Abstract
Intracellular copper-binding proteins (metallothionein I/II) and a copper exporter (Menkes copper-transporting ATPase) are upregulated in duodenal enterocytes from iron-deficient rats, consistent with copper accumulation in the intestinal mucosa. How copper enters enterocytes during iron deficiency is, however, not clear. Divalent metal transporter 1 (Dmt1), the predominant iron importer in the mammalian duodenum, also transports other metal ions, possibly including copper. Given this possibility and that Dmt1 expression is upregulated by iron deprivation, we sought to test the hypothesis that Dmt1 transports copper during iron deficiency. Two model systems were utilized: the Belgrade (b) rat, expressing mutant Dmt1, and an inducible Dmt1-overexpression cell culture system. Mutant rats (b/b) were fed a semipurified, AIN93G-based control diet and phenotypically normal littermates (+/b) were fed control or iron-deficient diets for ∼14 wk. An everted gut sleeve technique and a colorimetric copper quantification assay were utilized to assess duodenal copper transport. The control diet-fed +/b rats had normal hematological parameters, whereas iron-deprived +/b and b/b rats were iron deficient and Dmt1 mRNA and protein levels increased. Importantly, duodenal copper transport was similar in the control +/b and b/b rats; however, it significantly increased (∼4-fold) in the iron-deprived +/b rats. Additional experiments in Dmt1 overexpressing HEK-293 cells showed that copper (64Cu) uptake was stimulated (∼3-fold) in the presence of an iron chelator. Dmt1 transcript stabilization due to a 3′ iron-responsive element was also documented, likely contributing to increased transport activity. In summary, these studies suggest that Dmt1 enhances copper uptake into duodenal enterocytes during iron deprivation.
Introduction
Copper accumulates during iron deficiency in tissues important for iron homeostasis in many mammalian species, suggesting that copper may influence iron metabolism. For example, it was previously documented that copper increases in the duodenal mucosa (1), liver, and serum of iron-deprived rats (2–4). Increased serum copper coincides with enhanced serum ferroxidase activity and ceruloplasmin expression (2). Recent studies, focused on molecular events in the duodenal mucosa where dietary iron and copper are absorbed, have begun to provide mechanistic insight into how enterocytes increase copper transport during iron deprivation. Initially, induction of metallothoinein I and II (MtI/II5; intracellular copper-binding proteins) and Menkes copper-transporting ATPase (Atp7a; a copper exporter) mRNA expression was noted in the intestinal epithelium of iron-deprived rats (5). Subsequent studies confirmed that these genes were also upregulated in iron-deprived mice (6) and IEC-6 cells (7). Interestingly, induction of MtI/II and Atp7a paralleled the upregulation of several genes encoding iron transport-related proteins, including duodenal cytochrome b (Dcytb; an apically-expressed ferrireductase), divalent metal transporter 1 (Dmt1; the principal iron importer), and ferroportin 1 (Fpn1; the basolateral iron exporter) (5, 8). We thus hypothesized that iron and copper homeostasis may be coordinately regulated in enterocytes and further that copper may be important for regulation and/or maintenance of iron absorption.
Increased MtI/II expression in duodenal enterocytes likely reflected increased apical entry of dietary copper, but the specific mechanism of copper uptake has remained unclear. Genome-wide gene expression screens, demonstrating that copper homeostasis-related genes were upregulated during iron deprivation (5), did not reveal changes in expression of the principal intestinal copper importer, copper transporter 1 (Ctr1). Unchanged Ctr1 expression has subsequently been confirmed by qRT-PCR experiments in different rodent and cell culture models (L. Jiang, S. Gulec, J. Collins, unpublished data). Although it is conceivable that Ctr1 protein levels increase in the absence of changes in mRNA expression, perhaps explaining increased copper import, a more likely scenario was that divalent metal transporter 1 (Dmt1) transported copper during iron deficiency. Dmt1 was so named because it was originally shown to transport multiple divalent cations (9). The physiological relevance of this observation is not clear with respect to all transported metal ions, but subsequent studies have most strongly supported a role for Dmt1 in iron and manganese metabolism (10–13). Moreover, Dmt1 mRNA expression is strongly upregulated during iron deprivation in rodent intestine (often 10-fold or more) coincident with copper accumulation. The apical location of Dmt1 would place it in presumed proximity to a candidate cupric reductase (14, 15) that might be encoded by Dctyb.
Transport of copper by Dmt1 was considered plausible, because some studies have shown that Dmt1 can transport copper (16, 17); however, there is controversy about whether Dmt1 does transport copper (12, 18). Whether Dmt1 transports cupric (Cu2+) or cuprous (Cu1+) copper is an important question, but copper could be available in both forms at the apical surface of enterocytes; so whether the unknown cupric reductase is absolutely required for copper absorption is currently unknown. At least one study has, however, shown that Dmt1 can transport cuprous copper (16). Moreover, rats expressing a mutant Dmt1 protein (Belgrade) (19), although iron deficient, were recently shown not to have alterations in hepatic or serum copper concentrations, whereas iron-deprived, phenotypically normal littermates did (consistent with previous studies in wild-type rats) (20). Given this background, our intent for this investigation was to test the hypothesis that Dmt1 can transport copper, particularly during iron deficiency. We thus utilized 2 distinct models, one in vivo (the Belgrade rat) and one in vitro (Dmt1 overexpressing HEK-293 cells). Results of the present investigation confirm that Dmt1 can participate in copper transport during iron deficiency.
Methods
Belgrade rats.
Breeding pairs were originally obtained from the University at Buffalo and a breeding colony was established on the University of Florida campus. Mutant (b/b) male and phenotypically normal (+/b) female rats were used for breeding and were housed in standard shoe-box cages. Breeders were maintained with an iron-supplemented diet (360 ppm), which enhances fertility of the males and decreases neonatal mortality of mutant offspring. All diets used for this study were modified AIN-93–based diets with various amounts of iron added in the form of ferrous sulfate (Dyets). With the exception of iron content, the diets were otherwise identical. These custom diets have been used by us for >10 y (2, 3, 5). Animals were given ad libitum access to diets. At weaning (19–21 d of age), DNA was isolated from tail clips using the DNeasy Blood and Tissue kit (Qiagen). Genotype was confirmed by genomic PCR (19). Weaned pups were housed in overhanging, stainless steel cages (to avoid coprophagia) and assigned to a dietary treatment group. The groups were as follows: 1) +/b rats (phenotypically normal) fed a control diet (198 ppm Fe; 4 times more than the standard AIN-93–based diet but modeling standard rodent chow); 2) +/b rats fed a low-iron diet (∼3 ppm Fe); and 3) b/b rats (which are naturally iron deficient) fed the control diet. After weaning, dietary treatments ensued for ∼3.5 mo and then rats were anesthetized by CO2 exposure and killed by performing a thoracotomy. The upper small intestine and liver tissue were then collected for mRNA and protein analysis. In addition, hemoglobin and hematocrit were measured by standard methods (20) from blood obtained by cardiac puncture just before death. All animal studies were approved by the University of Florida Institutional Animal Care and Use Committee.
qRT-PCR.
Total RNA was purified from rat duodenal mucosal scrapings or HEK-293 cell lysates with TRIzol reagent (Invitrogen), and qRT-PCR was performed by routine methods (21). Standard curve reactions validated each primer pair and melt curves routinely demonstrated a single amplicon. RT reactions were run in duplicate for 18S rRNA (in all samples), Dmt1 [rat (r) in enterocytes; mouse (m) in cell lysates] and human transferrin receptor 1 (hTFR1; in cell lysates). Next, 18S average was subtracted from the experimental gene average to generate the Ct value. ΔΔCt values were calculated for Dmt1 and hTFR1 for each treatment group. Mean fold induction equates to 2−ΔΔCt. Primer sequences were as follows: rDmt1 For- 5′ catgctttaccggtcaactacatc 3′ rDmt1 Rev- 5′ tcacagtttggagcagcacttg 3′ mDmt1 For- 5′ gtgatcctgacccggtctactg 3′ mDmt1 Rev- 5′ tgaggatgggtagaagcaaagg 3′ hTFR1 For- 5′ tcagagcgtcgggatatcgg 3′ hTFR1 Rev- 5′ cttgatccatcattctgaactgcc 3′. Dmt1 primers were designed to quantify all transcript variants.
Western-blot analysis.
The Dmt1 antibody (called anti-1A) used for HEK-293 cell studies was previously described (22). Validation of this antibody included studies in HEK-293 cells overexpressing Dmt1 (22), experiments with brain-specific Dmt1 knockout mice, as well as studies performed in control and iron-deficient rats and in b/b compared with +/b rats (data not shown). All indications are that this reagent specifically recognizes the rat Dmt1 protein. Our supply of this antibody was unfortunately exhausted after the HEK-293 cell studies, so we utilized another anti-Dmt1 antibody for experiments with the rat samples (Alpha Diagnostics, catalog no. 1082627A). Proteins were purified from cell extracts by routine methods and then separated by SDS/PAGE and blotted onto polyvinylidene difluoride membranes. After blots were blocked in 5% nonfat milk, they were reacted with a 1:4000 dilution of Dmt1 antibody followed by a 1:6000 dilution of HRP-conjugated anti-rabbit (secondary) antibody followed by exposure to X-ray film. Blots were subsequently stained with Ponceau S solution to confirm equal sample loading and efficient transfer. The OD of bands on film and proteins on stained blots was determined using the digitizing software UN-SCAN-IT (Silk Scientific).
Everted gut sac assay and copper uptake measurements.
Given the difficulty in obtaining radiolabeled copper (64Cu) and its very short half-life (<12 h), an alternative to oral gavage for measuring copper absorption was sought. An everted gut sac assay (23, 24), in combination with the bicinchoninic acid (BCA) assay (described below), was thus utilized to test the difference in copper transport across the intestinal epithelium among the 3 experimental groups. After dietary treatments, rats were killed and a ∼10-cm section from the upper small intestine (just distal to the pyloric sphincter) was collected and flushed with ice-cold PBS. The intestinal sac was everted using a glass rod to expose the mucosal surface, followed by tying one end with a thread and then filling it with 1 mL Kreb’s-Ringer (KR) buffer (pH 5.5–6.0) (25). The sac was then tied at the other end and incubated for 40 min in gassed KR buffer (95% O2) containing 600 μmol/L CuCl2 at room temperature (∼24°C). The tissue remains viable for at least 40 min under these conditions (26). The gut sacs were then washed in PBS to remove excess Cu2+ and untied at one end to allow collection of the solution inside for quantification of copper concentrations. Permeability was monitored by adding 0.002% phenol red to the KR buffer outside the gut sac and then measuring the absorbance (A562) of the solution inside the sac after the incubation period (26).
The BCA assay was subsequently utilized to measure the copper content of fluids collected from within the everted gut sacs (27). A 500-μL fluid sample was placed into a 1.5-mL microfuge tube and 250 μL of 30% (wt:v) trichloroacetic acid was added. The sample was mixed and then centrifuged at 16,000 × g for 5 min. Then, 500 μL of the supernatant was transferred to a clean tube and 100 μL of 0.0352% (wt:v) L-dihydroascorbic acid was added, followed by brief mixing. HEPES-buffered BCA solution (400 μL) was then added to the sample (6 mg BCA disodium salt, 3.6 g NaOH, and 15.6 g HEPES dissolved in 90 mL double-distilled H2O). Next, a 200-μL sample was transferred to a 96-well plate and the absorbance was read at 354 nm (27). To estimate the copper concentration of experimental samples, standard curve reactions were prepared in BCA solution by the same procedure using the following copper concentrations: 0, 5, 10, 15, 20, and 25 μmol/L. A standard curve was also established for phenol red, with the following concentrations: 0, 0.5%, 1%, 1.5%, 2%, and 2.5%. The same point-to-point relation for the 2 sets of standards was plotted to estimate experimental sample concentrations.
HEK-293 cell Dmt1 overexpression model.
Four Dmt1 isoforms have been reported, varying at the 5′ and 3′ ends (28). Two variants are derived from alternative promoters, leading to transcripts with 2 different 5′ exons (exons 1A and 1B). The other 2 are generated from alternative polyadenylation at the 3′ end (10), which produces transcripts with or without an iron-responsive element [(+IRE) or (−IRE)]. The IRE might stabilize the transcript when intracellular iron is low, mediating interaction with iron-regulatory proteins (IRPs), but there is controversy on this issue (29–35). The isoform of Dmt1 that is predominantly expressed in rat intestine is transcribed from exon 1A and it contains the 3′ IRE (1A/+IRE). HEK-293 cells stably transfected with the rat Dmt1(1A+) (containing the IRE) or the mouse Dmt1(1B-) (lacking the IRE) cDNAs were used in the current investigation (previously described in detail) (22). These cells were engineered so that the Dmt1 cDNA was expressed from a strong cytomegalovirus promoter only when cells were treated with doxycycline (DOX; a tetracycline analogue, i.e., a ‘tet-on’ system). Culture conditions were previously described (22). For some experiments, cells were exposed to desferrioxamine (DFO; an iron chelator) to induce an iron-deficiency response or treated with additional iron (ferric ammonium citrate).
Dmt1-overexpressing HEK-293 cells were grown to ∼60% confluence in 6-well poly-lysine–coated plates and then incubated for 24 h with or without DOX (50 nmol/L), DFO (100 μmol/L), or ferric ammonium citrate (200 μmol/L). Cells were subsequently washed twice in prewarmed, serum-free DMEM medium and then incubated in BSA-containing DMEM medium for 1 h at 37°C to deplete transferrin. 59Fe2+ and 64Cu1+ were generated from 59FeCl3 and 64CuCl2, respectively, by combining the radiolabeled compounds with uptake buffer containing ascorbic acid (a reducing agent). In addition to the labeled iron and copper compounds, the uptake buffer contained the following: 10 mmol/L HEPES, 10 mmol/L MES (pH 5.5–6.0), 150 mmol/L NaCl, 1 mmol/L CaCl2, 2 μmol/L unlabeled FeCl3 or CuCl2, and 2 mmol/L ascorbic acid. After incubating cells for 30 min in this buffer at 37°C, uptake was terminated by replacing the uptake buffer with prewarmed, serum-free DMEM medium containing 2 mmol/L DFO (for iron uptake) or 2 mmol/L bathocuproine disulphonate (a copper chelator; for copper uptake). The medium was then removed and 600 μL cell lysis buffer (0.2 mol/L NaOH and 0.2% SDS) was added to solubilize the cells. Five hundred μL of cell lysate was subsequently transferred into a γ-counting tube and radioactivity counted in a PerkinElmer 2480 Automatic Gamma Counter. The remaining cell lysates were used to determine protein concentration.
Statistical analysis.
All data were analyzed by 1-factor ANOVA followed by Tukey’s post hoc test to determine differences between groups. We routinely transformed qRT-PCR data by log2 before ANOVA; otherwise, when Bartlett’s test for equal variances failed, we transformed the data by log10 for the ANOVA. All such occasions are plotted as log10 for the y-axis. We also ran multivariate ANOVA to identify interactions but report only the 1-factor P values. P < 0.05 was considered significant.
Results
Hematological status of experimental rats.
Hemoglobin and hematocrit amounts were significantly lower in the b/b rats and +/b rats fed the low-iron diet compared with +/b rats consuming the control diet (Table 1).
TABLE 1.
Hematological parameters of control (+/b), iron-deprived (14 wk) control, and b/b rats1
| Genotype, diet | Hemoglobin | Hematocrit (v:v) |
| g/L | ||
| +/b, control | 166 ± 6a | 0.5 ± 0.004a |
| +/bD, low-iron | 88 ± 1.4c | 0.4 ± 0.0003b |
| b/b, control | 66 ± 1.6b | 0.3 ± 0.001b |
Values are means ± SEMs, n = 4. Labeled means in a row with superscripts without a common letter differ, P < 0.05. b/b, mutant; +/b, phenotypically normal; +/bD, iron-deprived control.
Quantification of Dmt1 expression.
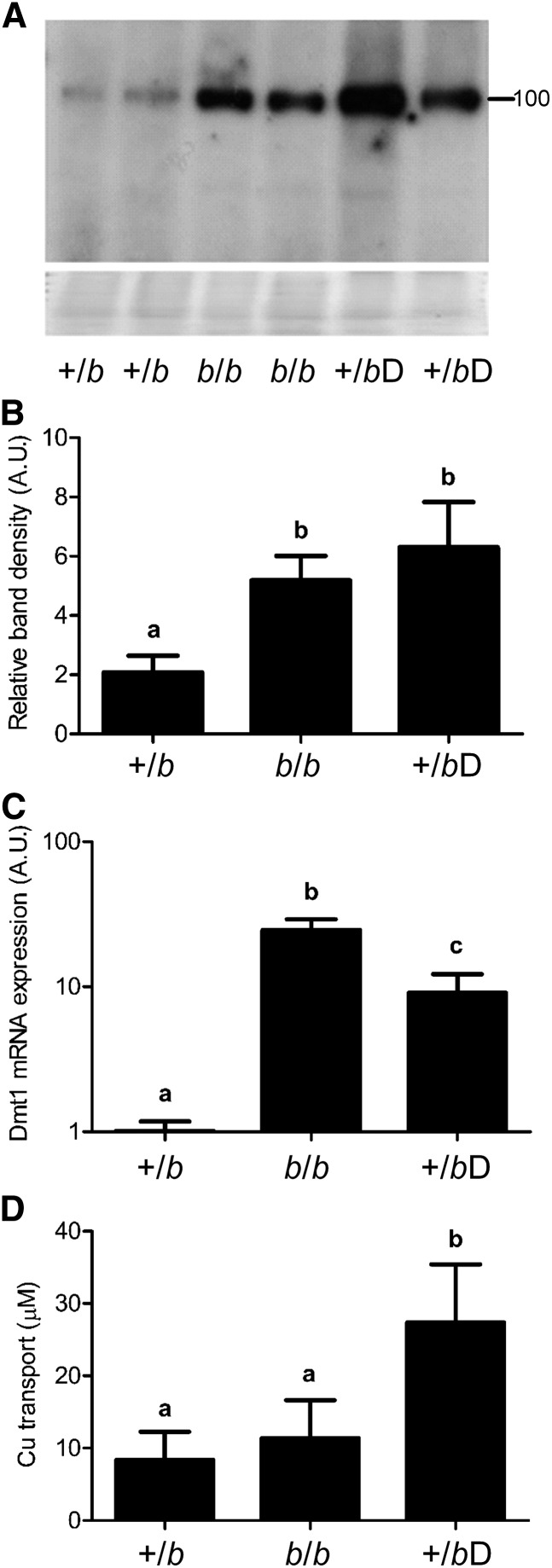
Duodenal Dmt1 protein expression in b/b rats and +/b rats consuming the low-iron diet was significantly higher than in +/b rats consuming the control diet (Fig. 1A,B). Several immunoreactive bands were detected; these bands were specific, because they were absent in the uninduced cells. Detection of several bands was not unexpected given that different forms of Dmt1 protein have routinely been detected by immunoblot analyses (22), possibly representing variants with different post-translational modifications. Similarly, Dmt1 mRNA expression was induced in b/b rats and iron-deprived +/b rats compared with +/b rats consuming the control diet (Fig. 1C). The induction was greater in the b/b rats, perhaps reflecting more significant iron deficiency.
FIGURE 1.
Dmt1 mRNA and protein expression and duodenal mutant (b/b) copper transport in control (+/b), iron-deprived (14 wk) control (+/bD), and b/b rats. A representative Western blot from duodenal tissue is shown (A) along with quantitative data (B) from all experimental rats. Band intensities were normalized to total protein on the stained blots (shown below the Western-blot image). The number beside the blot indicates the placement of the closest molecular weight marker (in kDa). Means without a common letter differ, P < 0.005. (C) qRT-PCR analysis of Dmt1 mRNA expression. Means without a common letter differ, P < 0.00005. (D) Copper transport studies in everted gut sacs derived from duodenum of experimental rats. Shown is the amount of copper transported from the mucosal to serosal side. (B–D) Values are means ± SEMs. Means without a common letter differ, P < 0.01, n = 4–5. A.U., arbitrary units; Dmt1, divalent metal transporter 1.
Copper transport assay.
An ex vivo approach using everted duodenal sleeves was utilized to assess copper transport. Mucosal integrity was assessed by phenol red transport, which was very low (∼0.15%) and did not differ among groups (data not shown), indicating intact tight junctions and proper sealing of the gut sac ends. Results showed that +/b rats consuming the low-iron diet showed significantly increased copper transport in relation to iron-deficient b/b and +/b rats fed the control diet, as indicated by higher copper accumulation in the fluid inside the sealed gut sacs (Fig. 1D).
Validation and extension of the HEK-293 cell model.
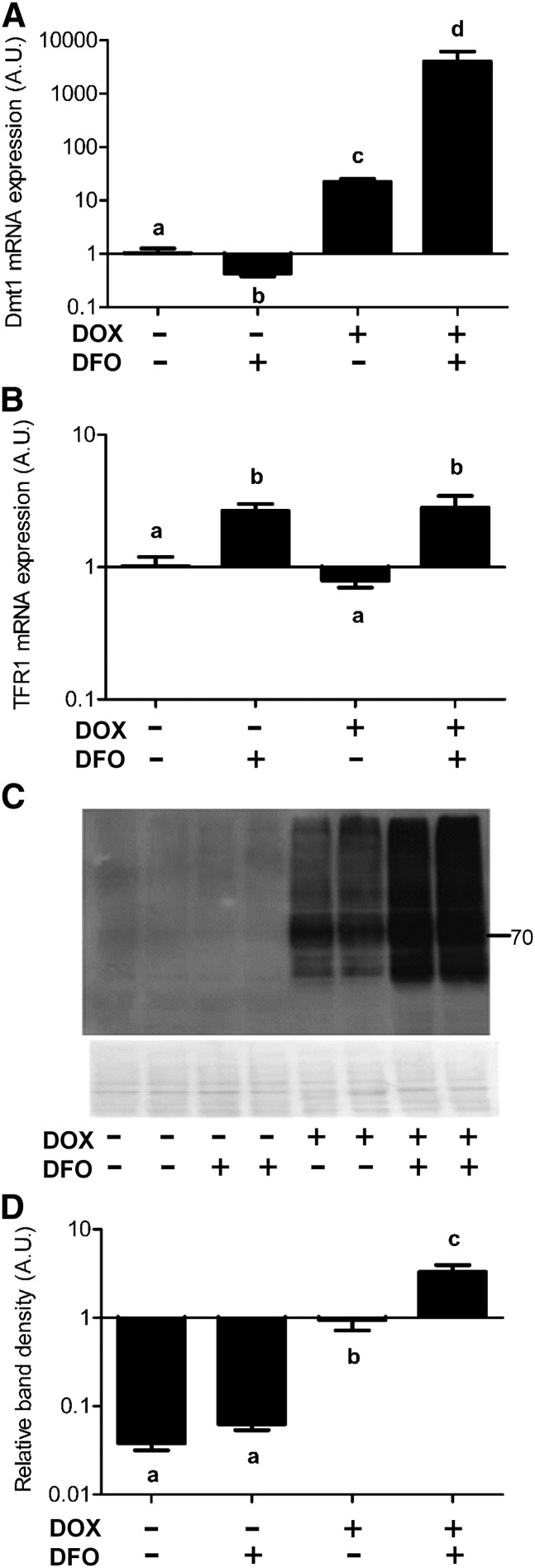
As cells were transferred from Buffalo to Gainesville and experiments were carried out by a new investigator (L. Jiang), we first revalidated, and then extended, the model. To confirm Dmt1 overexpression, real-time PCR and Western blotting were employed. Results showed that Dmt1 mRNA expression was significantly higher in cells treated with DOX (Fig. 2A). Remarkably, mRNA expression shot up to much higher levels when both DOX and DFO were present. As expected, endogenous TFR1 mRNA expression increased when cells were treated with the iron chelator (DFO) whether or not the inducer (DOX) was present (Fig. 2B), demonstrating that cells were indeed iron deficient. TFR1 mRNA levels vary inversely with intracellular iron levels and are thus a reliable indicator of cellular iron content (36). Immunoblot analyses also showed that after adding the DOX inducer, Dmt1 protein expression was significantly higher than in untreated cells. Furthermore, DOX plus DFO treatment enhanced protein expression even more dramatically although not fully reflecting mRNA expression, whereas DFO only (as a control treatment) did not induce Dmt1 protein expression (Fig. 2C,D).
FIGURE 2.
DOX induces expression of stably transfected Dmt1 in HEK-293 cells and iron chelation with DFO further potentiates expression. qRT-PCR quantification of rat Dmt1 (A) and hTFR1 (B) mRNA expression levels. (C) A representative Western blot of Dmt1 protein expression and quantitative blot data (D) from all experiments. Band intensities on film were normalized to total protein on stained blots (shown below the Western blot) with that for DOX only set to 1. The number beside the image indicates the placement of the closest molecular weight marker (in kDa). Values are means ± SEMs, n = 4. Means without a common letter differ; P < 0.0005 for a and b and P < 0.00005 for the rest (A); P < 0.001 (B); and P < 0.0005 for b and c and P < 0.00005 for the rest (D). A.U., arbitrary units; DFO, desferrioxamine; Dmt1, divalent metal transporter 1; DOX, doxycycline; hTFR1, human transferrin receptor 1.
Iron and copper uptake studies in the HEK-293 cell model.
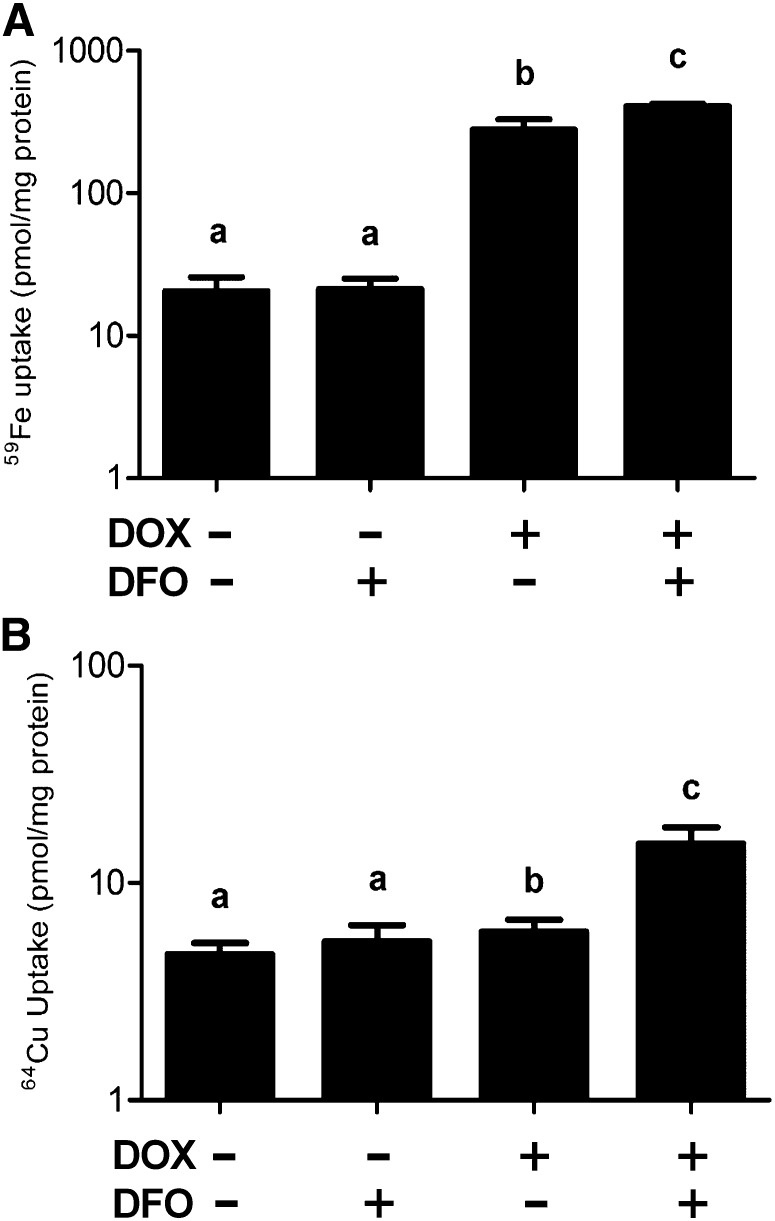
Iron uptake studies indicated that adding DOX augmented iron transport as previously reported (22) and that this increase was greater in cells treated with DOX plus DFO. DFO alone had no effect on subsequent iron uptake (Fig. 3A). These data confirmed that the overexpression system worked properly at the functional level. Importantly, copper uptake significantly increased when Dmt1 expression was induced in the presence of an iron chelator (Fig. 3B) but was similar in the other 3 groups (likely reflecting copper influx not mediated by Dmt1).
FIGURE 3.
DOX induction of Dmt1 expression in combination with iron chelation by DFO increases copper transport in HEK293 cells. 59Fe (A) or 64Cu (B) transport was assessed in cells stably transfected with the rat Dmt1/1A(+IRE) cDNA. Values are means ± SEMs, n = 4. Means without a common letter differ; P < 0.0005 for b and c and P < 0.00005 for the rest (A); P < 0.005 for a and b and P < 0.00005 for the rest (B). DFO, desferrioxamine; Dmt1, divalent metal transporter 1; DOX, doxycycline; IRE, iron-responsive element.
Iron-dependent regulation of Dmt1 expression constructs with and without an IRE in HEK-293 cells.
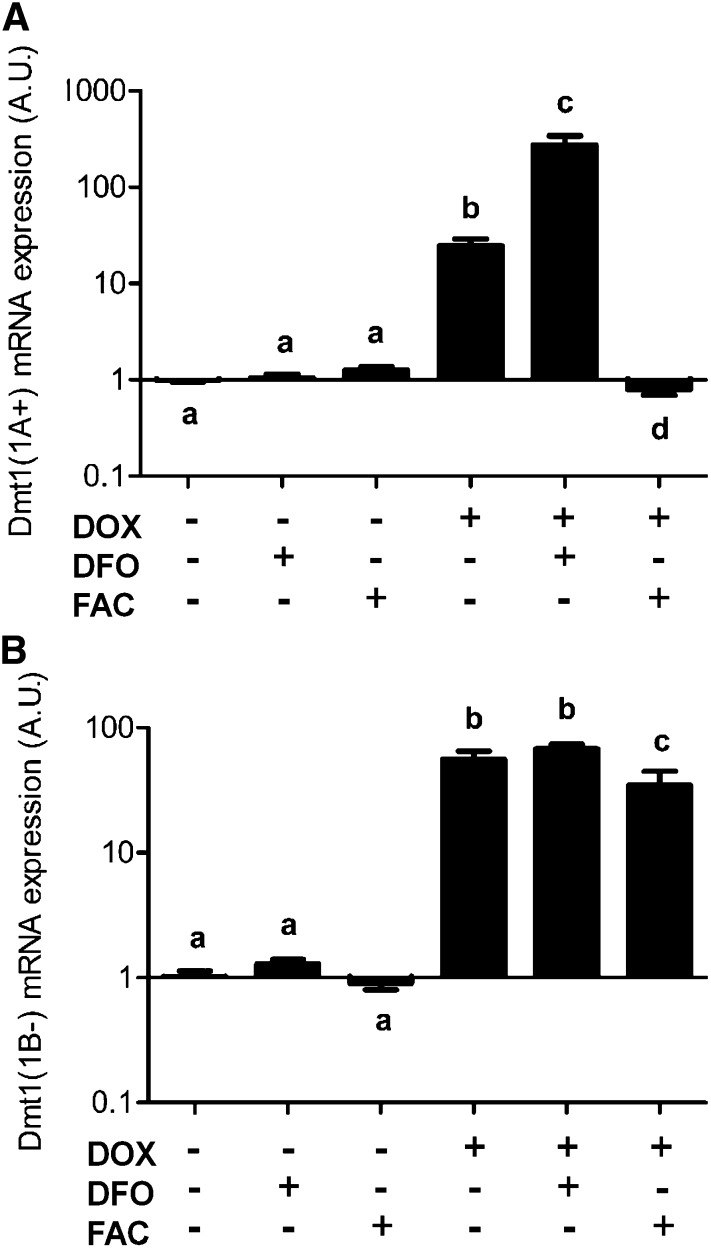
The remarkable additional increase in +IRE Dmt1 transcript amounts when cells were treated with an iron chelator (Fig. 3A) could reflect the presence of a functional IRE in the 3′ portion of the mRNA. Therefore, and because functionality of the IRE in the Dmt1 transcript is an unresolved issue, we tested mRNA stabilization using 2 different Dmt1 overexpression constructs (Fig. 4A,B). Again, a significant additional increase in +IRE transcript amounts was noted with DFO treatment, whereas adding excess iron decreased expression to levels seen in uninduced cells (even in the presence of the DOX inducer) (Fig. 4A). Conversely, −IRE transcript amounts were not altered by iron chelation and were only modestly affected by treating the cells with additional iron (Fig. 4B). These results indicate that Dmt1 mRNA synergistically increases after induction during DFO treatment when it contains an IRE but not when the IRE is absent.
FIGURE 4.
The increase in Dmt1 expression in stably transfected HEK-293 cells is mediated by the 3′ IRE. Cells were stably transfected with either the Dmt1(1A+) cDNA (containing the IRE) (A) or the Dmt1(1B-) cDNA (lacking the IRE) (B) and Dmt1 mRNA expression was quantified by qRT-PCR. Values are means ± SEMs, n = 4. Means without a common letter differ, P < 0.00005 (A); P < 0.01 for b and c and P < 0.00005 for the rest (B). A.U., arbitrary units; DFO, desferrioxamine; Dmt1, divalent metal transporter 1; DOX, doxycycline; FAC, ferric ammonium citrate; IRE, iron-responsive element.
Discussion
Previous studies reported that Belgrade rats did not have increased hepatic or serum copper concentrations despite significant iron deficiency (20), leading to the postulate that altered tissue copper concentrations (or copper redistribution) during iron deprivation relates to Dmt1 function. The present studies were designed to test this hypothesis by using complementary in vivo and in vitro models. The in vivo model used was the Belgrade rat model of genetic iron deficiency. The Belgrade (b) mutation is a single nucleotide transversion in the Dmt1 gene, resulting in a glycine to arginine substitution in the Dmt1 protein, which significantly decreases activity (19, 20, 37, 38). Heterozygous Belgrade rats (+/b) are phenotypically normal, whereas homozygous mutant rats (b/b) are significantly iron deficient. The iron deficiency in the b/b rats stems from defects in intestinal iron absorption and transferrin-bound iron assimilation by erythroid precursors (19, 39). The rationale for the current studies related to the function of Dmt1 in dietary acquisition of iron and/or copper on the apical surface of duodenal enterocytes. Comparing copper transport in b/b rats (with Dmt1 function impaired) with +/b rats (with active Dmt1) fed a low-iron diet allows one to assess the role of Dmt1 in copper uptake during iron deficiency. Although Dmt1 is required for intestinal iron absorption, during iron-replete conditions, it probably is not a physiological copper transporter. We thus considered the possibility that Dmt1 could transport copper specifically during iron deficiency, possibly as a result of a massive increase in expression or via an unknown mechanism that could alter its affinity for copper ions.
The classic everted gut sac transport technique was thus utilized to assess copper transport. Copper accumulation inside the everted sacs was quantified using the BCA assay, which is highly sensitive and specific for reduced (Cu1+) copper (27). The addition of ascorbate to the KR buffer used for these studies ensured that transported copper would be Cu1+. The BCA-Cu reaction rapidly forms an intense purple-colored complex that can be spectrophotometrically quantified. Standard curves using various Cu1+ concentrations showed linearity and all experimental values fell within the range of the standard curve. Importantly, copper accumulation inside the gut sacs was not a result of a leaky epithelial barrier, because phenol red transport was extremely low in all experiments. It is thus likely that the increased duodenal copper transport noted in the iron-deprived +/b rats was mediated via Dmt1, because copper transport did not increase in comparably iron-deficient b/b rats expressing mutant Dmt1.
The in vitro model used was DOX-inducible Dmt1 overexpressing HEK-293 cells. These cells were utilized to determine whether Dmt1 can transport copper. Untreated cells absorbed very little iron, indicating low expression and/or activity of endogenously expressed Dmt1 and providing an excellent background in which to perform these studies. Induced expression of Dmt1, while dramatically increasing iron uptake, barely increased copper uptake, suggesting that most copper enters these cells via a distinct pathway. When Dmt1 was expressed in the presence of an iron chelator, however, copper transport was enhanced. Because treatment of uninduced cells with DFO did not affect iron or copper uptake, this result can best be explained by highly induced expression of Dmt1 (and/or modification of the protein under iron-deprived conditions) rather than an unexpected effect of DFO.
These studies also provide data demonstrating functionality of the 3′ IRE in the Dmt1 transcript. Previous studies have provided conflicting results regarding the role of the 3′ IRE, with some supporting its functionality (31–33) and others providing opposing data (29, 30, 34, 35). Experiments were thus designed to confirm that the robust induction of Dmt1 mRNA expression caused by DFO treatment was mediated by the IRE. Accordingly, studies were undertaken using stably transfected HEK-293 cells expressing 2 isoforms of Dmt1, one containing the 3′ IRE and the other not. Results showed that expression of the +IRE form of Dmt1 was highly responsive to intracellular iron levels, whereas expression of the transcript variant without the IRE was not (Fig. 4). The differences cannot be attributed to changes in transcription rates, because both cell lines relied on the same strong cytomegalovirus promoter to express Dmt1. These data demonstrate that the 3′ end IRE in the Dmt1 transcript is indeed functional. It is of note that the Dmt1 isoform that exhibits the striking increase in mRNA and protein levels with a clear increase in Cu transport in these cells is the 1A/+IRE form. The same form is the predominant isoform in intestinal epithelia, where we also detected Cu transport associated with Dmt1 in everted gut sacs.
In summary, the current investigation provides evidence that Dmt1, at least in part, mediates the increase in copper uptake by enterocytes during iron deprivation under conditions where Dmt1 abundance increases many fold. Whether increased Dmt1 protein abundance permits copper transport or if the protein is modified during iron deprivation (e.g., by phosphorylation), perhaps changing its functional properties, is unknown. The fact that similar results were obtained using 2 different models, one ex vivo derived from experimental rats and the other an in vitro expression system, supports the argument that these findings are of physiological importance. This investigation further increases our understanding of the molecular events that occur in duodenal enterocytes to maintain iron homeostasis during states of deficiency. Although the functional role of increased intracellular copper in enterocytes is not fully understood, we speculate that one effect would be to increase activity of the multi-copper ferroxidase hephaestin (Heph). Heph couples iron oxidation with export via Fpn1 to promote ferric iron binding to transferrin in the interstitial fluid. This prediction is based upon the fact that expression and activity of another multi-copper ferroxidase, ceruloplasmin, expressed in liver and secreted into blood, increases when hepatocytes load copper during iron deficiency (2). It was hypothesized (but not experimentally tested) that increased metallation of the apo form of the enzyme stabilized the protein. Further experimentation will, however, be required to assess the impact of increasing intracellular copper on Heph expression and activity as well as to determine whether additional copper-dependent mechanisms may be involved in maintenance of iron homeostasis.
Acknowledgments
L.J., M.D.G., L.M.G., and J.F.C. designed research and analyzed data; L.J. conducted research; L.J. and M.D.G. performed statistical analysis; M.D.G., L.M.G., and L.Z. provided essential reagents; L.J., M.D.G., L.M.G., L.Z., and J.F.C. wrote the manuscript; and J.F.C. had primary responsibility for final content. All authors have read and approved the final manuscript.
Footnotes
Abbreviations used: Atp7a, Menkes copper-transporting ATPase; b/b, mutant; BCA, bicinchoninic acid; Ctr1, copper transporter 1; Dcytb, duodenal cytochrome B; DFO, desferrioxamine; Dmt1, divalent metal transporter 1; DOX, doxycycline; Fpn1, ferroportin 1; Heph, hephaestin; hTFR1, human transferrin receptor 1; IRE, iron-responsive element; IRP, iron-regulatory protein; KR- Kreb’s-Ringer; MtI/II, metallothionein I/metallothionein II; +/b, phenotypically normal.
Literature Cited
- 1.El-Shobaki FA, Rummel W. Binding of copper to mucosal transferrin and inhibition of intestinal iron absorption in rats. Res Exp Med (Berl). 1979;174:187–95. [DOI] [PubMed] [Google Scholar]
- 2.Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF. Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood. 2011;118:3146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes Copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem. 2005;280:36221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman AR, Moran PE. Copper metabolism in iron-deficient maternal and neonatal rats. J Nutr. 1984;114:298–306. [DOI] [PubMed] [Google Scholar]
- 5.Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G964–71. [DOI] [PubMed] [Google Scholar]
- 6.Gulec S, Collins JF. Investigation of iron metabolism in mice expressing a mutant Menke's copper transporting ATPase (Atp7a) protein with diminished activity (brindled; MoBr/y). PLoS ONE. 2013;8:e66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L, Collins JF. Transcriptional regulation of the Menkes copper ATPase (Atp7a) gene by hypoxia-inducible factor (HIF2a) in intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;300:C1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins JF, Hu Z. Promoter analysis of intestinal genes induced during iron-deprivation reveals enrichment of conserved SP1-like binding sites. BMC Genomics. 2007;8:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. [DOI] [PubMed] [Google Scholar]
- 10.Garrick MD. Human iron transporters. Genes Nutr. 2011;6:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie B, Garrick MD. Iron imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G981–6. [DOI] [PubMed] [Google Scholar]
- 12.Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H(+)-Coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Curr Top Membr. 2012;70:169–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knöpfel M, Solioz M. Characterization of a cytochrome b(558) ferric/cupric reductase from rabbit duodenal brush border membranes. Biochem Biophys Res Commun. 2002;291:220–5. [DOI] [PubMed] [Google Scholar]
- 15.Wyman S, Simpson RJ, McKie AT, Sharp PA. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett. 2008;582:1906–16. [DOI] [PubMed] [Google Scholar]
- 16.Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol. 2003;284:C1525–30. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza A, Le Blanc S, Olivares M, Pizarro F, Ruz M, Arredondo M. Iron, copper, and zinc transport: inhibition of divalent metal transporter 1 (DMT1) and human copper transporter 1 (hCTR1) by shRNA. Biol Trace Elem Res. 2012;146:281–6. [DOI] [PubMed] [Google Scholar]
- 18.Knöpfel M, Zhao L, Garrick MD. Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry. 2005;44:3454–65. [DOI] [PubMed] [Google Scholar]
- 19.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Ranganathan P, Lu Y, Kim C, Collins JF. Exploration of the copper-related compensatory response in the Belgrade rat model of genetic iron deficiency. Am J Physiol Gastrointest Liver Physiol. 2011;301:G877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins JF, Hu Z, Ranganathan PN, Feng D, Garrick LM, Garrick MD, Browne RW. Induction of arachidonate 12-lipoxygenase (Alox15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am J Physiol Gastrointest Liver Physiol. 2008;294:G948–62. [DOI] [PubMed] [Google Scholar]
- 22.Garrick MD, Kuo HC, Vargas F, Singleton S, Zhao L, Smith JJ, Paradkar P, Roth JA, Garrick LM. Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochem J. 2006;398:539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam MA, Al-Jenoobi FI, Al-Mohizea AM. Everted gut sac model as a tool in pharmaceutical research: limitations and applications. J Pharm Pharmacol. 2012;64:326–36. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TH, Wiseman G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954;123:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handy RD, Musonda MM, Phillips C, Falla SJ. Mechanisms of gastrointestinal copper absorption in the African walking catfish: copper dose-effects and a novel anion-dependent pathway in the intestine. J Exp Biol. 2000;203:2365–77. [DOI] [PubMed] [Google Scholar]
- 26.Cassidy MM, Tidball CS. Cellular mechanism of intestinal permeability alterations produced by chelation depletion. J Cell Biol. 1967;32:685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner AJ, Harris ED. A quantitative test for copper using bicinchoninic acid. Anal Biochem. 1995;226:80–4. [DOI] [PubMed] [Google Scholar]
- 28.Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci USA. 2002;99:12345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardrop SL, Wells C, Ravasi T, Hume DA, Richardson DR. Induction of Nramp2 in activated mouse macrophages is dissociated from regulation of the Nramp1, classical inflammatory genes, and genes involved in iron metabolism. J Leukoc Biol. 2002;71:99–106. [PubMed] [Google Scholar]
- 30.Tchernitchko D, Bourgeois M, Martin ME, Beaumont C. Expression of the two mRNA isoforms of the iron transporter Nramp2/DMTI in mice and function of the iron responsive element. Biochem J. 2002;363:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–16. [DOI] [PubMed] [Google Scholar]
- 32.Kato J, Kobune M, Ohkubo S, Fujikawa K, Tanaka M, Takimoto R, Takada K, Takahari D, Kawano Y, Kohgo Y, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879–87. [DOI] [PubMed] [Google Scholar]
- 33.Galy B, Ferring-Appel D, Kaden S, Grone HJ, Hentze MW. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab. 2008;7:79–85. [DOI] [PubMed] [Google Scholar]
- 34.Sharp P, Tandy S, Yamaji S, Tennant J, Williams M, Singh Srai SK. Rapid regulation of divalent metal transporter (DMT1) protein but not mRNA expression by non-haem iron in human intestinal Caco-2 cells. FEBS Lett. 2002;510:71–6. [DOI] [PubMed] [Google Scholar]
- 35.Zoller H, Theurl I, Koch R, Kaser A, Weiss G. Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cells Mol Dis. 2002;29:488–97. [DOI] [PubMed] [Google Scholar]
- 36.Jeffrey GP, Basclain KA, Allen TL. Molecular regulation of transferrin receptor and ferritin expression in the rat gastrointestinal tract. Gastroenterology. 1996;110:790–800. [DOI] [PubMed] [Google Scholar]
- 37.Canonne-Hergaux F, Fleming MD, Levy JE, Gauthier S, Ralph T, Picard V, Andrews NC, Gros P. The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood. 2000;96:3964–70. [PubMed] [Google Scholar]
- 38.Garrick MD, Dolan KG. An expression system for a transporter of iron and other metals. Methods Mol Biol. 2002;196:147–54. [DOI] [PubMed] [Google Scholar]
- 39.Garrick MD, Gniecko K, Liu Y, Cohan DS, Garrick LM. Transferrin and the transferrin cycle in Belgrade rat reticulocytes. J Biol Chem. 1993;268:14867–74. [PubMed] [Google Scholar]