Abstract
Very little is known about the effects of gestational diabetes mellitus (GDM) on lactation and milk components. Recent reports suggested that hyperglycemia during pregnancy was associated with altered breast milk immune factors. Human milk oligosaccharides (HMOs) and N-glycans of milk immune-modulatory proteins are implicated in modulation of infant immunity. The objective of the current study was to evaluate the effect of GDM on HMO and protein-conjugated glycan profiles in breast milk. Milk was collected at 2 wk postpartum from women diagnosed with (n = 8) or without (n = 16) GDM at week 24–28 in pregnancy. Milk was analyzed for HMO abundances, protein concentrations, and N-glycan abundances of lactoferrin and secretory immunoglobulin A (sIgA). HMOs and N-glycans were analyzed by mass spectrometry and milk lactoferrin and sIgA concentrations were analyzed by the Bradford assay. The data were analyzed using multivariate modeling confirmed with univariate statistics to determine differences between milk of women with compared with women without GDM. There were no differences in HMOs between milk from women with vs. without GDM. Milk from women with GDM compared with those without GDM was 63.6% lower in sIgA protein (P < 0.05), 45% higher in lactoferrin total N-glycans (P < 0.0001), 36–72% higher in lactoferrin fucose and sialic acid N-glycans (P < 0.01), and 32–43% lower in sIgA total, mannose, fucose, and sialic acid N-glycans (P < 0.05). GDM did not alter breast milk free oligosaccharide abundances but decreased total protein and glycosylation of sIgA and increased glycosylation of lactoferrin in transitional milk. The results suggest that maternal glucose dysregulation during pregnancy has lasting consequences that may influence the innate immune protective functions of breast milk.
Introduction
In addition to nutrients, breast milk delivers complex carbohydrates and proteins that possess a wide range of biological activities that promote the normal development and maturation of specific systems, the innate immune system, and gut microbiota (1, 2). Human milk glycans composed of free oligosaccharides (HMOs)9 and conjugated to proteins impart protective benefits to the neonate. HMO is the third most abundant component of human milk, is completely indigestible to the neonate, and is reported to enrich the growth of beneficial strains of infant gut bifidobacteria (2). Human milk glycoproteins exert protection against infectious diseases via antimicrobial and immunomodulatory activities that confer passive immunity to the breast-fed infant (1, 3).
Human milk glycans play key roles in mediating milk’s immune protection through cell signaling and cell-to-cell recognition events, enrichment of protective gut microbiota, and modulation of microbial adhesion and invasion of the infant intestinal mucosa (2, 4, 5). Human milk lactoferrin, the major milk glycoprotein, binds to pathogenic gram-positive (6) and gram-negative (7) bacteria to exert antimicrobial activity via iron depletion and/or bacterial membrane disruption. Secretory IgA (sIgA), the predominant Ig in human milk (8), provides protection to neonates by coating microorganisms and inhibiting colonization and neutralizing viral and bacterial endotoxins (1). Glycosylation of lactoferrin and sIgA in milk renders the glycoproteins resistant to digestion and is critical for their biological functions, including pathogen decoy and prebiotic activities (9, 10) and nonspecific innate immune defense against invading pathogens in the gut by inhibiting pathogen adhesion and infection (11, 12), respectively.
Gestational diabetes mellitus (GDM), a complex disease characterized by elevated blood glucose, affects a mean 7% and up to 14% of pregnancies in the United States (13). GDM has immediate and lasting consequences in women and their infants exposed to maternal diabetes in utero (14–16). Hyperglycemia results in aberrant carbohydrate metabolism that contributes to the pathogenesis of the disease in part through increased flux through the hexosamine biosynthetic pathway (17, 18) and altered activities of cellular glycosyltransferases and glycosidases (19, 20). Yet, very little is known about the effect of GDM on lactation and milk components. Recently, colostrum from women diagnosed with diabetes mellitus was reported to contain lower concentrations of IgA and IgG (21, 22) and complement C3 protein (22) compared with colostrum from normoglycemic women, suggesting a link between maternal insulin sensitivity and the immune functions of milk. Glycomic profiling of human milk in which HMOs and conjugated N-glycan residues of milk proteins can be measured using high-sensitivity, quantitative MS has led to the discovery of structure-function relations in neonates (2, 23).
The objective of this study was to determine the effects of GDM on the glycosylation of HMOs and protective proteins of breast milk. We hypothesized that lactoferrin and sIgA N-glycans will be altered in milk from women with GDM compared with women without GDM. In the current study, HMOs, human milk lactoferrin, and sIgA and their N-glycans from women at 2 wk postpartum, with and without GDM, were measured by MS using a system-wide glycomics approach. The findings suggest the potential for characterizing milk for immune functionality. The findings document differences in milk due to GDM in mothers and illustrate the potential for characterizing milk for immune functionality.
Participants and Methods
Participant enrollment.
Participants diagnosed with (n = 8) or without (n = 16) GDM were enrolled in the study. GDM was screened for during each participant’s routine 24- to 28-wk prepartum clinical visit using a 50-g, 1-h oral glucose tolerance test (OGTT). Participants whose 1-h OGTT was <7.2 mmol/L comprised the normal glycemic participant pool. One participant declined the OGTT screening at 28 wk but was considered glucose tolerant based on her 1-wk fasting and 2-h postprandial normal glucose responses. Participants whose 1-h OGTT exceeded 7.2 mmol/L completed a 3-h, 100-g OGTT. One participant declined the 3-h, 100-g OGTT and was retained in the study, because her 50-g, 1-h OGTT was 8.0 mmol/L. Five of the 8 women with GDM reported their blood sugar was controlled with diet and the remaining 3 women reported their blood sugar was controlled with medication (specifically, glyburide). Two of the participants diagnosed with GDM in this study were diagnosed with GDM in past pregnancies. Maternal insulin sensitivity was not followed-up postpartum.
Milk sample collection and processing.
Milk samples were collected using a published method at 2 wk postpartum from 8 women with GDM and 16 women without GDM delivering at full-term (24). In short, milk was collected by a research assistant at participants’ homes or in the laboratory using a hospital-grade breast pump between 0800 and 1200 h; milk collected at participants’ homes was transported to the laboratory on ice. At the laboratory, milk was warmed in water and gently swirled to mix before aliquoted and stored at −80 C. Anthropometric and health history data were obtained from self-reported health history questionnaires. The Louisiana State University and University of California Davis Institutional Review Boards approved all aspects of the study and informed consent was obtained from all participants. Samples were de-identified to protect patient privacy and ensure blinding during glycan analysis.
Free oligosaccharide extraction and purification.
Sample preparation and analysis for HMOs and milk N-glycans are summarized in Supplemental Figure 1. Participants’ milk samples were thawed at room temperature and immediately defatted via centrifugation at 4000 × g for 30 min at 4°C. Lipids and proteins were removed from the skimmed milk by Folch extraction (25) followed by ethanol precipitation and centrifugation at 4 000 × g. The supernatant (aqueous phase containing free oligosaccharides) was evaporated and reconstituted in 200 μL of deionized water. HMOs were reduced to their alditol form with 1.0 mol/L NaBH4. Solid-phase extraction on a graphitized carbon cartridge (GCC) was used for desalting and enrichment. HMOs were eluted from GCC with 20% acetonitrile in water and 40% acetonitrile in 0.05% trifluoracetic acid (v:v). The eluents from both fractions were combined and evaporated. The HMO samples were reconstituted in 200 μL water and diluted for mass spectrometric analysis.
Lactoferrin purification from human milk.
Milk lactoferrin purification from individual milk samples was performed following a procedure described by Lönnerdal et al. (26) with slight modifications. Milk samples (0.5 mL) were centrifuged at 4000 × g for 30 min at 4°C and the lower phase was acid-precipitated using CaCl2 solution (pH 4.6) to reach a final concentration of 60 mmol/L. The whey fractions were loaded onto heparin-Sepharose resin columns and the flow-through was collected and reloaded onto the column twice. Lactoferrin bound to heparin-Sepharose was eluted and collected fractions were collected, dialyzed using a dialysis membrane with a molecular weight cutoff of 10,000 in 10 mmol/L ammonium bicarbonate, concentrated, and stored at −20°C.
sIgA purification from human milk.
Milk sIgA was acquired from the flow-through described above for lactoferrin. Purification of sIgA was similar to the purification of lactoferrin except the columns for sIgA were packed with Staphylococcus aureus super antigen-like protein 7 (SSL 7)/agarose (27). Lactoferrin and sIgA protein concentrations were determined using the Bradford assay and 5-μL aliquots were assayed by SDS-PAGE.
N-glycan release and purification from milk lactoferrin and sIgA.
Lactoferrin and sIgA (50 μg) purified from milk from all participants were reduced in 50 μL of 10 mmol/L dithiothreitol in 200 mmol/L NH4HCO3. An endoglycosidase (PNGase F) (2 μL) was added to all samples and glycans were released by incubation in a microwave reactor at 20 W at 60°C for 10 min. Released glycans were purified by solid-phase extraction using GCC, as described in HMO extraction and purification.
HPLC/CHIP-time of flight MS.
The analysis of both free HMOs and enzymatically released N-glycans was performed on an Agilent 6200 series nanoLC-CHIP/TOF MS system equipped with both a capillary pump for sample loading and a nanopump for sample separation. The HPLC unit was coupled to an Agilent 6210 series TOF mass spectrometer via a chip-cube interface. The microfluidic chip consisted of a 40-nL enrichment column and a 75-μm × 43-mm analytical column, both packed with porous graphitized carbon. Data were collected in the positive mode and calibrated by a dual nebulizer electrospray source with internal calibrant ions ranging from m/z 118.086 to m/z 2721.895.
Free HMO separation.
Oligosaccharide samples were separated by an enrichment column using a binary gradient consisting of an aqueous solvent A [3% acetonitrile/water (v:v) in 0.1% formic acid solution] and an organic solvent B [90% acetonitrile/water (v:v) in 0.1% formic acid solution] delivered at 0.4 μL/min. A 60-min gradient optimized for separating HMO mixtures developed by Wu et al. (28) was used for this analysis. HMO in one sample from a mother with GDM was unable to reduce to the alditol form, was not analyzed by MS, and therefore was not included in the statistical analyses.
N-glycan separation.
N-glycan samples were loaded onto the enrichment and separated using aqueous solvent A and organic solvent B, as described above. A 31.5-min gradient optimized for separating glycan mixtures was used for the analysis.
HPLC/CHIP-time of flight MS data analysis.
N-Glycan and free HMO identification and quantitation were performed using Agilent Mass Hunter Qualitative Analysis software (Version B.03.01, Agilent Technologies). Individual HMO structures were rapidly identified by matching retention times and exact, deconvoluted glycan masses obtained experimentally to calculated masses compiled in annotated and published HMO libraries (28). N-glycan composition were rapidly identified by matching exact, deconvoluted glycan masses obtained experimentally to calculated masses compiled in annotated in-house glycan libraries. The relative quantities of each glycan species for HMO and for lactoferrin and IgA N-glycans were calculated by normalizing the absolute abundance of the individual species to the total oligosaccharide abundance in each sample, yielding a relative abundance expressed as a percentage of the total [percentage of total abundance = (ion count of the glycan species/total ion count of all glycans) × 100].
Data analysis and statistics.
Multivariate data analysis was used to explore the relation between maternal phenotype (women with vs. without GDM) and the human milk glycome. N-glycan data were log10-transformed, mean centered, and scaled to unit variance. An orthogonal signal corrected partial least-squares discriminant analysis (O-PLS-DA) (29) model was developed to explore the variation in milk glycans and determine their importance in predicting maternal phenotype. Spearman’s ρ (P < 0.05) was used to determine the correlation between the original 47 variables and the O-PLS-DA model scores. Variables that were significantly correlated with the model scores and displayed absolute loadings [contribution of each variable to the model on the first dimension or latent variable (LV)] that were >60th percentile for all of the loadings were retained in the final model. The maximum difference between milk glycans from women with and without GDM was captured in the first dimension or LV of the model. The O-PLS-DA model scores were plotted to visualize the discrimination achieved by the model between the 2 maternal phenotypes. Variable loadings (LV1) were plotted to display the magnitude and direction of the differences for the selected variables between the 2 phenotypes.
The quality of the final model was evaluated by predicting maternal phenotype using the final model’s selected variables through cross-validation and maternal phenotype permutation testing. Cross-validation was conducted by randomly selecting two-thirds of the participants as a training set (to build models) and using one-third of them to test the models in 100 iterations. Permutation testing was conducted by applying the training/testing procedure described above to predict randomly assigned (permuted) phenotype labels. To validate the final model and evaluate model over-fitting, the distributions in performance statistics (cross-validated fit, root mean squared error of prediction, and area under the receiver operator characteristic curve) between the final and permuted models were compared.
Power calculations were conducted on the full data set (47 variables) using the effect sizes based on Cohen’s distance for the measured variables, a sample size of n = 8 for GDM and n = 16 for non-GDM, with an α set at 0.05 and adjustment for the false discovery rate (FDR). O-PLS-DA was combined with feature selection to limit hypothesis testing to only those variables correlated with differences between maternal phenotype (GDM vs. non-GDM). This approach was used to reduce the multiple hypotheses testing to only the selected features (12 variables), which were interrogated using univariate statistics with inclusion of updated FDR penalties.
ANOVA was used to test for differences of the final model’s selected variables between women with GDM and without GDM. The probability level for the test statistics was set at α = 0.05 and was adjusted for multiple hypotheses testing using Benjamini Hochberg (30) to allow for a maximum 5% probability (q = 0.05) in false positive detection.
A correlation-based network was used to analyze interrelationships among the milk glycans retained in the final model to predict maternal phenotype. In this network, vertices represent milk glycans, which are colored according to the direction in the difference between the 2 phenotypes and sized to represent their importance in the multivariate model (O-PLS-DA loadings on LV1). Vertices are connected to each other by colored lines based on their correlations (Spearman’s ρ, P < 0.001) and the thickness of the lines was used to display the absolute magnitude of the coefficient of correlation. Relations between sIgA with lactoferrin identified by the correlation-based network were confirmed with multiple linear regression with adjustment for maternal phenotype. All analyses were carried in a combination of R statistical programming environment (31) and SPSS version 20.0 for Windows, imDEV (v1.42) (32), and the network visualization was executed using Cytoscape (33).
Results
Participant characteristics.
Participant characteristics are reported in Table 1. Maternal age, prepregnancy BMI, and infant gestational age, weight, and length at birth did not significantly differ between women with and without GDM. Participants without GDM gained 64% [(12.1 -7.4)/7.4 x 100] more weight during pregnancy than those without GDM (P < 0.05). The lower weight gain during pregnancy in women with GDM compared with women without GDM is atypical for GDM. This finding is likely a reflection of the unbalanced number of participants in each group and small sample size of study participants with GDM. All participants exclusively breast-fed their infants by 2 wk postpartum when milk was collected for analysis, except for one woman without GDM who supplemented her breast milk with infant formula.
TABLE 1.
Characteristics of women with and without GDM1
| Characteristic | GDM (n = 8) | Non-GDM (n = 16) |
| Maternal age, y | 29.9 ± 3.8 (25–36) | 29.6 ± 4.6 (21–38) |
| Maternal ethnicity | 6 Caucasian, 1 African American, 1 Asian | 12 Caucasian, 4 African American |
| Delivery mode | ||
| C-section | 2 | 5 |
| Vaginal | 6 | 11 |
| Parity | ||
| Primiparous | 3 | 3 |
| Multiparous | 5 | 13 |
| Maternal prepregnancy BMI, kg/m2 | 24.2 ± 6.1 (17.5–36.5) | 24.9 ± 4.8 (20.9–40.9) |
| Maternal pregnancy weight gain, kg | 7.4 ± 3.1 (0.9–10.5)* | 12.1 ± 4.9 (4.5–24.5) |
| Gestational age of infant at birth, wk | 38.9 ± 0.9 (37.1–40.3) | 39.6 ± 0.7 (38.5–40.6) |
| Infant birth weight, kg | 3.41 ± 0.31 (2.99–3.88) | 3.46 ± 0.31 (3.09–4.09) |
| Infant birth length, cm | 50.8 ± 2.0 (48.3–54.0) | 51.7 ± 2.1 (48.3–55.8) |
Values are means ± SDs (ranges). *Different from non-GDM, P < 0.05. GDM, gestational diabetes mellitus.
Milk N-glycan data analyses.
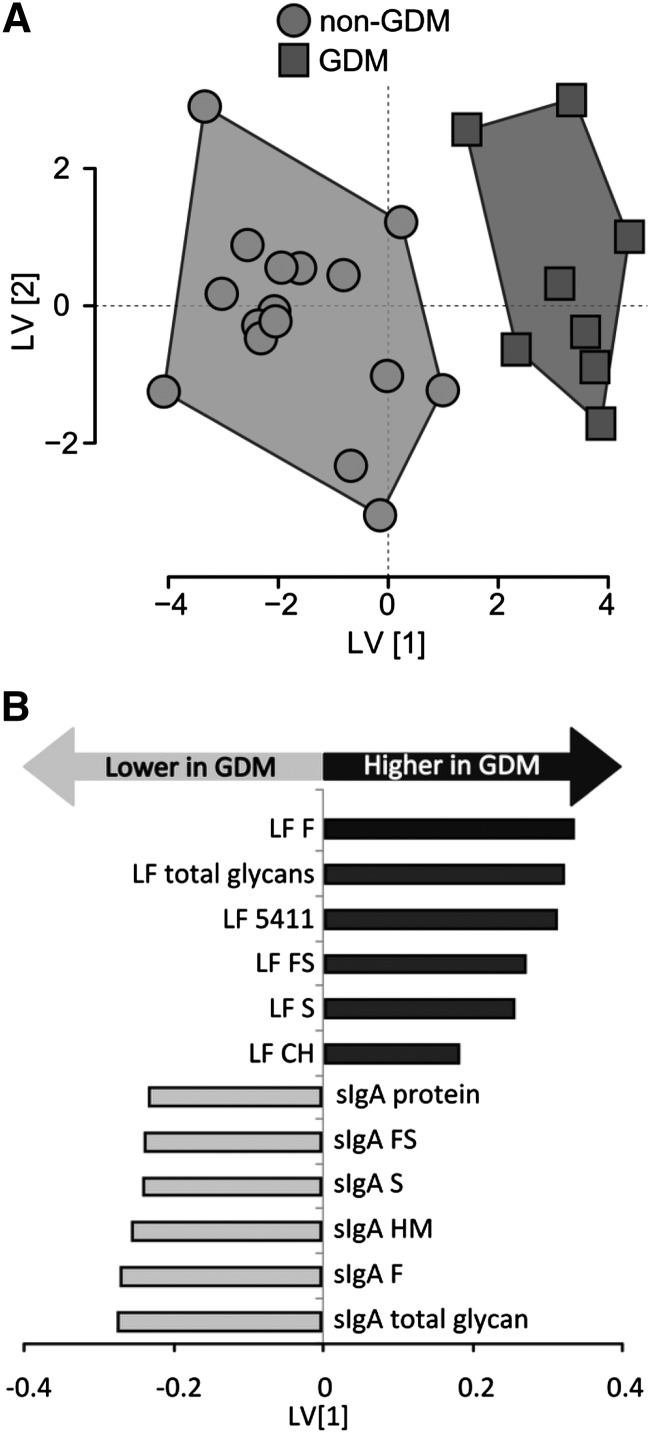
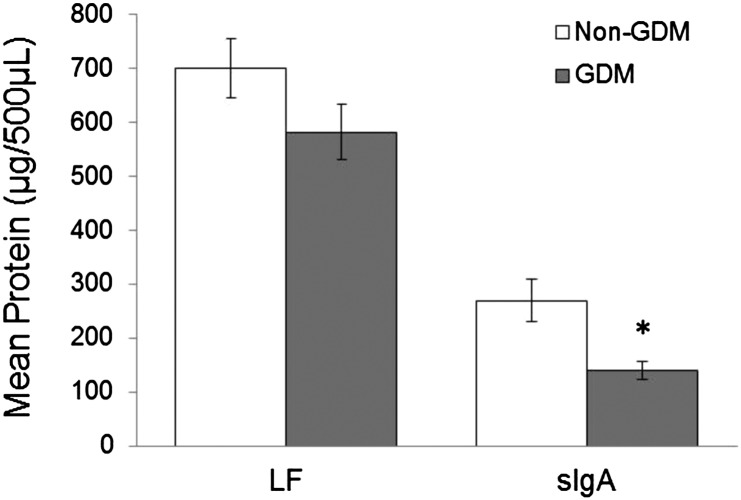
When power calculations were conducted on the full data set (47 variables), the mean power reached 0.22 and N-glycans of lactoferrin [5411 (N-glycan containing 5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, 1 sialic acid), sialic acid, fucose, fucose and sialic acid, and total N-glycans] reached a power ≥0.8 at the FDR-adjusted α of 0.05. O-PLS-DA model optimization was used to retain 12 of the 47 original variables, which displayed the highest inter- and lowest intra-individual variation for predicting maternal phenotype. O-PLS-DA was combined with feature selection to limit hypothesis testing to only those variables correlated with differences between experimental groups. This approach was used to reduce the multiple hypothesis testing to only the selected features (12 variables), which were interrogated using univariate statistics with inclusion of FDR penalties. A follow-up power analysis on only the 12 selected variables suggested a mean power of 0.82 at the FDR-adjusted α of 0.05 (Table 2). O-PLS-DA scores from a model based on the 12 selected variables demonstrate clear visual separation between the 2 phenotypes (Fig. 1A). Model cross-validation and permutation testing were used to examine over-fitting and validate the performance of the final model. Based on these evaluations, the final model performed better than expected by random chance, which is supported by its area under the receiver operating characteristic curve (0.99) compared with that of the permuted model (0.54) (Table 3). Analysis of the model loadings suggests that the N-glycans of lactoferrin are higher, but total sIgA protein and N-glycans of sIgA are lower in milk from women with GDM compared with women without GDM (Fig. 1B). There were no differences in the absolute quantitation or relative percentage of HMO compositions between the 2 groups (Supplemental Fig. 2). ANOVA was used to confirm that the 12 selected variables of importance were different between the 2 phenotypic groups (Table 2). The amount of lactoferrin protein in milk was not different, yet the total amount of sIgA protein in milk was lower by 63.6% (P < 0.05; power = 0.68) in women with compared with those without GDM (Fig. 2). The N-glycosylation of lactoferrin from the milk of women with vs. without GDM was higher. The N-glycans of lactoferrin in milk from women with compared with those without GDM were higher by 57% for total N-glycans (P < 0.0005) and 55% for 5411 (5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, 1 sialic acid) (P < 0.001); 80% for complex-hybrid N-glycans (P < 0.05); and 113, 70, and 44% for fucosylated (P < 0.0005), sialylated and fucosylated, and sialylated abundances (P < 0.01), respectively (Table 2). The N-glycosylation of sIgA from women with GDM was lower compared with women without GDM. The N-glycans of sIgA were lower by 46% for total N-glycans and 54, 54, 39, and 40% for fucosylated, sialylated; fucosylated, and sialylated, and high-mannose abundances, respectively (P < 0.05) in milk from women with compared with those without GDM (Table 2).
TABLE 2.
Final and validated model based on breast milk glycans that predicts GDM in study participants1
| Glycan | Non-GDM (n = 16) | GDM (n = 8) | FDR-adjusted P value | Power |
| 1 × 104 | 1 × 104 | |||
| Lactoferrin | ||||
| Complex hybrid | 3.31 ± 2.31 | 7.72 ± 4.52 | 0.023 | 0.66 |
| Fucosylated | 11.6 ± 4.01 | 41.8 ± 22.7 | 0.0001 | 1.0 |
| Sialylated | 14.4 ± 9.37 | 29.7 ± 12.1 | 0.009 | 0.87 |
| Fucosylated and sialylated | 112 ± 33.6 | 176 ± 36.1 | 0.004 | 0.94 |
| Total | 142 ± 40.8 | 256 ± 32.0 | 0.0001 | 1.0 |
| 5411 | 55.7 ± 19.6 | 97.6 ± 14.4 | 0.001 | 0.99 |
| sIgA | ||||
| High mannose | 33.6 ± 10.6 | 22.4 ± 11.2 | 0.020 | 0.73 |
| Fucosylated | 85.2 ± 36.3 | 48.9 ± 17.5 | 0.011 | 0.82 |
| Sialylated | 116 ± 59.6 | 66.3 ± 42.2 | 0.020 | 0.70 |
| Fucosylated + sialylated | 222 ± 104 | 150 ± 81.4 | 0.041 | 0.54 |
| Total | 519 ± 204 | 324 ± 175 | 0.016 | 0.77 |
Values are means ± SDs. FDR, false discovery rate; GDM, gestational diabetes mellitus; sIgA, secretory IgA; 5411, N-glycan containing 5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, 1 sialic acid.
FIGURE 1.
(A) O-PLS-DA from the final model based on the 12 selected variables demonstrate clear visual separation between women with and without GDM. (B) O-PLS-DA variable loadings for the LV1 display the relative levels of milk N-glycans from women with vs. without GDM. CH, complex hybrid; F, fucose; FS, fucose and sialic acid; GDM, gestational diabetes mellitus; HM, high mannose; LF, lactoferrin; LV, latent variable; O-PLS-DA, orthogonal signal corrected partial least-squares discriminant analysis; S, sialic acid; sIgA, secretory IgA; 5411, N-glycan containing 5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, 1 sialic acid.
TABLE 3.
Performance statistics for the training/testing cross-validated permuted and final models generated from breast milk glycans to predict GDM in study participants1
| Model | LV | Q2 | RMSEP | AUROCC |
| O-PLS-DA | 2 | 0.62 ± 0.1 | 0.28 ± 0.1 | 0.99 ± 0.02 |
| Permuted2 | 2 | −0.52 ± 0.4 | 0.53 ± 0.1 | 0.54 ± 0.3 |
Values are means ± SDs. AUROCC, area under the receiver operating characteristic curve; GDM, gestational diabetes mellitus; LV, latent variable; O-PLS-DA, orthogonal signal corrected partial least-squares discriminant analysis; Q2, cross-validated model fit; RMSEP, root mean squared error of prediction.
Randomly permuted class label models, n = 100.
FIGURE 2.
Concentrations of human milk lactoferrin (LF) and sIgA from women with compared with those without GDM. Means ± SEMs for human milk LF and sIgA concentrations from 500 μL of milk from women with GDM (n = 8) vs. non-GDM (n = 16). ANOVA, different from non-GDM, *P < 0.05. GDM, gestational diabetes mellitus; LF, lactoferrin; sIgA, secretory IgA.
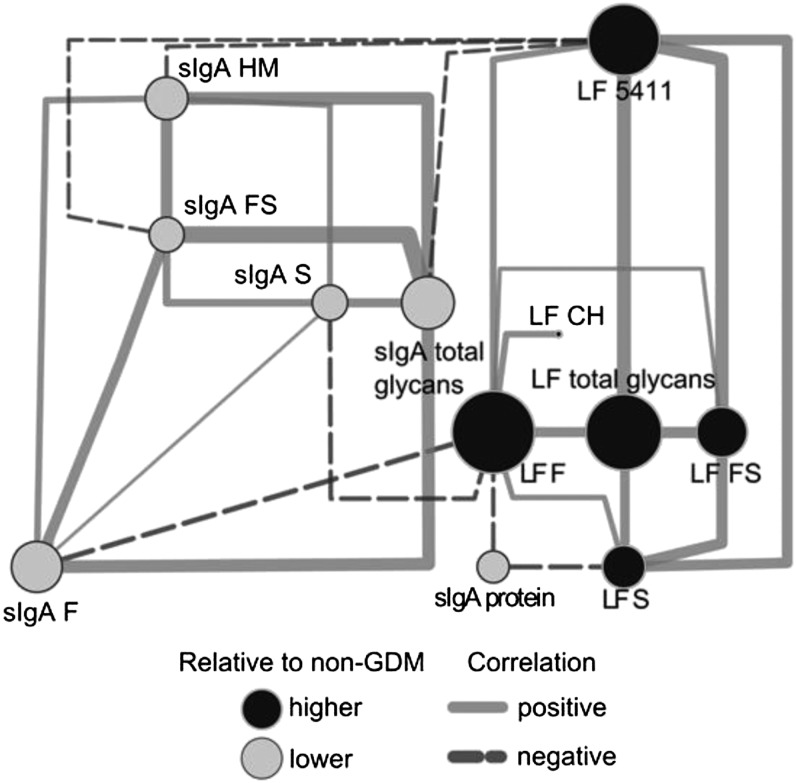
The correlation network developed to investigate the statistical and multivariate results in the context of the interrelationships among the milk glycans demonstrated high collinearity among sIgA and lactoferrin N-glycan abundances with one another, respectively. Additionally, there were significant inverse associations between the abundance of fucosylated lactoferrin and the amount of sIgA protein; and lactoferrin N-glycan 5411 and sIgA total N-glycans (ρ = 0.57) (Fig. 3). Using multiple linear regression, fucosylated lactoferrin could no longer predict the amount of sIgA with adjustment for maternal phenotype (data not shown). Additionally, lactoferrin N-glycan 5411 predicted the abundance of sIgA total N-glycans (P < 0.05) with adjustment for maternal phenotype, a relation largely explained by maternal phenotype based on collinearity diagnostics of the adjusted model (Eigenvalue of 0.000 and condition index of 101.2).
FIGURE 3.
Correlation network among milk glycans from women with, compared with those without, GDM selected by O-PLS-DA. Using Spearman’s ρ, P < 0.001, relations and strength of the relations (thickness of the lines; thickest lines, ρ = 0.97; thinnest lines, ρ = 0.5) among the milk components, represented by vertices, are annotated to show the relative difference and importance (vertex size, variable loading on LV1) of the glycans in milk from women with vs. without GDM (non-GDM). CH, complex hybrid; F, fucose; FS, fucose and sialic acid; GDM, gestational diabetes mellitus; HM, high mannose; LF, lactoferrin; LV, latent variable; O-PLS-DA, orthogonal signal corrected partial least-squares discriminant analysis; S, sialic acid; sIgA, secretory IgA; 5411, N-glycan containing 5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, 1 sialic acid.
Discussion
The effects of maternal glycemic dysregulation during pregnancy increase a mother’s risk of developing type 2 diabetes mellitus in the postpartum period (14, 34) and lead to long-term metabolic consequences in neonates (15, 35). Yet very little is understood regarding the role of GDM in lactation and breast milk components. Much of what is known about the effects of glycemic dysregulation on milk components comes from research on lactating women diagnosed with insulin-dependent diabetes mellitus and animal models. In this study of breast milk from women with and without GDM, multivariate modeling was used to select 12 of the 47 measured milk glycans as key variables for explaining the observed differences in maternal phenotype. Statistical multiple hypothesis testing with FDR adjustments was used to confirm the significance of differences in milk glycans identified using the multivariate approach. We discuss the possible role of maternal phenotype in milk composition and implications in neonatal immunity.
In the current experiment, there was no significant difference in HMO, nor did it contribute to multivariate discrimination between classes, yet our findings on the total amount and variation for HMO are within previously reported values for breast milk in healthy individuals (36). Neither the mechanisms in nor the implications for infant health for this regulation have been elucidated, but our finding points to tight control, which deserves further examination. On the other hand, we provide evidence for perturbation in breast milk content of the N-glycans in GDM.
The total concentration of sIgA in the breast milk from women with GDM was 63.6% lower compared with women without GDM. These data suggested that the dysregulated state of GDM in this cohort had lasting consequences on milk sIgA concentrations by at least 14 d postpartum. This finding is consistent with other reports in which IgA, IgG, and IgM of colostrum from hyperglycemic women diagnosed with clinical diabetes (abnormal prepregnancy 100-g OGTT) was reduced by up to 30% compared with normoglycemic women (21, 22). The reduction in Igs in colostrum and milk from women with diabetes could be a result of reduced B-cell lymphocyte function associated with hyperglycemia in diabetes. This proposal is supported by the finding that incubation of peripheral blood B cells in increased concentrations of glucose impaired cellular function, reduced cell viability, increased apoptosis, and induced oxidative stress (37–39). Another mechanism to explain the reduction in milk and colostral Igs in GDM could stem from the reduced circulating concentrations of prolactin associated with obesity and diabetes, as demonstrated in insulin-dependent diabetes mellitus (40, 41). Prolactin plays a role in regulating humoral immunity by binding to prolactin receptors on B lymphocytes and stimulating ornithine decarboxylase, the rate-limiting enzyme in polyamine biosynthesis required for Ig synthesis and secretion (42). In the current study, glucose regulation or prolactin concentrations were not measured at the time of milk collection and with this limitation, we were unable to confirm the women in the GDM group had aberrant carbohydrate metabolism or altered prolactin concentrations.
The total N-glycosylation, mannose, fucose, and sialylated residues of sIgA in transitional milk were lower in milk from women with compared with women without GDM. No reports to date demonstrate dysregulated glycosylation patterns of milk glycoproteins from women with GDM. Yet our results are in line with the finding of Lee et al. (20), who reported reduced sialic acid, mannose residues, and impaired immunomodulatory functions of the endometrial glycoprotein, glycodelin A, isolated from amniotic fluid from women with compared with those without GDM. These data suggest that regulated glycemia is required for normal glycosyl transferase activity involving the terminal sialylation of N-linked glycoproteins. Reduced sialylation of Igs secreted by mammary epithelial cells has implications for the nonspecific innate immune defense against pathogen adhesion and infection. For example, the sialylation of the tail region of sIgA was found to independently inhibit the adhesion of Escherichia coli to human epithelial cells (12). We did not compare the functional characteristics of milk sIgA isolated from women with compared with women without GDM, yet our finding that GDM leads to reduced N-glycosylation of breast milk glycoproteins that participate in immunomodulation deserves further investigation.
The total N-glycosylation, fucosylation, and sialylation of lactoferrin in transitional milk were higher from women with compared with those without GDM. Human lactoferrin N-glycosylation influences the glycoprotein’s susceptibility to proteolysis (43, 44), thus affecting the production of potent active peptides and glycopeptides involved in its biological activities (9). Much of the antimicrobial functions of lactoferrin are exerted by the peptides formed during the digestion of lactoferrin (45). The higher glycosylation of lactoferrin observed in milk from women with GDM could result from altered glycan metabolizing enzymes linked to the pathophysiology of diabetes. Work by Itoh et al. (46) revealed that in the db/db mouse model for type 2 diabetes mellitus, fucosylation of serum glycoproteins was higher compared with controls and associated with a 1.7-fold higher expression of hepatic α1,6–fucosyltransferase mRNA. In humans, the placental transferrin receptor isolated from women with diabetes mellitus during pregnancy had reduced binding kinetics to transferrin, which was explained by increased N-glycosylation and associated with increased cord c-peptide concentrations (19). These data suggest that hyperinsulinemia in diabetes alters glycosylation. To our knowledge, the influence of GDM on breast milk lactoferrin and its glycans have not previously been reported. Mechanistic research is needed to explore how systemic hyperinsulinemia alters the activities of mammary gland glycosidase and glycosyltransferase activities, which influence milk glycan composition and structure.
Herein, we report that diabetes mellitus during pregnancy had an enduring impact on transitional breast milk total and N-glycosylation of milk proteins. Furthermore, the reduced N-glycosylation of sIgA and increased N-glycosylation of lactoferrin suggests differential dysregulation in plasma B cells and mammary epithelial cells by hyperglycemia and/or increased oxidative stress and hyperinsulinemia of GDM. This was an observational study demonstrating proof of concept that glycobiological alterations in GDM have lasting implications for both maternal and infant immune function and gut microbial ecology. These implications should be further explored in large prospective cohort studies. Glycoproteins serve as decoys and block pathogens from invading mucosa and exert immune-modulatory activities through both innate and adaptive immune responses. Glycosylation is critical to the biological activities of these proteins. The findings from this study should now be extended into functional studies to determine if GDM alters human milk lactoferrin and sIgA activities in resisting digestion and/or protecting infant epithelial cells against pathogen adherence or invasion.
Supplementary Material
Acknowledgments
C.J.L.-K. and H.A.D. designed the clinical study, collected participant characteristic data, and provided milk samples; S.M.T. and J.H. generated the data; J.T.S., S.M.T., J.H., and D.G. analyzed and interpreted the data; J.T.S., J.B.G., and C.L. conceived the project; J.T.S. wrote the report; and S.M.T., J.H., D.G., J.B.G., C.L., C.J.L.-K., and H.A.D. made revisions to the report. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: FDR, false discovery rate; GCC, graphitized carbon cartridge; GDM, gestational diabetes mellitus; HMO, human milk oligosaccharide; OGTT, oral glucose tolerance test; O-PLS-DA, orthogonal signal corrected partial least-squares discriminant analysis; sIgA, secretory IgA; SSL 7, Staphylococcus aureus super antigen-like protein 7; 5411, N-glycan containing 5 hexoses, 4 N-acetyl-hexosamines, 1 fucose, 1 sialic acid.
Literature Cited
- 1.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156:S8–15. [DOI] [PubMed] [Google Scholar]
- 2.Sela DA, Chapman J, Adeuya A, Kim J, Chen F, Whitehead T, Lapidus A, Rokhsar D, Lebrilla C, German J. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105:18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lönnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009;12:293–7. [DOI] [PubMed] [Google Scholar]
- 4.Barboza M, Pinzon J, Wickramasinghe S, Froehlich JW, Moeller I, Smilowitz JT, Ruhaak LR, Huang J, Lönnerdal B, German JB, et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol Cell Proteomics. 2012;11:M111.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chichlowski M, De Lartigue G, German JB, Raybould HE, Mills DA. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastroenterol Nutr. 2012;55:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu J, Hendrixson DR, Baker EN, Murphy TF, Geme JWS, Plaut AG. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA. 1998;95:12641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochoa TJ, Noguera-Obenza M, Ebel F, Guzman CA, Gomez HF, Cleary TG. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect Immun. 2003;71:5149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirico G, Marzollo R, Cortinovis S, Fonte C, Gasparoni A. Antiinfective properties of human milk. J Nutr. 2008;138:S1801–6. [DOI] [PubMed] [Google Scholar]
- 9.Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. Adv Exp Med Biol. 2008;606:163–94. [DOI] [PubMed] [Google Scholar]
- 10.Liepke C, Adermann K, Raida M, Mägert HJ, Forssmann WG, Zucht HD. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem. 2002;269:712–8. [DOI] [PubMed] [Google Scholar]
- 11.Murthy AK, Chaganty BKR, Troutman T, Guentzel MN, Yu JJ, Ali SK, Lauriano CM, Chambers JP, Klose KE, Arulanandam BP. Mannose-containing oligosaccharides of non-specific human secretory immunoglobulin A mediate inhibition of Vibrio cholerae biofilm formation. PLoS ONE. 2011;6:e16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroten H, Stapper C, Plogmann R, Köhler H, Hacker J, Hanisch FG. Fab-independent antiadhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infect Immun. 1998;66:3971–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34 Suppl 1:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9. [DOI] [PubMed] [Google Scholar]
- 15.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes. Diabetes Care. 2008;31:340–6. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Ouyang F, Story RE, Pongracic JA, Hong X, Wang G, Pearson C, Ortiz K, Bauchner H, Wang X. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol. 2009;124:1031–8. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert LF, Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine: fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgieff MK, Petry C, Mills M, McKay H, Wobken J. Increased N-glycosylation and reduced transferrin-binding capacity of transferrin receptor isolated from placentae of diabetic women. Placenta. 1997;18:563–8. [DOI] [PubMed] [Google Scholar]
- 20.Lee CL, Chiu PCN, Pang PC, Chu IK, Lee KF, Koistinen R, Koistinen H, Seppälä M, Morris HR, Tissot B. Glycosylation failure extends to glycoproteins in gestational diabetes mellitus. Diabetes. 2011;60:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.França EL, Calderon IMP, Vieira EL, Morceli G, Honorio-França AC. Transfer of maternal immunity to newborns of diabetic mothers. Clin Dev Immunol. 2012;2012:928187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morceli G, França E, Magalhães V, Damasceno D, Calderon I, Honorio-França A. Diabetes induced immunological and biochemical changes in human colostrum. Acta Paediatr. 2011;100:550–6. [DOI] [PubMed] [Google Scholar]
- 23.Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Ag Food Chem. 2010;58:5334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris AM, Jensen RG. Lipids in human milk: a review. 1: Sampling, determination, and content. J Pediatr Gastroenterol Nutr. 1984;3:108. [DOI] [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 26.Carlsson J, Porath J, Lönnerdal B. Isolation of lactoferrin from human milk by metal-chelate affinity chromatography. FEBS Lett. 1977;75:89. [DOI] [PubMed] [Google Scholar]
- 27.Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-FcαRI binding and serum killing of bacteria. J Immunol. 2005;174:2926. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemometr. 2002;16:119–28. [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 31.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2011 [cited 2011]. Available from: http://www.R-project.org/.
- 32.Grapov D, Newman JW. imDEV: a graphical user interface to R multivariate analysis tools in Microsoft Excel. Bioinformatics. 2012;28:2288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care. 2002;25:1862. [DOI] [PubMed] [Google Scholar]
- 35.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290. [DOI] [PubMed] [Google Scholar]
- 36.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–80. [DOI] [PubMed] [Google Scholar]
- 37.Rubinstein R, Genaro A, Motta A, Cremaschi G, Wald M. Impaired immune responses in streptozotocin -induced type I diabetes in mice. Involvement of high glucose. Clin Exp Immunol. 2008;154:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakowicz-Burkiewicz M, Pawelczyk T. Recent advances in understanding the relationship between adenosine metabolism and the function of T and B lymphocytes in diabetes. J Physiol Pharmacol. 2011;62:505. [PubMed] [Google Scholar]
- 39.Sakowicz-Burkiewicz M, Grden M, Maciejewska I, Szutowicz A, Pawelczyk T. High glucose impairs ATP formation on the surface of human peripheral blood B lymphocytes. Int J Biochem Cell Biol. 2013;45:1246–54. [DOI] [PubMed] [Google Scholar]
- 40.Ostrom KM, Ferris AM. Prolactin concentrations in serum and milk of mothers with and without insulin-dependent diabetes mellitus. Am J Clin Nutr. 1993;58:49–53. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113:e465. [DOI] [PubMed] [Google Scholar]
- 42.Russell DH, Kibler R, Matrisian L, Larson DF, Poulos B, Magun BE. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985;134:3027–31. [PubMed] [Google Scholar]
- 43.van Berkel PH, Geerts M, Van Veen H, Kooiman P, Pieper F, De Boer H, Nuijens J. Glycosylated and unglycosylated human lactoferrins both bind iron and show identical affinities towards human lysozyme and bacterial lipopolysaccharide, but differ in their susceptibilities towards tryptic proteolysis. Biochem J. 1995;312:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Veen HA, Geerts M, van Berkel P, Nuijens J. The role of N-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur J Biochem. 2004;271:678–84. [DOI] [PubMed] [Google Scholar]
- 45.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–6. [DOI] [PubMed] [Google Scholar]
- 46.Itoh N, Sakaue S, Nakagawa H, Kurogochi M, Ohira H, Deguchi K, Nishimura S-I, Nishimura M. Analysis of N-glycan in serum glycoproteins from db/db mice and humans with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;293:E1069–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.