Abstract
Scientific opinion on the relationship between selenium and the risk of cancer has undergone radical change over the years, with selenium first viewed as a possible carcinogen in the 1940s then as a possible cancer preventive agent in the 1960s–2000s. More recently, randomized controlled trials have found no effect on cancer risk but suggest possible low-dose dermatologic and endocrine toxicity, and animal studies indicate both carcinogenic and cancer-preventive effects. A growing body of evidence from human and laboratory studies indicates dramatically different biological effects of the various inorganic and organic chemical forms of selenium, which may explain apparent inconsistencies across studies. These chemical form-specific effects also have important implications for exposure and health risk assessment. Overall, available epidemiologic evidence suggests no cancer preventive effect of increased selenium intake in healthy individuals and possible increased risk of other diseases and disorders.
Keywords: Selenium, cancer, epidemiologic studies, prevention, risk assessment
INTRODUCTION
Few health issues have elicited as intense debate as the relationship between selenium (Se) and the risk of cancer [1–4]. Synthesizing the scientific evidence on the Se-cancer relationship is difficult—there is a large abundance of literature reporting effects ranging from nutritional to toxic even for the same chemical form of Se, amount of exposure, and target species [2, 5]—and carries the risk of being labeled a selenophobic [6] or a selenophilic [7], depending on one's conclusions. Yet attempts to reconcile the current evidence are critically important, not only to meet the information needs of key stakeholders, including the general population and regulatory agencies, but also because the attempt can yield important lessons for epidemiology, public health, and oncology. The time trend in the number of Medline-indexed citations returned when using the search terms “selenium” and “cancer” indicates an enduring interest in this issue (Figure 1).
Figure 1:
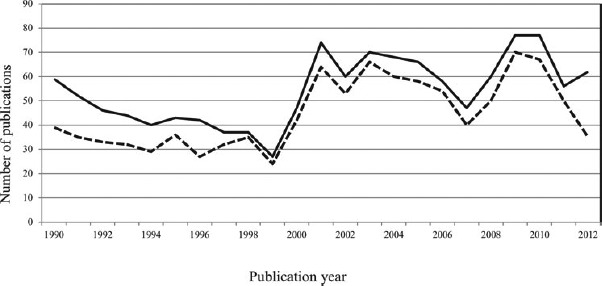
Number of publications per year yielded a Medline search using the terms “selenium” and “cancer” (solid line) and “selenium,” “cancer,” and “humans” (dotted line).
Debates about the health effects of particular substances are not unusual in biomedical research, but the disputes surrounding Se have been particularly heated. At various time points in recent history, Se has been hypothesized to be a carcinogen, a cancer preventive agent, a cancer therapeutic agent, and to have no effect on human cancer. The suggestion of opposite effects of Se on health is not confined to cancer. Se has been shown to have both antioxidant and pro-oxidant activity [5], to be both neuroprotective and neurotoxic [8], a cardiovascular health promotor as well as a cardiovascular health risk factor [9], and has been suggested to be both an antidiabetogenic and a prodiabetogenic agent [10]. Overlaid on this confusing state of affairs is the fact that Se is an essential nutrient for humans; it is a constituent of more than 20 selenoproteins that play critical roles in reproduction, thyroid hormone metabolism, DNA synthesis, and protection from oxidative damage and infection [11].
The aims of this review are to briefly summarize past literature to provide historical perspective and critically evaluate the most recent findings on the Se-human cancer relationship emerging from epidemiologic and clinical studies and their coherence with laboratory evidence. We also briefly discuss exposure limits for Se and the prospects for Se as a cancer therapeutic agent.
EARLY LABORATORY STUDIES ON SE AND CANCER
Se was first implicated in the etiology of cancer in 1943 by the seminal study of Nelson and colleagues, who reported its ability to promote liver cell adenoma and carcinoma in rats [12]. This was followed by other reports of carcinogenic effects [13, 14], but also several studies in laboratory animals suggesting the ability of Se to inhibit cancer cell growth [15], leading to a large literature on the ability of Se compounds to inhibit cancer progression [1, 16]. More recent laboratory investigations have also found evidence of both cancer-enhancing and cancer-inhibiting effects of Se, depending on the amount of exposure and chemical forms [17–20].
EARLY ECOLOGIC STUDIES ON SE AND CANCER
The first hypothesis concerning the relationship of Se and human cancer arose from ecologic studies carried out in the United States and were published starting in the late 1960s. These investigations, initiated by Shamberger and colleagues [21, 22] and followed by others [23–26], consistently suggested an inverse association between environmental indicators of Se exposure such as forage and water Se levels or population levels of human biomarkers (Se dietary intake or blood levels) and area-level cancer rates, and were the first studies to bring the possible relationship between Se intake and cancer risk to the attention of the scientific community and the general public. Surprisingly, the weaknesses of such studies, inherent in their ecologic study design, were given little attention, and these data, together with animal studies suggesting a cancer preventive effect, had a major role in promoting the hypothesis of cancer chemoprevention by selenium.
Most ecologic studies of the Se-cancer relationship, particularly the earlier ones [27], had little or no control of potential confounders, and had indicators of community-level Se exposure that were of questionable reliability. More recent studies have had improved control of confounders [2, 27], and also found inverse relationships between Se exposure and risk of several site-specific cancers as well as overall cancer risk; however, the large potential for bias associated with ecologic study designs remains. Findings from ecologic studies have not been uniformly in favor of cancer chemoprevention. A direct association with environmental indicators of Se exposure has been suggested for some site-specific cancers, such as leukemia and lymphoma [26]. More recently, and unexpectedly, direct association between Se exposure indicators and cancer risk have been suggested by other ecologic studies, such as a direct association between breast cancer mortality and serum Se levels in 65 Chinese rural counties [28] and between esophageal cancer incidence and soil Se in northeastern Iran [29], in contrast with other recent reports [30], further highlighting the need for caution in evaluating the validity and reliability of the results of such studies.
In the case of Se, the weaknesses of ecologic studies far exceed those of most ecologic studies. Se local water, soil, and forage content has little influence on the Se exposure of local residents, since most individuals, particularly in Western countries including the United States, do not consume mainly locally produced foodstuffs, and therefore their diet is not directly influenced by local soil Se. Further, water Se levels may vary considerably across local wells even in very small areas, thus precluding reliable evaluation of local exposure using geographical aggregate estimates. In addition, other factors such as occupational exposures, smoking habits, and intake of methionine may greatly influence Se intake and bioavailability, further weakening the reliability of any epidemiologic studies based on aggregate assessment of exposure and outcomes.
THE NEXT STAGE: COHORT AND CASE-CONTROL STUDIES
Following the early ecologic studies, numerous observational studies with individual-level Se exposure assessment were conducted [2, 27]. Many of these studies had a prospective design, for example, a cohort-nested case-control study design, in an attempt to avoid one of the most common weaknesses of case-control studies, reverse causality—a disease-induced change of Se status. However, as we describe, exposure assessment is still a major issue for such studies, limiting their value in resolving the Se-cancer relationship. Given its importance, we first discuss exposure assessment issues for such studies.
Many individual-level epidemiologic studies have used biological indicators of exposure, generally toenail or blood Se content or whole blood glutathione-peroxidase activity, in specimens collected at baseline to assess overall Se exposure [2, 31]. A limitation of these common biomarkers is that Se content in specific compartments such as blood or toenails may not represent levels of Se species in tissues relevant to cancer risk; in this, we can take a lesson from studies of the Se distribution in the human central nervous system [8, 32], which have found that Se concentrations in cerebrospinal fluid may be uncorrelated with peripheral circulating Se levels (Table 1).
Table 1:
Median Values (μg/l) of Se Chemical Species Identified in Blood (Serum) and Cerebrospinal Fluid (CSF) of 24 Human Subjects, with Their Squared Correlation Coefficients (r2) (Published and Unpublished Data from Solovyev, Berthele, and Michalke [32])
| Se Species | Serum | CSF | r2 |
|---|---|---|---|
| Se-cysteine | <LoD1 | <LoD | |
| Selenoprotein P | 5.19 | 0.474 | 0.037 |
| Glutathione peroxidase | 4.27 | 0.036 | 0.384 |
| Se-methionine | 0.23 | <LoD | |
| Thioredoxin reductase | 1.64 | 0.035 | 0.629 |
| Human serum albumin Se (HSA-Se) | 18.03 | 0.068 | 0.172 |
| Selenite | 12.25 | 0.046 | 0.040 |
| Selenate | <LoD | <LoD | 0.009 |
| UF2−1 | <LoD | <LoD | |
| UF-2 | <LoD | <LoD | |
| UF-3 | <LoD | <LoD | |
| UF-4 | 6.34 | <LoD | |
| Total | 58.39 | 0.861 | 0.0002 |
Limit of detection.
Unknown form.
Estimation of Se dietary intake has also been used for exposure assessment. This approach has been considered by some investigators as less reliable than biological indicators, due to large variations of Se content in foodstuffs over space and time, individual differences in absorption, and the role of other sources of exposure, that is, dermal absorption and inhalation (e.g., with smoking, in occupational environments, or in coal-polluted areas). However, many epidemiologic studies [33–37], although not all [38–40], have found that estimated Se dietary intake is correlated with tissue level, such as blood or toenail Se content. It could be argued that dietary intake is superior to Se tissue content as an indication of exposure, since the different Se species are retained at very different rates in the body and they also modify their biological activity on the basis of concurrent intake of dietary factors such as methionine and other elements such as Zn, Hg, and Cd [5, 41]. Furthermore, lower persistence of some Se species in the body compared with others does not per se indicate less biological availability or lower toxicity, since carefully conducted studies in animals have demonstrated that Se retained in the body after administration of its inorganic forms can be more toxic than Se administered as organic species, for equivalent amounts of intake [42, 43]. Under this perspective, careful assessment of Se intake from diet and other sources and whenever possible its speciation would allow a better estimate of actual exposure than simple determination of overall Se content through biomarkers, which is mainly influenced by intake of organic Se.
Exposure assessment using Se-containing enzymes such as plasma selenoprotein-P or whole blood glutathione-peroxidase activity has also been suggested [44]. Each such indicator has particular strengths and limitations; however, in general, the use of indirect indicators such as Se-containing enzymes has serious limitations, since the relationship between their tissue levels and Se exposure is complex and not well-defined, is heavily influenced by other dietary factors, and is inducible by factors such as oxidative stress (including Se itself) [5, 45–48].
We now turn to the findings of these case-control and observational cohort studies, originally designed to elucidate the promising findings of the ecologic investigations and some of the laboratory studies. A huge number of these studies have been carried out in different countries and populations, and most of them have found either inverse or null associations between baseline Se exposure and subsequent cancer risk [2, 49–52], while a few have reported direct associations for specific cancer sites [53–58]. For prostate cancer, in particular, most though not all studies have indicated a beneficial effect of Se [2, 50]. The reasons of such inconsistencies are unclear. They cannot be readily ascribed to differences in Se exposure levels, since even in populations in the lower range of exposure, inverse, null, and direct associations have been found for the same cancer site, for example, lung cancer [57–60]. Unmeasured confounding is a key methodological issue that may explain these conflicting results of observational studies, despite the efforts of investigators to adjust for or stratify on measured potential confounders. Unmeasured confounding is a well-known problem in nutritional epidemiology due to the large number of dietary and lifestyle factors, which may co-vary with the primary variables under study or act as effect modifiers. However, the dietary or lifestyle factors responsible for the inconsistencies among observational studies have not been convincingly identified. Another important methodological issue that may explain these inconsistencies is exposure misclassification. Most observational studies have been based on determination of total Se in peripheral biomarkers such as blood (generally plasma or serum) or toenails. However, Se may be found in environmental matrices, including foods, usually by far the main source of exposure, as organic and inorganic compounds [3, 61], and these different chemical forms of Se have different and in some cases even opposite toxicological and nutritional effects [5, 62–70]. Moreover, some dietary factors such as methionine and trace elements such as heavy metals may considerably modify Se biological activity and metabolism [41, 42, 71, 72], and the chemical forms of Se may influence its distribution in different body tissues [27, 32, 73–75]; in addition, the levels found in some body tissues may not correlate with those detected by other biological indicators. Taking into account all these aspects, lack of determination of specific Se species in target organs/tissues for disease onset, and of other circulating factors influencing Se biological activity, may have created substantial misclassification of exposure, which, in the worse cases—particularly for Se compounds having lower concentrations but the highest toxicological activity such as the inorganic forms selenite and selenite [76–78]—may have biased the overall results. On the other hand, analytical methodologies for determination of individual Se species have only recently been developed [32, 79, 80] and are complex, expensive, and time-consuming. In case-control investigations based on biomarkers, exposure misclassification may also have been generated by an effect of disease itself (particularly at its advanced stages) on Se biological indicators, generally inducing a decrease in circulating Se levels but in some instances increasing Se concentrations (as the neoplastic tissue). This raises the issue of reverse causality, whose risk is inherent in this type of studies. Finally, an additional possible explanation of the different findings may be the different genetic compositions of the underlying populations, since variants of genes coding selenoproteins might modify the risk of cancer, as well as of cancer survival, associated with Se exposure [81–83]. Specific genotypes may modify the selenium-human cancer association [51] and may even switch Se from being protective to being detrimental in influencing cancer risk [84, 85].
MOVING FORWARD: RANDOMIZED CONTROLLED TRIALS
Considering the conflicting results of observational studies and their limitations, and the strong chemopreventive effect of Se suggested by some of them, it is no surprise that randomized controlled trials (RCTs) were designed to confirm such findings. The key features of these studies and their main results are summarized in Table 2 and Figure 2a–c.
Table 2:
Summary of Effect of Se Supplementation on Human Cancer Risk by Cancer Site (Dose of 200 µg/Day as Organic Se When Not Otherwise Specified)
| Placebo Group |
Selenium Supplementation Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Study | Population | Events | Total | Events | Total | RRa | 95% Confidence Interval |
| Any cancer | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 119 | 659 | 77 | 653 | 0.63 | (0.47–0.85) |
| NPC | Duffield-Lillico et al., 2002 [93] | Patients with history of basal or squamous cell skin cancer | 137 | 629 | 105 | 621 | 0.75 | (0.58–0.97) |
| NPC | Reid et al., 2008 [123] | Patients with history of basal or squamous cell skin cancer | 21 | 161 | 15 | 154 | 0.73 | (0.35–1.48) |
| NPC | Reid et al., 2008(400mcg/day Se) [123] | Patients with history of basal or squamous cell skin cancer | 19 | 213 | 21 | 210 | 1.10 | (0.57–2.17) |
| SELECT | Lippman et al., 2009 [99] | >50 yrs. men not at risk for prostate cancer, US, Canada and Puerto Rico | 824 | 8696 | 837 | 8752 | 1.01 | (0.89–1.15)b |
| SELECT | Klein et al., 2011 [100] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 1108 | 8696 | 1132 | 8752 | 1.02 | (0.92–1.14)b |
| Prostate cancer | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 35 | 659 | 13 | 653 | 0.37 | (0.18–0.71) |
| NPC | Duffield-Lillico et al., 2003 [95] | Patients with history of basal or squamous cell skin cancer | 42 | 470 | 22 | 457 | 0.48 | (0.28–0.80) |
| SELECT | Lippman et al., 2009 [99] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 416 | 8696 | 432 | 8752 | 1.04 | (0.87–1.24)b |
| SELECT | Klein et al., 2011 [100] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 529 | 8696 | 575 | 8752 | 1.09 | (0.93–1.27)b |
| SELECT | Klein et al., 2011 [100] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico High-grade disease (Gleason score ≥7) | 133 | 8696 | 161 | 8752 | 1.21 | (0.90–1.63)b |
| Marshall et al., 2011 [96] | Men at high risk for prostate cancer, U.S. | 49 | 134 | 48 | 135 | 0.91c | (0.55–1.52)c | |
| Marshall et al., 2011 [96] | Men at high risk for prostate cancer, U.S. (only subjects for which plasma Se levels were available) | 47 | 130 | 45 | 125 | 0.97 | (0.68–1.39) | |
| Algotar et al., 2013 [97] | Men at high risk for prostate cancer, U.S. and New Zealand | 26 | 232 | 24 | 234 | 0.94 | (0.52–1.70) | |
| Algotar et al., 2013 (400 mcg/day Se) [97] | Men at high risk for prostate cancer, U.S. and New Zealand | 26 | 232 | 23 | 233 | 0.90 | (0.48–1.66) | |
| Bladder cancer | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 6 | 659 | 8 | 653 | 1.32 | (0.40–4.61) |
| NPC | Duffield-Lillico et al., 2002 [93] | Patients with history of basal or squamous cell skin cancer | 8 | 629 | 10 | 621 | 1.28 | (0.50–3.25) |
| SELECT | Lotan et al., 2012 [113] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 53 | 8696 | 60 | 8752 | 1.13 | (0.78–1.63)b |
| Lung cancer | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 31 | 659 | 17 | 653 | 0.54 | (0.30–0.98) |
| NPC | Duffield-Lillico et al., 2002 [93] | Patients with history of basal or squamous cell skin cancer | 35 | 629 | 25 | 621 | 0.74 | (0.44–1.24) |
| SELECT | Lippman et al., 2009 [99] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 67 | 8696 | 75 | 8752 | 1.12 | (0.73–1.72)b |
| SELECT | Klein et al., 2011 [100] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 92 | 8696 | 94 | 8752 | 1.02 | (0.70–1.50)b |
| Colorectal cancer | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 19 | 659 | 8 | 653 | 0.42 | (0.18–0.95) |
| NPC | Duffield-Lillico et al., 2002 [93] | Patients with history of basal or squamous cell skin cancer | 19 | 629 | 9 | 621 | 0.46 | (0.21–1.02) |
| SELECT | Lippman et al., 2009 [99] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 60 | 8696 | 63 | 8752 | 1.05 | (0.66–1.67)b |
| SELECT | Klein et al., 2011 [100] | >50 yrs. men not at risk for prostate cancer, U.S., Canada and Puerto Rico | 75 | 8696 | 74 | 8752 | 0.96 | (0.63–1.46)b |
| Breast cancer | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 3 | 659 | 9 | 653 | 2.88 | (0.72–16.5) |
| NPC | Duffield-Lillico et al., 2002 [93] | Patients with history of basal or squamous cell skin cancer | 6 | 629 | 11 | 621 | 1.89 | (0.69–5.14) |
| Lubinski et al., 2011 [101] (250 µg/day inorganic Se) | Women with a BRCA1 mutation | 45 | -d | 60 | -d | 1.4 | (0.9–2.0) | |
| Melanoma | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 8 | 659 | 8 | 653 | 0.97 | (0.32–2.96) |
| NPC | Duffield-Lillico et al., 2002 [93] | Patients with history of basal or squamous cell skin cancer | 9 | 629 | 11 | 621 | 1.18 | (0.49–2.85) |
| Nonmelanoma skin cancer | ||||||||
| NPC | Duffield-Lillico et al., 2003 [94] | Patients with history of basal or squamous cell skin cancer | not provided | 629 | not provided | 621 | 1.17 | (1.02–1.34) |
| Dreno et al., 2007 [98] | Recent organ transplant patients | 2 | 93 | 6 | 91 | 3.07 | (0.5–31.1) | |
| NPC | Reid et al., 2008 [123] | Patients with history of basal or squamous cell skin cancer | 80 | 161 | 99 | 154 | 1.49 | (1.10–2.03) |
| NPC | Reid et al., 2008(400mcg/day Se) [123] | Patients with history of basal or squamous cell skin cancer | 108 | 213 | 98 | 210 | 0.88 | (0.66–1.16) |
| NM skincancer—Squamouscell carcinoma | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 190 | 659 | 218 | 653 | 1.14 | (0.93–1.39) |
| NPC | Duffield-Lillico et al., 2003 [94] | Patients with history of basal or squamous cell skin cancer | not provided | 629 | not provided | 621 | 1.25 | (1.03–1.51) |
| NPC | Reid et al., 2008 [123] | Patients with history of basal or squamous cell skin cancer | 42 | 161 | 65 | 154 | 1.76 | (1.18–2.66) |
| NPC | Reid et al., 2008 (400mcg/day Se) [123] | Patients with history of basal or squamous cell skin cancer | 53 | 213 | 56 | 210 | 1.05 | (0.71–1.56) |
| NM skin cancer—Basal cellcarcinoma | ||||||||
| NPC | Clark et al., 1996 [102] | Patients with history of basal or squamous cell skin cancer | 350 | 659 | 377 | 653 | 1.10 | (0.95–1.28) |
| NPC | Duffield-Lillico et al., 2003 [94] | Patients with history of basal or squamous cell skin cancer | not provided | 629 | not provided | 621 | 1.09 | (0.94–1.26) |
| NPC | Reid et al., 2008 [123] | Patients with history of basal or squamous cell skin cancer | 69 | 161 | 75 | 154 | 1.20 | (0.85–1.68) |
| NPC | Reid et al., 2008(400mcg/day Se) [123] | Patients with history of basal or squamous cell skin cancer | 83 | 213 | 76 | 210 | 0.90 | (0.65–1.24) |
Relative risk of cancer in in supplemented vs. nonsupplemented individuals.
99% confidence interval.
Unpublished data (courtesy of Dr. James Marshall).
1135 women with a BRCA1 mutation randomized to Se or placebo.
Figure 2:
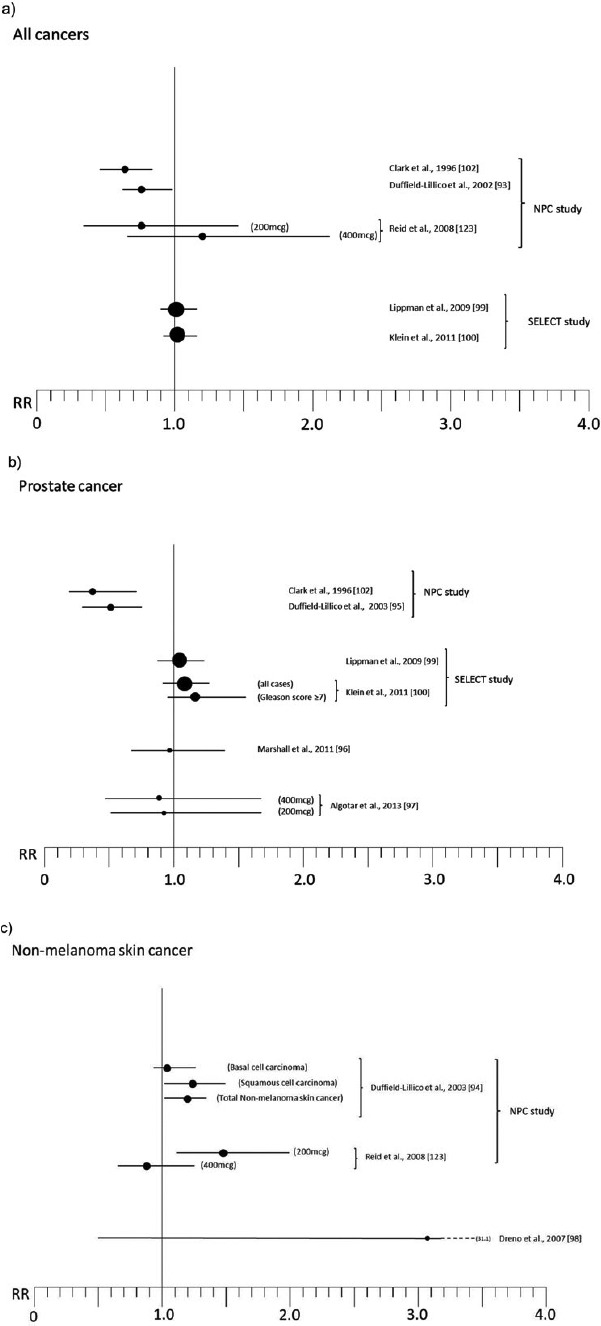
Relative risk (RR) for selected cancers in randomized, placebo-controlled trials (with 200 μg of organic Se when not otherwise specified; see Table 2 for references numbers).
Some trials have been designed as multiple intervention cohort studies, administering other trace elements and vitamins or different substances [86–89] to participants in addition to Se, thus precluding identification of its specific activity. Other experimental studies having a “community” design selectively focused on Se activity but were characterized by lack of adequate information about potential confounders or individual-based follow-up and ascertainment of both exposure and outcome [90–92], thus hampering interpretation of results. We therefore do not take these studies into further consideration in the present review. Rather, we focus on RCTs that were specifically designed to estimate the effects of Se administration on cancer risk, the majority of which were carried out in specific patient populations in the United States [93–97] and in France [98], and in one case in the general U.S. population [99, 100]. The patient populations involved in such studies were nonmelanoma skin cancer patients [93–95], individuals at high risk of prostatic neoplasm [96, 97] or breast cancer [101], and organ transplant recipients [98]. Among these trials, the two largest, the Nutritional Prevention of Cancer (NPC) Trial and the Selenium and Vitamin E Cancer Prevention Trial (SELECT), have received the greatest attention from both the general population and the scientific community. We discuss these trials in detail.
The Nutritional Prevention of Cancer (NPC) Trial
The NPC trial [93–95, 102–104] was designed to test the hypothesis that dietary supplementation with Se at 200 µg/day may decrease cancer risk. The study participants (n = 1312) were patients with recent (1-year) diagnosis of basal or squamous cell skin cancer, life expectancy of at least 5 years, and no treatment for internal cancers in the past 5 years. Se was administered as selenized baker's yeast, which contains organic Se in the form of selenomethionine (∼60%) and other selenoproteins [103]. The primary outcomes were the incidence of basal cell and squamous cell skin cancer; secondary outcomes included incidence of all cancers and of some major site-specific cancers. Patient recruitment occurred in 1983–1991, and the official end of the blinded treatment period was February 1, 1996. Final results were published in 2002–2003 [93–95]. A preliminary analysis was published in 1996 based on follow-up data through December 1, 1993, following the recommendation of the trial's Safety Monitoring and Advisory Committee in 1994 to unblind the study and present the results for the first 10-year period [102]. We focus on the final results, which are based on longer follow-up.
The final results of the NPC trial, reported in Table 2 and Figure 2a–c, are complex to interpret. There was an excess risk of squamous cell carcinoma (RR 1.25, 95% CI 1.03–1.51) and a slightly increased risk of basal cell carcinoma (RR 1.09, 95% 0.94–1.26), yielding an excess risk of overall nonmelanoma skin cancer of 17% (95% CI 2%–34%) and leading to the conclusion that Se increased the risk of total nonmelanoma skin cancer [94]. In contrast, the risk of all cancers (RR 0.75, 95% CI 0.58–0.97), prostate (RR 0.48, 95% CI 0.28–0.80), lung (RR 0.74, 95% CI 0.44–1.24), and colorectal (RR 0.46, 95% CI 0.21–1.02) cancers [93, 103] was decreased. For lung cancer, an analysis according to baseline plasma Se showed that the inverse association was confined to subjects in the lowest tertile, which was <106 μg/l [103]. Some cancer sites suggested an increased risk associated with Se supplementation, but estimates were generally statistically unstable due to the low number of cases: among these were breast cancer (RR 1.89, 95% CI 0.69–5.14) and, with much lower RRs, melanoma, bladder, and lymphoma and leukemia. Further analyses on prostate cancer revealed very different RRs according to baseline plasma Se, ranging from RR values as low as 0.14 (95% CI 0.03–0.61) among subjects in the lowest tertile of plasma Se (≤106.4 μg/l) up to 1.14 (95% CI 0.51–2.59) for plasma Se > 123.2 μg/l, but the risk reduction was nearly entirely confined to subjects with prostate specific antigen (PSA) ≤4 ng/ml [95]. In addition, further analysis stratified by treatment arm showed a weak inverse association between baseline Se and subsequent prostate cancer risk among placebo participants but a direct association among Se-treated participants [105]. After adjusting for some differences between treatment groups, the positive association between Se administration and prostate cancer risk remained strong [105], thus suggesting an increased risk among subjects with higher baseline plasma Se, in sharp contrast to the preliminary results of the trial [102] and the findings in placebo-treated subjects [105].
The NPC results, particularly the preliminary ones, received widespread attention from both the public and the scientific community (more than 2500 citations in Google Scholar for its first 1996 report as of July 2013), with emphasis placed on the “beneficial” findings for all cancers and prostate cancer incidence and the concerning results for the primary outcome, nonmelanoma skin cancer, either overlooked or dismissed. This lack of attention probably occurred because such results were not consistent with preexisting expectations: Se was one of most promising dietary factors in chemoprevention, the first prospective studies carried out during the 1980s had suggested an association between Se exposure indicators at baseline and subsequent cancer risk [60, 106–109], and Se had proven effective in inhibiting cancer cell growth in several animal models (although such reasoning ignored the carcinogenic activity of some Se compounds suggested by a few of these studies).
The Selenium and Vitamin E Cancer Prevention Trial (SELECT)
The SELECT trial was a large-scale attempt to evaluate the activity of two promising chemopreventive agents, Se and vitamin E, for prostate cancer prevention [110–112]. This trial recruited more than 35,000 men from 427 sites in the United States, Canada, and Puerto Rico; eligibility criteria were ≥50 years for black men and ≥55 for all others, a PSA value of 4.0 ng/l or less, and a digital rectal examination not suspicious for prostate cancer. Recruitment was from August 22, 2001 through June 24, 2004, and three reports of the study results have been published to date: one concerning the main outcomes after follow-up to Oct 23, 2008 [99], the second one updating the results with an (unblinded) follow-up until July 5, 2011 [100], and the third one reporting RR of bladder cancer [113].
SELECT study participants were randomized to oral Se as selenomethionine (200 μg/day), vitamin E, Se plus vitamin E or placebo. The Se only and placebo arms included 8752 and 8696 patients who could be included in the primary analysis, respectively, and who were also followed in the updated follow-up with a person-time increase of 23%. The follow-up was extended beyond the primary outcome, prostate cancer incidence, to include cardiac events, diabetes, severe or life-threatening (grade 3 or 4) events, and minor side effects potentially related to the study supplements: alopecia, dermatitis, fatigue, halitosis, nail changes, and nausea. The originally planned minimum and maximum follow-up was 7 and 12 years, but the actual average follow-up periods were 5.5 years until the end of the blinded follow-up with discontinuation of Se supplementation and 7.1 until the end of subsequent follow-up to report additional postexposure events [99, 100]. This shorter follow-up compared to that originally planned was due to an unanticipated interruption of the trial, due to the recommendation of the independent Data Safety Monitoring Committee to discontinue the study because of its inefficacy in risk reduction and concern about increased risk of prostate cancer in vitamin E-supplemented participants and of type 2 diabetes in the Se-treated participants [99, 100], an observation that echoed the marked increase in diabetes risk observed after Se administration in the NPC trial [114].
Overall, the results of the SELECT trial, summarized in Table 2 and Figure 2a–b, have so far provided strong evidence against any cancer-prevention effect in this study population [115]. Overall relative risk of cancer was 1.01 (99% CI 0.89–1.15), with a slightly and statistically imprecise increased risk of prostate cancer (RR 1.04, 99% CI 0.87–1.24), lung cancer (RR 1.12, 99% CI 0.73–1.72), and colorectal cancer (RR 1.05, 99% CI 0.66–1.67), and little evidence of altered risk for the other primary neoplasms (excluded nonmelanoma skin cancer) (RR 0.99, 95% CI 0.77–1.17) [99, 100]. An analysis limited to the risk of aggressive prostate cancer (Gleason score ≥7) showed an excess risk of high-grade disease among Se-treated subjects (RR = 1.21, 99% CI 0.90–1.63) [100], this possibly being the most concerning finding of the SELECT study [115].
Bladder cancer incidence, despite being one of the site-specific cancers most strongly associated with Se status in observational cohort studies, was not decreased by Se treatment but rather was slightly increased (RR 1.13), although the estimate was statistically very imprecise (99% CI 0.78–1.63) [113].
NPC vs. SELECT: Why the Conflicting Results?
Why did the Se-supplemented participants in the NPC trial exhibit a reduced risk of cancer while those in the second larger SELECT trial did not? One possibility is the underlying difference in populations; the NPC trial enrolled patients with nonmelanoma skin cancer whereas the SELECT trial participants, although male only, more closely resembled the general population, presenting the possibility of specific risk factors or behavioral profiles interacting with Se to modify some cancer preventive effect.
Another possibility is the different ranges of Se exposure in the two study populations, with the patients enrolled in NPC exhibiting a lower average exposure than SELECT participants. This hypothesis is interesting but unconvincing for several reasons. The first is the small difference in median intake between the two populations, of about 23 µg/l, which corresponds to about 15 µg/day in dietary intake [116], a small difference when compared to the large range of intake across different Western populations, ranging from an average of 20–40 µg/day in Italian, Austrian, and French populations to ≥100 µg/day in some U.S. and Japanese populations (excluding supplements) [116–118]. Under the same perspective, the very different results obtained in the NPC across tertiles of plasma Se for all cancers and for prostate cancer risk in Se-treated subjects, ranging from −76% in the bottom category to +14% in the highest category despite an inter-tertile range as small as 16.8 µg/l (around 11 µg/day of dietary Se intake), make it biologically implausible to ascribe such extreme variations in cancer RR to such small differences in exposure, unless we assume that 120 µg/l of plasma Se represents a critical threshold for cancer risk. Any small change of a few µg of dietary Se intake from this amount would therefore imply remarkable changes in cancer risk, but no such effects have ever been described in ecologic or individual-based epidemiologic studies despite variations in individual intakes that range into the hundreds of micrograms. This hypothesis also finds little support from observational studies concerning the effects of Se supplementation in populations with much lower average intakes of Se, such as the trends in prostate cancer incidence in Finland following nationwide Se supplementation compared with other Nordic countries, which did not show any beneficial effect [27]; from the lack of any reduction in mortality from prostate cancer within an Italian population naturally exposed to inorganic hexavalent Se [119]; and from the results of a recent phase III trial by Marshall and colleagues in high-risk individuals for prostate cancer, focusing on RRs observed in those with baseline plasma Se in the <106 and 106–132 μg/l categories [96].
Further weakening the possibility that such small differences in average intake may explain the different results of the two trials is the observation that toxicity of the Se supplements occurred in both the NPC and SELECT trials: an excess risk of glaucoma and diabetes in the NPC study [114, 120], and of dermatologic abnormalities and diabetes in the SELECT trial [99], the latter being one of the reasons for interrupting the trial; the risk of diabetes subsequently decreased following discontinuation [100]. Occurrence of toxicity indicates a comparable activity of Se in the two trials, an observation that weakens the hypothesis of marked differences in exposure.
To facilitate interpretation of the results of the two trials, some further analyses of the SELECT trial data would be highly informative. In particular, we urge analyses as to whether the overall risk of cancer and risk of site-specific cancers varies by baseline Se exposure level, and whether the risk of nonmelanoma and melanoma skin cancer is associated with Se supplementation [94, 98, 121].
Last, a potentially severe bias has been acknowledged by the NPC trial investigators, and it may at least partially explain the impressive results obtained in that trial for prostate cancer and subsequently unconfirmed by the other experimental studies [95, 96]. For unknown reasons, presumably by chance, the NPC participants randomized to Se were later found to have been less biopsied than individuals on placebo following a PSA test at baseline or in the follow-up (14% vs. 35% in the Se and placebo groups, respectively) [95, 96], thus exposing the Se supplementation arm of the trial to risk of under-detection of prostate cancer. The investigators tried to adjust the analysis for this potentially serious bias [95], but the extent to which this bias may have occurred is unclear and remains a critical limitation of the NPC study. A role of unmeasured confounders and of the limitations of secondary analyses (all outcomes other than nonmelanoma skin cancer primary occurrence) have also been hypothesized as potentially explaining at least some of the beneficial results on prostate cancer reported by the NPC trial [96]. However, these factors are unlikely to fully explain the trial results since ascertainment of the secondary outcomes was apparently satisfactory and unbiased in the NPC trial, and since claims about limitations of multiple comparisons are not convincing from an epidemiologic perspective [122].
Other Randomized Controlled Trials
A small ancillary study of NPC, involving patients enrolled at the Macon, GA site, added an arm assigning 400 μg/day Se supplementation in order to evaluate the effects of high Se exposure and possible dose-response trends [123]. The results showed a possible limited decrease of risk of nonmelanoma skin cancer in the 400 μg Se supplemented individuals (RR 0.88, 95% CI 0.66–1.16), compared with the excess incidence observed in the 200 μg/day participants (RR 1.49, 95% CI 1.10–2.03), and no indication of decreased all-cancer incidence as seen in the NPC main results, with a RR of 1.10 (95% CI 0.57–2.17) (Figure 2–c). These results appear to weaken the NPC findings, although they lack statistical precision.
A trial carried out in a French population of 184 organ graft recipients, considered to be at high risk of premalignant and malignant epithelial lesions, unexpectedly detected a higher incidence of skin cancer in the 91 Se-treated patients (6 cases, 6.6%) than in the 93 placebo patients (2 cases, 2.2%, P = 0.15) during 5-year follow-up, the first 3 of which including daily supplementation with 200 μg Se as selenized yeast [98] (Figure 2–c). Thus this study suggests an increased risk of skin cancer, mirroring the results of the NPC study [94] and of some although not all observational studies [55, 121].
Another randomized double-blind trial of considerable interest was conducted in Poland with a group of healthy women who were carriers of the BRCA1 mutation and thus at high risk for breast cancer [101]. Lubinski and colleagues randomized 1135 female BRCA1 mutation carriers to 250 μg/day of Se as selenite or placebo for 6 to 62 months (median follow-up 35 months). During follow-up, 60 incident breast cancers were diagnosed among Se-supplemented women in comparison with 45 cases among placebo-administered women (RR 1.4, 95% CI 0.9–2.0) [101]. Such results, which were mirrored in findings on primary breast cancer, contralateral breast cancer, and ovarian cancer, suggest not just a lack of any beneficial effect of Se on breast cancer in these high-risk women but the possibility of increased risk.
Two phase III trials have published findings on the effect of Se on prostate cancer risk for high-risk individuals, which distinguishes these studies from the SELECT trial. Results of these studies, and more generally of the trials investigating prostate cancer risk, are reported in Figure 2–b. The first study, coordinated by the Southwest Oncology Group [96], randomized 423 men with high-grade prostatic intraepithelial neoplasia to 200 μg/day Se as selenomethionine or placebo. After a treatment period with follow-up of 3 years, the incidence of prostate cancer in the two groups was nearly identical (48/135 [35.6%] in the Se group vs. 49/134 [36.6%] in the placebo group). There was little evidence of modifying effects of baseline plasma Se status on prostate cancer risk. In fact, lower risk emerged in the bottom (<106 μg/l) and top (>162 μg/l) quartiles of plasma Se, where RRs were 0.82 (95% CI 0.40–1.69) and 0.91 (95% CI 0.45–1.84), respectively, while individuals in the second quartile (106–132 μg/l) showed a RR of 1.38 (95% CI 0.68–2.78). When the study sample was stratified by Gleason score of diagnosed prostate cancer, there was a tendency toward more aggressive tumors among Se-treated subjects. Overall, the authors concluded there was no effect of Se supplementation on prostate cancer risk in this high-risk population, although they did not entirely rule out the possibility of a beneficial effect in the lowest category of Se exposure. These results are similar to results from a recently reported Canadian phase III trial of patients with high-grade prostatic intraepithelial neoplasia, in which 3-year supplementation with vitamin E, soy, and Se (100 μg/day) was associated with a prostate cancer RR of 1.12 (95% CI 0.71–1.76) [124].
A second phase III trial, the Negative Biopsy Study, enrolled individuals from the United States and New Zealand at high risk of prostate cancer (PSA >4 ng/ml and/or suspicious digital rectal examination and/or PSA velocity >0.75 ng/ml/year) but with a prostate biopsy negative for cancer or high-grade intraepithelial neoplasia, who were randomized to 200 or 400 μg/day Se as selenized yeast or placebo [125]. The results of this 5-year trial [97] suggested no effect of Se treatment on prostate cancer incidence, which was 11.3% (26 cases) among 228 placebo-randomized subjects, 10.3% (24/233) among subjects receiving 200 μg/day Se, and 10% (23/230) among those supplemented with 400 μg/day Se. No effect of Se status at baseline on risk emerged, despite the fact that the lowest category had a plasma Se concentration of less than 101.1 μg/l. Additionally, little evidence of any effect of the Se treatment on PSA velocity emerged. Therefore, it is difficult not to agree with authors' conclusion that “Selenium supplementation appeared to have no effect on the incidence of prostate cancer in men at high risk. In conjunction with results of other studies, these data indicate that selenium supplementation may not have a role in prostate cancer chemoprevention” [97].
Lack of replication of the original NPC results by the SELECT trial, the results of two phase III trials of high prostate cancer risk patients, and the weakening results of the NPC trial itself over time indicate that prospective observational studies suggesting a beneficial effect of baseline Se status on subsequent prostate cancer risk [50] were likely biased by unmeasured confounders, and that Se does not modify prostate cancer risk. However, the possibility of modifying effects of specific genotypes [126] or of possible adverse effects of very low and very high Se exposures deserve further investigation. This may be possible using SELECT trial data and biological specimens (blood and toenails) to assess baseline Se status in study participants.
Over and above these null findings, recent findings of observational studies suggest extreme caution is needed when considering the effect of Se-containing supplements on prostate cancer risk. In fact, cohort and case-control studies have shown an unexpectedly increased prostate cancer risk among Se-treated subjects with the highest baseline exposure levels [105], excess risk associated with multivitamin use only among Se supplements use [127], an excess prostate cancer risk associated with Se self-supplementation (OR 2.2 with 95% CI 1.2–4.2 among consumers for ≥10 yrs. of Se supplements) [128], and finally a higher risk of aggressive disease in patients with higher plasma Se levels [85].
Results of a Natural Experiment on Selenium Exposure
Natural experiments, of which Snow's study on cholera remains the archetype, have long enjoyed a privileged position in environmental epidemiology, since they allow the study of toxic substances that cannot be administered to humans for ethical reasons or that may require such long periods of exposure as to make them infeasible [129]. Such a natural experiment occurred in the Northern Italy community of Reggio Emilia, where the Rivalta neighborhood was supplied from 1972 to 1988 with municipal tap water containing unusually high levels of Se in its inorganic hexavalent form, selenate, which was the only distinctive chemical characteristics compared to drinking water distributed in the remaining municipal territory [119, 130]. The Rivalta residents also had a very similar distribution of demographic characteristics compared with the municipal population, suggesting limited potential for bias [119, 130]. Results of cohort studies carried out among long-term consumers of this high-Se tap water are summarized in Figure 3. Overall, no beneficial effect on mortality from cancer or other chronic diseases emerged: cancer mortality, in fact, was slightly increased (RR 1.20, 95% CI 1.01–1.41), with excess mortality from melanoma and colorectal cancer in the whole cohort, kidney cancer in males and lympho-hematopoietic malignancies in females [119], and further confirmation of the increased melanoma risk from an incidence study [121]. Prostate cancer mortality was not decreased, but rather increased, among exposed residents (RR 1.40, 95% CI 0.45–3.39).
Figure 3:
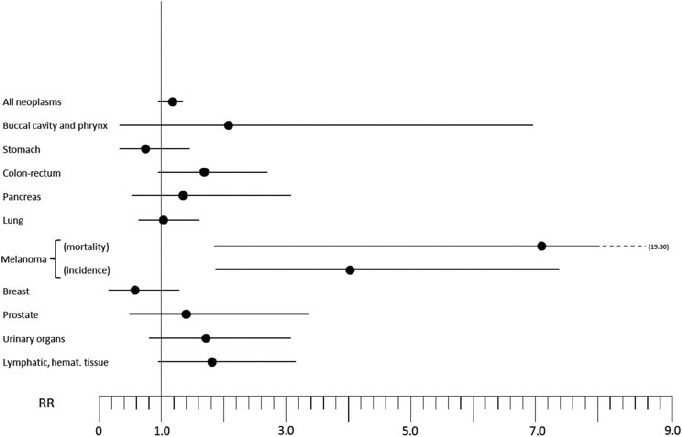
Relative risk (RR) for selected cancers in a natural experiment investigated in Reggio Emilia, northern Italy, where residents consumed drinking water with high inorganic hexavalent Se content (around 8 μg/l) as only distinctive feature (Vinceti et al., 1995, 1998, and 2000 [119, 121, 130]).
Overall, these findings suggest no beneficial effect of Se on cancer risk, but rather the potential for adverse effects. These results, however, have to be considered specific for the inorganic hexavalent form of Se, whose biological properties may be markedly different from those of other chemical forms of Se such as selenomethionine, an organic form commonly used in human trials. Absolute amounts of Se exposure across the different chemical species cannot be directly compared, as demonstrated by laboratory studies that show that toxicity of inorganic (tetravalent) Se greatly exceeds that of organic Se [131]. However, these results are of interest since they allow assessment of the effect of the Se form usually found in drinking water, the inorganic hexavalent one, on cancer risk, and allow risk assessment of such sources of exposure [132].
WHAT LEVEL OF SE EXPOSURE IS “SAFE”?
Given the nutritional role of Se and its possible low-dose toxic effects, the questions arise: What level of Se exposure in humans is needed for adequate nutrition, and at what level does Se exposure carry health risks? These are open questions, as demonstrated by the different upper and lower safe limits that have been proposed by various agencies, institutions, and investigators [133, 134], for both nutritional [135] and toxicological [5] effects, such as for Se drinking water standards [132].
Many current standards are based on outdated approaches and evidence. For example, one frequently used indicator of a “safe” Se exposure level and of a nutritional range of intake is the Se-induced increases and maximization of selenoproteins such as the Se-containing glutathione-peroxidases. We agree with a World Health Organization research group that such an approach should be regarded with extreme caution [135]. Recent studies indicate the complexity of this issue at the molecular level [136]. Maximization of Se-containing glutathione-peroxidases has never been demonstrated to be beneficial, and more generally, maximal expression of antioxidant enzymes should not be considered beneficial for human health unless a sound epidemiologic basis has been provided for this [5, 137]. Epidemiologic data suggest adverse health effects of Se at levels lower than those required to maximize selenoprotein expression [114, 119, 138, 139], and biochemical studies have long indicated that increased selenoprotein activity, such as in the case of glutathione-peroxidases, is driven not only by increased Se availability but also by oxidative stress induced by Se itself or by other environmental or biological stressors [45–48, 140, 141]. In line with this hypothesis, Se supplementation has been shown to increase levels of some proteins in adult men including clusterin [142], an enzyme that may be induced by oxidative stress [143, 144], and recent cross-sectional studies confirmed a direct relationship between Se exposure and oxidative stress biomarkers [145, 146].
Perhaps the best available evidence on toxic effects of Se and the levels of intake associated with them comes from observations—sometimes entirely unexpected—in human experimental studies [94, 99, 104, 114, 120]. Currently, the main concerns of adverse effects of Se exposure [115, 147] are an excess of skin cancer [94, 98], diabetes [99, 114, 139], and dermatologic alterations [99]; increased risk of glaucoma [120, 148, 149] and amyotrophic lateral sclerosis [8, 150] may also occur after low-dose overexposure to organic and inorganic Se, respectively, consistent with laboratory evidence [45, 151, 152]. Toxicity of inorganic Se appears to be higher than toxicity of organic forms, in line with observations from laboratory studies [67, 131, 153], possibly being as low as around 20 μg/day on the basis of epidemiologic observations in long-term consumers of drinking water with high content of hexavalent Se [119, 154], which represents one of the few natural sources of exposure to this inorganic Se form. Additional consequences of low-dose Se overexposure may be other endocrine abnormalities or adverse effects to other organs [5, 155] and down-regulation of phase 2 genes [156], although evidence for these effects and identification of the thresholds of exposure involved have not been convincingly provided so far.
Currently proposed “safe” upper limits are not convincingly “safe” by current evidence; for example, the safe upper limit of intake of 400 μg/day in adults suggested in 2000 by the Institute of Medicine [133] appears inadequate to protect human health in light of the SELECT trial results showing toxic effects of (organic) Se exposure at around 300 μg/day [99], and other epidemiologic studies for organic Se and inorganic Se, with the latter being especially of concern for toxicity at such levels [5, 114, 139, 150, 157, 158]. Therefore, although the 2009 statement by Platz and Lippman that “At present, we do not know enough to determine how much selenium any man or woman should receive from the diet or a supplement” [159] is certainly and unfortunately true, the epidemiologic evidence from the most recent studies suggest that the safe range of intake of dietary Se is much lower than anticipated for the organic Se forms and much, much lower for inorganic species [5].
SELENIUM AS A POTENTIAL CANCER THERAPEUTIC AGENT
The fading promise of a cancer-preventive effect of Se has been accompanied by rising interest in its potential ability to reduce cancer recurrence or to directly treat cancer in diagnosed patients, in line with a larger interest in the possible therapeutic properties of Se for various diseases [160].
The objective of preventing cancer recurrence is relatively new and differs from the objective of the NPC trial, which aimed to assess whether Se could reduce the occurrence of new cancers in nonmelanoma skin cancer patients rather than the risk of recurrence [102]. One study evaluating this new objective is the SELEnium and BLAdder cancer Trial (SELEBLAT), a phase III randomized, placebo-controlled, double-blind trial designed to assess the ability of Se to reduce recurrences of noninvasive transitional bladder carcinoma [161]. This trial is currently ongoing, and its findings, whether negative or positive, will certainly add to the debate about Se.
The ability of certain Se species to exert toxic effects on cancer cell growth has been demonstrated in a number of laboratory studies [162–171], leading to interest in the use of Se compounds as anticancer therapeutic agents, possibly as additions to standard chemotherapeutic regimens [165, 172–174]. Some Se compounds have been shown to exert specific activities against cancer cells compared to normal cell [175], although these findings have not always been confirmed [176]. The number of human studies in this area is still very limited. A trial conducted in non-Hodgkin lymphoma patients reported that the addition of selenite to chemotherapy appeared to reduce recurrence risk [177]. On the other hand, a study involving stage-I nonsmall cell lung cancer patients failed to show beneficial results on risk of second primary tumors [178], and self-administration of Se supplements did not reduce recurrence outcomes among prostate cancer patients enrolled in the TAX 327 study [179]. On a cautionary note, a review of the use of dietary antioxidant supplementation during conventional chemotherapy and radiation therapy concluded that the use of such supplements should be discouraged because of the possibility of tumor protection and reduced survival [180].
The current evidence is too limited and inconsistent [181] to allow for an adequate assessment of Se as an anticancer agent and to justify its administration in cancer therapy, although this issue remains relevant and merits further evaluation. The complexity of this issue is highlighted by a recent study in mice, which showed that organic Se supplementation reduced and delayed breast cancer metastasis while selenite exacerbated it [182], confirming again that it is of utmost importance to consider the chemical form-specific effects of Se. Further studies and particularly human trials, taking into account clinical disease stage and genetic factors, are required to better investigate the utility and safety of Se compounds as cancer therapeutic agents.
FINAL CONSIDERATIONS
The trajectory of opinion on the relationship between Se and cancer over the years could be encapsulated in the study of lung cancer. Studies suggested a beneficial effect of Se on lung cancer risk until a decade ago [183], when further studies [99] failed to substantiate such an effect. Further, a recent observational study found a direct relationship between Se exposure and risk [58], mirroring previous results obtained in a U.S. study [53], and Se has now been added to the list of initially promising but ultimately unsuccessful—and possibly harmful—preventive agents for lung cancer [178, 184]. In Se, we have witnessed what has now become a common occurrence in nutritional epidemiology, the failure of micronutrient dietary supplements to show beneficial effects on human health [185–187].
Overall, experimental human studies indicate that Se does not reduce cancer risk and may increase risk of some site-specific cancers such as skin neoplasms, and no convincing evidence has so far been provided that individuals with “low” Se intake, that is, in the order of 20–70 μg/day, may reduce cancer risk by increasing their Se exposure. Taking into account the potential for harm at very low levels of intake, no dietary or environmental supplementation with this metalloid may be currently recommended for cancer prevention from either an individual or a public health perspective.
REFERENCES
- 1.Brozmanova J, Manikova D, Vlckova V, Chovanec M. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010;84:919–938. doi: 10.1007/s00204-010-0595-8. [DOI] [PubMed] [Google Scholar]
- 2.Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MP, Horneber M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2011;5:CD005195. doi: 10.1002/14651858.CD005195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar M, Konieczka P, Namiesnik J. The properties, functions, and use of selenium compounds in living organisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30:225–252. doi: 10.1080/10590501.2012.705164. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrenner H, Speckmann B, Sies H. Towards understanding success and failures in the use of selenium for cancer prevention. Antioxid Redox Signal. 2013;19:181–191. doi: 10.1089/ars.2013.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinceti M, Maraldi T, Bergomi M, Malagoli C. Risk of chronic low-dose selenium overexposure in humans: insights from epidemiology and biochemistry. Rev Environ Health. 2009;24:231–248. doi: 10.1515/reveh.2009.24.3.231. [DOI] [PubMed] [Google Scholar]
- 6.Frost DV. The two faces of selenium—can selenophobia be cured? CRC Crit Rev Toxicol. 1972;1:467–514. doi: 10.3109/10408447209103467. [DOI] [PubMed] [Google Scholar]
- 7.Casey CE. Selenophilia. Proc Nutr Soc. 1988;47:55–62. doi: 10.1079/pns19880010. [DOI] [PubMed] [Google Scholar]
- 8.Vinceti M, Solovyev N, Mandrioli J, Crespi CM, Bonvicini F, Arcolin E, Georgoulopoulou E, Michalke B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology. 2013;38:25–32. doi: 10.1016/j.neuro.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD009671. doi: 10.1002/14651858.CD009671.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocourt CR, Wu M, Chen BP, Cheng WH. The catalytic subunit of DNA-dependent protein kinase is downstream of ATM and feeds forward oxidative stress in the selenium-induced senescence response. J Nutr Biochem. 2012;24:781–787. doi: 10.1016/j.jnutbio.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Sunde RA. Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Selenium. Modern Nutrition in Health and Disease. 11th Ed. Philadelphia: Lippincott Williams & Wilkins; 2012:225–237. [Google Scholar]
- 12.Nelson AA, Fitzhugh OG, Calvery HO. Liver tumors following cirrhosis caused by selenium in rats. Cancer Res. 1943;3:230–236. [Google Scholar]
- 13.Birt DF, Julius AD, Runice CE, White LT, Lawson T, Pour PM. Enhancement of BOP-induced pancreatic carcinogenesis in selenium-fed Syrian golden hamsters under specific dietary conditions. Nutr Cancer. 1988;11:21–33. doi: 10.1080/01635588809513966. [DOI] [PubMed] [Google Scholar]
- 14.National Toxicology Program. Selenium sulfide. Rep Carcinog. 2011;12:376–377. [PubMed] [Google Scholar]
- 15.Clayton CC, Baumann CA. Diet and azo dye tumors: effect of diet during a period when the dye is not fed. Cancer Res. 1949;9:575–582. [PubMed] [Google Scholar]
- 16.Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–726. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- 17.Csallany AS, Su LC, Menken BZ. Effect of selenite, vitamin E and N,N'-diphenyl-p-phenylenediamine on liver organic solvent-soluble lipofuscin pigments in mice. J Nutr. 1984;114:1582–1587. doi: 10.1093/jn/114.9.1582. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Jiang C, Kaeck M, Ganther H, Vadhanavikit S, Ip C, Thompson H. Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochem Pharmacol. 1995;50:213–219. doi: 10.1016/0006-2952(95)00119-k. [DOI] [PubMed] [Google Scholar]
- 19.Hatfield DL, Yoo MH, Carlson BA, Gladyshev VN. Selenoproteins that function in cancer prevention and promotion. Biochim Biophys Acta. 2009;1790:1541–1545. doi: 10.1016/j.bbagen.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasaikina MV, Lobanov AV, Malinouski MY, Lee BC, Seravalli J, Fomenko DE, et al. Reduced utilization of selenium by naked mole rats due to a specific defect in GPx1 expression. J Biol Chem. 2011;286:17005–17014. doi: 10.1074/jbc.M110.216267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamberger RJ, Frost DV. Possible protective effect of selenium against human cancer. Can Med Assoc J. 1969;100:682. [PMC free article] [PubMed] [Google Scholar]
- 22.Shamberger RJ, Tytko SA, Willis CE. Antioxidants and cancer. Part VI. Selenium and age-adjusted human cancer mortality. Arch Environ Health. 1976;31:231–235. doi: 10.1080/00039896.1976.10667225. [DOI] [PubMed] [Google Scholar]
- 23.Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies—III: statistical associations with dietary selenium intakes. Bioinorg Chem. 1977;7:23–31. doi: 10.1016/s0006-3061(00)80126-x. [DOI] [PubMed] [Google Scholar]
- 24.Jansson B, Jacobs MM, Griffin AC. Gastrointestinal cancer: epidemiology and experimental studies. Adv Exp Med Biol. 1978;91:305–321. doi: 10.1007/978-1-4684-0796-9_21. [DOI] [PubMed] [Google Scholar]
- 25.Cech I, Holguin A, Sokolow H, Smith V. Selenium availability in Texas: possible clinical significance. South Med J. 1984;77:1415–1420. doi: 10.1097/00007611-198411000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Clark LC, Cantor KP, Allaway WH. Selenium in forage crops and cancer mortality in U.S. counties. Arch Environ Health. 1991;46:37–42. doi: 10.1080/00039896.1991.9937427. [DOI] [PubMed] [Google Scholar]
- 27.Vinceti M, Rovesti S, Bergomi M, Vivoli G. The epidemiology of selenium and human cancer. Tumori. 2000;86:105–118. doi: 10.1177/030089160008600201. [DOI] [PubMed] [Google Scholar]
- 28.Guo W, Chow W, Zheng W, Li J, Blot WJ. Diet, serum markers and breast cancer mortality in China. Jpn J Cancer Res. 1994;85:572–577. doi: 10.1111/j.1349-7006.1994.tb02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semnani S, Roshandel G, Zendehbad A, Keshtkar A, Rahimzadeh H, Abdolahi N, Besharat S, Moradi A, Mirkarimi H, Hasheminasab S. Soils selenium level and esophageal cancer: an ecological study in a high risk area for esophageal cancer. J Trace Elem Med Biol. 2010;24:174–177. doi: 10.1016/j.jtemb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Lv J, Liao C. Dietary exposure estimates of 14 trace elements in Xuanwei and Fuyuan, two high lung cancer incidence areas in China. Biol Trace Elem Res. 2012;146:287–292. doi: 10.1007/s12011-011-9252-1. [DOI] [PubMed] [Google Scholar]
- 31.Krogh V, Pala V, Vinceti M, Berrino F, Ganzi A, Micheli A, et al. Toenail selenium as biomarker: reproducibility over a one-year period and factors influencing reproducibility. J Trace Elem Med Biol. 2003;17(Suppl 1):31–36. [PubMed] [Google Scholar]
- 32.Solovyev N, Berthele A, Michalke B. Selenium speciation in paired serum and cerebrospinal fluid samples. Anal Bioanal Chem. 2013;405:1875–1884. doi: 10.1007/s00216-012-6294-y. [DOI] [PubMed] [Google Scholar]
- 33.Swanson CA, Longnecker MP, Veillon C, Howe M, Levander OA, Taylor PR, McAdam PA, Brown CC, Stampfer MJ, Willett WC. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. Am J Clin Nutr. 1990;52:858–862. doi: 10.1093/ajcn/52.5.858. [DOI] [PubMed] [Google Scholar]
- 34.van den Brandt PA, Goldbohm RA, van't Veer P, Bode P, Hermus RJ, Sturmans F. Predictors of toenail selenium levels in men and women. Cancer Epidemiol Biomarkers Prev. 1993;2:107–112. [PubMed] [Google Scholar]
- 35.Ovaskainen ML, Virtamo J, Alfthan G, Haukka J, Pietinen P, Taylor PR, Huttunen JK. Toenail selenium as an indicator of selenium intake among middle-aged men in an area with low soil selenium. Am J Clin Nutr. 1993;57:662–665. doi: 10.1093/ajcn/57.5.662. [DOI] [PubMed] [Google Scholar]
- 36.Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7:384–390. doi: 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Pestitschek M, Sonneck-Koenne C, Zakavi SR, Li S, Knoll P, Mirzaei S. Selenium intake and selenium blood levels: a novel food frequency questionnaire. Wien Klin Wochenschr. 2013;125:160–164. doi: 10.1007/s00508-013-0334-2. [DOI] [PubMed] [Google Scholar]
- 38.Hunter DJ, Morris JS, Chute CG, Kushner E, Colditz GA, Stampfer MJ, Speizer FE, Willett WC. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132:114–122. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
- 39.Karita K, Sasaki S, Ishihara J, Tsugane S. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study to assess selenium intake: comparison with dietary records and blood levels. J Epidemiol. 2003;13:S92–97. doi: 10.2188/jea.13.1sup_92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satia JA, King IB, Morris JS, Stratton K, White E. Toenail and plasma levels as biomarkers of selenium exposure. Ann Epidemiol. 2006;16:53–58. doi: 10.1016/j.annepidem.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Salbe AD, Levander OA. Effect of various dietary factors on the deposition of selenium in the hair and nails of rats. J Nutr. 1990;120:200–206. doi: 10.1093/jn/120.2.200. [DOI] [PubMed] [Google Scholar]
- 42.Salbe AD, Levander OA. Comparative toxicity and tissue retention of selenium in methionine-deficient rats fed sodium selenate or L-selenomethionine. J Nutr. 1990;120:207–212. doi: 10.1093/jn/120.2.207. [DOI] [PubMed] [Google Scholar]
- 43.Panter KE, Hartley WJ, James LF, Mayland HF, Stegelmeier BL, Kechele PO. Comparative toxicity of selenium from seleno-DL-methionine, sodium selenate, and Astragalus bisulcatus in pigs. Fund Appl Toxicol. 1996;32:217–223. [PubMed] [Google Scholar]
- 44.Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr. 2009;89:2025S–2039S. doi: 10.3945/ajcn.2009.27230F. [DOI] [PubMed] [Google Scholar]
- 45.Maraldi T, Riccio M, Zambonin L, Vinceti M, De Pol A, Hakim G. Low levels of selenium compounds are selectively toxic for a human neuron cell line through ROS/RNS increase and apoptotic process activation. Neurotoxicology. 2011;32:180–187. doi: 10.1016/j.neuro.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Thomas JK, Janz DM. Dietary selenomethionine exposure in adult zebrafish alters swimming performance, energetics and the physiological stress response. Aquat Toxicol. 2011;102:79–86. doi: 10.1016/j.aquatox.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Zhang A, Li C, Zhang P, Su X, Li Y, Mu C, Li T. The link between selenium binding protein from Sinonovacula constricta and environmental pollutions exposure. Fish Shellfish Immunol. 2013;35:271–277. doi: 10.1016/j.fsi.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 48.Pacitti D, Wang T, Page MM, Martin SA, Sweetman J, Feldmann J, Secombes CJ. Characterization of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase genes in rainbow trout (Oncorhynchus mykiss) and their modulation by in vitro selenium exposure. Aquat Toxicol. 2013;130–131:97–111. doi: 10.1016/j.aquatox.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Lee EH, Myung SK, Jeon YJ, Kim Y, Chang YJ, Ju W, Seo HG, Huh BY. Effects of selenium supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutr Cancer. 2011;63:1185–1195. doi: 10.1080/01635581.2011.607544. [DOI] [PubMed] [Google Scholar]
- 50.Hurst R, Hooper L, Norat T, Lau R, Aune D, Greenwood DC, et al. Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr. 2012;96:111–122. doi: 10.3945/ajcn.111.033373. [DOI] [PubMed] [Google Scholar]
- 51.Takata Y, Kristal AR, King IB, Song X, Diamond AM, Foster CB, et al. Serum selenium, genetic variation in selenoenzymes, and risk of colorectal cancer: primary analysis from the Women's Health Initiative Observational Study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1822–1830. doi: 10.1158/1055-9965.EPI-11-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen RD, Albieri V, Tjonneland A, Overvad K, Andersen KK, Raaschou-Nielsen O. Effects of smoking and antioxidant micronutrients on risk of colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:406–415. e3. doi: 10.1016/j.cgh.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 53.Menkes MS, Comstock GW, Vuilleumier JP, Helsing KJ, Rider AA, Brookmeyer R. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N Engl J Med. 1986;315:1250–1254. doi: 10.1056/NEJM198611133152003. [DOI] [PubMed] [Google Scholar]
- 54.van Noord PA, Maas MJ, van der Tweel I, Collette C. Selenium and the risk of postmenopausal breast cancer in the DOM cohort. Breast Cancer Res Treat. 1993;25:11–19. doi: 10.1007/BF00662396. [DOI] [PubMed] [Google Scholar]
- 55.Garland M, Morris JS, Stampfer MJ, Colditz GA, Spate VL, Baskett CK, Rosner B, Speizer FE, Willett WC, Hunter DJ. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst. 1995;87:497–505. doi: 10.1093/jnci/87.7.497. [DOI] [PubMed] [Google Scholar]
- 56.Hartman TJ, Albanes D, Pietinen P, Hartman AM, Rautalahti M, Tangrea JA, Taylor PR. The association between baseline vitamin E, selenium, and prostate cancer in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 1998;7:335–340. [PubMed] [Google Scholar]
- 57.Ratnasinghe D, Tangrea JA, Forman MR, Hartman T, Gunter EW, Qiao YL, et al. Serum tocopherols, selenium and lung cancer risk among tin miners in China. Cancer Causes Control. 2000;11:129–135. doi: 10.1023/a:1008977320811. [DOI] [PubMed] [Google Scholar]
- 58.Suadicani P, Hein HO, Gyntelberg F. Serum selenium level and risk of lung cancer mortality: a 16-year follow-up of the Copenhagen Male Study. Eur Respir J. 2012;39:1443–1448. doi: 10.1183/09031936.00102711. [DOI] [PubMed] [Google Scholar]
- 59.Salonen JT, Alfthan G, Huttunen JK, Puska P. Association between serum selenium and the risk of cancer. Am J Epidemiol. 1984;120:342–349. doi: 10.1093/oxfordjournals.aje.a113898. [DOI] [PubMed] [Google Scholar]
- 60.Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Hakama M, Hakulinen T, Peto R, Teppo L. Serum selenium and subsequent risk of cancer among Finnish men and women. J Natl Cancer Inst. 1990;82:864–868. doi: 10.1093/jnci/82.10.864. [DOI] [PubMed] [Google Scholar]
- 61.Combs GF., Jr Selenium in global food systems. Br J Nutr. 2001;85:517–547. doi: 10.1079/bjn2000280. [DOI] [PubMed] [Google Scholar]
- 62.Valdiglesias V, Pasaro E, Mendez J, Laffon B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch Toxicol. 2010;84:337–351. doi: 10.1007/s00204-009-0505-0. [DOI] [PubMed] [Google Scholar]
- 63.Nogueira CW, Rocha JB. Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Arch Toxicol. 2011;85:1313–1359. doi: 10.1007/s00204-011-0720-3. [DOI] [PubMed] [Google Scholar]
- 64.Benko I, Nagy G, Tanczos B, Ungvari E, Sztrik A, Eszenyi P, Prokisch J, Banfalvi G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ Toxicol Chem. 2012;31:2812–2820. doi: 10.1002/etc.1995. [DOI] [PubMed] [Google Scholar]
- 65.Weekley CM, Aitken JB, Finney L, Vogt S, Witting PK, Harris HH. Selenium metabolism in cancer cells: the combined application of XAS and XFM techniques to the problem of selenium speciation in biological systems. Nutrients. 2013;5:1734–1756. doi: 10.3390/nu5051734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hazane-Puch F, Champelovier P, Arnaud J, Garrel C, Ballester B, Faure P, Laporte F. Long-term selenium supplementation in HaCaT cells: importance of chemical form for antagonist (protective versus toxic) activities. Biol Trace Elem Res. 2013;154:288–298. doi: 10.1007/s12011-013-9709-5. [DOI] [PubMed] [Google Scholar]
- 67.Borella P, Bargellini A, Medici CI. Chemical form of selenium greatly affects metal uptake and responses by cultured human lymphocytes. Biol Trace Elem Res. 1996;51:43–54. doi: 10.1007/BF02790146. [DOI] [PubMed] [Google Scholar]
- 68.Kim CY, Kim GN, Wiacek JL, Chen CY, Kim KH. Selenate inhibits adipogenesis through induction of transforming growth factor-beta1 (TGF-beta1) signaling. Biochem Biophys Res Commun. 2012;426:551–557. doi: 10.1016/j.bbrc.2012.08.125. [DOI] [PubMed] [Google Scholar]
- 69.Kipp AP, Frombach J, Deubel S, Brigelius-Flohe R. Selenoprotein w as biomarker for the efficacy of selenium compounds to act as source for selenoprotein biosynthesis. Methods Enzymol. 2013;527:87–112. doi: 10.1016/B978-0-12-405882-8.00005-2. [DOI] [PubMed] [Google Scholar]
- 70.Davis TZ, Stegelmeier BL, Welch KD, Pfister JA, Panter KE, Hall JO. Comparative oral dose toxicokinetics of selenium compounds commonly found in selenium accumulator plants. J Anim Sci. 2013;91:4501–4509. doi: 10.2527/jas.2012-6101. [DOI] [PubMed] [Google Scholar]
- 71.Zwolak I, Zaporowska H. Selenium interactions and toxicity: a review: Selenium interactions and toxicity. Cell Biol Toxicol. 2012;28:31–46. doi: 10.1007/s10565-011-9203-9. [DOI] [PubMed] [Google Scholar]
- 72.George CM, Gamble M, Slavkovich V, Levy D, Ahmed A, Ahsan H, Graziano J. A cross-sectional study of the impact of blood selenium on blood and urinary arsenic concentrations in Bangladesh. Environ Health. 2013;12:52. doi: 10.1186/1476-069X-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiobara Y, Yoshida T, Suzuki KT. Effects of dietary selenium species on Se concentrations in hair, blood, and urine. Toxicol Appl Pharmacol. 1998;152:309–314. doi: 10.1006/taap.1998.8537. [DOI] [PubMed] [Google Scholar]
- 74.Behne D, Gessner H, Kyriakopoulos A. Information on the selenium status of several body compartments of rats from the selenium concentrations in blood fractions, hair and nails. J Trace Elem Med Biol. 1996;10:174–179. doi: 10.1016/S0946-672X(96)80029-0. [DOI] [PubMed] [Google Scholar]
- 75.Scharpf M, Schweizer U, Arzberger T, Roggendorf W, Schomburg L, Kohrle J. Neuronal and ependymal expression of selenoprotein P in the human brain. J Neural Transm. 2007;114:877–884. doi: 10.1007/s00702-006-0617-0. [DOI] [PubMed] [Google Scholar]
- 76.Antunes Soares F, Farina M, Boettcher AC, Braga AL, Batista TRJ. Organic and inorganic forms of selenium inhibited differently fish (Rhamdia quelen) and rat (Rattus norvergicus albinus) delta-aminolevulinate dehydratase. Environ Res. 2005;98:46–54. doi: 10.1016/j.envres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 77.Xiao R, Qiao JT, Zhao HF, Liang J, Yu HL, Liu J, Guo AM, Wang W. Sodium selenite induces apoptosis in cultured cortical neurons with special concomitant changes in expression of the apoptosis-related genes. Neurotoxicology. 2006;27:478–484. doi: 10.1016/j.neuro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 78.Ayaz M, Dalkilic N, Tuncer S, Bariskaner H. Selenium-induced changes on rat sciatic nerve fibers: compound action potentials. Methods Find Exp Clin Pharmacol. 2008;30:271–275. doi: 10.1358/mf.2008.30.4.1166220. [DOI] [PubMed] [Google Scholar]
- 79.Nischwitz V, Berthele A, Michalke B. Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinal fluid samples: an approach to investigate the permeability of the human blood-cerebrospinal fluid-barrier. Anal Chim Acta. 2008;627:258–269. doi: 10.1016/j.aca.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 80.Michalke B, Halbach S, Nischwitz V. JEM spotlight: metal speciation related to neurotoxicity in humans. J Environ Monit. 2009;11:939–954. doi: 10.1039/b817817h. [DOI] [PubMed] [Google Scholar]
- 81.Steinbrecher A, Meplan C, Hesketh J, Schomburg L, Endermann T, Jansen E, Akesson B, Rohrmann S, Linseisen J. Effects of selenium status and polymorphisms in selenoprotein genes on prostate cancer risk in a prospective study of European men. Cancer Epidemiol Biomarkers Prev. 2010;19:2958–2968. doi: 10.1158/1055-9965.EPI-10-0364. [DOI] [PubMed] [Google Scholar]
- 82.Slattery ML, Lundgreen A, Welbourn B, Corcoran C, Wolff RK. Genetic variation in selenoprotein genes, lifestyle, and risk of colon and rectal cancer. PLoS One. 2012;7:e37312. doi: 10.1371/journal.pone.0037312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meplan C, Hesketh J. The influence of selenium and selenoprotein gene variants on colorectal cancer risk. Mutagenesis. 2012;27:177–186. doi: 10.1093/mutage/ger058. [DOI] [PubMed] [Google Scholar]
- 84.Jablonska E, Gromadzinska J, Sobala W, Reszka E, Wasowicz W. Lung cancer risk associated with selenium status is modified in smoking individuals by Sep15 polymorphism. Eur J Nutr. 2008;47:47–54. doi: 10.1007/s00394-008-0696-9. [DOI] [PubMed] [Google Scholar]
- 85.Chan JM, Oh WK, Xie W, Regan MM, Stampfer MJ, King IB, Abe M, Kantoff PW. Plasma selenium, manganese superoxide dismutase, and intermediate- or high-risk prostate cancer. J Clin Oncol. 2009;27:3577–3583. doi: 10.1200/JCO.2008.18.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: mortality rates by vitamin-mineral intervention group. Am J Clin Nutr. 1995;62:1424S–1426S. doi: 10.1093/ajcn/62.6.1424S. [DOI] [PubMed] [Google Scholar]
- 87.Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098–2105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- 88.Ezzedine K, Latreille J, Kesse-Guyot E, Galan P, Hercberg S, Guinot C, Malvy D. Incidence of skin cancers during 5-year follow-up after stopping antioxidant vitamins and mineral supplementation. Eur J Cancer. 2010;46:3316–3322. doi: 10.1016/j.ejca.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 89.Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, Sun PH, Xie XY. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J (Engl) 2004;117:1155–1160. [PubMed] [Google Scholar]
- 90.Yu SY, Zhu YJ, Li WG, Huang QS, Huang CZ, Zhang QN, Hou C. A preliminary report on the intervention trials of primary liver cancer in high-risk populations with nutritional supplementation of selenium in China. Biol Trace Elem Res. 1991;29:289–294. doi: 10.1007/BF03032685. [DOI] [PubMed] [Google Scholar]
- 91.Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56:117–124. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 92.Li W, Zhu Y, Yan X, Zhang Q, Li X, Ni Z, Shen Z, Yao H, Zhu J. [The prevention of primary liver cancer by selenium in high risk populations] Zhonghua Yu Fang Yi Xue Za Zhi. 2000;34:336–338. [PubMed] [Google Scholar]
- 93.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GFJ, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 94.Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF, Jr, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 95.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, Marshall JR, Clark LC. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 96.Marshall JR, Tangen CM, Sakr WA, Wood DP, Jr, Berry DL, Klein EA, et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila) 2011;4:1761–1769. doi: 10.1158/1940-6207.CAPR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, Thompson PA, et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dreno B, Euvrard S, Frances C, Moyse D, Nandeuil A. Effect of selenium intake on the prevention of cutaneous epithelial lesions in organ transplant recipients. Eur J Dermatol. 2007;17:140–145. doi: 10.1684/ejd.2007.0127. [DOI] [PubMed] [Google Scholar]
- 99.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lubinski J, Jaworska K, Durda K, Jakubowska A, Huzarski T, Byrski T, et al. Selenium and the risk of cancer in BRCA1 carriers. Hered Cancer Clin Pract. 2011;9(Suppl 2):A5. doi: 10.1186/1897-4287-9-S2-A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark LC, Combs GFJ, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 103.Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;11:1285–1291. [PubMed] [Google Scholar]
- 104.Reid ME, Stratton MS, Lillico AJ, Fakih M, Natarajan R, Clark LC, Marshall JR. A report of high-dose selenium supplementation: response and toxicities. J Trace Elem Med Biol. 2004;18:69–74. doi: 10.1016/j.jtemb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 105.Dalkin BW, editor; Lillico AJ, editor; Reid ME, editor; Jacobs ET, editor; Combs GF, editor; Slate E, editor; Turnbull BW, Marshall JR, editors. Selenium and chemoprevention against prostate cancer: an update of the Clark results. 92nd Meeting of the American Association for Cancer Research. New Orleans, LA: American Association for Cancer Research; 2001. [Google Scholar]
- 106.Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, Rosner B, Taylor JO, Schneider K, Hames CG. Prediagnostic serum selenium and risk of cancer. Lancet. 1983;2:130–134. doi: 10.1016/s0140-6736(83)90116-2. [DOI] [PubMed] [Google Scholar]
- 107.Salonen JT, Salonen R, Lappetelainen R, Maenpaa PH, Alfthan G, Puska P. Risk of cancer in relation to serum concentrations of selenium and vitamins A and E: matched case-control analysis of prospective data. Br Med J. 1985;290:417–420. doi: 10.1136/bmj.290.6466.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peleg I, Morris S, Hames CG. Is serum selenium a risk factor for cancer? Med Oncol Tumor Pharmacother. 1985;2:157–163. doi: 10.1007/BF02934543. [DOI] [PubMed] [Google Scholar]
- 109.Kok FJ, de Bruijn AM, Hofman A, Vermeeren R, Valkenburg HA. Is serum selenium a risk factor for cancer in men only? Am J Epidemiol. 1987;125:12–16. doi: 10.1093/oxfordjournals.aje.a114493. [DOI] [PubMed] [Google Scholar]
- 110.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr, Kristal AR, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 111.Goodman PJ, Hartline JA, Tangen CM, Crowley JJ, Minasian LM, Klein EA, et al. Moving a randomized clinical trial into an observational cohort. Clin Trials. 2013;10:131–142. doi: 10.1177/1740774512460345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kristal AR, Lippman SM. Nutritional prevention of cancer: new directions for an increasingly complex challenge. J Natl Cancer Inst. 2009;101:363–365. doi: 10.1093/jnci/djp029. [DOI] [PubMed] [Google Scholar]
- 113.Lotan Y, Goodman PJ, Youssef RF, Svatek RS, Shariat SF, Tangen CM, Thompson IM, Jr, Klein EA. Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT. J Urol. 2012;187:2005–2010. doi: 10.1016/j.juro.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 115.Moyad MA. Heart healthy = prostate healthy: SELECT, the symbolic end of preventing prostate cancer via heart unhealthy and over anti-oxidation mechanisms? Asian J Androl. 2012;14:243–244. doi: 10.1038/aja.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haldimann M, Venner TY, Zimmerli B. Determination of selenium in the serum of healthy Swiss adults and correlation to dietary intake. J Trace Elem Med Biol. 1996;10:31–45. doi: 10.1016/S0946-672X(96)80006-X. [DOI] [PubMed] [Google Scholar]
- 117.World Health Organization. Trace elements in human nutrition and health. Geneva, Switzerland: WHO press; 1996. [Google Scholar]
- 118.Leblanc JC, Guerin T, Noel L, Calamassi-Tran G, Volatier JL, Verger P. Dietary exposure estimates of 18 elements from the 1st French Total Diet Study. Food Addit Contam. 2005;22:624–641. doi: 10.1080/02652030500135367. [DOI] [PubMed] [Google Scholar]
- 119.Vinceti M, Nacci G, Rocchi E, Cassinadri T, Vivoli R, Marchesi C, Bergomi M. Mortality in a population with long-term exposure to inorganic selenium via drinking water. J Clin Epidemiol. 2000;53:1062–1068. doi: 10.1016/s0895-4356(00)00233-x. [DOI] [PubMed] [Google Scholar]
- 120.Marshall JR, Reid ME, Lillico A. Nutritional prevention of cancer: review of glaucoma data. Tucson: University of Arizona; 2002. [Google Scholar]
- 121.Vinceti M, Rothman KJ, Bergomi M, Borciani N, Serra L, Vivoli G. Excess melanoma incidence in a cohort exposed to high levels of environmental selenium. Cancer Epidemiol Biomarkers Prev. 1998;7:853–856. [PubMed] [Google Scholar]
- 122.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 123.Reid ME, Duffield-Lillico AJ, Slate E, Natarajan N, Turnbull B, Jacobs E, Combs GF, Jr, Alberts DS, Clark LC, Marshall JR. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155–163. doi: 10.1080/01635580701684856. [DOI] [PubMed] [Google Scholar]
- 124.Fleshner NE, Kapusta L, Donnelly B, Tanguay S, Chin J, Hersey K, et al. Progression from high-grade prostatic intraepithelial neoplasia to cancer: a randomized trial of combination vitamin-E, soy, and selenium. J Clin Oncol. 2011;29:2386–2390. doi: 10.1200/JCO.2010.32.0994. [DOI] [PubMed] [Google Scholar]
- 125.Stratton MS, Reid ME, Schwartzberg G, Minter FE, Monroe BK, Alberts DS, Marshall JR, Ahmann FR. Selenium and prevention of prostate cancer in high-risk men: the Negative Biopsy Study. Anticancer Drugs. 2003;14:589–594. doi: 10.1097/00001813-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 126.Penney KL, Schumacher FR, Li H, Kraft P, Morris JS, Kurth T, Mucci LA, Hunter DJ, Kantoff PW, Stampfer MJ, Ma J. A large prospective study of SEP15 genetic variation, interaction with plasma selenium levels, and prostate cancer risk and survival. Cancer Prev Res (Phila) 2010;3:604–610. doi: 10.1158/1940-6207.CAPR-09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, Leitzmann MF. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–764. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer Causes Control. 2009;20:691–698. doi: 10.1007/s10552-008-9282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rothman KJ, Greenland S, Lash TL. In: Modern Epidemiology. Edition T, editor. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 130.Vinceti M, Rovesti S, Gabrielli C, Marchesi C, Bergomi M, Martini M, Vivoli G. Cancer mortality in a residential cohort exposed to environmental selenium through drinking water. J Clin Epidemiol. 1995;48:1091–1097. doi: 10.1016/0895-4356(95)00014-u. [DOI] [PubMed] [Google Scholar]
- 131.Ammar EM, Couri D. Acute toxicity of sodium selenite and selenomethionine in mice after ICV or IV administration. Neurotoxicology. 1981;2:383–386. [PubMed] [Google Scholar]
- 132.Vinceti M, Crespi CM, Bonvicini F, Malagoli C, Ferrante M, Marmiroli S, Stranges S. The need for a reassessment of the safe upper limit of selenium in drinking water. Sci Total Environ. 2013;443:633–642. doi: 10.1016/j.scitotenv.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 133.Institute of Medicine. Dietary references intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 134.Nordic Nutrition Recommendations 2004. 4th Edition ed. Copenhagen: Nordic Council of Ministers; 2004. [Google Scholar]
- 135.Levander OA. Selenium requirements as discussed in the 1996 joint FAO/IAEA/WHO expert consultation on trace elements in human nutrition. Biomed Environ Sci. 1997;10:214–219. [PubMed] [Google Scholar]
- 136.Reszka E, Jablonska E, Gromadzinska J, Wasowicz W. Relevance of selenoprotein transcripts for selenium status in humans. Genes Nutr. 2012;7:127–137. doi: 10.1007/s12263-011-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Macallan DC, Sedgwick P. Selenium supplementation and selenoenzyme activity. Clin Sci. 2000;99:579–281. doi: 10.1042/cs0990579. [DOI] [PubMed] [Google Scholar]
- 138.Brätter P, Negretti de Brätter VE. Influence of high dietary intake on the thyroid hormone level in human serum. J Trace Elem Med Biol. 1996;10:163–166. doi: 10.1016/s0946-672x(96)80027-7. [DOI] [PubMed] [Google Scholar]
- 139.Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, Muti P, Berrino F, Krogh V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health. 2010;10:564. doi: 10.1186/1471-2458-10-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 141.Kramer GF, Ames BN. Mechanisms of mutagenicity and toxicity of sodium selenite (Na2SeO3) in Salmonella typhimurium. Mutat Res. 1988;201:169–180. doi: 10.1016/0027-5107(88)90123-6. [DOI] [PubMed] [Google Scholar]
- 142.Sinha R, Sinha I, Facompre N, Russell S, Somiari RI, Richie JP, Jr, El-Bayoumy K. Selenium-responsive proteins in the sera of selenium-enriched yeast-supplemented healthy African American and Caucasian men. Cancer Epidemiol Biomarkers Prev. 2010;19:2332–2340. doi: 10.1158/1055-9965.EPI-10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Loison F, Debure L, Nizard P, le Goff P, Michel D, le Drean Y. Up-regulation of the clusterin gene after proteotoxic stress: implication of HSF1-HSF2 heterocomplexes. Biochem J. 2006;395:223–231. doi: 10.1042/BJ20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trougakos IP. The molecular chaperone apolipoprotein J/clusterin as a sensor of oxidative stress: implications in therapeutic approaches—a mini-review. Gerontology. 2013. (in press) [DOI] [PubMed]
- 145.Chen Q, Wang Z, Xiong Y, Zou X, Liu Z. Comparative study of p38 MAPK signal transduction pathway of peripheral blood mononuclear cells from patients with coalcombustion-type fluorosis with and without high hair selenium levels. Int J Hyg Environ Health. 2010;213:381–386. doi: 10.1016/j.ijheh.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Karunasinghe N, Han DY, Zhu S, Yu J, Lange K, Duan H, et al. Serum selenium and single-nucleotide polymorphisms in genes for selenoproteins: relationship to markers of oxidative stress in men from Auckland, New Zealand. Genes Nutr. 2012;7:179–190. doi: 10.1007/s12263-011-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koyama H, Mutakin, Abdulah R, Yamazaki C, Kameo S. Selenium supplementation trials for cancer prevention and the subsequent risk of type 2 diabetes mellitus. Nihon Eiseigaku Zasshi. 2013;68:1–10. doi: 10.1265/jjh.68.1. [DOI] [PubMed] [Google Scholar]
- 148.Lillico A, Jacobs E, Reid ME. Selenium supplementations and risk of glaucoma in the NPC trial. Tucson: University of Arizona; 2002. [Google Scholar]
- 149.Bruhn RL, Stamer WD, Herrygers LA, Levine JM, Noecker RJ. Relationship between glaucoma and selenium levels in plasma and aqueous humour. Br J Ophthalmol. 2009;93:1155–1158. doi: 10.1136/bjo.2007.125997. [DOI] [PubMed] [Google Scholar]
- 150.Vinceti M, Bonvicini F, Rothman KJ, Vescovi L, Wang F. The relation between amyotrophic lateral sclerosis and inorganic selenium in drinking water: a population-based case-control study. Environ Health. 2010;9:77. doi: 10.1186/1476-069X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conley SM, Bruhn RL, Morgan PV, Stamer WD. Selenium's effects on MMP-2 and TIMP-1 secretion by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2004;45:473–479. doi: 10.1167/iovs.03-0767. [DOI] [PubMed] [Google Scholar]
- 152.Casteignau A, Fontan A, Morillo A, Oliveros JA, Segales J. Clinical, pathological and toxicological findings of a iatrogenic selenium toxicosis case in feeder pigs. J Vet Med A Physiol Pathol Clin Med. 2006;53:323–326. doi: 10.1111/j.1439-0442.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 153.Tsunoda M, Johnson VJ, Sharma RP. Increase in dopamine metabolites in murine striatum after oral exposure to inorganic but not organic form of selenium. Arch Environ Contam Toxicol. 2000;39:32–37. doi: 10.1007/s002440010076. [DOI] [PubMed] [Google Scholar]
- 154.Vinceti M, Guidetti D, Pinotti M, Rovesti S, Merlin M, Vescovi L, Bergomi M, Vivoli G. Amyotrophic lateral sclerosis after long-term exposure to drinking water with high selenium content. Epidemiology. 1996;7:529–532. [PubMed] [Google Scholar]
- 155.Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G. Adverse health effects of selenium in humans. Rev Environ Health. 2001;16:233–251. doi: 10.1515/reveh.2001.16.4.233. [DOI] [PubMed] [Google Scholar]
- 156.Ravn-Haren G, Krath BN, Overvad K, Cold S, Moesgaard S, Larsen EH, Dragsted LO. Effect of long-term selenium yeast intervention on activity and gene expression of antioxidant and xenobiotic metabolising enzymes in healthy elderly volunteers from the Danish Prevention of Cancer by Intervention by Selenium (PRECISE) pilot study. Br J Nutr. 2008;99:1190–1198. doi: 10.1017/S0007114507882948. [DOI] [PubMed] [Google Scholar]
- 157.Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Despres C, Gingras S, Muckle G. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology. 2006;27:567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 158.Berthold HK, Michalke B, Krone W, Guallar E, Gouni-Berthold I. Influence of serum selenium concentrations on hypertension: the Lipid Analytic Cologne cross-sectional study. J Hypertens. 2012;30:1328–1335. doi: 10.1097/HJH.0b013e32835414df. [DOI] [PubMed] [Google Scholar]
- 159.Platz EA, Lippman SM. Selenium, genetic variation, and prostate cancer risk: epidemiology reflects back on selenium and vitamin E cancer prevention trial. J Clin Oncol. 2009;27:3569–3572. doi: 10.1200/JCO.2009.22.2117. [DOI] [PubMed] [Google Scholar]
- 160.Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011;364:1920–1931. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 161.Goossens ME, Buntinx F, Joniau S, Ackaert K, Ameye F, Billiet I, et al. Designing the selenium and bladder cancer trial (SELEBLAT), a phase lll randomized chemoprevention study with selenium on recurrence of bladder cancer in Belgium. BMC Urol. 2012;12:8. doi: 10.1186/1471-2490-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bhattacharya A, Turowski SG, San Martin ID, Rajput A, Rustum YM, Hoffman RM, Seshadri M. Magnetic resonance and fluorescence-protein imaging of the anti-angiogenic and antitumor efficacy of selenium in an orthotopic model of human colon cancer. Anticancer Res. 2011;31:387–393. [PMC free article] [PubMed] [Google Scholar]
- 163.Desai D, Sinha I, Null K, Wolter W, Suckow MA, King T, Amin S, Sinha R. Synthesis and antitumor properties of selenocoxib-1 against rat prostate adenocarcinoma cells. Int J Cancer. 2010;127:230–238. doi: 10.1002/ijc.25033. [DOI] [PubMed] [Google Scholar]
- 164.Nguyen N, Sharma A, Sharma AK, Desai D, Huh SJ, Amin S, Meyers C, Robertson GP. Melanoma chemoprevention in skin reconstructs and mouse xenografts using isoselenocyanate-4. Cancer Prev Res (Phila) 2011;4:248–258. doi: 10.1158/1940-6207.CAPR-10-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng Y, Sk UH, Zhang Y, Ren X, Zhang L, Huber-Keener KJ, et al. Rational incorporation of selenium into temozolomide elicits superior antitumor activity associated with both apoptotic and autophagic cell death. PLoS One. 2012;7:e35104. doi: 10.1371/journal.pone.0035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qi Y, Fu X, Xiong Z, Zhang H, Hill SM, Rowan BG, Dong Y. Methylseleninic acid enhances paclitaxel efficacy for the treatment of triple-negative breast cancer. PLoS One. 2012;7:e31539. doi: 10.1371/journal.pone.0031539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng S, Li X, Zhang Y, Xie Q, Wong YS, Zheng W, Chen T. PEG-nanolized ultrasmall selenium nanoparticles overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int J Nanomedicine. 2012;7:3939–3949. doi: 10.2147/IJN.S30940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gowda R, Madhunapantula SV, Desai D, Amin S, Robertson GP. Simultaneous targeting of COX-2 and AKT using selenocoxib-1-GSH to inhibit melanoma. Mol Cancer Ther. 2013;12:3–15. doi: 10.1158/1535-7163.MCT-12-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li F, Jiang Q, Shi KJ, Luo H, Yang Y, Xu CM. RhoA modulates functional and physical interaction between ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells. Cell Death Dis. 2013;4:e708. doi: 10.1038/cddis.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dos Santos ED, Hamel E, Bai R, Burnett JC, Tozatti CS, Bogo D, et al. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorg Med Chem Lett. 2013;23:4669–4673. doi: 10.1016/j.bmcl.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang Y, He L, Liu W, Fan C, Zheng W, Wong YS, Chen T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials. 2013;34:7106–7116. doi: 10.1016/j.biomaterials.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 172.Kim YW, Bae SM, Liu HB, Kim IW, Chun HJ, Ahn WS. Selenium enhances the efficacy of Radachlorin mediated-photodynamic therapy in TC-1 tumor development. Oncol Rep. 2012;28:576–584. doi: 10.3892/or.2012.1820. [DOI] [PubMed] [Google Scholar]
- 173.Chintala S, Najrana T, Toth K, Cao S, Durrani FA, Pili R, Rustum YM. Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition. BMC Cancer. 2012;12:293. doi: 10.1186/1471-2407-12-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sonaa E, Usha S, Ja In J. An ex vivo study of selenium, genistein on the morphological and nuclear changes in anticancer drug-induced apoptosis in human peripheral blood lymphocytes. Biofactors. 2013;39:279–293. doi: 10.1002/biof.1069. [DOI] [PubMed] [Google Scholar]
- 175.Zeng H, Briske-Anderson M, Wu M, Moyer MP. Methylselenol, a selenium metabolite, plays common and different roles in cancerous colon HCT116 cell and non-cancerous NCM460 colon cell proliferation. Nutr Cancer. 2012;64:128–135. doi: 10.1080/01635581.2012.630555. [DOI] [PubMed] [Google Scholar]
- 176.Weiller M, Latta M, Kresse M, Lucas R, Wendel A. Toxicity of nutritionally available selenium compounds in primary and transformed hepatocytes. Toxicology. 2004;201:21–30. doi: 10.1016/j.tox.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 177.Asfour IA, El-Tehewi MM, Ahmed MH, Abdel-Sattar MA, Moustafa NN, Hegab HM, Fathey OM. High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin's lymphoma. Biol Trace Elem Res. 2009;127:200–210. doi: 10.1007/s12011-008-8240-6. [DOI] [PubMed] [Google Scholar]
- 178.Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, Belinsky SA, Johnson DH, Johnston MR, Goodman G, Clamon G, Okawara G, Marks R, Frechette E, McCaskill-Stevens W, Lippman SM, Ruckdeschel J, Khuri FR. Randomized, Double-Blind, Placebo-Controlled, Phase III Chemoprevention Trial of Selenium Supplementation in Patients With Resected Stage I Non-Small-Cell Lung Cancer: ECOG 5597. J Clin Oncol. 2013. (in press) [DOI] [PMC free article] [PubMed]
- 179.Niraula S, Pond G, de Wit R, Eisenberger M, Tannock IF, Joshua AM. Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study. Can Urol Assoc J. 2013;7:E74–81. doi: 10.5489/cuaj.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 181.Buntzel J, Riesenbeck D, Glatzel M, Berndt-Skorka R, Riedel T, Mucke R, et al. Limited effects of selenium substitution in the prevention of radiation-associated toxicities. results of a randomized study in head and neck cancer patients. Anticancer Res. 2010;30:1829–1832. [PubMed] [Google Scholar]
- 182.Chen YC, Prabhu KS, Das A, Mastro AM. Dietary selenium supplementation modifies breast tumor growth and metastasis. Int J Cancer. 2013;133:2054–2064. doi: 10.1002/ijc.28224. [DOI] [PubMed] [Google Scholar]
- 183.Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev. 2004;13:771–778. [PubMed] [Google Scholar]
- 184.Cortes-Jofre M, Rueda JR, Corsini-Munoz G, Fonseca-Cortes C, Caraballoso M, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2012;10:CD002141. doi: 10.1002/14651858.CD002141.pub2. [DOI] [PubMed] [Google Scholar]
- 185.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008. p. CD007176. [DOI] [PubMed]
- 186.Marik PE, Flemmer M. Do dietary supplements have beneficial health effects in industrialized nations: what is the evidence? JPEN J Parenter Enteral Nutr. 2012;36:159–168. doi: 10.1177/0148607111416485. [DOI] [PubMed] [Google Scholar]
- 187.Mayne ST, Ferrucci LM, Cartmel B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annu Rev Nutr. 2012;32:369–390. doi: 10.1146/annurev-nutr-071811-150659. [DOI] [PubMed] [Google Scholar]


