Abstract
Peripheral nerve lesions caused sensory and motor deficits along the distribution of the injured nerve. Numerous researches have been carried out to enhance and/or accelerate the recovery of such lesions. The objective of this study was to assess the functional recovery of sciatic nerve in rats subjected to different fluences of low-level laser therapy (LLLT). Thirty-six animals were randomly divided into four groups: one consisting of sham rats and three others irradiated with progressive fluencies of 10 J/cm2, 40 J/cm2 and 80 J/cm2 of laser AsGaAl (830 nm) for 21 consecutive days. They were evaluated by the Sciatic Functional Index (SFI) method. The crush injury was performed by using a portable device with dead weight of 5,000 g whose load was applied for 10 min. A digital camera was used to record the footprints left on the acrylic track, before surgery and after, on the 7th, 14th, and 21st days. The results also showed that on the 7th day, there was a difference between the groups irradiated with 40 J/cm2, when compared with the sham group (p < 0.05). On the 14th day the groups irradiated with 40 J/cm2 and 80 J/cm2 also presented better results when compared with sham, however, on the 21st day, no inter-group difference was found (p > 0.05). It was possible to observe that the LLLT at fluency of 40 J/cm2 and 80 J/cm2 had a positive influence on the acceleration of the functional nerve recovery.
Keywords: Sciatic nerve, Nerve regeneration, Nerve crush, Low-level laser therapy
Introduction
Peripheral nervous injuries have high incidence among traumatic lesions resulting in nerve crush or transection, causing significant functional disability with life-long consequences [1]. These injuries were classified by Seddon into three types, neuropraxis, axonotmesis and neurotmesis [2, 3] and also by Sunderland [4], who subdivided them into 5 levels according to the structures involved.
Several studies have been conducted to determine the types of stimuli and parameters that can accelerate regeneration and functional recovery of the peripheral nerves, aiming to minimise further dysfunctions. Nowadays, the most studied physiotherapeutic resources [5–15] with the objective to analyse the process of nerve regeneration and functional recovery are the electric stimulation [16], ultrasound therapy [17] and the low-level laser therapy (LLLT)
LLLT has demonstrated positive effects when related to the regeneration of injured peripheral nerves [5, 8, 9, 18, 19]. On the other hand, Bagis et al. [20] observed no beneficial effects of LLLT on nerve lesions. This unsuccessful result could be related with the brief experiment time and with the short laser pulse emission. Several parameters such as: wavelength, energy density, pulse mode, and laser potency, should be considered for peripheral nerves regeneration using to LLLT. Regarding the selection of the best biostimulation dose, an analysis of the previous experimental studies was made and it showed that laser treatment on nerves could exert detectable effects at quite different doses [21, 22]; also, the existing literature does not allow any definitive conclusion about it. Thus, in light of the promising results, future studies should be designed to find the best treatment protocol, with special attention to the selection of the stimulation dose [23]. Gigo-Benato et al. [24] showed that the technique of point contact application and continuous beam is more efficient for treatment of peripheral nerve lesions. In fact, researches on peripheral nerve injuries presented better results for longer periods of irradiation, reinforcing the fact that the evaluation of the first week showed poor functional results [7, 18, 19].
Based on this, the objective of this study was to assess the functional recovery of sciatic nerve in rats submitted to different energy density of LLLT, following the sciatic nerve crushing.
Materials and Methods
The experimental study was approved by the Ethics Committee on Experimental Use of Animals of Ribeirão Preto School of Medicine, University of São Paulo, Brazil.
Animals
Thirty-six Wistar adult, male, rats (Rattus norvergicus: var. albinus, Rodentia, Mammalia) aged 3 months old, weighting 280–310 g were obtained from the Bioterium Central of Ribeirão Preto’s School of Medicine, University of São Paulo, Brazil. They were maintained in collective cages with four animals each and were fed with commercial rations and water ad libitum. The rats were weighed and randomly assigned into four groups of nine animals each. All animals were submitted to the same surgical procedure. Group 1 – simulation of laser irradiation (sham); Group 2 – laser irradiation with 10 J/cm2 fluency Group 3 - irradiation with 40 J/cm2 fluency and Group 4 - irradiation with 80 J/cm2 fluency.
Operative Procedure
All the animals were anesthetized with an intraperitoneal injection of 1:4 combination with 5 % of Ketamine (0.1 ml/100 g body weight) and 2 % of Xylazine (0.07 ml/ 100 g body weight). The right sciatic nerve was exposed through a 3 cm long posterolateral longitudinal incision of the thigh followed by blunt dissection between the gluteus maximus and quadriceps muscles. The crush injury was carried with a portable device being characterized by making the crush process easier and more reliable, regarding the load used [25, 26]. The 5.000 g load was applied for 10 min, causing a severe crush injury circumscribed to a 5 mm intermediate long segment and a 5 mm proximal to the trifurcation of the nerve sciatic. At the end of the programmed time, the nerve was carefully removed from the portable device and repositioned to its original place. The wound was closed with a non-absorbable suture and the animal was allowed to recover from anaesthesia. The surgical procedure was identical for all animals.
Low-Level Laser Irradiation
A portable Aluminium Gallium Arsenide (AlGaAs) Laser Diode (Ibramed®) was used on this study, with wavelengths of 830 nm, 30 mW power and continuous wave beam with a spot size of 0.116 cm2, in fluencies of 10 J/cm2 with energy (E) of 1.16 J and exposure time of 38.66 s, 40 J/cm2 (E = 4.64 J and exposure time of 154.66 s) and 80 J/cm2 (E = 9.28 J and exposure time of 309.33 s).
For the laser radiation transmission analysis, a digital power analyzer, model LaserCheck (Coherent, Staunton, VA) was used.
Laser irradiation was performed in all experimental groups focusing on the area of damaged nerve, which had been pre-determined surgically. A laser pen was positioned at a 90o angle in relation to the skin according the contact point technique, immediately after surgery and in the 21 subsequent days.
Monte-Raso et al. [27] have evaluated this method as being quantitative, reliable, and reproducible under the functional condition of the sciatic nerve in rats.
Footprint Recording and Sciatic Functional Index (SFI) Analysis
A digital video camera (Sony® Handycam, model DVD 203) recorded the images of the animal’s footprint through an acrylic static runway [28]. Footprints were analyzed before and after the surgery on the 7th, 14th and 21st days.
Footprints images obtained in the recording process were appropriate in the right size using the Adobe Photoshop® CS3 software version and edited to change to the ideal size for the analysis program use allowing the calculation of the Sciatic Functional Index (SFI). These images were entered into a computer program, which allows the identification and analysis of them according the parameters previously selected and data storage with the help of graphical analysis program specially developed for this purpose [17, 27].
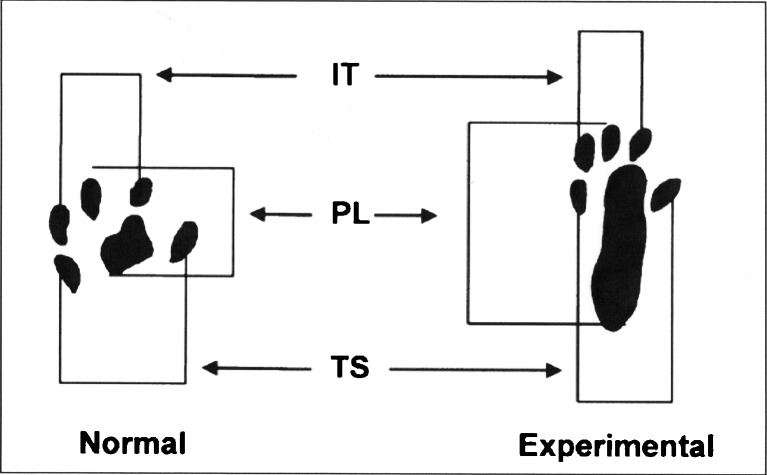
Once established the footprints on the program, the parameters were measured by simply clicking the mouse cursor on the points corresponding to each parameter, as pre-determined. The measured parameters were the length of the footprint (PL, or print length), the full opening of the fingers, from 1 to 5 (TS or total spread of toes) and the opening of the middle fingers, the 2nd to 4th (IT or intermediate toes) [29, 30]. As suggested by Bain et al., [31] once registered the parameters; the program automatically calculates the value of the SFI, which also automatically store on file in order to enable analysis of the curves as a function of regeneration of the time.
The SFI is a negative indicator of the degree of the nerve dysfunction and varies from zero to −100, with zero corresponding to normal function and −100 indicating a complete dysfunction of the studied segment (Fig. 1).
Fig. 1.
Footprint image with Sciatic Functional Index parameters delimitation (PL-print length, TS-total spread, IT-intermediate toes)
Records of the footprints obtained before and after surgery (on days 7, 14, and 21) were used for functional analysis of gait and totaled 144 footprints. The images were analyzed according to the SFI proposed by Bain et al. [31], and the following formula was employed to calculate the SFI through the program:
Formula:
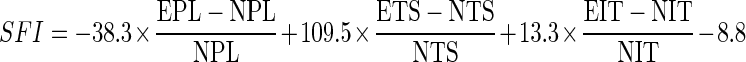 |
Were:
- SFI
Sciatic Functional Index
- N
Stands for Normal
- TS
Stands for the total Spread
- E
Stands for Experimental
- PL
Standsfor the Print Length
- IT
Stands for Intermediate Toes
Statistical Analysis
The analysis of variance (ANOVA) was proposed for the data evaluation obtained in this study, with repeated measures for two factors (group × period), although the assumption of normality was an accepted waste. Post hoc test – orthogonal contrasts. In this study, a significance level of 5 % was set [32–34].
Results
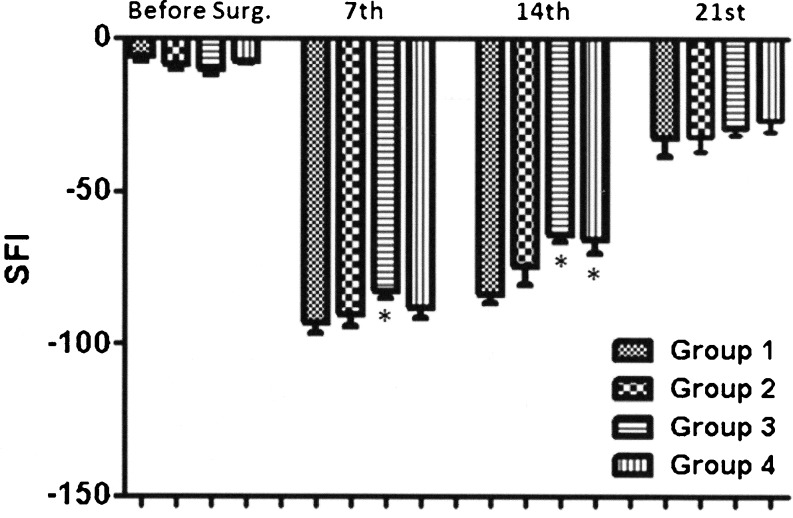
The mean values and the standard deviation of SFI obtained in each group along with the periods analyzed are described in Fig. 2.
Fig. 2.
Mean values for the Sciatic Functional Index (SFI) before and after 7th, 14th and 21st postoperative days in the four groups: Sham, Laser 10 J/cm2, Laser 40 J/cm2 and Laser 80 J/cm2. (n = 9). *p < 0,05 when compared with the sham group
The results obtained with the SFI average value and standard deviation of the 4 groups were: pre-operative, group 1 (−6,10 ± 3,82), group 2 (−8,89 ± 4,03), group 3 (−10,42 ± 4,19) and group 4 (−7,36 ± 2,24); on the 7th postoperative (PO), group 1 (−92,94 ± 10,59), group 2 (−90,50 ± 11,65), group 3 (−82,85 ± 6,15) and group 4 (−88,19 ± 10,41); on the 14th PO, group 1 (−83,70 ± 8,04), group 2 (−74,43 ± 19,14), group 3 (−64,36 ± 6,60) and group 4 (−65,79 ± 12,94) e on the 21st PO, group 1 (−32,58 ± 18,63), group 2 (−31,89 ± 15,03), group 3 (−29,38 ± 5,86) and group 4 (−26,63 ± 10,89).
The data obtained in the seventh postoperative day only showed a statistically significant difference when compared with the sham group irradiated with the 40 J/cm2 (p < 0.04).
On 14th postoperative day a statistical difference between the sham group and the groups irradiated with 40 J/cm2 and 80 J/cm2 (p < 0.01) appeared.
When the comparison was made between the groups submitted to LLLT a statistically significant difference between the irradiated group with 10 J/cm2 compared to the irradiated group with 40 J/cm 2 (p <0.04) was found. However, in the 21st postoperative day there were no differences between groups.
Discussion
Wistar rats were chosen for this study because they are easy to obtain and to handle, have a low cost and their morphology, physiology, and peripheral nerve regeneration is similar to human beings [7, 15, 17, 25, 26, 29]. This LLLT is a therapeutic resource that has been largely used to stimulate regeneration and to accelerate functional recovery of the peripheral nerve [5, 7, 11, 15, 18–20, 24, 35, 36].
The aim of this study was to compare three different laser fluencies (10 J/cm2, 40 J/cm2 and 80 J/cm2) at a pre-established wavelength of 830 nm. Rochkind et al. [37] used a laser fluency of 10 J/cm2 with a 632.8 nm wavelength, whereas Gigo-Benato et al. [23] used 40 J/cm2 and 904 nm respectively. The fluency of 80 J/cm2 was added in the present study to allow comparisons with other studies that suggested the use of higher laser fluencies for the treatment of peripheral nerve injuries, as demonstrated by Rochkind et al. [6].
In the present study, SFI was analysed up to the 21st day in order to observe differences between the groups over time. This study corroborated Monte-Raso et al. [24] and Endo et al. [7] therefore; there is no need for longer studies. In fact, on the 21st day, the animals were close to normal, taking into account the rapid rat’s sciatic nerve regeneration. Based on the results obtained during this experimental period, it was observed that the functional recovery was stimulated by the use of LLLT. Oliveira et al. [38] reported a high correlation between functional recovery and morphological/morphometric regeneration of the injured peripheral nervous tissue. Indeed, some peripheral nerve injuries researches demonstrated that longer radiation periods provides better results, reinforcing the poor functional results obtained in the first week [7, 18, 19]. These data corroborated functional results observed in the present study on the 7th day after surgery, where no differences were observed among groups irradiated with a fluency of 10 J/cm2 and 80 J/cm2, when compared with the control group.
The results of this study confirmed the accelerated effect of phototherapy in functional recovery on the 14th postoperative day, supporting the current literature [18, 19]. Fluencies of 40 J/cm2 and 80 J/cm2 showed differences (p < 0.05) in functional recovery compared with sham group in this period. The fact that the fluency of 40 J/cm2 has also presented satisfactory results with 7 days may be related to the neovascularisation, according to Salate et al. [39] in 40 J/cm2 fluency o with 7 days of treatment, as well as a partial existence of an inflammatory process on that period
These results seem to characterize a dose dependent response to reflect changes in the repair process, claimed by Longo and Master [40] and Basford [41], where LLLT can stimulate or inhibit healing, depending on the parameters used. Accordingly, there is need for further studies to clarify the morphological and physiological mechanisms that justify such actions.
Anders et al. [42] and Rochkind [43] have described the laser mechanisms involved on nerve regeneration: immediate protective effect and increase in functional activity, longer maintenance of functional activity in nerve lesion, influence of healing tissue formation on the lesion area, prevention or decrease of degeneration in the motor neuron corresponding to spinal cord, and influence of both axonal growth and myelin sheath.
Several studies report the effects of irradiation on the action of LLLT in nerve tissues; however, the mechanisms are not well understood. There are some suggestions as increasing newly formed blood vessels [35], increasing the number of axons [11, 35], positive response in the sprouting stage in nerve regeneration [36] or increasing the number of myelinated axons [14]. Endo et al. [7] relates to functional improvement of rats after sciatic nerve crush, according to the effects described above.
By comparing the SFI results and using the analysis of variance (ANOVA) with repeated measures, laser therapy with fluencies of 40 J/cm2 (E = 4.64 J) and 80 J/cm2 (E = 9.28 J) was found to be more efficient compared with the one using a fluency of 10 J/cm2 (E = 1.16 J). The non-satisfactory results of the 10 J/cm2 group can be explained by the fact that this amount of energy was not recommended by the World Association for Laser Therapy (WALT) for peripheral nerve compression, such as the carpal tunnel syndrome (E = 6 J each). The fluency of 10 J/cm2 was used to mimic the clinical practice of physiotherapy in cases where the tissue repair was needed.
There is not a consensus about the optimal dose to treat peripheral nerve injuries. This research in rats, which were submitted to higher energy (Group 3 to 4.64 J and Group 4 to 9.28 J) can observe an acceleration in functional recovery. However, the group that used 10 J/cm2 (E - 1,16 J) did not observe a positive effect on accelerating functional with LLLT (830 nm).
Enwemeka [44] reported, in a recent literature review, that about 30 % of the manuscripts related to low-intensity laser have a lack of relevant information about the dose and energy used (J/cm2 or J) or inform imprecise data. The author suggested that dose-related mistakes could be associated with common errors found in clinical findings. The same author also pointed out the existence of a great number of research studies on low-intensity laser, but without any standardization of the parameters used. He also stated that the lack of reliable data made it difficult to compare the results and to understand some of the mechanisms involved.
Another aspect to be considered is the experimental procedure for the peripheral nerve injury. Literature shows some methods which are applied to crush the nerve tissue experimentally by using the universal testing machine [16, 38] or jeweller’s forceps [45], which need constant adjustment of the load being applied. Mazzer et al. [25] reported that, because of the nervous tissue viscoelasticity, these devices are susceptible to load accommodation. On the other hand, the portable device with dead weight employed to crush the sciatic nerve in rats is characterized to be a more rapid crushing procedure, with load being applied more easily and reliably [26].
In this way, further studies are needed not only to compare different methodologies of nerve lesions, considering the degree and condition of the lesion, the importance and the dependence between these laser parameters, but also the possible influences of biological responses, which might improve laser therapy specificity and help elaborating safer and more efficient treatment protocols.
Conclusion
Based on our samples and parameters and methods used, it was possible to observe that LLLT with 830 nm at the fluency of 40 J/cm2 and 80 J/cm2 had a positive influence on the acceleration of the functional nerve recovery.
Acknowledgments
This project received financial support from the State of São Paulo Research Foundation (FAPESP), Protocol number - 2007/00490-7, Brazil.
References
- 1.Marcolino AM, Barbosa RI, Fonseca MCR, Mazzer N, Elui VMC. J Phys Ther Mov. 2008;21:53–61. [Google Scholar]
- 2.Seddon HJ. Brain. 1943;66:237–288. doi: 10.1093/brain/66.4.237. [DOI] [Google Scholar]
- 3.Novak CB, Mackinnon SE. J Hand Ther. 2005;18:230–240. doi: 10.1197/j.jht.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Sunderland S. Muscle Nerve. 1990;13:771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- 5.Rochkind S, Barrnea L, Razon N, Bartal A, Schwartz M. Neurosurgery. 1987;20:843–847. doi: 10.1227/00006123-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Rochkind S, Nissan M, Alon M, Shamir M, Salame K. Lasers Surg Med. 2001;28:216–219. doi: 10.1002/lsm.1041. [DOI] [PubMed] [Google Scholar]
- 7.Endo C, Barbieri CH, Mazzer N, Fazan VS. Acta Ortop Bras. 2008;16:305–310. doi: 10.1590/S1413-78522008000500011. [DOI] [Google Scholar]
- 8.Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE. Photomed Laser Surg. 2007;25:436–442. doi: 10.1089/pho.2007.2093. [DOI] [PubMed] [Google Scholar]
- 9.Rochkind S, Leider-Trejo L, Nissan M, Shamir MH, Kharenko O, Alon M. Photomed Laser Surg. 2007;25:137–143. doi: 10.1089/pho.2007.2076. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed IFR, AL-Mustawfi NBV, Kaka LN. Photomed Laser Surg. 2007;25:107–111. doi: 10.1089/pho.2006.1090. [DOI] [PubMed] [Google Scholar]
- 11.Stainki DR, Raiser AG, Graça DL, Becker C. Braz J Vet Res Anim Sci. 1998;35:37–40. doi: 10.1590/S1413-95961998000100007. [DOI] [Google Scholar]
- 12.Ozen T, Orhan K, Gorur I, Ozturk A. Head Face Med. 2006;2:1–9. doi: 10.1186/1746-160X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khullar SM, Brodin P, Messelt EB, Haanaes HR. Eur J Oral Sci. 1995;103:299–305. doi: 10.1111/j.1600-0722.1995.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen YS, Hsu SF, Chiu CW, Lin JG, Chen CT, Yao CT. Microsurgery. 2005;25:83–89. doi: 10.1002/micr.20079. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa RI, Marcolino AM, Guirro RRJ, Mazzer N, Barbieri CH, Fonseca MCR. Lasers Med Sci. 2010;25:423–430. doi: 10.1007/s10103-009-0750-8. [DOI] [PubMed] [Google Scholar]
- 16.Mendonça AC, Barbieri CH, Mazzer N. J Neurosci Methods. 2003;129:183–190. doi: 10.1016/S0165-0270(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 17.Monte-Raso VV, Barbieri CH, Mazzer N, Fazan VS. J Neurosci Methods. 2005;142:185–192. doi: 10.1016/j.jneumeth.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Reis FA, Belchior ACG, Carvalho PTC, Silva BAK, Pereira DM, Silva IS, Nicolau RA. Lasers Med Sci. 2008;24:741–747. doi: 10.1007/s10103-008-0634-3. [DOI] [PubMed] [Google Scholar]
- 19.Belchior ACG, Reis FA, Nicolau RA, Silva IS, Perreira DM, Carvalho PTC. Lasers Med Sci. 2009;24:893–899. doi: 10.1007/s10103-008-0642-3. [DOI] [PubMed] [Google Scholar]
- 20.Bagis S, Comelekoglu U, Coskun B, Milcan A, Buyukakilli B, Sahin G, Ozisik S, Erdogan C. Lasers Med Sci. 2003;18:83–88. doi: 10.1007/s10103-003-0258-6. [DOI] [PubMed] [Google Scholar]
- 21.Shamir MH, Rochkind S, Sandbank J, Alon M. J Reconstr Microsurg. 2001;17:133–137. doi: 10.1055/s-2001-12702. [DOI] [PubMed] [Google Scholar]
- 22.Shin DH, Lee E, Hyun JK, Lee SJ, Chang YP, Kim JW, Choi YS, Kwon BS. Neurosci Lett. 2003;344:71–74. doi: 10.1016/S0304-3940(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 23.Gigo-Benato D, Geuna S, Rodrigues AC, Tos P, Fornaro M, Boux E, Battiston B, Giacobini-Robecchi MG. Lasers Med Sci. 2004;19:57–65. doi: 10.1007/s10103-004-0300-3. [DOI] [PubMed] [Google Scholar]
- 24.Gigo-Benato D, Geuna S, Rochkind S. Muscle Nerve. 2005;31:609–701. doi: 10.1002/mus.20305. [DOI] [PubMed] [Google Scholar]
- 25.Mazzer PYCN, Barbieri CH, Mazzer N, Fazan VPS. J Neurosci Methods. 2008;173:249–258. doi: 10.1016/j.jneumeth.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Pachioni CAS, Mazzer N, Barbieri CH, Fazan VPS, Moro CA, Silva CAA. Acta Ortop Bras. 2006;14:203–207. doi: 10.1590/S1413-78522006000400005. [DOI] [Google Scholar]
- 27.Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G. J Neurosci Methods. 2008;170:255–261. doi: 10.1016/j.jneumeth.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Gasparini ALP, Barbieri CH, Mazzer N. Acta Ortop Bras. 2007;15:285–289. doi: 10.1590/S1413-78522007000500011. [DOI] [Google Scholar]
- 29.De Medinacelli L, Derenzo E, Wyatt RJ. Rat sciatic functional index data management system with digited input. Comput Biomed Res. 1984;17:185–192. doi: 10.1016/0010-4809(84)90031-4. [DOI] [PubMed] [Google Scholar]
- 30.De Medinacelli L, Freed WJ, Wyatt RJ. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 31.Bain JR, Mackinnon SE, Hunter RT. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Liang KV, Zeger SL. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 33.Mc Cullagh P, Neder JA. Generalized linear models. 2. London: Chapman and Hall; 1989. [Google Scholar]
- 34.(2002–2003) SAS/STAT® User’s Guide, Version 9, Cary, NC, USA: SAS Institute Inc
- 35.Sotelo PR, Sosa VMR, Martinez RT, Barry HG. Rev Cubana Cir. 1996;35(2):84–88. [Google Scholar]
- 36.Rochkind S, El-Ani D, Nevo Z, Shahar A. Lasers Surg Med. 2009;41:277–281. doi: 10.1002/lsm.20757. [DOI] [PubMed] [Google Scholar]
- 37.Rochkind S, Vogler I, Barr-Nea L. Spine. 1990;15:6–10. doi: 10.1097/00007632-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira EF, Mazzer N, Barbieri CH, Selli M. J Reconstr Microsurg. 2001;17:69–75. doi: 10.1055/s-2001-12691. [DOI] [PubMed] [Google Scholar]
- 39.Salate ACB, Barbosa G, Gaspar P, Koeke PU, Parizotto NA, Benze BG, Foschiani D. Photomed Laser Surg. 2005;23(5):470–475. doi: 10.1089/pho.2005.23.470. [DOI] [PubMed] [Google Scholar]
- 40.Longo L, Mester A (1998) Present and future of laser cicatrization. In: Proceeding 2nd Congress World Association for Laser Therapy. Kansas City, Missouri, USA, September 2–5, 10–11
- 41.Basford JR. Laser Surg Med. 1995;16:331–342. doi: 10.1002/lsm.1900160404. [DOI] [PubMed] [Google Scholar]
- 42.Anders JJ, Geuna S, Rochkind S. Neurol Res. 2004;26:233–239. doi: 10.1179/016164104225013914. [DOI] [PubMed] [Google Scholar]
- 43.Rochkind S. Photomed Laser Surg. 2006;24:151–157. doi: 10.1089/pho.2006.24.151. [DOI] [PubMed] [Google Scholar]
- 44.Enwemeka CS. Photomed Laser Surg. 2009;27:387–393. doi: 10.1089/pho.2009.2503. [DOI] [PubMed] [Google Scholar]
- 45.Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, Hertl C. Nerve crush injuries: model for axonotmesis. Exp Neurol. 1994;127:284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]