Abstract
S-(-)equol, an intestinally derived metabolite of the soy isoflavone daidzein, is proposed to enhance the efficacy of soy diets. Adults differ in their ability to produce equol when consuming soy foods for reasons that remain unclear. Therefore, we performed a comprehensive dietary analysis of 143 macro- and micronutrients in 159 healthy adults in the United States (n = 89) and Australia (n = 70) to determine whether the intake of specific nutrients favors equol production. Three-d diet records were collected and analyzed using Nutrition Data System for Research software and S-(-)equol was measured in urine by mass spectrometry. Additionally, in a subset of equol producers and nonproducers (n = 10/group), we examined the long-term stability of equol producer status by retesting 12, 18, and 24 mo later. Finally, the effect of oral administration of the antibiotic metronidazole (500 mg/d for 7 d) on equol production was examined in 5 adults monitored during a 4-mo follow-up period. Equol producers accounted for 30.3% and 28.6% of the United States and Australian participants, respectively (overall frequency, 29.6%). No significant differences were observed for total protein, carbohydrate, fat, saturated fat, or fiber intakes between equol producers and nonproducers. However, principal component analysis revealed differences in several nutrients, including higher intakes of polyunsaturated fatty acids (P = 0.039), maltose (P = 0.02), and vitamins A (P = 0.01) and E (P = 0.035) and a lower intake of total cholesterol (P = 0.010) in equol producers. During a 2-y period, equol producer status remained unchanged in all nonproducers and in 80% of equol producers, whereas metronidazole abolished equol production in only 20% of participants. In conclusion, these findings suggest that major differences in the macronutrient content of the diet appear not to influence equol production, but subtle differences in some nutrients may influence the ability to produce equol, which was a relatively stable phenomenon.
Introduction
Interest in the role of soy and isoflavones in preventing or treating chronic disease has in recent years gravitated away from soy protein and isoflavones toward a focus on the natural intestinal isoflavone metabolite S-(-)equol (1, 2). There is now sufficient interest in S-(-)equol for its potential to prevent or treat estrogen- and androgen-mediated diseases that it is under development as a nutraceutical (3, 4) and pharmaceutical agent (5) targeted at adults who cannot naturally make equol when consuming soy foods (6). S-(-)equol is formed from daidzin/daidzein by bacteria in the distal region of the intestine and colon (1, 2, 7, 8); however, it is not produced by all adults after consuming soy foods (6, 9–11). Numerous studies show that the frequency of “equol producers” in adult Western populations is ~25–30% (6, 9–11) and this is considerably lower than the frequency of 50–70% reported for adult equol producers in Japan, China, and Korea, where traditional soy foods are regularly consumed (12–16). Collectively, these data show that the frequency of equol producers differs among different population groups. It is presently unclear what factors determine S-(-)equol production, but understanding why equol production varies among individuals is important, because of the increasing evidence supporting the hypothesis that the ability to produce equol contributes to the overall efficacy of a soy food-based diet (17–19). Although a primary factor is the presence of specific intestinal bacteria capable of converting daidzein/daidzein to S-(-)equol, of which numerous species have been identified [reviewed in (1)], other dietary factors may play a role in S-(-)equol formation even if those intestinal bacteria are present (11, 20–24). If specific dietary factors can be identified as important in contributing to S-(-)equol production, it may then be possible to develop strategies to stimulate equol production in non–equol producers.
We describe a comprehensive study of the relation between equol producer status and dietary composition in a large population of free-living, healthy adults consuming a typical Western diet from 2 different geographical centers, one in the United States and the other in Australia. The objective was to determine the extent to which the macro- or micronutrient composition of the diet is associated with equol production by rigorous analysis of 3-d diet records for 143 different dietary constituents and to simultaneously test each participant for equol producer status by a standardized test (6). As a corollary to this study, we addressed whether equol production is a stable phenomenon in an individual, as was previously suggested (10, 17) by a longitudinal follow-up during a 2-y period of a subset of equol producers and non–equol producers. Finally, we describe the effects of administration of the commonly used antibiotic metronidazole on equol production.
Materials and Methods
Study design
Three interrelated studies were performed in free-living, healthy adults as follows.
Study 1: associations between the macro- and micro-composition of the diet and equol production.
The objective of this study was to determine in detail the relation between diet composition and equol production. Healthy men and women (n = 159) between 21 and 65 y of age with a BMI <30 were enrolled at 2 sites: Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (n = 89) and the Sydney Adventist Hospital, Wahroonga, New South Wales, Australia (n = 70). The study participants consisted of omnivores, self-proclaimed vegetarians, or people consuming a low-carbohydrate/high-protein diet. None of the study participants had evidence of pre-existing chronic liver, kidney, or gastrointestinal diseases and none were taking cholesterol-lowering drugs, bile acid binders, or statins, nor had antibiotics been prescribed within the 3 mo preceding enrollment.
On the initial screening visit, height and body weight were recorded and a general health questionnaire completed. Each participant meeting the entry criteria was tested for the ability to produce S-(-)equol (6). In brief, participants consumed 240 mL of soymilk (So Good soymilk brand, obtained from Sanitarium Health and Wellbeing) twice daily for 3.5 d and a 24-h urine collection was obtained beginning after the morning void on day 3 up to and including the morning void on d 4. Urine samples were stored at −20°C until analyzed. One week prior to this soy challenge, each participant kept a detailed 3-d diet diary that covered 2 weekdays and 1 weekend day. A dietitian instructed participants on how to assess and record food portions and how to complete a 3-d diet record for analysis of diet composition. All diet records were analyzed using the Nutrition Data System for Research software (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
Study 2: assessment of the stability of equol producer status.
This study was a longitudinal study of a subset of the participants (n = 20) retained from study 1 comprising documented equol producers (n = 10) and non–equol producers (n = 10) followed for 2 y, during which time their ability to produce equol was determined again after 12, 18, and 24 mo by the same standardized soy food challenge described above (6). Equol producer status was determined from urine analysis (6) and in the week preceding this testing, a 3-d diet diary was completed for reassessment of dietary composition in the event of a change in equol producer status.
Study 3: influence of antibiotics on equol producer status.
The objective of this observational study was to determine whether administration of a commonly used antibiotic, metronidazole, would inhibit equol production in documented equol producers and, if abolished, to then determine the time course for recovery of equol production.
Established equol producers (n = 5) identified from study 1 were rechallenged with soymilk to again confirm their equol producer status. After testing, the participants continued to consume soy milk, 240 mL twice daily, for the next 10 d. At that time, each participant was administered metronidazole (7.5 mg/kg body weight; three times a day) for 7 consecutive days. Throughout antibiotic administration and for a further 3 d, consecutive 24-h urine collections were obtained. Equol producer status was then reassessed at 14 d and then 1, 2, 3, and 4 mo later by the soy challenge test (6).
The study protocols were approved by the human Institutional Review Board of the Children’s Hospital Medical Center (protocol CCHMC no. 05–08–27) in the United States and the Ethics Committee of the Seventh Day Adventist Hospital, Wahrooga, New South Wales in Australia. Written informed consent was obtained from all participants. These studies were initiated in September 2005, which predated requirements for registration of clinical studies.
Data analysis
Data are expressed as means ± SDs unless otherwise stated. For study 1, the demographics and geographic location were compared between equol producer status groups using t tests for continuous variables and chi-square test for categorical variables. In study 2, the changes over time in macro-nutrient composition of the diet were compared for equol producers and non–equol producers using repeated-measures ANOVA with time (in months) modeled as a continuous covariate. Changes over time within each group were evaluated using this same statistical model. Study 3 was an observational study only. In studies 1 and 2, for each of the dietary components, intakes were totaled for the 3-d diet records for each participant. Data were log transformed, if needed, to attain symmetry. The 143 different dietary components were grouped in specific macromolecular categories. Principal component analysis (PCA) was conducted, including the specified standardized dietary components for each macromolecular category to identify the factors that explained ~95% of the variance of that category (Supplemental Table 1). The principal components (PCs) were described based upon the loadings. Each PC was then analyzed separately within each category to determine if there were any significant differences between equol producers and non–equol producers using the Wilcoxon’s rank-sum test. This was also done for other major dietary groups and vitamin groups: lipids, proteins, vitamins A, B, C, D, E, and K, and minerals. Pearson’s chi-square test was used to compare percentage of equol producers for the total fiber intake categories. P values < 0.05 were considered significant.
Results
Demographics
Study 1.
Of the 159 participants that met the entry criteria and completed the study, 91 (57.2%) were female and 68 (42.8%) were male. The age range of the cohort was 21–61 y and the mean age was 36.8 ± 11.8 y for the females and 35.4 ± 9.4 y for males. The mean BMI for the women was 23.5 ± 3.2 and for the men was 24.9 ± 2.8.
The racial and ethnic characteristics of the cohort were 88.7% Caucasian, 8.8% Asian, and 2.5% African American; all were non-Hispanic. Demographics did not significantly differ between the equol producers and nonproducers with the exception of BMI, where the producers had a slightly higher BMI (24.9 ± 2.7 kg/m2) than that of the nonproducers (23.8 ± 3.2; P = 0.03), although both were within the normal range.
Study 2.
A total of 10 equol producers and 10 non–equol producers enrolled and completed the study. The mean age of the group comprising equol producers was 35.2 ± 11.8 y and for non–equol producers was 42.0 ± 11.5 y. The mean BMIs were 24.8 ± 1.1 kg/m2 and 24.9 ± 3.4 kg/m2, respectively, for these 2 groups. Among the 10 equol producers, 7 were female and 3 were male and in the non–equol producers, there were 4 females and 6 males. The race and ethnicity of the 20 study participants was 70% Caucasian, 5% African American, and 25% Asian, and all were non-Hispanic.
Study 3.
The mean age of the 5 participants (4 female and 1 male) enrolled was 34.4 ± 8.1 y (range, 27–47 y) and the BMI was 25.3 ± 3.4 (range, 20.7–29.7). Of these, there were 3 Caucasian, 1 African American, and 1 Asian.
Frequency of equol producer status in healthy adults
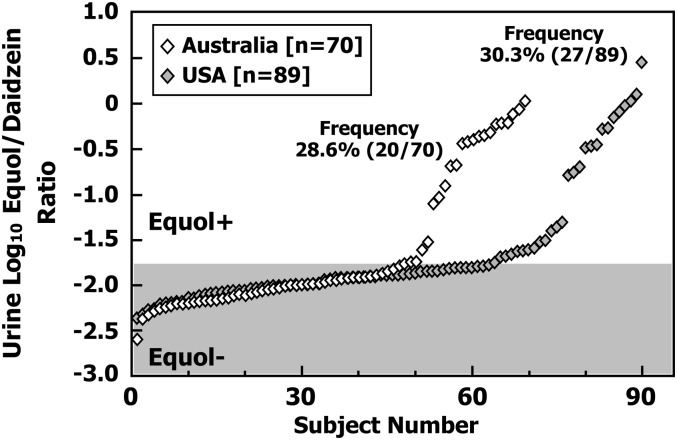
The log10-transformed urinary equol:daidzein ratios for the individual participants from the United States and Australia are plotted separately and in rank order of value in Figure 1. Using the threshold value of −1.75 as previously defined (6) to differentiate equol producers from nonproducers, 27 of the 89 American adults (30.3%) and 20 of the 70 Australian adults (28.6%) were classified as equol producers. The overall frequency of equol producers was 29.6%, of which 29.7% of the females (27 of 91) and 29.4% of the males (20 of 68) were equol producers, showing no gender bias in equol production. However, with regard to gender differences, there were more females than males who were equol producers in the United States (19 female, 8 mol/L) cohort, whereas the reverse trend was observed for the Australian cohort (8 females, 12 mol/L). The predominance of male equol producers in the Australian participants is in agreement with a previous study of 69 Australian adults (6). Neither the geographical nor gender difference in frequency of equol producers proved significant.
FIGURE 1.
Log10-transformed urinary S-(-)equol:daidzein ratios in rank order of the individual participants grouped according to study site, Australia (n = 70) and United States (n = 89). Points falling in the shaded area were classified as non–equol producers and those in the unshaded area were classified as equol producers. Equol+, equol producer; Equol−, non–equol producer.
Dietary composition: association with equol producer status
A total of 156 of the 159 study participants completed the 3-d diet records and analysis of these data found no significant associations between equol producer status and intake of the macronutrients of total fats, total carbohydrates, or total protein when these were expressed as a percent of energy (Table 1). This finding was consistent irrespective of whether data were analyzed separately as United States and Australia cohorts or combined. For this reason, further detailed analysis of the diet was performed on the combined groups using PCA. Box plots are shown in Figure 2 for those nutrients showing the most significant differences between equol producers and nonproducers.
TABLE 1.
Data from study 1 comparing the dietary intakes of 143 macro- and micronutrients in healthy adults classified as equol producers and non–equol producers determined from analysis of 3-d diet records using Nutrition Data System for Research software1
| Equol producer (n = 47) | Non–equol producer (n = 109) | P value2 | |
| Macronutrient | |||
| Total, g/d | 2040 ± 103 | 2060 ± 57 | 0.55 |
| Energy, kcal/d | 2070 ± 98 | 2050 ± 54 | 0.83 |
| Total fat, g/d | 73.4 ± 3.6 | 73.5 ± 2.4 | 0.86 |
| Calories from fat, %/d | 31.1 ± 1.0 | 31.2 ± 0.6 | 0.94 |
| PUFA:SFA ratio | 0.27 ± 0.02 | 0.22 ± 0.01 | 0.002 |
| Total PUFAs, g/d | 16.8 ± 0.9 | 14.7 ± 0.5 | 0.04 |
| Calories from PUFA, %/d | 7.1 ± 0.3 | 6.4 ± 0. | 0.02 |
| Total carbohydrate, g/d | 273 ± 17 | 265 ± 8.2 | 0.76 |
| Calories from carbohydrate, %/d | 50.9 ± 1.5 | 50.7 ± 0.8 | 0.82 |
| Maltose, g/d | 6.0 ± 0.7 | 4.4 ± 0.4 | 0.02 |
| Total dietary fiber, g/d | 27.1 ± 1.9 | 25.0 ± 1.0 | 0.49 |
| Total protein, g/d | 83.8 ± 4.9 | 84.1 ± 2.4 | 0.50 |
| Calories from protein, %/d | 16.1 ± 0.7 | 16.6 ± 0.4 | 0.30 |
| Micronutrient | |||
| Vitamins | |||
| Total vitamin A activity, IU/d | 14,000 ± 1200 | 10,600 ± 622 | 0.008 |
| Total vitamin A activity (retinol activity equivalents), μg/d | 1360 ± 116 | 1070 ± 56 | 0.03 |
| Total vitamin A activity (retinol equivalents), μg/d | 1930 ± 155 | 1490 ± 82 | 0.01 |
| β-Carotene (provitamin A carotenoid), μg/d | 6080 ± 588 | 4410 ± 361 | 0.005 |
| β-Carotene equivalents (derived from provitamin A carotenoids), μg/d | 6810 ± 672 | 5030 ± 422 | 0.008 |
| Retinol, μg/d | 794 ± 97 | 654 ± 45 | 0.31 |
| Thiamin (vitamin B-1), μg/d | 2.7 ± 0.2 | 2.4 ± 0.1 | 0.66 |
| Vitamin B-6 (pyridoxine, pyridoxyl, and pyridoxamine), mg/d | 3.5 ± 0.2 | 2.9 ± 0.2 | 0.39 |
| Vitamin C (ascorbic acid), mg/d | 237 ± 29 | 145 ± 15 | 0.20 |
| Vitamin E, IU/d | 46.3 ± 10 | 24.6 ± 1.4 | 0.08 |
| Vitamin E (total α-tocopherol), mg/d | 23.9 ± 4.7 | 13.6 ± 1.0 | 0.06 |
| Total α-tocopherol equivalents, mg/d | 43.6 ± 10 | 22.6 ± 2.0 | 0.06 |
| Natural α-tocopherol (RRR-α-tocopherol or d-α-tocopherol), mg/d | 9.3 ± 0.8 | 7.7 ± 0.3 | 0.16 |
| Synthetic α-tocopherol (all rac-α-tocopherol or dl α-tocopherol), mg/d | 32.4 ± 10 | 13.2 ± 2.0 | 0.12 |
| Minerals | |||
| Magnesium, mg/d | 440 ± 35 | 383 ± 14 | 0.33 |
| Calcium, mg/d | 1220 ± 89 | 1050 ± 38 | 0.16 |
| Iron, mg/d | 25.8 ± 3.2 | 22.2 ± 1.2 | 0.96 |
Values are means ± SEs, n = 156.
Wilcoxon’s rank-sum test.
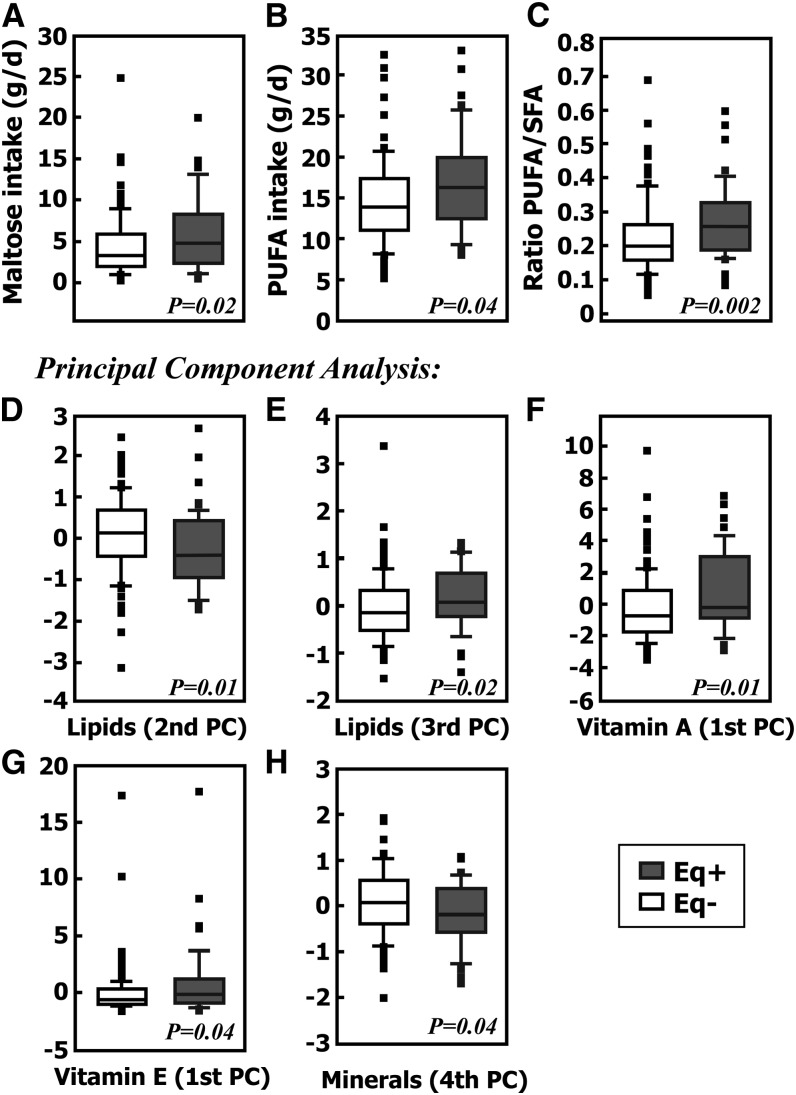
FIGURE 2.
Box-plots for study 1 comparing equol producers (n = 47) and non–equol producers (n = 109) for individual nutrients or PCs of specific macromolecular categories that demonstrated significance at P < 0.05. For PCs, positive values indicate higher intake and negative values indicate lower intake. (A) Dietary intake (g/d) of the disaccharide maltose. (B) Dietary intake of total PUFAs (g/d). (C) Ratio of PUFA:SFA intake. (D) PC representing the difference between intake of total cholesterol relative to PUFAs. (E) PC representing the difference between the intake of total cholesterol and PUFAs relative to SFAs. (F) PC representing weighted mean of vitamin A components. (G) PC representing weighted mean of total α-tocopherol, vitamin E components. (H) PC representing weighted sum of copper, sodium, and manganese relative to the intake of calcium. The shaded boxes represent equol producers and the white boxes represent non–equol producers. The line in the middle of the box represents the median or 50th percentile of the data and the box extends from the 25th to the 75th percentile. The lines emerging from the box extend to the upper and lower adjacent values, i.e., the largest data point ≤75th percentile plus 1.5 times the IQR and the smallest data point ≥25th percentile minus 1.5 times the IQR, respectively. If the examined data come from a normal distribution, one would expect the interval between the adjacent values to include 99.3% of the data. Observed points more extreme than the upper or lower adjacent values, if any, are individually plotted. Eq+, equol producer; Eq−, non–equol producer; PC, principal component.
In the carbohydrate category, which included the mono-, di-, and polysaccharides of glucose, galactose, fructose, sucrose, lactose, and maltose, total dietary fiber, soluble fiber, insoluble dietary fiber, and pectin, there were 5 PCs that accounted for most of the variability (typically 95%) in the group and dietary intake did not significantly differ between equol producers and nonproducers for any of these PCs. However, when individual carbohydrates were separately analyzed to compare the amount present in the diet of equol producers with nonproducers, the disaccharide maltose was greater in abundance in the diet of equol producers (P = 0.021) (Table 1; Fig. 2A).
The lipids grouped together for PCA included SFAs, MUFAs, PUFAs, cholesterol, and total fat and resulted in 3 PCs.
The first PC was an overall weighted mean of the components, but this was not significantly different between equol producers and nonproducers. The second PC represented the difference between the amount of total cholesterol in the diet relative to PUFAs, with higher values in nonproducers (P = 0.010) compared with equol producers (Fig. 2D). The third PC represented the sum of cholesterol and PUFA relative to SFA, which was higher for equol producers compared with nonproducers (P = 0.02) (Fig. 2E). Likewise, when individual components were separately analyzed to compare the amount present in the diet of equol producers with nonproducers, PUFA intake was greater for equol producers (P = 0.039) than for non–equol producers (Fig. 2B). Additionally, the ratio of PUFA:SFA was significant (Fig. 2C). These analyses indicate that individuals with more PUFAs in the diet relative to SFA tended to be equol producers and those with more PUFAs relative to total cholesterol also tended to be equol producers. Hence, higher amounts of PUFAs alone and relative to the amount of SFA and total cholesterol indicate that PUFAs play an important role in the diet of equol producers, and this may be driving the differences we observe between the 2 groups.
The third area identified for analysis was proteins, and when all amino acids were included in a PCA, only one PC resulted, which was not significantly different between equol producers and non–equol producers. The second PC evaluated included total protein and animal and vegetable protein. The PC did not differ between equol producers and nonproducers; neither did any of the individual components when analyzed separately.
Vitamins A, B C, D, E, and K were analyzed separately, but within each category, all possible forms of the each vitamin were included. PCA of vitamin A resulted in 5 PCs. The first PC was a weighted mean of all vitamin A components and resulted in a difference between equol producers and nonproducers (P = 0.012), indicating that the intake of vitamin A was significantly higher in individuals who were equol producers (Fig. 2F). None of the remaining 4 PCs resulted in significant differences. Analysis of vitamin B included thiamin (vitamin B-1), riboflavin (vitamin B-2), niacin (vitamin B-3), niacin equivalents, total folate, pantothenic acid, vitamin B-6 (pyridoxine, pyridoxyl, and pyridoxamine), and vitamin B-12 (cobalamin), and PCA resulted in 3 PCs, none of which were significant. PCA of vitamin E included total α-tocopherol, β-tocopherol, γ-tocopherol, and Δ-tocopherol and resulted in 4 PCs. The first PC was the weighted mean of these components and dietary intake in equol producers was significantly higher than in nonproducers (P = 0.035) (Fig. 2G). None of the remaining 3 PCs reached significance. When individual members of the tocopherol group were separately analyzed, no specific one was found to be present in greater amounts in the diet of equol producers than in the diet of nonproducers. PCA found no significant differences in the vitamin C, D, or K intakes of equol producers compared with nonproducers.
When mineral content was analyzed by PCA, 5 PCs were identified. The first 3 PCs did not yield any significant difference between equol producers and nonproducers. The fourth PC showed the weighted sum of the intake of copper, sodium, and manganese relative to calcium was higher in nonproducers than in equol producers (P = 0.038) (Fig. 2H).
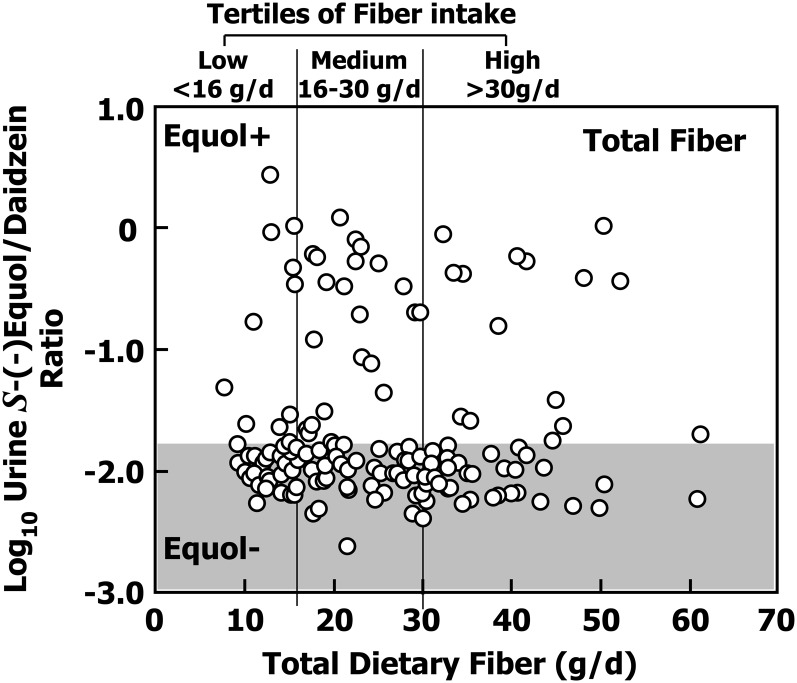
Fiber intake varied considerably among individuals (range, 7.7–61.4 g/d). When the participants were subdivided into 3 groups based on total dietary fiber intake being low (<16 g/d, n = 36), moderate (16–30 g/d, n = 71), or high (>30 g/d, n = 49), the frequency of equol producers was similar among the tertiles of fiber intake (28%, 30%, and 31%) (Fig. 3).
FIGURE 3.
The correlation between the urinary log10-transformed S-(-)equol:daidzein ratio and daily total fiber intakes for 156 healthy adults. Points falling in the shaded area were classified as non–equol producers (n = 109) and those in the unshaded area were classified as equol producers (n = 47). The vertical lines represent tertiles of total fiber intake: low (<16 g/d), medium (16–30 g/d), and high (>30 g/d). Equol+, equol producer; Equol−, non–equol producer.
Long-term stability in equol production
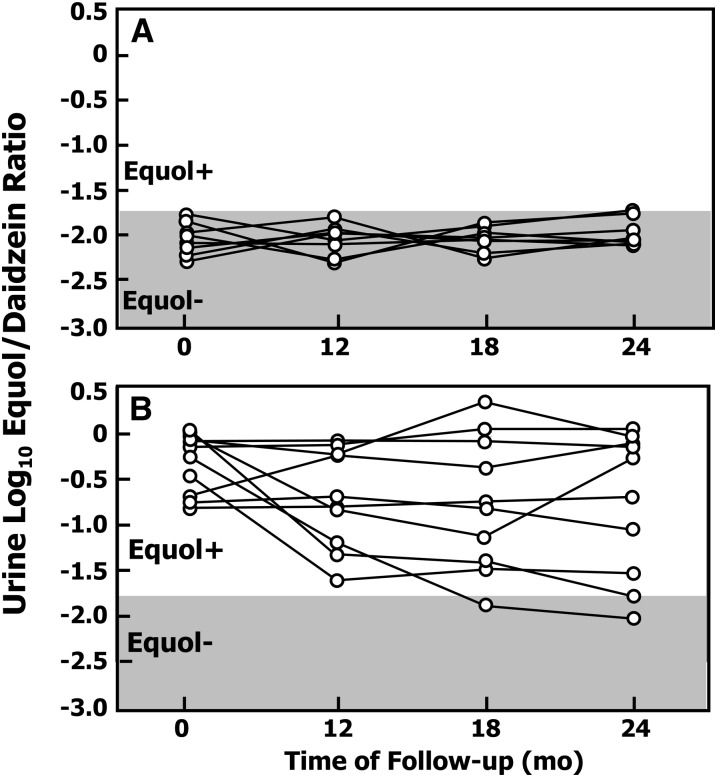
The log10-transformed urinary equol:daidzein ratio, determined in 20 healthy participants comprising 10 equol producers and 10 non–equol producers selected from the 159 adults enrolled in study 1 who were repeat tested for equol producer status after 12, 18, and 24 mo, is shown in Figure 4. In the 10 adults that were classified as non–equol producers, no significant change in the log10-transformed urinary equol:daidzein ratio was observed during the 2-y follow-up period and consequently, all 10 participants remained classified as non–equol producers for up to 2 y after the initial testing.
FIGURE 4.
Changes in the urinary log10 S-(-)equol:daidzein ratio for 10 defined equol producers (A) and 10 non–equol producers (B) that underwent testing for equol production at baseline and after 12, 18, and 24 mo. Equol+, equol producer; Equol−, non–equol producer.
When repeated-measures ANOVA was conducted on the 3-d diet records, no significant differences were seen in these non–equol producers over time in the mean intake of total energy (kcal), total fat, carbohydrates, or fiber when expressed as absolute values and as a percentage of energy. However, for total protein, a trend toward a decrease in protein consumption per day was observed (Table 2).
TABLE 2.
Changes in the dietary macro-nutrient composition of equol producers and non–equol producers monitored during a 2-y period in study 21
| Energy | Total fat | Carbohydrates | Protein | Total fiber | |
| kcal | g | g | g | g | |
| Equol producers | |||||
| Baseline | 1940 ± 150 | 76.7 ± 6.9 (34.0 ± 2.4) | 234.6 ± 19.8 (48.2 ± 3.0) | 82.1 ± 9.2 (16.4 ± 1.1) | 21.5 ± 2.3 |
| 12 mo | 2140 ± 175 | 81.6 ± 8.2 (33 ± 1.6) | 268.9 ± 21.4 (49.2 ± 1.1) | 89.3 ± 9.2 (16.7 ± 1.0) | 20.0 ± 2.9 |
| 18 mo | 1880 ± 144 | 76.1 ± 7.1 (34.9 ± 1.8) | 226.7 ± 20.3 (47.4 ± 2.4) | 79.5 ± 7.4 (17.3 ± 1.0) | 17.4 ± 2.5 |
| 24 mo | 2060 ± 174 | 80.3 ± 7.1 (34.1 ± 1.6) | 249.7 ± 25.0 (47.8 ± 1.8) | 81.2 ± 6.8 (16.2 ± 0.9) | 19.8 ± 2.6 |
| Non–equol producers | |||||
| Baseline | 2010 ± 170 | 76.7 ± 6.3 (34.1 ± 2.7) | 226.1 ± 31.3 (42.7 ± 3.9) | 99.1 ± 10.7 (20.5 ± 1.8) | 22.0 ± 3.2 |
| 12 mo | 1700 ± 217 | 65.1 ± 8.7 (33.8 ± 2.0) | 195.7 ± 25.1 (45.3 ± 2.5) | 84.0 ± 14.3 (19.1 ± 1.2) | 18.6 ± 3.4 |
| 18 mo | 1760 ± 177 | 67.1 ± 8.7 (33.2 ± 2.7) | 205.9 ± 27.7 (46.6 ± 3.6) | 69.4 ± 6.7 (16.1 ± 1.0) | 17.5 ± 2.1 |
| 24 mo | 1860 ± 200 | 68.8 ± 8.0 (33.2 ± 3.0) | 220.0 ± 28.3 (47.0 ± 3.5) | 76.7 ± 11.1 (16.4 ± 0.8) | 19.0 ± 2.9 |
Values are means ± SEs (percentage of total calories), n = 10 for equol producers and 10 for non–equol producers. Data were calculated from 3-d diet records completed at baseline and after 12, 18, and 24 mo while retesting for equol producer status.
Equol production was maintained for the next 2 y in 8 of the 10 defined equol producers when they were retested with a soy challenge (Fig. 4). However, in 2 of these adults, the log10-transformed urinary equol:daidzein ratio changed during the 2-y follow-up period, with these participants becoming non–equol producers. Analysis of their 3-d diet records during the 2-y period of follow-up showed one participant had a decrease in total energy and total fat intake and a significant decrease in mean PUFA intake (23.3 to 8.3 g/d). Furthermore, this participant had been prescribed multiple courses of antibiotics (amoxicillin) during the 2-y follow-up, which may have accounted for the abolition of equol production. The other participant showed no significant variation in dietary intake of nutrients during the 2-y follow-up period and also reported no antibiotic use. However, overall, the repeated-measures ANOVA for all participants showed no significant changes over time for equol producers.
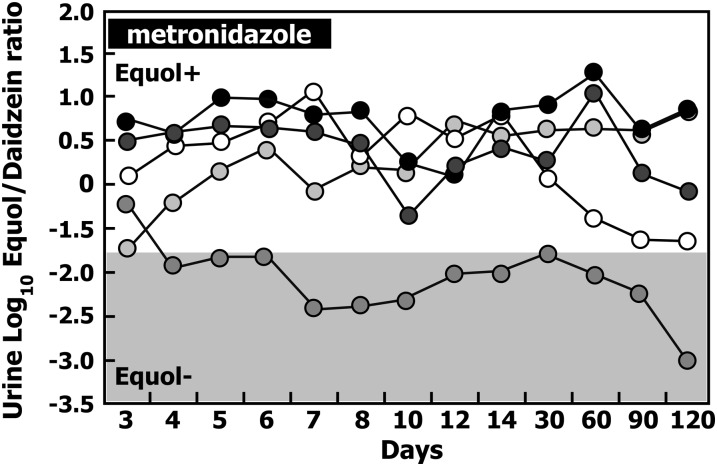
Effect of antibiotic administration on equol production
Oral administration of the antibiotic metronidazole had no effect on equol producer status in 4 of the 5 healthy adults classified as equol producers prior to administration, as confirmed from the log10-transformed urinary equol:daidzein ratio after repeated exposure to soy milk (Fig. 5). These 4 participants continued to test positive as equol producers on all 7 d of metronidazole administration and continued to be equol producers for an addition 4 mo, during which time each participant was retested at 30-d intervals. In one individual, metronidazole administration virtually abolished equol production, as evidenced by a change in the urine log10-transformed equol:daidzein ratio after a soy challenge and equol production was not restored during the next 4 mo when this participant was retested (Fig. 5).
FIGURE 5.
Changes in the urinary log10 S-(-)equol:daidzein ratio in 5 healthy adults that were equol producers measured at baseline and daily for 7 consecutive days, during which the antibiotic metronidazole (7.5 mg/kg body weight; three times a day) was administered and then again 12, 14, 30, 60, and 90 d after completion of the course of antibiotics. Equol producer status was measured after a standard soy challenge at each time point. Equol+, equol producer; Equol−, non–equol producer.
Discussion
This comprehensive diet study was an attempt to determine whether differences in the composition of the diet influence the propensity of an individual to produce the isoflavone metabolite S-(-)equol when consuming soy foods containing isoflavones. Unlike rodents and most other animal species that efficiently and exclusively convert daidzin:daidzein into S-(-)equol (17, 25–27), the frequency of equol producers in human adults is lower and more variable. In this study of adults living in the United States and Australia who were given a soy challenge (6), the frequency of equol producers was similar between these geographical locations, with a mean overall frequency of 29.6% testing positive for equol production. This finding is in agreement with previous reports for adults living in Western countries (6, 9–11, 28) and considerably lower than the reported frequency for Asian adults living in Asian countries (12–16).
The clinical relevance of this conversion is evident by the fact that S-(-)equol is a biologically more potent molecule than its precursor and for this reason, inter-individual variability in the conversion of daidzein to S-(-)equol may in part explain some of the variability in clinical responses to soy-containing diets (17). In support of this concept, a gene expression study reported striking differences in expression of a number of estrogen-responsive genes in postmenopausal women who were equol producers compared with those that were non–equol producers after chronic administration of a soy isoflavone supplement (29). S-(-)equol possesses unique properties. It preferentially binds to ERβ (8, 30, 31) and therefore can be considered to have selective estrogen receptor modulator-like properties, but it also antagonizes the action of the potent androgen dihydrotestosterone (32). In principle, therefore, it should play a role in numerous hormone-dependent diseases and conditions, as was originally proposed following its identification in human urine almost 30 y ago (9).
The large disparity in the frequency of equol producers between adults living in Asia and Western countries remains a mystery, but dietary differences are one potential explanation. Many studies have reported associations between equol producer status and a number of dietary components and nutrients, including fat and carbohydrate composition (11, 21, 23), PUFAs (22), dairy intake (33), lactose (34), green tea consumption (35), seaweed (36), and soy food intake (13, 15, 33), but no clear conclusions can be made. Prolonged soy food consumption appears not to be a factor driving equol formation (37), but clearly intake of foods containing the substrate daidzein/daidzein, such as soy foods, is essential in conjunction with the presence of appropriate bacteria (1, 38) in the intestinal tract. Indeed, the accurate assessment of an individual for equol producer status requires a sustained exposure to isoflavone-containing foods, because relying on endogenous isoflavone intakes in Westerners, who consume very little soy food (39–44) or Asians who may or may not have had recent intake of soy, is unreliable in assigning equol producer status (6). For this reason, we challenged all adults for 3.5 d with soymilk to achieve a steady-state isoflavone concentration before analyzing urine for equol and daidzein (6). This standard approach has been used by others and the practical limitations are discussed elsewhere (6, 10, 45).
It is conceivable that all adults possess the specific bacteria for S-(-)equol formation in their gastrointestinal tract but that in nonproducers, the intraluminal conditions may not be optimal for the multiple enzymatic reactions to occur. Diet can influence the microflora and the intraluminal milieu (46, 47). More than a decade ago, it was reported that equol production was associated with a higher intake of daily calories as carbohydrates and lower proportion as saturated fat (11, 21), an observation supporting in vitro studies of cultured human fecal flora showing a high proportion of nonstarch polysaccharides, which increased the fermentation rate, resulting in the rapid conversion of daidzein to equol (13). Furthermore, increased hydrogen production, butyrate, and propionate have all been associated with more efficient formation of equol (48, 49). However, a more recent diet study did not corroborate the association between equol production and total carbohydrate intake (23). In our study, dietary analysis was based on a 3-d diet record completed by each participant in the period of testing for equol producer status rather than relying on diet history questionnaires, also called FFQs, as has been done in numerous previous studies. Interestingly, in those adults (n = 20) that were prospectively followed and repeat tested 12, 18, and 24 mo later, analysis of their 3-d diet records revealed the intake of macronutrients to be relatively constant during this 2-y period (Table 2).
In the data reported here, we did not find any significant differences between equol producers and nonproducers in the intake of total carbohydrates expressed as a percentage of total calories and this was also the case for other macronutrients, including total protein, total fat, saturated fat, and total fiber (Table 1). Because fiber potentially could influence equol production by altering intestinal fermentation, we analyzed the data according to tertiles of fiber intake [low (>16 g/d), medium (16–30 g/d), and high (>30 g/d)] (Fig. 3). Equol production appeared not to be influenced by fiber intake. This finding is consistent with that previously reported by Lampe et al. (20) where no association between equol producer status and fiber intake was observed and furthermore, the addition of 16 g/d of fiber in the form of wheat bran did not alter equol production in women. It also does not support the findings from a report of an Italian cohort that suggested equol producers were associated with a diet of less fiber, vegetables, and cereals, although this study used diet histories to calculate intakes of nutrients (23) rather than 3-d diet records that we used.
A more detailed analysis of our data for the intake of 143 nutrients using PCA revealed several significant differences in the intake of a number of nutrients when comparing equol producers and nonproducers (Fig. 2). PCA spotlighted a higher intake of PUFAs, maltose, vitamins A and E, and calcium as playing a particularly important role in differentiating the 2 groups. Although total carbohydrate intake showed no association with equol production, it was found that equol producers had a significantly higher intake of maltose. Whether such a subtle difference in carbohydrate composition could influence the activity of the bacterial enzymes is unclear. At least one strain of bacteria linked to S-(-)equol production shows growth in the presence of maltose (50) and it may be possible that subtle differences in the carbohydrate composition of the diet, as observed in this study, could contribute to S-(-)equol production. A higher PUFA intake was previously implicated as being associated with equol producers (22), but to our knowledge, this is the first time micronutrient differences have been noted between the groups. It was suggested that the higher intake of PUFA in Japanese and Korean diets could explain the higher frequency of equol producers (50–70%) in these countries (22). It remains to be seen whether increasing PUFA intake by supplementation could facilitate equol production and such a study is warranted based on these findings. The abundance of vitamins A and E in the diet of equol producers may represent unique metabolic needs of the bacteria associated with equol production. Bacteria responsible for conversion of daidzein to equol have been identified in only the last 10 y and, intriguingly, these are not the most abundant bacterial species that colonize the human gastrointestinal tract [reviewed in (1)]. Although historically, bacteria were considered able to synthesize all the macromolecules they needed, as early as 1975 vitamins essential to bacterial metabolism were recognized (51). More recently, instances of complex medium and vitamin supplementation have been reported as being essential to bacterial function (52–54). Whether vitamins A and E reflect the needs of bacteria responsible for the metabolism of daidzein is not known; however, it is a distinct possibility. Alternatively, these components may alter the intestinal milieu in a manner that enhances colonization of the bacteria essential for conversions of daidzein to S-(-)equol.
The longitudinal follow-up of 20 adults that were repeat tested on 3 occasions during a 2-y period showed that all the nonproducers and 8 of the 10 equol producers maintained this status during this period. Repeat testing of these participants further highlights the stability of equol producers (Fig. 4). Collectively, these data support the previously held belief that equol producer status is a relatively stable phenomenon (6, 17, 20, 55) despite a recent study proposing that this may not always be the case for pre- and postmenopausal women (45, 56). In our study 2, careful monitoring of antibiotic use during this period confirmed that 1 of the 2 equol producers who became a nonproducer had been administered a course of antibiotics. It was not possible to ascertain the reason equol production was abolished in the other participant, but it appeared unrelated to dietary changes during this 2-y period (Table 2).
Because bacteria are responsible for the formation of S-(-)equol, certain antibiotics might be expected to abolish S-(-)equol production. In a study of cynomologous monkeys, kanamycin, doxycyclin, vancomycin, and metronidazole all significantly reduced plasma equol concentrations in this species (57). In vitro studies of cultured human fecal flora have shown inter-individual variability in responses to antibiotic administration in equol production (58). Some strains of equol-producing bacteria have shown antibiotic resistance (50). In a small subset of our participants (study 3) who were administered the commonly prescribed antibiotic metronidazole and followed for 4 mo, metronidazole abolished S-(-)equol production in only 1 of 5 participants and it was not restored during the 120 d following administration of the first dose of antibiotic (Fig. 5). These data indicate that the effect of this antibiotic can be variable among individuals, which corroborates a similar observation from Franke et al. (45, 56) showing inconsistent effects of antibiotic use on equol production in pre- and postmenopausal women. Furthermore, once equol production is abolished by antibiotic use, it appears to take a considerable time to restore its production, which is presumed to reflect the time it takes for recolonization of the microflora. This was also the case for the production of the mammalian lignans, enterolactone and enterodiol, which are formed by intestinal bacteria from the dietary flaxseed lignan, secoisolariciresinol, by analogous chemical reactions (59).
In summary, this comprehensive analysis of the equol producer status and its association with diet of a large cohort of adults living in 2 different countries again confirmed the frequency of equol producers to be ~30%, which is consistently lower than that reported for adults living in Asian countries, where soy foods are commonly consumed. No major differences in the total macronutrient composition of the diet were associated with equol producer status, but differences in the intake of some micronutrients were observed. Specifically, equol production appeared to be associated with a greater intake of PUFA, maltose, and vitamins A and E. Therefore, future strategies to stimulate equol production by supplementation of PUFAs or vitamins A and E might be worth considering. Previously used strategies to stimulate equol production have included the administration of probiotics or prebiotics, but these have been largely unsuccessful (20, 60–63). There is evidence to suggest that foods higher in isoflavone aglycons, rather than glycosides, may facilitate S-(-)equol production (1, 64, 65). Although the overriding determinant of equol production is the requirement of the intestinal bacteria capable of catalyzing a series of reactions to convert daidzein to S-(-)equol, it appears that subtle differences in the micronutrient composition of the diet may be important as cofactors in stimulating bacterial activity. Our studies also indicate that equol production is a relatively stable phenomenon in most individuals and that the response to antibiotic treatment may be variable among individuals, which, when abolished, may be difficult to restore.
Supplementary Material
Acknowledgments
The authors thank Linda Zimmer-Nechemias, Xueheng Zhao, and Brian Wolfe for their technical support. K.D.R.S. and N.M.B. designed research; N.M.B., S.C., and T.G. conducted research; S.S. and T.G. provided food databases and analyzed diet data; E.C.K. analyzed data; B.H. and J.E.H. were the physicians supervising patients in Australia and the United States, respectively; N.M.B. and K.D.R.S. wrote the manuscript; and K.D.R.S. has primary responsibility for the final content. All authors read and approved the final manuscript.
Literature Cited
- 1.Setchell KDR, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:S1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setchell KDR, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:S1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, Itoh T, Shimomura Y, Ueno T. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem Toxicol. 2008;46:2713–20. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KDR, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr. 2009;139:2037–43. [DOI] [PubMed] [Google Scholar]
- 5.Schwen RJ, Nguyen L, Plomley JB, Jackson RL. Toxicokinetics and lack of uterotropic effect of orally administered S-equol. Food Chem Toxicol. 2012;50:1741–8. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136:2188–93. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–7. [DOI] [PubMed] [Google Scholar]
- 8.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Zimmer-Nechemias L, Brown NM, Lund TD, et al. S-(-)equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. [DOI] [PubMed] [Google Scholar]
- 9.Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–78. [DOI] [PubMed] [Google Scholar]
- 10.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–9. [DOI] [PubMed] [Google Scholar]
- 11.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, Mazur W, Wähälä K, Adlercreutz H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J Nutr. 1998;128:1710–5. [DOI] [PubMed] [Google Scholar]
- 13.Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10:127–35. [DOI] [PubMed] [Google Scholar]
- 14.Akaza H, Miyanaga N, Takahima N, Naito S, Hirao Y, Tsukamoto T, Fujioka T, Mori M, Kim W-J, Song JM, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–9. [DOI] [PubMed] [Google Scholar]
- 15.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wähälä K, Thomas WK, Lampe JW. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136:1347–51. [DOI] [PubMed] [Google Scholar]
- 16.Hong KW, Ko KP, Ahn Y, Kim CS, Park SJ, Park JK, Kim SS, Kim Y. Epidemiological profiles between equol producers and nonproducers: a genomewide association study of the equol-producing phenotype. Genes Nutr. 2012;7:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 18.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:S1664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr Rev. 2011;69:432–48. [DOI] [PubMed] [Google Scholar]
- 20.Lampe JW, Skor HE, Li S, Wähälä K, Howald WN, Chen C. Wheat bran and soy protein feeding do not alter urinary excretion of the isoflavone equol in premenopausal women. J Nutr. 2001;131:740–4. [DOI] [PubMed] [Google Scholar]
- 21.Lydeking-Olsen E, Beck-Jensen JE, Setchell KDR, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss: a 2 year randomized, placebo-controlled trial. Eur J Nutr. 2004;43:246–57. [DOI] [PubMed] [Google Scholar]
- 22.Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, Verbruggen M, Depypere H, De Keukeleire K, Bracke M, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137:2242–6. [DOI] [PubMed] [Google Scholar]
- 23.Gardana C, Canzi E, Simonetti P. The role of diet in the metabolism of daidzein by human faecal microbiota sampled from Italian volunteers. J Nutr Biochem. 2009;20:940–7. [DOI] [PubMed] [Google Scholar]
- 24.Hanna KL, O'Neill S, Lyons-Wall PM. Intake of isoflavone and lignan phytoestrogens and associated demographic and lifestyle factors in older Australian women. Asia Pac J Clin Nutr. 2010;19:540–9. [PubMed] [Google Scholar]
- 25.Lundh T. Metabolism of estrogenic isoflavones in domestic animals. Proc Soc Exp Biol Med. 1995;208:33–9. [DOI] [PubMed] [Google Scholar]
- 26.Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136:1215–21. [DOI] [PubMed] [Google Scholar]
- 27.Setchell KDR, Brown NM, Zhao X, Lindley SL, Heubi JE, King EC, Messina M. Soy isoflavone phase II metabolism differs between rodents and humans: implications for the effect on breast cancer risk. Am J Clin Nutr. 2011;94:1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood). 2005;230:155–70. [DOI] [PubMed] [Google Scholar]
- 29.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem. 2007;18:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–6. [DOI] [PubMed] [Google Scholar]
- 31.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67. [DOI] [PubMed] [Google Scholar]
- 32.Lund TD, Munson DJ, Haldy ME, Setchell KDR, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95. [DOI] [PubMed] [Google Scholar]
- 33.Nagata C, Ueno T, Uchiyama S, Nagao Y, Yamamoto S, Shibuya C, Kashiki Y, Shimizu H. Dietary and lifestyle correlates of urinary excretion status of equol in Japanese women. Nutr Cancer. 2008;60:49–54. [DOI] [PubMed] [Google Scholar]
- 34.Tamura A, Shiomi T, Hachiya S, Shigematsu N, Hara H. Low activities of intestinal lactase suppress the early phase absorption of soy isoflavones in Japanese adults. Clin Nutr. 2008;27:248–53. [DOI] [PubMed] [Google Scholar]
- 35.Miyanaga N, Akaza H, Takashima N, Nagata Y, Sonoda T, Mori M, Naito S, Hirao Y, Tsukamoto T, Fujioka T. Higher consumption of green tea may enhance equol production. Asian Pac J Cancer Prev. 2003;4:297–301. [PubMed] [Google Scholar]
- 36.Teas J, Hurley TG, Hebert JR, Franke AA, Sepkovic DW, Kurzer MS. Dietary seaweed modifies estrogen and phytoestrogen metabolism in healthy postmenopausal women. J Nutr. 2009;139:939–44. [DOI] [PubMed] [Google Scholar]
- 37.Védrine N, Mathey J, Morand C, Brandolini M, Davicco MJ, Guy L, Remesy C, Coxan V, Manach C. One-month exposure to soy isoflavones did not induce the ability to produce equol in postmenopausal women. Eur J Clin Nutr. 2006;l60:1039–45. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama Y, Masumori N, Fukuta F, Yoneta A, Hida T, Yamashita T, Minatoya M, Nagata Y, Mori M, Tsuji H, et al. Influence of isoflavone intake and equol-producing intestinal flora on prostate cancer risk. Asian Pac J Cancer Prev. 2013;14:1–4. [DOI] [PubMed] [Google Scholar]
- 39.Messina M, Nagata C, Wu AH. Estimated asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. [DOI] [PubMed] [Google Scholar]
- 40.Valentín-Blasini L, Sadowski MA, Walden D, Caltabiano L, Needham LL, Barr DB. Urinary phytoestrogen concentrations in the U.S. population (1999–2000). J Expo Anal Environ Epidemiol. 2005;15:509–23. [DOI] [PubMed] [Google Scholar]
- 41.Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, Olsen A, Clavel-Chapelon F, Touillaud M, Boutron-Ruau MC, et al. Variations in plasma phytoestrogen concentrations in European adults. J Nutr. 2007;137:1294–300. [DOI] [PubMed] [Google Scholar]
- 42.Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res. 2008;10:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, Johnsen NF, Olsen A, Kaaks R, Linseisen J, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009;100:1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, Nomura AM, Henderson BE, Kolonel LN. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev Res (Phila). 2009;2:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G. Equol production changes over time in pre-menopausal women. Brit J Nutr. 2012;107:1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Floch MH. Intestinal microecology in health and wellness. J Clin Gastroenterol. 2011;45 Suppl:S108–10. [DOI] [PubMed] [Google Scholar]
- 48.Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol. 2005;183:45–55. [DOI] [PubMed] [Google Scholar]
- 49.Minamida K, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J Biosci Bioeng. 2006;102:247–50. [DOI] [PubMed] [Google Scholar]
- 50.Fortina MG, Ricci G, Foschino R, Picozzi C, Dolci P, Zeppa G, Cocolin L, Manachini PL. Phenotypic typing, technological properties and safety aspects of Lactococcus garvieae strains from dairy environments. J Appl Microbiol. 2007;103:445–53. [DOI] [PubMed] [Google Scholar]
- 51.Reissbrodt R, Kemmer G, Seltmann G. Abhangigkeit der Ausbildung bestimmter Antigene von Proteus mirabilis Stamm 1095/67 beim Wachstrum auf Minimalnahrmedien. Z Allg Mikrobiol. 1975;15:357–70. [PubMed] [Google Scholar]
- 52.Lopez RL, Garcia MT, Abriouel H, Ben Omar N, Grande MJ, Martinez-Canamero M, Galvez A. Semi-preparative scale purification of enterococcal bacteriocin enterocin EJ97, and evaluation of substrates for its production. J Ind Microbiol Biotechnol. 2007;34:779–85. [DOI] [PubMed] [Google Scholar]
- 53.Masurekar PS. Nutritional and engineering aspects of microbial process development. Prog Drug Res. 2008;65:291, 3–328. [DOI] [PubMed] [Google Scholar]
- 54.Terrade N, Mira de Orduna R. Determination of the essential nutrient requirements of wine-related bacteria from the genera Oenococcus and Lactobacillus. Int J Food Microbiol. 2009;133:8–13. [DOI] [PubMed] [Google Scholar]
- 55.Frankenfeld CL, Atkinson C, Thomas WK, Goode EL, Gonzalez A, Jokela T, Wähälä K, Schwartz SM, Li SS, Lampe JW. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp Biol Med (Maywood). 2004;229:902–13. [DOI] [PubMed] [Google Scholar]
- 56.Franke AA, Lai JF, Halm BM, Pagano I, Kono N, Mack WJ, Hodis HN. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2012;23:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blair RM, Appt SE, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J Nutr. 2003;133:2262–7. [DOI] [PubMed] [Google Scholar]
- 58.Atkinson C, Berman S, Humbert O, Lampe JW. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J Nutr. 2004;134:596–9. [DOI] [PubMed] [Google Scholar]
- 59.Setchell KDR, Lawson AM, Borriello SP, Harkness R, Gordon H, Morgan DM, Kirk DN, Adlercreutz H, Anderson LC, Axelson M. Lignan formation in man–microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7. [DOI] [PubMed] [Google Scholar]
- 60.Uehara M, Ohta A, Sakai K, Suzuki K, Watanabe S, Adlercreutz H. Dietary fructooligosaccharides modify intestinal bioavailability of a single dose of genistein and daidzein and affect their urinary excretion and kinetics in blood of rats. J Nutr. 2001;131:787–95. [DOI] [PubMed]
- 61. Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr. 2004;134:1998–2003. PubMed. [DOI] [PubMed]
- 62.Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Probiotic consumption does not enhance the cholesterol-lowering effect of soy in postmenopausal women. J Nutr. 2004;134:3277–83. [DOI] [PubMed] [Google Scholar]
- 63.Bonorden MJ, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, Kurzer MS. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur J Clin Nutr. 2004;58:1635–42. [DOI] [PubMed] [Google Scholar]
- 64.Clerici C, Setchell KDR, Battezzati PM, Pirro M, Giuliano V, Asciutti S, Castellani D, Nardi E, Sabatino G, Orlandi S, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007;137:2270–8. [DOI] [PubMed] [Google Scholar]
- 65.Clerici C, Nardi E, Battezzati PM, Asciutti S, Castellani D, Corazzi N, Giuliano V, Gizzi S, Perriello G, Di Matteo G, et al. Novel soy germ pasta improves endothelial function, blood pressure, and oxidative stress in patients with type 2 diabetes. Diabetes Care. 2011;34:1946–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.