature indicates that Skp1 stably binds the F-box domain and that free F-box proteins are too hydrophobic to exist in the cell in the absence of Skp1. Notably, in contrast to the strict conservation of Ser64 from insects to humans, Ser72 is not conserved in the mouse or lower vertebrates, indicating a non-essential role for this residue.
In conclusion, our data demonstrate that the phosphorylation of Ser72 does not control Skp2 binding to Skp1 or Cul1, has no influence on SCFSkp2 ubiquitin ligase activity, and does not affect the subcellular localization of Skp2. Importantly, two other groups independently reached the same conclusions.16, 17
Materials and Methods
Reagents, antibodies and DNA constructs
LY294002 was obtained from Cell Signaling Technology. FLAG-tagged Skp2 constructs were obtained by subcloning Skp2 wild type and mutants in the pcDNA3 vector. The phospho-specific antibody to phosphorylated Akt1 (Ser473) was from Cell Signaling. Antibodies to Skp2 and p27 were previously described.13 All other antibodies were described in.14
Biochemical methods
Extract preparation, immunoprecipitation, and immunoblotting were previously described.2,15 HEK293T were transfected by the calcium phosphate method. HeLa cells and U-2OS cells were transfected using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions.
Immunofluorescence
Cells were grown in CultureSlides (BD Falcon™), rinsed in PBS, and fixed for 20 minutes in 4% paraformaldehyde/PBS at room temperature. Fixed cells were permeabilized with PBS/0.2% Triton X-100 for 3 minutes, washed in PBS, and blocked with PTB buffer (PBS/0.2% Triton X-100/0.5% BSA) for 30 minutes at room temperature. Incubation with primary antibodies was then carried out for 2 hours. After three washes in PTB buffer, cells were incubated for 30 minutes with Alexa594-conjugated or Alexa488-conjugated secondary antibody. All antibody reactions were carried out at room temperature and dilutions were made in PTB buffer. Nuclei were visualised with DAPI. Samples were mounted in ProLong® Gold Antifade Reagent.
In vitro ubiquitylation assays
In vitro ubiquitylation reactions were performed as previously described.15 Briefly, ubiquitylation was performed in a volume of 10 μl containing 50 mM Tris pH 7.6, 5 mM MgCl2, 0.6 mM DTT, 2 mM ATP, 2 μl in vitro translated unlabelled Skp2, 1.5 ng/μl E1 (Boston Biochem), 10 ng/μl Ubc3, 10 ng/μl Ubc5, 2.5 μg/μl ubiquitin (Sigma), 1 μM ubiquitin aldehyde, 0.1 μl purified, recombinant Cdk2-cyclin E complex, 0.1 μl purified, recombinant Cks1, and 1 μl 35S-methionine-labelled in vitro translated p27.
Acknowledgments
We thank L. Cermak, R. Deshaies, G. Draetta, A. Hershko and J. Skaar for discussion and/or critically reading the manuscript, and Eva Hernando for reagents. M.P. is grateful to T.M. Thor for continuous support. This work was supported by a NHMRC fellowship to J.K.P., an AICF and a FIRC fellowship to L.B., and grants from the National Institutes of Health (R01-GM57587, R37-CA76584 and R21-CA125173) and the Multiple Myeloma Research Foundation to M.P. M.P. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations
- Skp2
S-phase-associated kinase 2
- APC/C
anaphase promoting complex/cyclosome
- CDK
cyclin-dependent kinase
- PKB
protein kinase B
References
- 1.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–49. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–3. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 3.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 4.Rodier G, Coulombe P, Tanguay PL, Boutonnet C, Meloche S. Phosphorylation of Skp2 regulated by CDK2 and Cdc14B protects it from degradation by APC(Cdh1) in G1 phase. EMBO J. 2008;27:679–91. doi: 10.1038/emboj.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–32. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji P, Goldin L, Ren H, Sun D, Guardavaccaro D, Pagano M, et al. Skp2 contains a novel cyclin A binding domain that directly protects cyclin A from inhibition by p27Kip1. J Biol Chem. 2006;281:24058–69. doi: 10.1074/jbc.M603105200. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein G, Ganoth D, Hershko A. Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc Natl Acad Sci USA. 2006;103:11515–20. doi: 10.1073/pnas.0603921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Nacusi L, Sheaff RJ, Liu X. Ubiquitination of p21Cip1/WAF1 by SCFSkp2: substrate requirement and ubiquitination site selection. Biochemistry. 2005;44:14553–64. doi: 10.1021/bi051071j. [DOI] [PubMed] [Google Scholar]
- 10.Zhu XH, Nguyen H, Halicka HD, Traganos F, Koff A. Noncatalytic requirement for cyclin A-cdk2 in p27 turnover. Mol Cell Biol. 2004;24:6058–66. doi: 10.1128/MCB.24.13.6058-6066.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyapina SA, Correll CC, Kipreos ET, Deshaies RJ. Human CUL1 forms an evolutionarily conserved ubiquitin ligase complex (SCF) with SKP1 and an F-box protein. Proc Natl Acad Sci USA. 1998;95:7451–6. doi: 10.1073/pnas.95.13.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol Cell. 2008;32:21–31. doi: 10.1016/j.molcel.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrano AC, Pagano M. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J Cell Biol. 2001;153:1381–9. doi: 10.1083/jcb.153.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge S, Skaar JR, Pagano M. APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle. 2009;8:167–71. doi: 10.4161/cc.8.1.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 16.Boutonnet C, Tanguay PL, Julien C, Rodier G, 1, Coulombe P, Meloche S. Phosphorylation of Ser72 does not regulate the ubiquitin ligase activiy and subcellular localization of Skp2. Cell Cycle. 2010 doi: 10.4161/cc.9.5.10915. this issue. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Cui J, Bauzon F, Zhu L. A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle. 2010 doi: 10.4161/cc.9.5.10916. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]