Abstract
Objectives
The blood-brain barrier is a selective diffusion barrier between brain parenchyma and the intravascular compartment. Tight junctions (TJs) are integral components of the blood-brain barrier. Pro-inflammatory cytokines are important in the pathogenesis of brain injury and could modify the protein constituents of TJs. We hypothesized that IL-6 down-regulates key protein constituents of endothelial TJs (e.g., occludin and claudin-5).
Methods
We examined the effects of IL-6 on TJ protein expression using an in vitro blood-brain barrier model. We isolated microvessels from yearling and adult ovine cerebral cortex and placed them into culture with IL-6 concentrations of 0 (control, phosphate buffered saline), 1, 10, and 100 ng/mL for 24 hours. Cerebral microvessels were harvested, Western immunoblot performed for occludin and claudin-5, densitometry performed, and results expressed as a ratio to control values.
Results
Western immunoblot analysis showed that treatment with 100 ng/ml of IL-6, but not the lower concentrations, reduced (P<0.05) occludin expression in microvessels from yearling and adult sheep, and claudin-5 in microvessels from adult sheep However, treatment with 10 ng/ml of IL-6 increased claudin-5 in microvessels from yearling sheep. The percent of lactate dehydrogenase released from the microvessels into the surrounding media was not increased by IL-6 treatment, suggesting that the reductions in TJ proteins did not result from cell death. Treatment of adult cerebral cortical microvessels with IL-6 pre-incubated with anti-IL-6 monoclonal antibodies partially attenuated the reduction in claudin-5.
Conclusion
We conclude that IL-6 modulates tight junction protein expression in cerebral cortical microvessels from yearling and adult sheep.
Keywords: blood-brain barrier, cerebral microvessels, IL-6, tight junction proteins, sheep
Introduction
The blood-brain barrier is a selective diffusion barrier that maintains central nervous system (CNS) homeostasis and limits the entry of substances that could alter neuronal function. This structure is composed of specialized endothelial cells that line cerebral microvessels and possess a low non-specific permeability to polar substances [1]. The cerebral microvascular endothelium, together with associated astrocytes, pericytes, neurons, and the components of the extracellular matrix, constitutes the neurovascular unit [1,2]. The neurovascular unit is essential for the health and function of the CNS in adult subjects and during development [2].
Although all components of the neurovascular unit, including the cellular constituents and basement membrane, contribute to the integrity of the blood-brain barrier, tight junctions of the endothelial cells represent the primary restrictive barrier [1,2]. Tight junction complexes form a continuous circumferential belt that separates the apical and basolateral plasma membrane domains, thus forming a fence within the plasma membrane [3,4]. Occludin and claudin are key transmembrane proteins that form the physical barrier characteristic of tight junctions [5] and serve to restrict paracellular diffusion between endothelial cells [2]. It is becoming increasingly apparent that blood-brain barrier dysfunction at the level of the tight junction is a critical event in the development and progression of several disorders that affect the CNS [2].
The role of pro-inflammatory cytokines in brain injury has become evident in many neurological disorders in the adult, such as Alzheimer’s disease, Parkinson’s disease, prion diseases, and AIDS-related dementia [6]. Additionally, Leviton et al, in a seminal study, showed that infants with postmortem bacteremia were more likely to exhibit brain damage than infants whose blood cultures were sterile, and postulated that noninfectious circulating inflammatory products could result in brain damage [7]. Furthermore, intrauterine infection or maternal chorioamnionitis has also been associated with umbilical cord endothelial activation, with upregulation of cell adhesion molecules, and systemic elevations in IL-6 levels in infants [8–10]. Importantly, elevated IL-6 levels have consistently been associated with brain damage in infants [9,11–13].
The blood-brain barrier is also an interactive interface that regulates the ways in which the CNS and the immune system communicate with one another [6]. Previous in vitro work has shown that pro-inflammatory cytokines can increase the permeability of some endothelial barriers [14–16]. Specifically, IL-6 has been shown to increase the permeability of bovine derived aortic endothelial monolayers [14] and confluent human umbilical vein endothelial cell monolayers in vitro [17].
Successful isolation of the brain microvasculature and development of in vitro models of the blood-brain barrier has facilitated understanding of the molecular characteristics of the blood-brain barrier. In fact, cerebral microvessels have been used as a reliable in vitro model of the blood-brain barrier and have been isolated from brain of a variety of animals including rat, bovine, and sheep [18–22]. Microvessel capillary fragments consist of endothelial cells ensheathed by basement membranes which contain pericytes to which astrocytic foot processes and nerve ending remnants can adhere, thus retaining many of the properties of the neurovascular unit [23]. In this model, three-dimensional aspects of the blood-brain barrier remain intact and several elements of the neurovascular unit may be maintained, unlike endothelial monolayers.
Sheep have been used extensively to investigate many aspects of CNS homeostasis [24,25]. The development of the ovine brain is similar to that of the human infant with respect to completion of neurogenesis, cerebral sulcation, and detection of the cortical component of auditory and somatosensory evoked potentials [26–28]. In addition, we have previously characterized the development of blood-brain barrier function in sheep [25]. Moreover, the brains of higher-level mammals, including humans and sheep, have extensive gyrations in order to maximize cortical surface area, whereas the rodent brain is almost completely agyric. Although microvessels have been isolated from a number of species, only a few studies have previously reported the use of microvessels isolated from ovine brain [19,20 ]. As in the bovine model, the ovine brain provides an ample amount of cerebral cortical tissue for isolation of microvessels in sufficient quantities for complex in vitro studies.
In the current study, we isolated microvessels from yearling and adult sheep to compliment our previous in vivo work [25,29]. We examined the effects of the pro-inflammatory cytokine, interleukin-6, on tight junction protein expression using in vitro microvessels, and tested the hypothesis that that IL-6 down-regulates key protein constituents of endothelial tight junctions.
Methods
Cerebral Cortical Microvessel Isolation
For each experimental procedure described below, the brains from five adult and five yearling sheep were obtained from a local supplier and transported to the laboratory in a cold transport solution (480 ml phosphate buffered saline, PBS, Bio-Rad Laboratories, Hercules, CA, USA; 10 ml Penicillin-Streptomycin liquid, GIBCO, Invitrogen, Carlsbad, CA, USA; and 10 ml Fungisone ,GIBCO, Invitrogen, Carlsbad, CA, USA). Based upon documentation by the farmers and dating by the supplier, the adult sheep were at least three years of age and the yearling sheep less than one year of age. Yearling sheep were used because they were commercially readily available and reflected a developmentally younger population than the adult sheep.
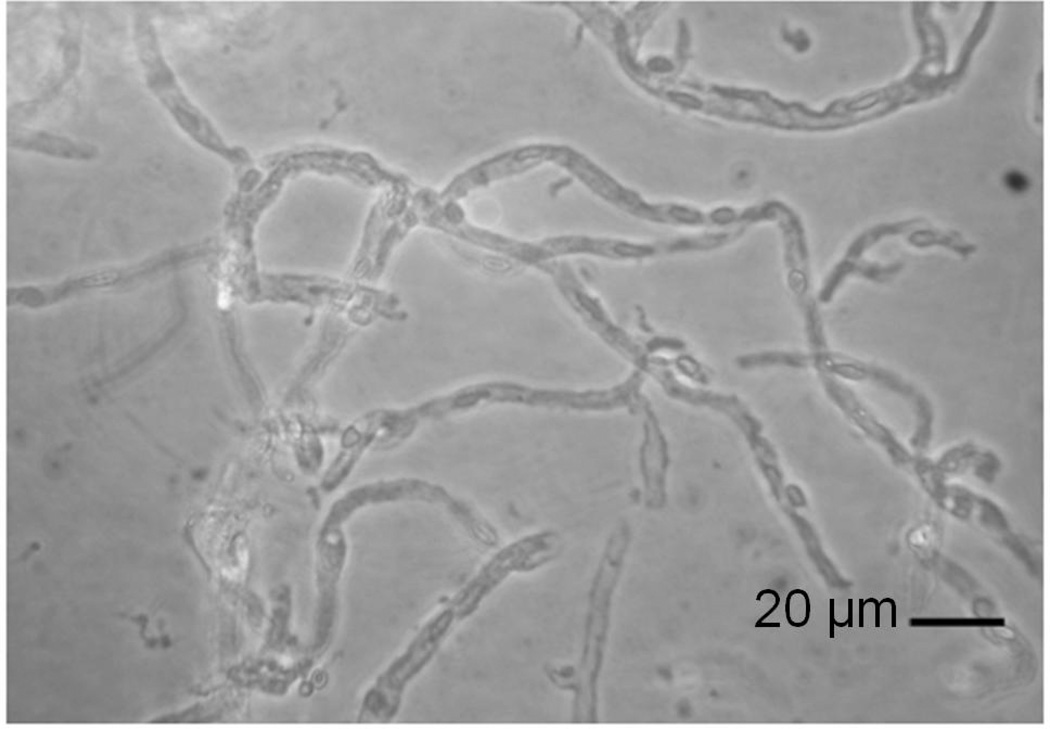
Microvessels were isolated from ovine brains using methods adapted with minor modifications from Sanchez del Pino et al [30]. Using sterile procedures, the meninges were carefully removed from the cerebral cortical grey matter. The cerebral cortical tissue was homogenized with cold PBS and 26% dextran solution (Sigma-Aldrich, St. Louis, MO, USA) in equal volumes with a blender (Hamilton Beach, Southern Pines, NC, USA) at minimum speeds for 9 five-second pulses separated by 10-second intervals. The resultant mixture was centrifuged at 6000 × g for 10 minutes at 4°C and the pellet was washed with cold HEPES buffer (5 g HEPES, Sigma-Aldrich, St. Louis, MO, USA; 1 g dextrose; Sigma-Aldrich, St. Louis, MO, USA, and one liter PBS). The pellet was then resuspended with cold PBS and homogenized using a motorized tissue grinder at approximately 1500 rpm for ten strokes (Wheaton Industries, Millville, NJ, USA). An equal amount of cold 26% dextran solution was added to the homogenate, and this mixture was centrifuged again at 6000 × g for 10 minutes at 4°C. The pellet was washed with cold HEPES buffer, resuspended in cold PBS and centrifuged at 3000 × g for 10 minutes at 4°C. The resulting pellet was again washed with cold HEPES buffer, resuspended in cold PBS, and homogenized using a motorized tissue grinder at 1500 rpm for ten strokes. The final homogenate was filtered over a 200-µm nylon mesh using approximately 2 liter of cold PBS. The resultant solution was again filtered over a 120-µm nylon mesh. The microvessel solution was filtered over a 20-µm nylon mesh using an additional 1 liter of cold PBS. The microvessels remaining on top of the 20-µm nylon mesh were collected and resuspended in cold PBS. This solution was inspected microscopically (Figure 1) for purity prior to centrifugation for 15 minutes at 4500 × g at 4°C. The resultant pellet was resuspended using 16 ml of complete media warmed to 37°C (186 ml Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12; Ham, GIBCO, Invitrogen, Carlsbad, CA, USA; 10 ml of Charcoal/Dextran Treated FBS; Hyclone, Logan, UT, USA; 2 ml Penicillin-Streptomycin liquid, and 2 ml of Fungizone). Two ml of resuspended microvessels were pipetted into sterile culture flasks in order to coat the bottom of the flask (75-cm2 cell culture flask, Corning Inc., Corning, NY). Then, 17 ml of complete media was pipetted into each culture flask and the microvessels remained at 37°C for 16 hours. The 16-hour rest period allowed for recovery of the microvessels after the extraction procedure. This rest period was based upon previous work suggesting that the energy state could be compromised inducing a metabolic shock-like phenomena in bovine microvessels after similar isolation procedures [31,32]. On the other hand, incubation of the metabolically stressed microvessels in enriched tissue culture media improved the metabolic status of the microvessels [32]. Therefore, the microvessels were allowed to recover from the isolation procedures before exposure to the IL-6 protein.
Figure 1.
40 × magnification of microvessels from adult ovine brain at the end of the isolation procedure.
Incubation of Microvessels with IL-6 Protein
Each flask was inspected visually to determine that the microvessels had adhered to the bottom of the flask and for microvessel density and purity before incubation with the experimental treatments. IL-6 (Cell Sciences, Canton, MA, USA) was reconstituted with PBS into concentrations of 20, 200, and 2000 ng/ml such that when the solutions were added to each flask, the final concentrations of IL-6 were 1, 10, or 100 ng/ml, respectively. Clinically relevant IL-6 concentrations were selected for the experimental conditions based upon reports suggesting similar serum concentrations in neonates that subsequently developed brain damage [13]. PBS was added to the media to replace the IL-6 protein, and used to represent the non-treated control condition in each experiment. Each flask containing the microvessels and PBS or one of the three experimental concentrations of IL-6 was incubated at 37°C for 24 hours in culture media.
Microvessel Co-Stimulation Assay with IL-6 Protein and Anti-IL-6 Antibody
Microvessels from adult sheep brains were used for this portion of the study. Each flask of microvessels was examined as described above. IL-6 was reconstituted using PBS and divided into aliquots with concentrations of 2000 ng/ml, such that when the solutions were added to each flask, the final concentrations of IL-6 was 100 ng/ml. In order to have adequate monoclonal antibodies (mAbs) to bind to the IL-6 protein, the concentrations of mAbs that we used were several orders of magnitude higher than the protein concentration. Anti-IL6 human antibody (Cell Sciences, Cat# 855.050.005, Canton, MA) was reconstituted using PBS and divided into aliquots with concentrations of 20 µg/ml. This concentration was designed to be 10-fold greater than the concentration of the IL-6 protein, which was 100 ng/ml. This mAb is an IgG1 neutralizing antibody made by the company (Cell Sciences, Canton, MA) that produced the human IL-6 protein used in the experiments above. It specifically recognizes the recombinant human IL-6 and exerts its biological activity by inhibiting IL-6 binding to its receptor.
The ovine microvessel co-stimulation assay was performed as follows: IL-6 protein was pre-incubated with anti-IL-6 antibody for 1 hour at 4°C. Subsequently, each of the four flasks of microvessels was incubated for 24 hours at 37°C in culture media with either PBS, IL-6 with a final concentration of 100 ng/ml, anti-human IL-6 antibody with a final concentration of 1 µg/ml, or the IL-6 protein (100 ng/ml) that had been pre-incubated with anti-IL-6 antibody (1 µg/ml). To test specificity of the anti-IL-6 antibody, we also pre-incubated IL-6 protein with a non-specific antibody at a concentration 10-fold greater than the concentration of IL-6 (Mouse IgG1 control, Cell Sciences, Canton, MA, USA). The additional control experiments were performed with the non-specific mouse anti-IgG1 antibodies to establish that the non-specific antibodies did not have the same effect as the specific anti-cytokine antibodies. At the end of the experiments, the microvessels were harvested from the culture flasks.
Lactate Dehydrogenase (LDH) Assay
LDH, an enzyme that is used as a marker for cell disruption and/or death [33]. The activity of extracellular, i.e., released LDH, was measured in the capillary-free supernatant, and compared to the total LDH activity achieved after lysis of the capillaries with Triton X-100 (1% in cell culture medium, 30 min at 37 °C). Samples containing 50 µg of protein were tested using a standard LDH assay protocol (OPS Diagnostics, LLC, Lebanon, NJ, USA). A 96-well microplate reader (Multiskan RC, Thermo Scientific, Portsmouth, NH, USA) was used for a colorimetric detection at an absorbance of 492 nm. Released LDH activity was expressed as a percentage of total LDH activity [34].
Western Immunoblotting
The expression of occludin and claudin-5 were measured by Western immunoblot. Cerebral cortical microvessel samples were homogenized in Triton/Deoxycholate/SDS (100mM NaCl, 1% Triton X, 0.5 Sodium Deoxycholate, 0.2% SDS, 2 mM EDTA, 1 mM benzamidine) buffer containing 1% protease inhibitor cocktail (Sigma, Saint Louis, MO, USA) on ice for 10 minutes, and centrifuged at 16,000 × g for 30 minutes to extract the membrane-associated proteins. The supernatants were collected and the protein content was determined using a bicinchoninic acid protein assay (BCA, Pierce, Rockford, IL, USA) with bovine serum albumin as a standard.
Different amounts of yearling and adult sheep protein from the microvessels were loaded due to preliminary findings of differences in intensity of the chemiluminescent signal (i.e., higher in yearling than adult sheep) when microvessels from the two age groups were loaded in similar concentrations. Hence, 4 µg of protein for the microvessels from yearling sheep and 12 µg of protein for the microvessels from the adult sheep proved optimal and were loaded on NuPAGE Novex 4-12% Bis-Tris Midi Gel (Invitrogen, Carlsbad, CA, USA) and immunoblotted to PVDF membranes (0.2 µm, Bio-Rad Laboratories, Hercules, CA, USA) using a semi-dry technique. The membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween (TBST) buffer for 1 hour at room temperature, washed in TBST three times for 10 minutes per wash, and incubated overnight at 4°C with the appropriate primary antibody solution. The proteins were probed with the anti-claudin-5 antibody (mouse polyclonal, Invitrogen, Carlsbad, CA, USA) and anti-occludin antibody (mouse polyclonal, Invitrogen, Carlsbad, CA, USA) at dilutions of 1:10000 and 1:2000, respectively. In addition, vinculin (mouse monoclonal, Thermo Scientific, Rockford, IL, USA) at a dilution of 1:10000 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, mouse monoclonal, Imgenex, San Diego, CA, USA) at a dilution of 1:5000 expressions were used as loading controls to ensure that equal amounts of protein were applied to each lane for the incubation of the microvessels with IL-6 protein, and for the microvessel co-stimulation assay with IL-6 protein and anti-IL-6 antibody, respectively. We used vinculin as the loading control in the studies of incubation of the microvessels with IL-6 protein (figs. 2 and 3). However, we were not able to achieve consistent results for vinculin due to technical difficulties in the costimulation assay studies (fig. 4). Consequently, GAPDH was used as a loading control for the costimulation assay studies (fig. 4).
Figure 2.
A Occludin expression in isolated cerebral microvessels after incubation with IL-6 for 24 hours. Results obtained from microvessels from the yearling lambs. Representative Western immunoblots for occludin and vinculin. Bar graphs represent results shown as ratio to PBS control treated microvessels. Open bar is control treated microvessels set to a value of 1, n=5; closed bars are IL-6 treated, n=5. Values are mean ± SEM, *P<0.05 vs. control.
B Occludin expression in isolated cerebral microvessels after incubation with IL-6 for 24 hours. Results obtained from microvessels from the adult sheep. Representative Western immunoblots for occludin and vinculin. Bar graphs, numbers, and statistics as for 2A.
Figure 3.
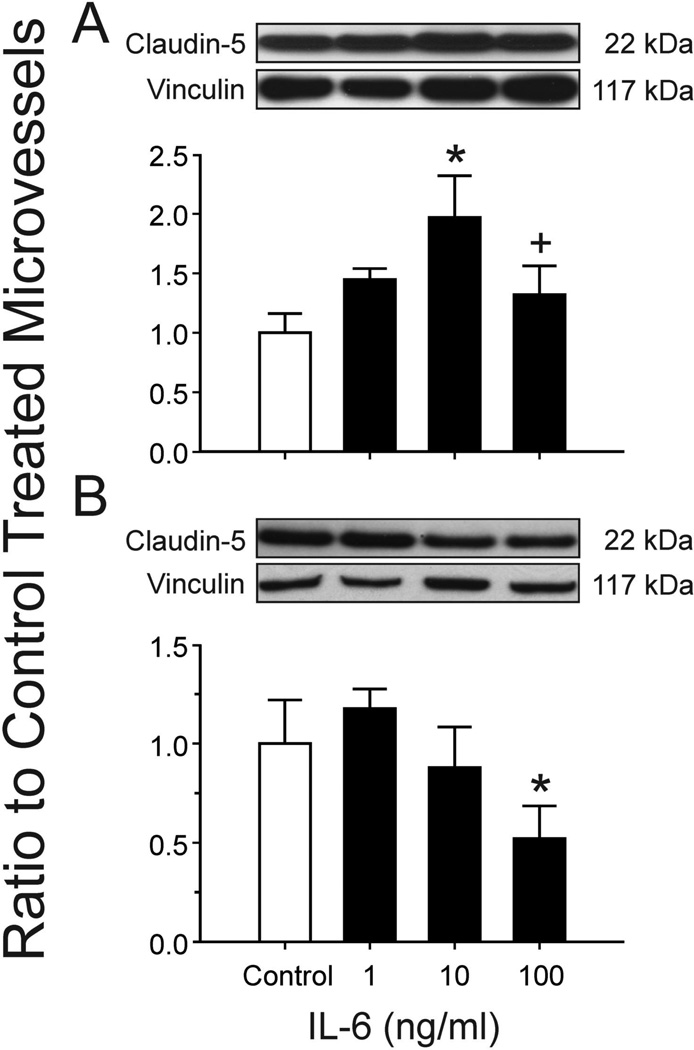
A Claudin-5 expression in isolated cerebral microvessels after incubation with IL-6 for 24 hours. Results obtained from microvessels from the yearling sheep. Representative Western immunoblots for claudin-5 and vinculin. Results shown as ratio to PBS control treated microvessels. Bar legends and numbers as for figure 2. Values are mean ± SEM,* P<0.05 vs. control. + P<0.05 vs. 10 ng/ml.
B Claudin-5 expression in isolated cerebral microvessels after incubation with IL-6 for 24 hours. Results obtained from microvessels from the adult sheep. Representative Western immunoblots for claudin-5 and vinculin. Results shown as ratio to PBS control treated microvessels. Bar legends and numbers as for figure 2. Values are mean ± SEM,* P<0.05 vs. control.
Figure 4.
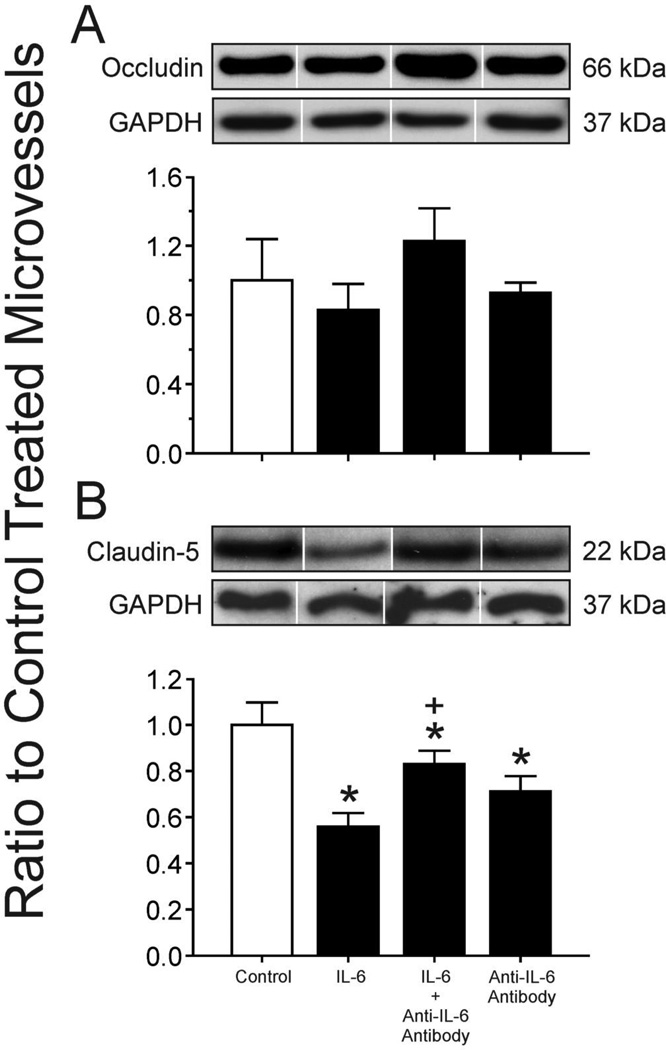
A Occludin expression in isolated cerebral microvessels from adult sheep after incubation with IL-6 protein (100 ng/ml), IL-6 protein (100 ng/ml) preincubated with IL-6 neutralizing monoclonal antibodies (1 µg/ml) and IL-6 neutralizing monoclonal antibodies (1 µg/ml) alone. Representative Western immunoblots for occludin and GAPDH. Results shown as ratio to PBS control treated microvessels. Open bar is control treated microvessels set to a value of 1; closed bars plotted for IL-6 alone, IL-6 plus anti-IL-6 antibody, and anti-IL-6 antibody alone as indicated on the x-axis. n=5 for each group. Values are mean ± SEM.
B Claudin-5 expression in isolated cerebral microvessels from adult sheep after incubation with IL-6 protein, IL-6 protein preincubated with IL-6 neutralizing monoclonal antibodies and IL-6 neutralizing monoclonal antibodies alone. IL-6 protein and IL-6 neutralizing monoclonal antibody concentrations as for figure 4 A. Representative Western immunoblots for claudin-5 and GAPDH. Results shown as ratio to PBS control treated microvessels. Open bar is control treated microvessels set to a value of 1; closed bars plotted for IL-6 alone, IL-6 plus anti-IL-6 antibody, and anti-IL-6 antibody alone as indicated on the x-axis. n=5 for each group. Values are mean ± SEM. * P< 0.05 vs. control, + P < 0.05 vs. IL-6 alone and anti-IL-6 antibody alone.
The immunoblots were washed three times in TBST for 10 minutes per wash and incubated for 1 hour at room temperature with goat anti-mouse secondary antibody (Zymed, San Francisco, CA, USA) at a dilution of 1:10,000 for claudin-5, occludin, vinculin, and GAPDH. After incubation with secondary antibodies, immunoblots were again washed four times in TBST for 10 minutes per wash. Binding of the secondary antibody was detected with enhanced chemiluminescence (ECL-plus) Western blotting detection reagents (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) and exposed to Hyperfilm ECL (Phenix, Candler, NC, USA). Uniformity in inter-lane loading was established by Coomassie blue (Sigma, St. Louis, MO, USA) staining of the polyacrylamide gels, and uniformity of transfer to the PVDF membranes confirmed by Ponceau S (Sigma, St. Louis, MO, USA).
Densitometrical Analysis
Band intensities were analyzed with a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD, USA). The final values represented an average of the densitometric values obtained from two to three different immunoblots. The densitometric values were presented as a ratio to the densitometric values of the microvessels that were exposed to the control PBS condition.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to compare values among the different treatment conditions (Statistica Software, Stat Soft Inc., Tulsa, OK, USA). Treatment conditions were compared separately for cerebral cortical microvessels from the yearling and adult sheep. If a significant difference was found by ANOVA, the Fisher least significant difference test was used to detect specific differences among the treatment conditions. Values are expressed as mean ± SEM. Differences are considered statistically significant if P<0.05.
Results
Figure 1 shows the ovine microvessels after isolation and purification.
Effects of Incubation with IL-6 on the Expression of Tight Junction Proteins
Compared to the control PBS-treated microvessels, incubation of microvessels obtained from the cerebral cortex of yearling sheep resulted in significant reductions in occludin expression after 24 h of exposure to 100 ng/ml of IL-6 protein, but not after exposure to 1 or 10 ng/ml (ANOVA: F=4.1, n=5, P<0.05, fig. 2 A). Likewise, incubation of microvessels from adult sheep resulted in significant reductions in occludin expression after exposure to 100 ng/ml of IL-6, but not to 1 or 10 ng/ml, (ANOVA: F=5.3, n=5, P<0.01, fig. 2 B). Incubation of microvessels obtained from cerebral cortices of yearling sheep resulted in an increase in claudin-5 expression after 24 h of exposure to 10 ng/ml of IL-6 protein (n=5, P<0.05) compared with the control condition, and a decrease after exposure to 100 ng/ml of IL-6 protein (n=5, P<0.05) compared with the 10 ng/ml treatment condition (ANOVA: F=3.4, n=5, P<0.05, fig. 3 A). In contrast, incubation of microvessels obtained from cerebral cortex of adult sheep resulted in significant reductions in claudin-5 expression after 24 h of exposure to 100 ng/ml of IL-6 protein (n=5, P<0.05), but not to 1 or 10 ng/ml compared to the control condition (ANOVA: F=4.9, n=5, P<0.01, fig. 3 B).
IL-6 Neutralizing Monoclonal Antibodies Attenuate the Effects of IL-6 on the Expression of Claudin-5
Incubation of microvessels obtained from adult sheep did not result in significant increases in occludin expression after 24 h of exposure to IL-6 protein that had been pre-incubated with the specific monoclonal antibody to IL-6 compared with IL-6 alone and anti-IL-6 antibody alone (ANOVA: F=1.84, n=5, P=0.18, fig. 4 A). Claudin-5 expression was significantly higher 24 h after treatment with IL-6 protein that had been pre-incubated with the anti-IL-6 neutralizing antibody compared with microvessels incubated with IL-6 protein alone and with anti-IL-6 neutralizing antibody alone (ANOVA: F= 12.3 n=5, P<0.01, fig. 4 B). However, after treatment with IL-6 protein that had been pre-incubated with anti-IL-6 neutralizing antibody, the claudin-5 expression remained significantly lower than the expression in control PBS-treated microvessels. In addition, exposure of microvessels to anti-IL-6 antibody alone was also associated with lower claudin-5 expression when compared with the control PBS-treated condition (P<0.01). Treatment with non-specific mouse IgG isotype did not alter the effects of IL-6 protein treatment on the expression of tight junction proteins (data not shown). However, it should be pointed out that our findings shown in fig. 2 B demonstrate a significant decrease in occludin expression in microvessels obtained from adult sheep after treatment with 100 ng of IL-6, whereas the same treatment did not result in similar findings in fig. 4B. Although we cannot entirely account for these discrepant findings, the standard error of the mean appears larger in fig. 4B than 2B. Another possibility is the microvessels were obtained from the brains of sheep from a local supplier and, consequently, were microvessels from outbred sheep. Therefore, the brains from different groups of sheep potentially could have responded differently to the same treatment.
IL-6 Mediated Effects are not Due to Cell Death as Assessed by Percent of LDH Release
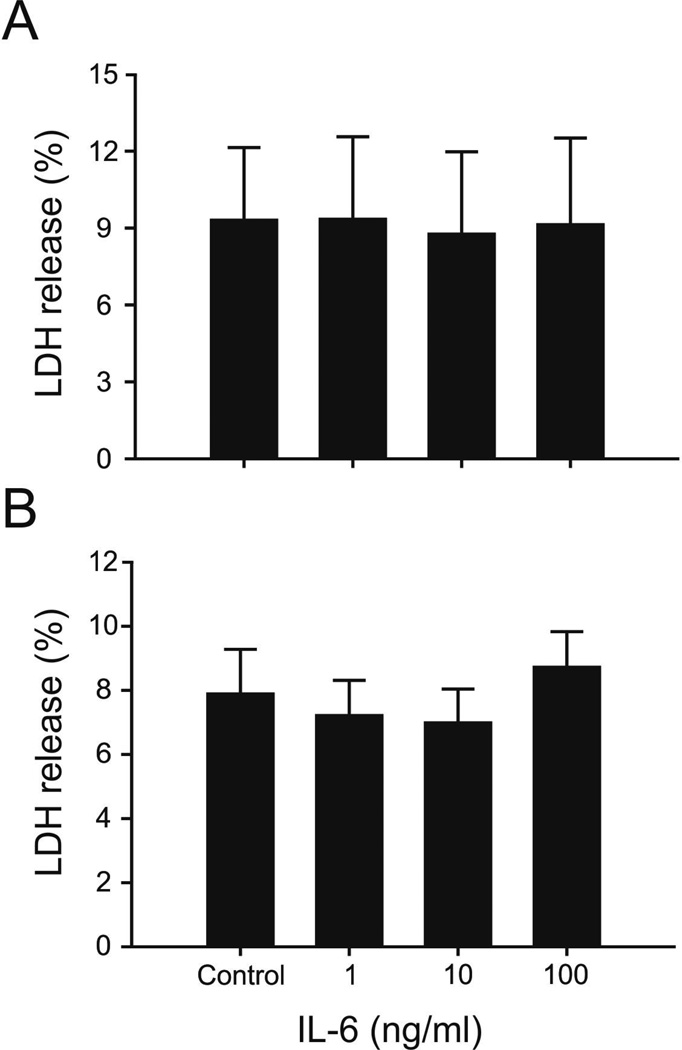
The percent of LDH release from the microvessels into the supernatant did not differ among the microvessels that had been exposed to control conditions (PBS), or 1, 10, or 100 ng/ml of IL-6 in yearling sheep (ANOVA: F=0.01, P=0.99, Figure 5 A) or adult sheep (F=0.44, P=0.72, Figure 5 B). These findings maybe interpreted suggest that exposure of the microvessels to IL-6 did not result cell disruption or death in the microvessels.
Figure 5.
A Values represent percent release from microvessels to supernatant in the yearling sheep. Values plotted for the control, 1, 10, and 100 ng/ml IL-6 treated microvessels on the x-axis, n=5 for each condition. Values are mean ± SEM. ANOVA: F=0.01, P=0.99. IL-6 mediated effects are not due to cell death as assessed by percent LDH release from microvessels.
B Values represent percent release from microvessels to supernatant in the adult sheep, conditions and numbers as for 5A. ANOVA: F=0.01, P=0.99. IL-6 mediated effects are not due to cell death as assessed by percent LDH release from microvessels.
Discussion
The purpose of the current study was to examine the effects of incubating ovine cerebral cortical microvessels with different concentrations of IL-6 on key protein constituents of endothelial tight junctions. Incubation of microvessels isolated from yearling and adult sheep brain with IL-6 protein resulted in decreased expression of the tight junction protein, occludin, and incubation of microvessels from adult sheep brain resulted in decreased expression of claudin-5. Incubation of microvessels from adult sheep with anti-IL-6 neutralizing antibodies attenuated the effects of IL-6 on the expression of claudin-5. The finding that IL-6 reduces expression of claudin-5 is important because claudin-5 forms the primary “seal” of the tight junction and restricts transfer of low molecular weight molecules from blood to brain [2,35]. Likewise, the finding that IL-6 reduces the expression of occludin is also important as this tight junction protein plays an important role as a support molecule and in modulating tight junction function [1].
Several previous studies support the contention that pro-inflammatory cytokines increase the permeability of endothelial cell confluent monolayers in vitro [14,16,17]. IL-6 has been shown to increase the permeability of bovine aortic endothelial cell confluent monolayers measured with albumin via mechanisms that appear to involve morphological changes in cell shape, gap formation between adjacent cells, and rearrangement of F-actin [14]. Similarly, IL-6 was shown to increase the permeability of human umbilical vein endothelial cell confluent monolayers via mechanisms associated with redistribution of the tight junctional protein ZO-1, redistribution of cytoskeletal actin, increased cell contraction, and disorganization of the intercellular borders [17]. In addition, IL-6 appears to contribute to increases in the vascular permeability of the retinal endothelial barrier, whose properties are similar to those of the blood-brain barrier [16]. Consistent with evidence suggesting that IL-6 increases the permeability of non-barrier endothelial monolayers [14,17] and of the retinal endothelial barrier [16], we have shown that IL-6 decreased occludin and claudin-5 expression in the endothelium of microvessels, which are known to possess the characteristics of an intact blood-brain barrier [20]. Hence, our findings, combined with the previous results, suggest that IL-6 increases barrier permeability by changes in cell shape, gap formation between adjacent cells, rearrangement in F-actin, redistribution in the tight junction protein ZO-1, disorganization of intercellular borders [14,17], and possibly by reductions in the expression of occludin and claudin-5. However, because we did not determine the microvessel permeability in our study, we cannot comment upon the potential contributions of the decreases in tight junction protein expression to increases in permeability that was observed in the former studies [14,16,17].
In the current study, the concentrations of IL-6 to which the cerebral cortical microvessels were exposed were selected based upon levels known to be associated with brain damage in infants [11–13]. We observed decreases in occludin expression in microvessels from the yearling and adult brains after 24 h of exposure to 100 ng/m, but not after exposure to the lower concentrations and similarly, decreases in claudin-5 expression in microvessels from adult brains after exposure to 100 ng/ml, but not after 1 or 10 ng/ml. These concentration related decreases in tight junction protein expression are also consistent with previous in vitro work showing increased permeability of bovine aortic and human umbilical vein endothelial cell monolayers after exposure to IL-6 in similar concentrations for comparable durations [14,17]. However, although previous studies have also shown that exposure to IL-6 both for shorter [14] and longer [17] durations was associated with changes in endothelial monolayer permeability, we only examined the effect of microvessel exposure to IL-6 for 24 h. Thus, we cannot comment upon the effects of different durations of IL-6 exposure on changes in microvessel tight junction protein expression. However, as an initial step in this study, we selected the 24 h exposure to IL-6 because this cytokine is elevated in the serum of neonates within 24 h after the development of sepsis, and IL-6 demonstrated the best sensitivity and specificity to predict sepsis [36]. In addition, sepsis/infection related inflammation is well known to be associated with significant neurodevelopmental impairment in infants [37].
It is also important to note that exposure of microvessels from the brains of yearling sheep to 10 ng/ml of IL-6 was actually associated with an increase in claudin-5 expression compared with control treated microvessels (fig. 3). In this regard, IL-6 is known to have both pro-inflammatory and anti-inflammatory or neuroprotective roles under normal and pathophysiological conditions [38]. Hence, it would appear that exposure of microvessels from yearling, but not adult sheep, to relatively low (10 ng/ml) concentrations of IL-6 actually increased the expression of claudin-5, potentially suggesting a neuroprotective role of this pro-inflammatory cytokine on microvessels from the younger animals. These findings suggest that the effects of IL-6 on the microvasculature also appear to be age related, even though the microvessels from both age groups were derived from relatively mature subjects.
TNF-α has been shown to play a critical role in the development of brain edema, increased blood-brain barrier permeability by disruption of tight junctions, and in the loss of the tight junction-associated protein occludin in an adult mouse model of acute liver failure [39]. These findings, taken together with the findings in our study, suggest that the pro-inflammatory cytokines, interleukin-6, and TNF-α both have the ability to alter the characteristics of the blood-brain barrier by their effects on the protein constituents of endothelial tight junctions. In addition, these findings suggest that occludin and claudin-5 are vulnerable to the effects of these pro-inflammatory cytokines and that the down-regulation of these tight junction proteins could affect the structural integrity of tight junctions [39,40].
To confirm that IL-6 alone down-regulated the expression of occludin and claudin-5, we investigated the ability of anti-IL-6 neutralizing antibodies to attenuate the effects of IL-6 on the expression of the tight junction proteins in the microvessels. Concordant with previous findings [14], incubation of microvessels from adult sheep with anti-IL-6 neutralizing antibodies attenuated the effects of IL-6 on the expression of claudin-5 (fig. 4 B). However, treatment with the neutralizing antibodies did not return claudin-5 expression to similar levels observed in control treated microvessels. In addition, anti-IL-6 antibody treatment also decreased claudin-5 expression. Other potential factors such as IL-6 mediated local increases in vascular endothelial growth factor and protein kinase C or up-regulation of other cytokines could also have contributed to the down-regulation of occludin and claudin-5 expression [17,40], there-by, limiting the efficacy of treatment with the anti-IL-6 neutralizing antibody. The neutralizing monoclonal (mAb) anti-human IL-6 antibody (Cell Sciences) that we used has specificity for the recombinant IL-6 that we used and exerts its biological activity by inhibiting IL-6 binding to its receptor, so that at least some of the IL-6-related down-regulation of the tight junction proteins probably resulted from IL-6 receptor-related events.
Claudin-5 expression also decreased after exposure to the anti-IL-6 neutralizing antibody alone. The treatment of the microvessels with anti-IL6 antibody alone could have decreased endogenous levels of IL-6 within the microvessels. IL-6 has both pro- and anti-inflammatory properties [38]. Therefore, treatment of the microvessels with anti-IL-6 antibody alone could have affected the endogenous levels of IL-6 within the microvessels, which could in turn could have affected other factors that could account for a lower claudin-5 expression compared to the control condition treated microvessels.
Although the pattern of change in the effects of the anti-IL-6 neutralizing antibodies on the expression of occludin (fig. 4 A) was similar to those of claudin-5, we did not detect similar significant attenuations on the effects of IL-6 protein on occludin expression, most likely, because of the larger standard errors of the mean in the latter. IL-6 treatment also did not result in a statistically significant decrease in occludin expression (fig. 4A) similar that observed in the dose-exposure study (fig. 2 B). Although we cannot entirely account for the discrepancy in the response of the microvessel to IL-6 exposure i.e. decrease in occludin after incubation with 100 ng/ml IL-6 in figure 2, and a lack of a significant decrease in fig. 4 A, inspection of figure 4 suggests relatively wide standard errors of the mean, potentially accounting for the discrepancy. In addition, the larger standard errors in for occludin (fig. 4 A) compared with claudin-5 (fig. 4 B), after exposure to same treatment conditions, could account for the significant findings for claudin-5, but not occludin. In addition, the microvessels were obtained from the brains of sheep from a local supplier and, consequently were microvessels from outbred sheep. Therefore, the brains from different groups of sheep potentially could have responded differently to the same treatment.
The mechanism(s) by which IL-6 down-regulates tight junction protein expression cannot be discerned by our study. However, occludin and claudin-5 are connected to cytoplasmic proteins such as zonula occludens-1, 2, and -3, which in turn are coupled to actin [5]. IL-6 results in an endothelial redistribution of zonula occludens-1 and actin [14,17] and, consequently the reductions in occludin and claudin-5 expression along with changes in zonula occludens-1 and actin could affect the integrity of tight junctions [40]. Moreover, consistent with previous work [14], the reductions in tight junction protein expression were not a result of cytotoxic microvascular cell death because we did not observe increases in the percent of LDH release from the microvessels into the supernatant in the treated microvessels (fig. 5).
There are several limitations to our study. Although we measured occludin and claudin-5 protein expression in the microvessels, we did not determine immunohistochemical localization of the tight junction proteins. It would be important to determine the effect of IL-6 on the distribution of tight junction proteins in microvessels in future studies. We do not know whether the effects of the changes in the levels of tight junction proteins are associated with changes in barrier permeability as has been previously reported using other models [14,17]. In addition, we recognize that incubation of the microvessels could have been associated with remodeling, cell division, or growth. However, it must be emphasized that the microvessels exposed to the control condition, and different amounts of IL-6 exposure were treated in precisely the same fashion and came from the same pool of brain tissue, thus validating our comparisons among the different treatment conditions. In addition, it would have been interesting to have evaluated different intervals of incubation times, which we will consider for future studies. Lastly, we would have preferred to perform these studies in microvessels from brains of immature sheep. However, as an initial step to establish this in vitro blood-brain barrier model in our laboratory, we used cadaveric brains from yearling and adult sheep that were available in sufficient quantities from a local supplier. Studies of the effects of inflammatory agents on the immature brain vasculature are particularly relevant to brain injury in the fetus and newborn [11–13]. Nonetheless, our present findings (fig. 3) suggest that examining the effects of IL-6 in the immature brain could yield interesting results that could differ from those in the adult brain (fig. 3).
Previous work has postulated that during maternal inflammation or infection cytokines originating in the uterus, amniotic fluid, or placenta could enter the fetal circulation and cross and/or damage the fetal/neonatal blood-brain barrier [41]. Based upon the findings of the current study, we speculate that pro-inflammatory cytokine-mediated brain damage could result, in part, by the down-regulation of tight junction proteins at the blood-brain barrier, impairing the integrity of the barrier and thereby rendering the blood-brain barrier more permeable to circulating substances toxic to the brain.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R01-HD-057100. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also would like to acknowledge the excellent editorial assistance of Courtney A. Hill, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 5.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson MA, Dohi K, Banks WA. Neuroinflammation: A common pathway in cns diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19:121–130. doi: 10.1159/000330247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leviton A, Gilles F, Neff R, Yaney P. Multivariate analysis of risk of perinatal telencephalic leucoencephalopathy. Am J Epidemiol. 1976;104:621–626. doi: 10.1093/oxfordjournals.aje.a112340. [DOI] [PubMed] [Google Scholar]
- 8.D'Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P, Speer CP. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57:263–269. doi: 10.1203/01.PDR.0000148713.48218.86. [DOI] [PubMed] [Google Scholar]
- 9.Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011;90:235–243. doi: 10.1016/j.jri.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 11.Foster-Barber A, Ferriero DM. Neonatal encephalopathy in the term infant: Neuroimaging and inflammatory cytokines. Ment Retard Dev Disabil Res Rev. 2002;8:20–24. doi: 10.1002/mrdd.10009. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Ancel A, Garcia-Alix A, Pascual-Salcedo D, Cabanas F, Valcarce M, Quero J. Interleukin-6 in the cerebrospinal fluid after perinatal asphyxia is related to early and late neurological manifestations. Pediatrics. 1997;100:789–794. doi: 10.1542/peds.100.5.789. [DOI] [PubMed] [Google Scholar]
- 13.Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics. 2002;110:673–680. doi: 10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- 14.Maruo N, Morita I, Shirao M, Murota S. Il-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 15.Gurkan OU, He C, Zielinski R, Rabb H, King LS, Dodd OJ, D'Alessio FR, Aggarwal N, Pearse D, Becker PM. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp Lung Res. 2011;37:575–584. doi: 10.3109/01902148.2011.620680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noma H, Funatsu H, Mimura T. Vascular endothelial growth factor and interleukin-6 are correlated with serous retinal detachment in central retinal vein occlusion. Curr Eye Res. 2011;37:62–67. doi: 10.3109/02713683.2011.614370. [DOI] [PubMed] [Google Scholar]
- 17.Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase c pathway. J Surg Res. 2002;104:118–123. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, Lytle C, O'Donnell ME. Il-6 secreted by astroglial cells regulates na-k-cl cotransport in brain microvessel endothelial cells. Am J Physiol. 1997;272:C1829–C1835. doi: 10.1152/ajpcell.1997.272.6.C1829. [DOI] [PubMed] [Google Scholar]
- 19.Jacob A, Hartz AM, Potin S, Coumoul X, Yousif S, Scherrmann JM, Bauer B, Decleves X. Aryl hydrocarbon receptor-dependent upregulation of cyp1b1 by tcdd and diesel exhaust particles in rat brain microvessels. Fluids Barriers CNS. 2011;8:23–35. doi: 10.1186/2045-8118-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernon H, Clark K, Bressler JP. In vitro models to study the blood brain barrier. Methods Mol Biol. 2011;758:153–168. doi: 10.1007/978-1-61779-170-3_10. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin SA, Cairns MT, Gardiner RM, Ruggier R. A d-glucose-sensitive cytochalasin b binding component of cerebral microvessels. J Neurochem. 1985;45:650–652. doi: 10.1111/j.1471-4159.1985.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 22.Beart PM, Sheehan KA, Manallack DT. Absence of n-methyl-d-aspartate receptors on ovine cerebral microvessels. J Cereb Blood Flow Metab. 1988;8:879–882. doi: 10.1038/jcbfm.1988.146. [DOI] [PubMed] [Google Scholar]
- 23.Joo F. The cerebral microvessels in culture, an update. J Neurochem. 1992;58:1–17. doi: 10.1111/j.1471-4159.1992.tb09272.x. [DOI] [PubMed] [Google Scholar]
- 24.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol. 1996;271:R1594–R1601. doi: 10.1152/ajpregu.1996.271.6.R1594. [DOI] [PubMed] [Google Scholar]
- 26.Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- 27.Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep. Prog Brain Res. 1967;26:60–77. doi: 10.1016/S0079-6123(08)61419-3. [DOI] [PubMed] [Google Scholar]
- 28.Cook CJ, Gluckman PD, Johnston BM, Williams C. The development of the somatosensory evoked potential in the unanaesthetized fetal sheep. J Dev Physiol. 1987;9:441–455. [PubMed] [Google Scholar]
- 29.Stonestreet BS, Sadowska GB, Leeman J, Hanumara RC, Petersson KH, Patlak CS. Effects of acute hyperosmolality on blood-brain barrier function in ovine fetuses and lambs. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1031–R1039. doi: 10.1152/ajpregu.00883.2005. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez del Pino MM, Hawkins RA, Peterson DR. Neutral amino acid transport by the blood-brain barrier. Membrane vesicle studies. J Biol Chem. 1992;267:25951–25957. [PubMed] [Google Scholar]
- 31.Sussman I, Carson MP, McCall AL, Schultz V, Ruderman NB, Tornheim K. Energy state of bovine cerebral microvessels: Comparison of isolation methods. Microvasc Res. 1988;35:167–178. doi: 10.1016/0026-2862(88)90060-x. [DOI] [PubMed] [Google Scholar]
- 32.McCall AL, Valente J, Cordero R, Ruderman NB, Tornheim K. Metabolic characterization of isolated cerebral microvessels: Atp and adp concentrations. Microvasc Res. 1988;35:325–333. doi: 10.1016/0026-2862(88)90087-8. [DOI] [PubMed] [Google Scholar]
- 33.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 34.Rauen U, Kerkweg U, Wusteman MC, de Groot H. Cold-induced injury to porcine corneal endothelial cells and its mediation by chelatable iron: Implications for corneal preservation. Cornea. 2006;25:68–77. doi: 10.1097/01.ico.0000167880.96439.c6. [DOI] [PubMed] [Google Scholar]
- 35.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 36.Bender L, Thaarup J, Varming K, Krarup H, Ellermann-Eriksen S, Ebbesen F. Early and late markers for the detection of early-onset neonatal sepsis. Dan Med Bull. 2008;55:219–223. [PubMed] [Google Scholar]
- 37.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birthweight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 38.Thorns V, Walter GF, Licastro F. Effects of il6 and il1beta on afgf expression and excitotoxicity in nt2n cells. J Neuroimmunol. 2002;127:22–29. doi: 10.1016/s0165-5728(02)00072-3. [DOI] [PubMed] [Google Scholar]
- 39.Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P. Tumour necrosis factoralpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010;30:1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 40.Forster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D. Differential effects of hydrocortisone and tnfalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol. 2008;586:1937–1949. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dammon O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatric Research. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]