Abstract
Lycopene (LYC) is the major tomato carotenoid and is the focus of substantial research. Phytoene (PE), a minor tomato carotenoid, is found in human blood and tissues in similar concentrations to LYC. To determine which metabolic differences underlie this phenomenon, Mongolian gerbils (Meriones unguiculatus, n = 56) were fed control or tomato powder (TP)-containing diets (to establish steady-state serum and tissue carotenoid concentrations similar to tomato-fed humans) for 26 d. The TP-fed gerbils were then provided either a single, oral, cottonseed oil (CO) vehicle dose and tissues were collected at 6 h or they were provided unlabeled PE or LYC in CO and tissues were evaluated at 6, 12, or 24 h. In vehicle-dosed, TP-fed gerbils, LYC was the major carotenoid (≥55% carotenoids) in liver, spleen, testes, and the prostate-seminal vesicle complex, whereas PE was the major serum and adipose carotenoid (≥37% total carotenoid) and phytofluene was the major carotenoid (≥38%) in adrenals and lungs. PE dosing increased hepatic, splenic, and serum PE concentrations compared with vehicle dosing (P < 0.05) from 6 to 24 h, whereas LYC dosing increased only serum LYC at 6 and 12 h (P < 0.05) compared with vehicle dosing. This suggested PE was more bioavailable and cleared more slowly than LYC. To precisely track absorptive and distributive differences, 14C-PE or 14C-LYC (n = 2/group) was provided to TP-fed gerbils. Bioavailability assessed by carcass 14C-content was 23% for PE and 8% for LYC. Nearly every extra-hepatic tissue accumulated greater dose radioactivity after 14C-PE than 14C-LYC dosing. Thus, LYC and PE, which structurally differ only by saturation, pharmacokinetically differ in bioavailability, tissue deposition, and clearance.
Introduction
The observed, disease-preventive properties of tomato consumption (1–5) are attributable in part to lycopene (LYC)7 and other non-provitamin A carotenoids (6–9), including neurosporene, γ-carotene, ζ-carotene (ZC), phytoene (PE), and phytofluene (PF). These carotenoids are deposited in many human tissues and have also demonstrated bioactivity in a range of laboratory studies (10).
Though PE, PF, and ZC are generally present in tomatoes at much lower concentrations than LYC, they are found in similar ng · g−1concentrations as LYC in human tissues and blood (11), which may be due to pharmacokinetic differences between these carotenoids. For instance, the relative plasma response to these tomato carotenoids was previously shown to differ. After a 4-wk tomato juice intervention providing high LYC (75 mg/d) and low PE, PF, and ZC (6, 5, and 0.5 mg/d, respectively), human PF and PE plasma concentrations increased more (167% and 174%, respectively) than those of ZC (98%) and LYC (29%) (12). The resultant plasma concentrations of PF and LYC were very similar (0.72 and 0.75 μmol · L−1, respectively), as were PE and ZC (0.30 and 0.26 μmol · L−1, respectively) (12). However, PE and PF were largely found in VLDL and LDL, whereas LYC was primarily in LDL and HDL, suggesting that carotenoid distribution is linked to the dynamics of lipoprotein metabolism (12). Additional animal feeding studies showed that PE, PF, and ZC are relatively enriched in the adrenals, liver, prostate-seminal vesicle complex, and spleen compared with LYC (13, 14). Thus, tomato product consumption leads to substantial circulating and tissue concentrations of PE, PF, and ZC, presenting these carotenoids, along with LYC, as potential mediators of biological actions associated with tomato feeding.
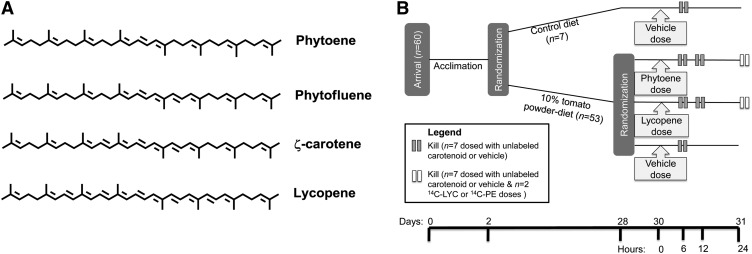
Carotenoid saturation and isomerization likely contributes to differences in bioavailability and/or metabolism (12–17). Although the tomato carotenoids bear the same tetraterpene, 40-carbon structures, PE, PF, and ZC are more saturated precursors of LYC (Fig. 1A) and such differences in carotenoid physicochemical properties have been shown to affect carotenoid bioaccessibility (the ability for a carotenoid be extracted from a matrix and transferred to mixed micelles) and plasma response (18). Although a number of studies have all shown unique tissue distribution patterns of the tomato carotenoids, the mechanisms that underlie these patterns remain to be clearly elucidated.
FIGURE 1.
Tomato carotenoid structures (A) and Expt. 1 and 2 designs (B). During acclimation, gerbils received a semipurified powdered diet for 2 d. On d 28, the experimental diets were replaced with a control diet for 2 d and on d 30, gerbils received a single oral dose of either PE or LYC (PE dosed or LYC dosed), a CO carrier dose (vehicle-dosed groups) (Expt. 1), or 14C-PE or 14C-LYC (Expt. 2). TP-fed and control-fed, vehicle-dosed gerbils were killed 6 h after dosing, PE-dosed and LYC-dosed gerbils at 6, 12, and 24 h postdosing (Expt. 1), and 14C-PE and 14C-LYC gerbils at 24 h after dosing (Expt. 2). CO, cottonseed oil; LYC, lycopene; PE, phytoene; TP, tomato powder.
Experimental model systems using labeled tracer technology may provide insight into pharmacokinetics relevant to humans and the design of future human studies. Among the rodent systems available for the evaluation of carotenoid pharmacokinetics, the Mongolian gerbil (Meriones unguiculatus) demonstrates tomato carotenoid absorption, metabolism, and distribution that more closely mimic human conditions (14, 19, 20). Thus, we compared the bioavailability, metabolism, and tissue distribution of LYC and its precursor, PE, in the gerbil system. This study utilized unlabeled and 14C-labeled carotenoids, produced from tomato cell culture biolabeling and commercial sources, provided to gerbils fed a chronic, fixed dietary concentration of tomato powder (TP) to better mimic the human steady state.
Materials and Methods
Gerbils and experimental design
The animal protocol was approved by the University of Illinois Institutional Animal Care and Use Committee and all necessary procedures were followed to ensure ethical treatment of gerbils. To examine tomato carotenoid absorption and metabolism in gerbils, in Expt. 1, male, 40-d-old gerbils (38.1 ± 0.4 g body weight, (Charles River Laboratories, n = 56) were acclimated with a nonpurified diet (Teklad 8640, Harlan Laboratories) for 2 d, followed by a previously described control, semipurified, powdered diet for 2 d (21), and then randomly assigned to either control (n = 7) or 10% TP diet (n = 49) (Fig. 1). The TP diet (TP donated by Futureceuticals) provided all-E-LYC (214 mg · kg−1), Z-LYC isomers (83 mg · kg−1), PE (12 mg · kg−1), PF (4 mg · kg−1), ζC (6 mg · kg−1), and βC (3 mg · kg−1) and diets were balanced for energy, macronutrient composition, and fiber (drum-dried TP contained ~15.4 kJ · g−1, 0.01 g protein · g−1, 0.03 g fat · g−1, 0.16 g fiber · g−1, and 0.52 g carbohydrate · g−1). Randomly assigned, individually housed gerbils began the study diets and were subsequently provided fresh diet 15 g) every other day with free access to water. After 26 d, gerbils were fed a control diet for 2 d to minimize potential competition for absorption (22) between dosed and dietary carotenoids. Gerbils fed the TP diet were randomly assigned to receive doses while under isoflurane anesthesia of either PE in cottonseed oil (CO) [PE dosed; n = 21, 1.00 ± 0.01 mg (1.84 ± 0.02 mmol) PE in 202 ± 3 μL of CO], LYC in CO [LYC dosed; n = 21, 1.31 ± 0.05 mg (2.44 ± 0.09 mmol) LYC in 206 ± 3 μL of CO], or CO alone (vehicle-dosed group; n = 7, 206 ± 4 μL CO) or control diet-fed gerbils received CO alone. After dosing, gerbils were housed in clean cages with fresh control diet to minimize coprophagy. PE-dosed and LYC-dosed gerbils were killed by carbon dioxide asphyxiation followed by cardiac exsanguination at 6, 12, or 24 h (n = 7 per time point) and both TP and control-fed, vehicle-dosed gerbils were killed by carbon dioxide asphyxiation followed by cardiac exsanguination 6 h postdosing as previously described (23). The liver, adrenal glands, gonadal adipose, spleen, lungs, testes, and the prostate-seminal vesicle complex were collected, weighed, and snap frozen in liquid nitrogen and then stored at −80°C for carotenoid analysis.
In Expt. 2, gerbils (n = 4) followed the same dietary acclimation and TP-feeding regimen as in Expt. 1. Gerbils were dosed as above with either 14C-PE (n = 2) or14C-LYC (n = 2) and killed 24 h later. In addition to the tissues listed above, the stomach, small intestine, and large intestine were flushed with cold PBS and these tissues and digesta were collected, as were the eyes, brain, thymus, skin, hind leg muscles, heart, the remaining carcass, and feces from cages.
Carotenoid analysis of diet
The TP and control diets (0.025 g) were analyzed by HPLC as previously published (14, 24, 25). Extinction coefficients and characteristic absorption spectra of carotenoids and respective geometrical isomers were previously published (26–31).
Preparation of purified carotenoid doses
Expt. 1. Unlabeled Doses.
General precautions were taken to minimize carotenoid degradation and artifact formation during the extraction and analytical processes (32). PE was isolated from PE-rich oil (derived from Blakeslea trispora and suspended in oil, donated by Vitan) and LYC was isolated from Redivivo 10% LYC beadlets (DSM) extracts using a previously described, semipreparatory HPLC system (33) to remove other noncarotenoid components. The geometric isomer composition of purified LYC was 52 ± 2% all-E with the remainder as various Z isomers, while 100% of the PE was 15-Z [the major isomer found in tomato (34)]. Carotenoid doses were transferred to CO as previously described (13). The targeted carotenoid dose was 1 mg in 200 μL of CO. Delivered doses were 1.00 ± 0.01 mg (1.84 ± 0.02 mmol) PE in 202 ± 3 μL CO, 1.31 ± 0.05 mg (2.44 ± 0.09 mmol) LYC in 206 ± 3 μL of CO, and 206 ± 4 μL CO.
Expt. 2. 14C-Labeled doses.
A previously described system for biolabeling and extracting tomato carotenoids was utilized (24, 35) to generate 14C-PE or 14C-LYC, which were not commercially available. PE was isolated in 2 steps, first as above (33), then by 10 × 250 mm, 5-μm particle size C30 (YMC) column using a published HPLC gradient method (flow rate, 3.8 mL · min−1) (31). Total radioactivity was determined by liquid scintillation counting (LSC) (LS6500, Beckman-Coulter) and chromatographic purity was determined by peak area at 286 nm for PE and 472 nm for LYC. Radiopurity was determined by LSC quantitation from 1-min fractions of the HPLC eluate obtained by a Waters Fraction Collector I mixed with 5 mL of Biosafe II scintillation cocktail (RPI). The targeted dose mass was lowered to 0.3 mg in 200 μL of CO to maximize absorption and the intended radioactivity was ∼0.35 μCi as limited by the tomato cell culture yields of 14C-PE and LSC detection. 14C-LYC yield from cell cultures was less than that of 14C-PE, so the cell culture-derived 14C-LYC was combined with a small mass of purified 14C-LYC previously donated by BASF. Delivered 14C-LYC doses were 0.76 μCi in 0.24 mg (0.45 nmol) in 202 μL oil and 14C-PE doses were 0.36 μCi in 0.32 mg (0.58 nmol) in 209 μL oil. Radioactivity results were normalized to the percent of dose radioactivity administered to correct for delivered radioactivity differences.
Serum and tissue carotenoid HPLC analyses
Serum and tissue carotenoid extraction methods were previously described (13). Carotenoids were identified and quantitated by HPLC-photodiode array detector (PDA) as described for dietary carotenoid analysis.
14C-tissue analysis and metabolite identification
To extract potential PE or LYC breakdown products in addition to intact carotenoids for 14C-analysis, a plasma LYC metabolite extraction protocol [HEAT (hexane/ethanol/acetone/toluene; 10:6:7:7 v:v:v:v) method] previously described by Kopec et al. (36) was modified for tissues. Briefly, liver (0.5 g) and lung (0.2 g) were homogenized (Powergen, Fisher Scientific) in 5 mL of saturated aqueous NaCl. Serum (300 μL) was extracted without homogenization. The homogenate was mixed with 5 mL of ethanol containing 0.1% butylated hydroxytoluene and extracted 3 times with 10 mL of the HEAT extraction solvent, with centrifugation between each step for 10 min at 4°C at 600 × g (CR3i, Jouan, Thermo-Scientific), and the upper layers were reserved, pooled, and dried under reduced pressure. The extracts were stored at −20°C, analyzed within 6 h by dissolution in 100 μL of mobile phase B, 80–95 μL was injected for HPLC analysis, and resultant 1-min fractions were analyzed by LSC.
Tissue and digesta 14C-analysis
Tissue radioactivity analysis by LSC was previously described with the exception of several modifications (37). Samples (0.05–0.1 g of minced tissue or 0.07–0.1 g ground feces) were prepared for solubilization by 1 mL of TS-2 Tissue and Gel Solubilizer (Research Products International) per the manufacturer’s instructions. Samples of gastric, small intestinal, and large intestinal digesta and the remaining carcass were homogenized and similarly solubilized. Total radioactivity for each tissue or compartment was calculated based on tissue weight or calculated plasma total volume (38). Carcass samples were analyzed in 5 replicates per gerbil, digestive tract tissues and digesta in triplicate, liver and testes in duplicate, and all other tissues by one sample per animal. Only one spleen was collected from the 14C-PE–dosed gerbils.
Statistical analyses
Tissue and serum carotenoid concentrations of PE-dosed and LYC-dosed gerbils at 6, 12, and 24 h postdosing were compared with those of the vehicle-dosed group (Expt. 1). Group comparisons were made by 1-factor ANOVA and significant differences were determined by Tukey’s studentized range test (α = 0.05) (Expt. 1). When the assumptions of normality and homogeneity of variance of ANOVA were not met due to a violation of homogeneity of variance for serum PE, spleen PE, and lung PE concentration data, the Kruskal-Wallis nonparametric t test was used (α = 0.05) (Expt. 1). The statistical analysis software SAS v. 7.1 (SAS Institute) was used. Results are presented as a mean of the analyses ± SEM when possible. In Expt. 2, limited 14C-PE from tomato cell cultures had to be considered in tandem with expected limitations of LSC sensitivity [estimated from (33, 39)], limiting group sizes to n = 2. Therefore, statistical mean separation tests could not be performed (Expt. 2), but averages were compared.
Results
Gerbil weight gain
Gerbil final weights in Expt. 1 did not differ by diet, dosing group, or kill time point (mean final weight, 67.8 ± 0.5 g). The final mean gerbil weight for Expt. 2 was 65.6 ± 1.2 g.
Expt. 1: tomato carotenoid biodistribution in vehicle-dosed gerbils
All of the tomato carotenoids were detectable in the vehicle-dosed serum but were more similar in concentration than those found in the diet, with PE being the major serum carotenoid followed by total LYC, PF, ζC, and trace amounts of βC (Table 1).
TABLE 1.
Serum and tissue carotenoid concentrations and proportions in vehicle-dosed, tomato-fed gerbils (Expt. 1)1
| Serum | Liver | Adrenals | Spleen | Lung | Gonadal adipose | Testes | Prostate-seminal vesicle complex | |
| nmol · L−1 (%) | nmol · g−1 (%) | nmol · g−1 (%) | nmol · g−1 (%) | nmol · g−1 (%) | nmol · g−1 (%) | nmol · g−1 (%) | nmol · g−1 (%) | |
| PE | 73 ± 16 (38) | 24 ± 6 (10) | 15.7 ± 2.1 (34) | 0.17 ± 0.06 (3) | 0.02 ± 0.01 (6) | 0.08 ± 0.04 (37) | n/d | n/d |
| Total LYC | 62 ± 11 (32) | 134 ± 30 (55) | 7.7 ± 1.3 (17) | 2.64 ± 0.30 (56) | 0.16 ± 0.03 (39) | 0.07 ± 0.02 (31) | 0.40 ± 0.08 (65) | 0.12 ± 0.02 (65) |
| PF | 39 ± 9 (20) | 67 ± 13 (28) | 19.1 ± 2.0 (42) | 1.71 ± 0.24 (36) | 0.15 ± 0.02 (38) | 0.03 ± 0.01 (14) | 0.17 ± 0.03 (28) | 0.02 ± 0.01 (13) |
| ZC | 20 ± 2 (10) | 12 ± 2 (5) | 2.9 ± 0.4 (6) | 0.22 ± 0.04 (5) | 0.07 ± 0.01 (17) | 0.04 ± 0.01 (18) | 0.05 ± 0.01 (7) | 0.04 ± 0.003 (23) |
| β-Carotene | Trace | 5 ± 1 (2) | 0.6 ± 0.04 (1) | n/d | n/d | n/d | n/d | n/d |
Values are means ± SEMs, n = 6–7. Lower limit of detection = 0.02 nmol PE ·g−1 tissue or 190 nmol PE ·g−1 and 0.5 nmol B-carotene ·g−1 tissue or 370 nmol B-carotene ·L−1 of injection carrier. LYC, lycopene; n/d, not detectable; PE, phytoene; PF, phytofluene; ZC, ζ-carotene.
LYC was the major carotenoid in 5 of the 7 tissues assayed, whereas PE was most prominent in the adipose and PF in the adrenals (Table 1). The total carotenoid concentrations in the liver and adrenals were ∼100- and 10-fold greater, respectively, than in the spleen. Carotenoids were most concentrated in the liver, where LYC was predominant, followed by the adrenals (Table 1). The proportions of tomato carotenoids accumulated in tissues (Table 1) substantially differed from those found in the TP (67% all-E LYC, 26% Z- LYC isomers, 3% PE, 2% ZC, 1% PF, and 1% β-carotene). Splenic tissue primarily accumulated LYC followed by PF. PF and LYC were the major carotenoids in the lung tissue and PE was primary in gonadal adipose. In both the testes and the prostate-seminal vesicle complex, LYC was most prominent, whereas PE was not detectable in either tissue (Table 1). Carotenoids were not detected in control-fed, vehicle-dosed gerbil serum or tissues or control diets and are not discussed further.
Expt. 1: Effect of PE or LYC dosing on serum and tissue carotenoid concentrations
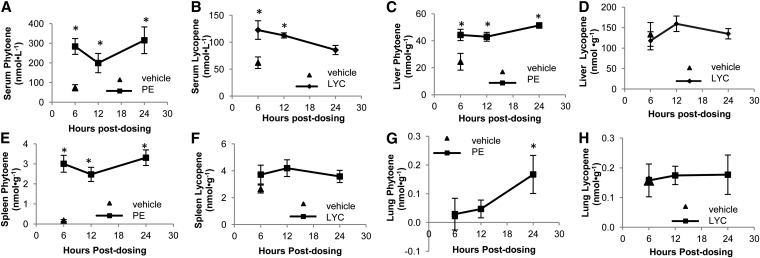
Serum PE concentrations in the PE-dosed gerbils (283 ± 40, 199 ± 49, and 315 ± 68 nmol · L−1, at 6, 12, and 24 h) were greater (P < 0.05) than those of vehicle-dosed gerbils (73 ± 16 nmol · L−1) (Fig. 2). Serum total LYC was greater (P < 0.05) in LYC-dosed gerbils 6 and 12 h after dosing (122 ± 17 and 113 ± 5 nmol · L−1, respectively) compared with vehicle-dosed gerbils (62 ± 11 nmol · L−1) but by 24 h had returned to the concentration found in the vehicle-dosed gerbils (Fig. 2). These changes in serum concentrations relate to elevation of the PE concentration in the serum by 570%, 340%, and 650% and LYC elevations of 97%, 80%, and 39% at 6, 12, and 24 h, respectively.
FIGURE 2.
Changes in carotenoid concentrations in serum (A,B), liver (C,D), spleen (E,F), and lung (G,H) in TP-fed gerbils that were administered vehicle, PE (A,C,E,G), or LYC (B,D,F,H) (Expt. 1). Values are means ± SEMs, n = 6 or 7. *Different from the vehicle-dosed group at 6 h, P < 0.05 by ANOVA between either PE-dosed or LYC-dosed gerbils at 6-, 12-, or 24-h time point compared with the vehicle-dosed, 6-h time point. Note that y-axes differ. LYC, lycopene; PE, phytoene; TP, tomato powder; vehicle, vehicle-dosed gerbils.
Hepatic and splenic PE increased in PE-dosed gerbils over 24 h (Fig. 2); however, LYC was unchanged in either tissue in LYC-dosed gerbils. Similarly, at 24 h, pulmonary PE was greater in PE-dosed gerbils than in those vehicle dosed (Fig. 2), whereas pulmonary LYC did not increase in LYC-dosed gerbils. PE was not detected in the testes or prostate-seminal vesicle complex in PE-dosed gerbils during the 24 h time-course.
Expt. 2: 24-h absorption of 14C-PE and 14C-LYC
Dose radioactivity distribution in tissues.
After 24 h, 23% of PE dose radioactivity was detected in the total carcass in contrast with 8% of the 14C-LYC dose radioactivity (Table 2). Forty-seven percent of the 14C-PE and 58% of the 14C-LYC dose radioactivity were recovered from the digesta and feces and presumed to have been unabsorbed. Twenty-nine percent of the 14C-PE dose and 34% of the 14C-LYC were not recovered.
TABLE 2.
Percent distribution of dosed 14C-PE and 14C-LYC radioactivity in digesta and tissues of TP-fed gerbils (Expt. 2)12
| Compartment | 14C-PE | 14C-LYC |
| % total dose radioactivity delivered | % total dose radioactivity delivered | |
| Feces | 29.4 | 42.3 |
| Large intestinal contents | 16.0 | 12.9 |
| Tissues and serum | 17.7 | 5.9 |
| Carcass | 4.9 | 1.6 |
| Stomach contents | 2.2 | 3.3 |
| Small intestinal contents | 0.4 | 0.2 |
| Unrecovered | 29.8 | 34.0 |
Values are means, n = 2. LYC, lycopene; PE, phytoene; TP, tomato powder.
24 h after dosing TP-fed gerbils.
The 24-h distribution of 14C-PE and 14C-LYC radioactivity differed by tissue. The small and large intestinal tissues contained the greatest percent of 14C-PE dose radioactivity, whereas the liver and stomach tissues contained the greatest percent of 14C-LYC radioactivity (Table 3). Across all tissue dose radioactivity uptake analyses, the mean within-group difference was 83% and 101% for 14C-PE– and 14C-LYC–dosed gerbils, respectively, whereas the mean absolute percent difference between 14C-PE– and 14C-LYC–dosed gerbil groups was 368%, indicating that between-group differences were larger than within-group variability. The percent of 14C-PE dose radioactivity was greater than that from 14C-LYC in nearly all tissues except the liver, stomach, and bladder (Table 3). Tissue deposition of 14C-PE radioactivity was greater than 14C-LYC by 9-fold in the peritoneal adipose and the adrenals, 6-fold in the lungs, and 4-fold in the hind leg muscle. The 14C-PE percent dose radioactivity per gram was greatest in adrenals (1.8 % · g−1 tissue) followed by the liver and the spleen (0.76 and 0.49% · g−1, respectively). The 14C-LYC percent dose radioactivity per gram was greatest in the liver (1% · g−1 tissue) followed by the spleen and the eyes (0.34 and 0.19% · g−1, respectively) (Table 3). Notably, the percent of 14C-PE dose radioactivity per gram in the skin was 12 times greater than that from 14C-LYC.
TABLE 3.
|
14C-PE |
14C-LYC |
|||
| Compartment | % dose radioactivity in compartment | % dose radioactivity ·g tissue−1 | % dose radioactivity in compartment | % dose radioactivity · g tissue−1 |
| Adrenals | 0.07 | 1.80 | 0.01 | 0.19 |
| Bladder | 0.00 | 0.02 | 0.00 | 0.08 |
| Brain | 0.02 | 0.02 | 0.01 | 0.01 |
| Eyes | 0.08 | 0.36 | 0.04 | 0.19 |
| Gonadal adipose | 0.03 | 0.04 | 0.01 | 0.02 |
| Heart | 0.02 | 0.06 | 0.01 | 0.04 |
| Kidneys | 0.10 | 0.16 | 0.02 | 0.04 |
| Large intestine | 6.87 | 0.85 | 0.90 | 0.11 |
| Liver | 2.36 | 0.76 | 3.20 | 1.04 |
| Lungs | 0.11 | 0.32 | 0.01 | 0.05 |
| Muscle | 0.07 | 0.02 | 0.02 | 0.00 |
| Peritoneal adipose | 0.02 | 0.13 | 0.00 | 0.01 |
| Prostate-seminal vesicle complex | 0.03 | 0.10 | 0.01 | 0.07 |
| Serum | 0.36 | 0.10 | 0.11 | 0.03 |
| Skin | 0.01 | 0.07 | 0.00 | 0.01 |
| Small intestinal mucosa | 0.33 | 1.34 | 0.09 | 0.24 |
| Small intestine | 6.95 | 0.99 | 1.12 | 0.15 |
| Spleen | 0.03 | 0.49 | 0.02 | 0.34 |
| Stomach | 0.21 | 0.40 | 0.26 | 0.48 |
| Testes | 0.03 | 0.03 | 0.01 | 0.01 |
| Thymus | 0.01 | 0.08 | 0.00 | 0.02 |
Values are means, n = 2. LYC, lycopene; PE, phytoene; TP, tomato powder.
In gerbils 24 h after 14C-PE or 14C-LYC dosing.
Tissue 14C-carotenoid HPLC-PDA-LSC analysis.
The hexane-extracted, radioactive, nonpolar compounds from the 14C-PE and 14C-LYC–dosed gerbil livers were determined to be largely associated with intact PE and LYC, respectively, based on carotenoid dose chromatograms and radioactivity plots (Supplemental Fig. 1). HEAT-extractable, polar, and nonpolar liver 14C-compounds were detected in 14C-PE–dosed gerbil liver (Supplemental Fig. 2) at elution times corresponding to intact PE (24 min) and a more polar compound eluting between 17 and 22 min, which was not present in the dose (Supplemental Fig. 1). Radioactivity of HEAT extract of 14C-LYC–dosed gerbil liver (Supplemental Fig. 3) was associated with intact LYC isomers (32–45 min) as well as more polar fractions (5 and 15–30 min), which were not present in the dose (Supplemental Fig. 1). The specific identities of these peaks could not be resolved by HPLC-PDA.
Radioactivity was detected in the HEAT extracts of from 14C-PE– but not 14C-LYC–dosed gerbil lung tissue (Supplemental Fig. 4). Radioactivity in the lungs of 14C-PE–dosed gerbils eluted near PE standard elution time (∼22 min); however, the characteristic PE absorption spectra could not be definitively identified, likely due to coeluting compounds. Serum was also HEAT extracted and HPLC fractionated; however, the radioactivity signal was below LSC detection.
Discussion
Here we demonstrate that PE and LYC, which structurally differ by only 4 double bonds, exhibit profound differences in their short-term distributive and metabolic fates. By examining the comparative pharmacokinetics of unlabeled and 14C-labeled PE and LYC doses, we found that a greater proportion of PE was absorbed than LYC; LYC appeared to be more rapidly cleared from the liver, the 24-h serum clearance of PE was slower than LYC, and radioactivity from 14C-PE was more highly deposited and retained than from 14C-LYC in nearly all extra-hepatic tissues.
Differential tissue biodistribution of tomato carotenoids in tomato-fed, vehicle-dose gerbils.
TP feeding led to distinct patterns of PE and LYC tissue and serum distribution. As in humans, the adrenals and liver of gerbils accumulated very high concentrations of LYC and other carotenoids (11, 40–42), potentially because both tissues express scavenger receptor class B member 1 (SRB1), a transporter also involved in intestinal LYC uptake (43, 44). In contrast to the adrenals, intact PE was not detected in the testes. In gerbils, PE was the major carotenoid in adipose tissue (Table 1), which is a major depot for lipophilic carotenoids (45). In gerbils, LYC was the prominent carotenoid in both the testes and prostate-seminal vesicle complex (Table 1), agreeing with previous reports in humans and animals (11, 13, 40, 41, 46).
Relative bioavailability and metabolism of PE vs. LYC.
Although the diets provided 3% PE and 93% LYC (percent total carotenoids), we observed that PE was a major carotenoid (>30%) in TP-fed, vehicle-dosed gerbils’ serum, adrenals, and adipose, yet it was not detected in the testes or prostate-seminal vesicle complex in Expt. 1. Accordingly, the relative pharmacokinetics of single doses of PE or LYC in tomato-fed gerbils substantially differed. The marked pharmacokinetic differences observed in Expt. 1 raised the following questions: 1) do hepatic and testicular tissue LYC and PE responses differ due to clearance or uptake; 2) is tissue uptake of PE greater than that of LYC; and 3) how does the total body uptake of PE and LYC differ? To help investigate these questions, gerbils were dosed with 14C-PE and 14C-LYC in Expt. 2.
First, we examined 14C-PE compared with 14C-LYC uptake into the liver and androgen-sensitive tissues. 14C was detectable in livers of 14C-LYC–dosed gerbils, suggesting uptake of either intact or metabolized 14C-LYC. In the liver, a large portion of this radioactivity was associated with intact LYC (Supplemental Fig. 3). Thus, although LYC dosing in Expt. 1 did not increase hepatic LYC concentrations (Fig. 2D), intact LYC is deposited in the liver within 24 h based on Expt. 2. This suggests that LYC is cleared from the liver at a rate such that dosing in Expt. 1 replaced metabolic losses but did not increase LYC concentrations. Evidence of extensive LYC metabolism in our model is supported by a recent report in which the 18.6-d plasma half-life of total radioactivity (from 14C-LYC and 14C-LYC metabolic products) in 2 men after consuming a microdose of 14C-LYC was substantially longer than the 5-d plasma half-life of 14C-LYC (47). Furthermore, the maximal serum concentration of total radioactivity in these men was 2.6-fold greater than the maximal serum concentration of radioactivity from 14C-LYC (47). LYC metabolism remains poorly understood; however, LYC may be an in vivo substrate for the β,β-carotene 9′,10’-oxygenase or β,β-carotene 15,15’-monooxygenase enzymes (23, 48, 49). In the current study, we could not definitively identify 14C-LYC metabolites in the liver tissue by HPLC-PDA, but LYC metabolites were previously detected in rat liver as well as human plasma (11, 36, 50).
Though intact PE was undetected in the testes and prostate-seminal vesicle complex in Expt. 1, in Expt. 2, these tissues actually accumulated a greater percent of 14C-PE dose radioactivity than of 14C-LYC (Table 3). This suggests PE is rapidly metabolized in these tissues or metabolites are retained or are taken up by these tissues. Previously, we found that in vitro prostate cancer cells accumulated polar 14C-PE products, suggesting PE is metabolized in cells or non-enzymatic oxidative PE products are taken up by these cells (34). To the authors’ knowledge, this is the first in vivo report suggesting that PE metabolites may be present in the prostate and testes.
Next, we found that all tissues except for the liver, bladder, and stomach, assayed in the Expt. 2, accumulated a greater percent of dose radioactivity from 14C-PE than from 14C-LYC (Table 3). To the authors’ knowledge, this is the first report indicating that PE or its products were accumulated, and to a greater extent than LYC, in the eyes, muscle, brain, heart, thymus, intestinal tissues, and adipose (Table 3). Extensive extra-hepatic distribution of PE and/or its metabolites warrants future investigation to identify metabolites and characterize PE and metabolite bioactivities in these tissues.
14C-PE was much more bioavailable than 14C-LYC based on greater total body deposition (23% vs. 8%, respectively) (Table 3). As in previous studies of 14C-LYC dosing in rats (33, 39), a large percent of both the 14C-PE and 14C-LYC radioactivity was unrecovered. Detectable 14C was found in the breath and urine of men who received 14C-LYC microdoses within hours of consumption (47), and nearly 1% of dose radioactivity from LYC was detected in rat urine collected during 24 h after 14C-LYC dosing (39). Although neither urine nor exhaled air was assayed in the current study, future tomato carotenoid mass-balance studies should measure radioactivity in these compartments.
Elevated plasma PE compared with LYC after dosing was likely due to several factors. First, Expt. 1 indicated a faster clearance rate of serum LYC than PE by steady serum LYC depletion during 24 h. Then, Expt. 2 indicated greater bioavailability of PE based on greater relative serum radioactivity response to 14C-PE than 14C-LYC. The relatively slower rate of PE clearance from the liver compared with LYC, suggested by a sustained elevation in liver PE concentrations after dosing (Fig. 2D) (Expt. 1), may have also contributed to a larger pool of intact PE for efflux into the circulation and subsequent uptake by extra-hepatic tissues. A much greater amount of 14C from the 14C-PE than from the 14C-LYC dose was also recovered in intestinal tissues, suggesting greater enterocyte uptake and a slow release of PE over time may have also contributed to the sustained plasma PE concentrations observed in Expt. 1. Extensive metabolism of LYC may have caused the higher percent of 14C-LYC dose radioactivity in the bladder, most likely in the form of water-soluble LYC metabolites in the urine (Table 3), as was previously observed in humans and rats (39, 47).
Limitations of the current study extend from the amount of 14C-labeled carotenoid available due to the expense and complexity of producing high yields of this unique and valuable tool. Thus, we are limited to small but strategic studies of limited statistical power to test bioavailability and tissue distribution. Methodological improvements to improve yield and the use of stable isotopes are already being made and will enhance the ability of investigators to elucidate the metabolism of bioactive carotenoids and their biological importance (25, 31, 51). Stable isotope carotenoids are better suited for future metabolite identification based on signal strength, potential for analysis in shared MS facilities, and safety in humans.
In summary, these results demonstrated that compared with LYC, PE was more bioavailable and more slowly cleared from serum and liver than LYC. Additionally, extensive extra-hepatic distribution of PE was shown. PE appears to be more bioavailable and more slowly cleared than LYC, which may suggest LYC is preferred to PE for enzymatic cleavage. These pharmacokinetic differences likely underlie the phenomenon of relatively lower amounts of dietary PE than LYC resulting in similar PE and LYC blood and tissue concentrations. Further studies to characterize the absorption of LYC and PE and identify the metabolites of LYC and PE should be pursued. Careful pharmacokinetic investigation of different dietary phytochemicals reveals that even structurally similar compounds are handled quite differently and likely result in different biological effects.
Supplementary Material
Acknowledgments
The authors thank Chi-Hua Lu for his assistance in analytical and preparatory chromatography. N.E.M., S.K.C., and J.W.E. Jr designed the research; N.E.M. conducted the research, analyzed data, and performed statistical analyses; N.E.M., S.K.C., and J.W.E. Jr wrote the paper; and J.W.E. Jr had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CO, cottonseed oil; HEAT, hexane/ethanol/acetone/toluene; LSC, liquid scintillation counting; LYC, lycopene; PDA, photodiode array detector; PE, phytoene; PF, phytofluene; SRB1, scavenger receptor class B member 1; TP, tomato powder; ZC, ζ-carotene.
Literature Cited
- 1.Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–5. [PubMed] [Google Scholar]
- 2.Wei MY, Giovannucci EL. Lycopene, tomato products, and prostate cancer incidence: a review and reassessment in the PSA screening era. J Oncol. 2012;2012:271063. [DOI] [PMC free article] [PubMed]
- 3.Karppi J, Laukkanen JA, Makikallio TH, Kurl S. Low serum lycopene and beta-carotene increase risk of acute myocardial infarction in men. Eur J Public Health. 2012;22:835–40. [DOI] [PubMed]
- 4.Karppi J, Laukkanen JA, Sivenius J, Ronkainen K, Kurl S. Serum lycopene decreases the risk of stroke in men: a population-based follow-up study. Neurology. 2012;79:1540–7. [DOI] [PubMed]
- 5.Jacques PF, Lyass A, Massaro JM, Vasan RS, D'Agostino RB., Sr Relationship of lycopene intake and consumption of tomato products to incident CVD. Br J Nutr. 2013;110:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86. [DOI] [PubMed] [Google Scholar]
- 7.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–43. [DOI] [PubMed]
- 8.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr The tomato as a functional food. J Nutr. 2005;135:1226–30. [DOI] [PubMed]
- 9.Campbell JK, Canene-Adams K, Lindshield BL, Boileau TW, Clinton SK, Erdman JW., Jr Tomato phytochemicals and prostate cancer risk. J Nutr. 2004;134:S3486–92. [DOI] [PubMed]
- 10.Engelmann NJ, Clinton SK, Erdman JW., Jr Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr. 2011;2:51–61. [DOI] [PMC free article] [PubMed]
- 11.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood). 2002;227:845–51. [DOI] [PubMed] [Google Scholar]
- 12.Paetau I, Khachik F, Brown ED, Beecher GR, Kramer TR, Chittams J, Clevidence BA. Chronic ingestion of lycopene-rich tomato juice or lycopene supplements significantly increases plasma concentrations of lycopene and related tomato carotenoids in humans. Am J Clin Nutr. 1998;68:1187–95. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JK, Engelmann NJ, Lila MA, Erdman JW., Jr Phytoene, phytofluene, and lycopene from tomato powder differentially accumulate in tissues of male Fisher 344 rats. Nutr Res. 2007;27:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conlon LE, King RD, Moran NE, Erdman JW., Jr Coconut oil enhances tomato carotenoid tissue accumulation compared to safflower oil in the Mongolian gerbil (Meriones unguiculatus). J Agric Food Chem. 2012;60:8386. [DOI] [PubMed] [Google Scholar]
- 15.Moran NE, Erdman JW, Clinton SK. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch Biochem Biophys. Epub 2013 July 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unlu NZ, Bohn T, Francis D, Clinton SK, Schwartz SJ. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-beta-carotene varieties of tomatoes. J Agric Food Chem. 2007;55:1597–603. [DOI] [PubMed] [Google Scholar]
- 17.Paetau I, Rao D, Wiley ER, Brown ED, Clevidence BA. Carotenoids in human buccal mucosa cells after 4 wk of supplementation with tomato juice or lycopene supplements. Am J Clin Nutr. 1999;70:490–4. [DOI] [PubMed] [Google Scholar]
- 18.Sy C, Gleize B, Dangles O, Landrier JF, Veyrat CC, Borel P. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol Nutr Food Res. 2012;56:1385–97. [DOI] [PubMed]
- 19.Huang CS, Chuang CH, Hu ML. Effects of lycopene supplementation on plasma and tissue lycopene levels in various rodent strains. Int J Vitam Nutr Res. 2006;76:377–84. [DOI] [PubMed] [Google Scholar]
- 20.Mills JP, Simon PW, Tanumihardjo SA. Beta-carotene from red carrot maintains vitamin A status, but lycopene bioavailability is lower relative to tomato paste in Mongolian gerbils. J Nutr. 2007;137:1395–400. [DOI] [PubMed] [Google Scholar]
- 21.Mills JP, Tumuhimbise GA, Jamil KM, Thakkar SK, Failla ML, Tanumihardjo SA. Sweet potato beta-carotene bioefficacy is enhanced by dietary fat and not reduced by soluble fiber intake in Mongolian gerbils. J Nutr. 2009;139:44–50. [DOI] [PubMed] [Google Scholar]
- 22.van den Berg H. Carotenoid interactions. Nutr Rev. 1999;57:1–10. [DOI] [PubMed] [Google Scholar]
- 23.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9′,10'-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr. 2010;140:2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu CH, Engelmann NJ, Lila MA, Erdman JW., Jr Optimization of lycopene extraction from tomato cell suspension culture by response surface methodology. J Agric Food Chem. 2008;56:7710–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelmann NJ, Campbell JK, Rogers RB, Rupassara SI, Garlick PJ, Lila MA, Erdman JW., Jr Screening and selection of high carotenoid producing in vitro tomato cell culture lines for [(13)C]-carotenoid production. J Agric Food Chem. 2010;58:9979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. Washington, DC: ILSI Press; 2001.
- 27.Britton G, Liaaen-Jensen S, Pfander H. editors. Carotenoids: handbook. Basel: Springer; 2004.
- 28.Frohlich K, Conrad J, Schmid A, Breithaupt DE, Bohm V. Isolation and structural elucidation of different geometrical isomers of lycopene. Int J Vitam Nutr Res. 2007;77:369–75. [DOI] [PubMed]
- 29.Neudert U, Martinez-Ferez IM, Fraser PD, Sandmann G. Expression of an active phytoene synthase from Erwinia uredovora and biochemical properties of the enzyme. Biochim Biophys Acta. 1998;1392:51–8. [DOI] [PubMed] [Google Scholar]
- 30.Breitenbach J, Sandmann G. zeta-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta. 2005;220:785–93. [DOI] [PubMed] [Google Scholar]
- 31.Moran NE, Rogers RB, Lu C, Conlon LE, Lila MA, Clinton SK, Erdman JW., Jr Biosynthesis of highly enriched 13C-lycopene for human metabolic studies using repeated batch tomato cell culturing with 13C-glucose. Food Chem. 2013;139:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiedt K, Liaaen-Jensen S. Isolation and analysis. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids: isolation and analysis. 1st ed. Basel: Birkhauser Verlag; 1995. p. 81.
- 33.Zaripheh S, Erdman JW., Jr The biodistribution of a single oral dose of [14C]-lycopene in rats prefed either a control or lycopene-enriched diet. J Nutr. 2005;135:2212–8. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JK, Rogers RB, Lila MA, Erdman JW., Jr Biosynthesis of 14C-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. J Agric Food Chem. 2006;54:747–55. [DOI] [PubMed] [Google Scholar]
- 35.Engelmann NJ, Rogers RB, Lila MA, Erdman JW., Jr Herbicide treatments alter carotenoid profiles for 14C tracer production from tomato (Solanum lycopersicum cv. VFNT cherry) cell cultures. J Agric Food Chem. 2009;57:4614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edinger MS, Koff WJ. Effect of the consumption of tomato paste on plasma prostate-specific antigen levels in patients with benign prostate hyperplasia. Braz J Med Biol Res. 2006;39:1115–9. [DOI] [PubMed]
- 38.Nicolosi RJ, Herrera MG, el Lozy M, Hayes KC. Effect of dietary fat on hepatic metabolism of 14C-oleic acid and very low density lipoprotein triglyceride in the gerbil. J Nutr. 1976;106:1279–85. [DOI] [PubMed] [Google Scholar]
- 39.Zaripheh S, Boileau TW, Lila MA, Erdman JW., Jr [14C]-lycopene and [14C]-labeled polar products are differentially distributed in tissues of F344 rats prefed lycopene. J Nutr. 2003;133:4189–95. [DOI] [PubMed] [Google Scholar]
- 40.Stahl W, Schwarz W, Sundquist AR, Sies H. Cis-trans isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem Biophys. 1992;294:173–7. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan LA, Lau JM, Stein EA. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem. 1990;8:1–10. [PubMed] [Google Scholar]
- 42.Schmitz HH, Poor CL, Wellman RB, Erdman JW., Jr Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121:1613–21. [DOI] [PubMed] [Google Scholar]
- 43.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–12. [DOI] [PubMed] [Google Scholar]
- 44.Moussa M, Landrier JF, Reboul E, Ghiringhelli O, Comera C, Collet X, Frohlich K, Bohm V, Borel P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J Nutr. 2008;138:1432–6. [DOI] [PubMed] [Google Scholar]
- 45.Canene-Adams K, Erdman JW., Jr Absorption, transport, distribution in tissues and bioavailability. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Volume 5: Nutrition and Health. 1st ed. Basel (Switzerland): Birkhauser Verlag; 2009. p. 115.
- 46.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW., Jr Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–33. [PubMed] [Google Scholar]
- 47.Ross AB, Vuong LT, Ruckle J, Synal HA, Schulze-König T, Wertz K, Rümbeli R, Liberman RG, Skipper PL, Tannenbaum SR, et al. Lycopene bioavailability and metabolism in humans: an accelerator mass spectrometry study. Am J Clin Nutr. 2011;93:1263–73. [DOI] [PubMed] [Google Scholar]
- 48.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6. [DOI] [PubMed] [Google Scholar]
- 49.Wang TT, Edwards AJ, Clevidence BA. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to beta-carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms. J Nutr Biochem. 2013;24:1538–46. [DOI] [PubMed]
- 50.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12'-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–7. [DOI] [PubMed]
- 51.Lu CH, Choi JH, Engelmann Moran N, Jin YS, Erdman JW., Jr Laboratory-scale production of 13C-labeled lycopene and phytoene by bioengineered Escherichia coli. J Agric Food Chem. 2011;59:9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.