Abstract
CD4 T cell function declines significantly during aging. While the mammalian target of rapamycin (TOR) has been implicated in aging, the roles of the TOR complexes (TORC1, TORC2) in the functional declines of CD4 T cells remain unknown. In this study, we demonstrate that aging increases TORC2 signaling in murine CD4 T cells, a change blocked by long-term exposure to rapamycin, suggesting that functional defects may be the result of enhanced TORC2 function. Using overexpression of Rheb to activate TORC1 and Rictor plus Sin1 to augment TORC2 in naïve CD4 T cells from young mice, we demonstrated that increased TORC2, but not TORC1, signaling results in aging-associated biochemical changes. Furthermore, elevated TORC2 signaling in naïve CD4 T cells from young mice leads to in vivo functional declines. The data presented herein suggest a novel model in which aging increases TORC2 signaling and leads to CD4 T cell defects in old mice.
Keywords: T cells, Aging, Rapamycin, mTOR, TRC signaling
Introduction
Data from diverse model organisms suggest that the mammal target of rapamycin (TOR) is a critical controller of aging (1–3), and rapamycin treatments can extend the lifespan of mice (4). TOR, a serine/threonine kinase, forms the catalytic core of at least 2 complexes, TOR complex 1 (TORC1) and TOR complex 2 (TORC2). The role of each complex in aging is not well understood (2). TORC1 is known to respond to nutrient environmental signals responsible for modulating cell growth and stress responses (5). The GTP-binding protein, Ras homolog enriched in brain (Rheb), represents a critical upstream activator of TORC1 (6). Rheb overexpression enhances TORC1 activity (7) and increases phosphorylation of its downstream substrates such as S6K1 (8). In turn, S6K1 enhances the phosphorylation of the ribosomal protein S6 kinase at Ser-235 (pS6(235)) a component of the 40 S ribosomal subunit that mediates translation (9). In lymphocytes, TCR/CD28 signaling leads to TORC1 activation via Rheb and AKT phosphorylation at Thr-308 (pAKT(308)) via PDK1 pathway (10); these events regulate CD4 T cell activation and differentiation of naïve CD4 T cells into Th-1 and Th-17 phenotypes (11). In contrast, TORC2 regulates naïve CD4 T cell differentiation into a Th-2 phenotype (11, 12) and it also regulates actin polymerization (13), cell size (14), Rac-GTPase and RhoA-GTPase activity (15, 16), and PKC function (17), and may also regulate the phosphorylation of Ezrin and Moesin (pERM) cytoskeleton proteins involved in immune synapse formation (18). In addition, inhibition of TORC2 can increase apoptosis (19, 20), suggesting a role for TORC1 in the regulation of lymphocyte survival. TORC2 contains the Rictor and Sin1 scaffolding proteins that are critical for its function (21). The best-characterized phosphorylation substrates of TORC2 are AKT at Ser473 (pAKT(473)), serum glucocorticoid kinase 1 at Ser-422 (pSGK1(422)), and the N-myc downstream-regulated (pNDRG1) gene at Thr-346 (pNDRG1(346)) via SGK1 activation (22).
Aging alters numerous aspects of CD4 T cell function (23, 24). These alterations include cytoskeletal changes and declines in TCR dependent activation (25–29), increased cell size (27), altered RhoA and Rac activity (30), decreased pERM(558) expression (30), and decreased proliferation in vivo (31). We recently demonstrated that some of these defects are the result of alterations in TCR/CD28 signaling (32). In addition, others have reported that aging decreases CD4 T cell proliferation and alters the expression of the Bcl-2 family of apoptotic proteins, including declines in pro-apoptotic BIM expression (33). However, the molecular mechanism(s) that regulate these changes remain unknown. Because many of these phenotypic changes in lymphocytes from old mice are controlled by TORC2, we postulated that the age-related defects in CD4 T cell function could be the result of alterations in TORC2 function. Here, using in vitro and in vivo models of CD4 T cell function, we evaluated TORC1 and TORC2 signaling in the context of the age-related functional changes of CD4 T lymphocytes.
Material and Methods
Animals, reagents, and rapamycin treatments
H-2(k/k) TCR-Vα11Vβ3 CD4+ mice (AND mice) and CD4 knockout (CD4KO) mice on a B10.BR background were bred in our facilities from stock generously provided by Susan Swain and Laura Haynes (Trudeau Institute, NY). Specific pathogen-free B10.BR and CB6F1 (BALB/c × C57BL/6) mice were purchased from the Charles River Laboratories (Kingston, NJ) and from the National Institute of Aging contract colonies at Harlan (Indianapolis, IN), respectively. The genetically heterogeneous mice population (UM-HET) generated by crossing CB6F1 females with C3D2F1 males (34) was also bred in our facilities. All experiments involving mice conformed to institutional and national standards and were approved by the University of Michigan’s Committee on Use and Care of Animals. All mice were given free access to food and water. Sentinel animals were examined quarterly for evidence of viral infection; all tests were negative during the course of these studies. Mice found to have splenomegaly or macroscopically visible tumors were not used for the experiments. AND mice were used at 4 months of age; B10.BR or CD4KO adoptive host mice were 2–3 months of age. CB6F1 mice used in the study were either 6–8 (young) or 20–22 (old) months of age. All chemical reagents were purchased from Sigma (www.sigmaldrich.com). Carboxifluorescein succinimidyl ester (CFSE), violet blue cell division trackers, and rabbit polyclonal anti-HA were purchased from Invitrogen (www.invitrogen.com). Flow cytometry antibodies to surface CD4 T cells molecules were purchased from Biolegends (www.biolegends.com) or Becton Dickson (www.bdbiosciences.com). Rabbit polyclonal anti-Myc, monoclonals anti-Sin1, anti-mTOR and anti-Rictor were purchased from Millipore (www.millipore.com). Rabbit anti-Glut1 was from Epitomics (www.epitomics.com). Protein antibodies, phospho-specific rabbit monoclonal antibodies, and rabbit polyclonal anti-Flag antibodies were purchased from Cell Signaling (www.cellsignal.com), with the exception of pSGK1(422) and NDRG, which were obtained from Santa Cruz (www.scb.com). Anti-rabbit FITC-conjugated antibodies were obtained from Jackson Immunoresearch (www.jacksonimmuno.com). Plasmids corresponding to the human pRK5-Rheb-Flag, pRK5-Rictor-HA, pRK5-Sin1-Myc, and pRK5 stocks were generously donated by Diane Fingar (University of Michigan, MI) and purified with Qiagen endotoxin-free purification kits (www.qiagen.com). Rapamycin treatments were performed as previously described (4)34) by mixing microencapsulated rapamycin at different doses in Purina 5LG6 chow as described in text and given ad libitum to the mice. Spleens from the rapamycin-treated mice were harvested and the lymphocytes were purified as previously described (25). Then, lymphocytes were stained for CD4 and CD44 and intracellular staining was performed as described elsewhere (30).
Transient transfection of CD4 T cells, intracellular staining, and western blot analysis
Naïve CD4 cells from the spleen and lymph nodes were obtained by negative selection using the Miltenyi CD4 purification kit II according to the manufacturer’s recommendations (www.miltenyibiotec.com). Analysis of a typical preparation showed the cells to be 90% positive for both CD3 and CD4. Then, 10 × 106 CD4 T cells were transfected with the different plasmids by electroporation using the Amaxa Nucleofector mouse CD4 T cell transfection kit (www.biolonza.com) as described elsewhere (35, 36). In each experiment, we performed electroporation of the following constructs: 5 μg of pRK5 (Vector), 5 μg of pRK5-Rheb-Flag (Rh), or a mix of 5 μg of pRK5-Rictor-HA plus 5 μg of pRK5-Sin1-Myc (R+S). Transfection efficiency controls were performed for each experiment using Pmax-green fluorescent protein (GFP) as recommended by the Amaxa Nucleofector systems. Then, cells were cultured in complete RPMI-1640 medium and after 24 hours the dead cells and debris were removed using the lympholyte-M method and the live cells were counted. For each analysis, 10 × 106 CD4 T cells were lysed and the aliquots were analyzed by western blotting using phospho-specific antibodies or total protein. The respective bands were quantified as described elsewhere (30). In other cases, analysis by flow cytometry using intracellular staining was performed as we previously described (30).
Adoptive transfer and analysis of in vivo CD4 T cell activation and proliferation
Purified splenic naïve CD4 T cells from young AND mice were transfected with the different plasmids according to the methods described above. Then, electroporated CD4 T cells (V, Rh, and R+S) were labeled with violet blue cell division tracker following the manufacturer’s recommendations (www.invitrogen.com). In addition, untransfected CD4 T cells (Controls, C) were labeled with CFSE (31). Then, 1 × 106 CD4 T cells from each of the transfected populations (Vector, Rheb, and R+S) were mixed with 1 × 106 untransfected CD4 T cells (C) and injected into the tail veins of CD4KO host mice. Twenty-four hours later, the mice were primed with 10 μg of PCC in 100 μL of PBS and the spleen and lymph nodes were harvested at the indicated time points. Analysis of the activation and proliferation of the adoptively transferred CD4 T cells was performed using flow cytometry as previously described (31).
Statistical analysis
Unless otherwise indicated, results are presented as mean ± standard error of the mean (SEM). Statistical significance was assessed using Mann-Whitney tests, with the significance level set at p = 0.05.
Results
In vivo rapamycin treatment can prevent age-related changes in CD4 T cells
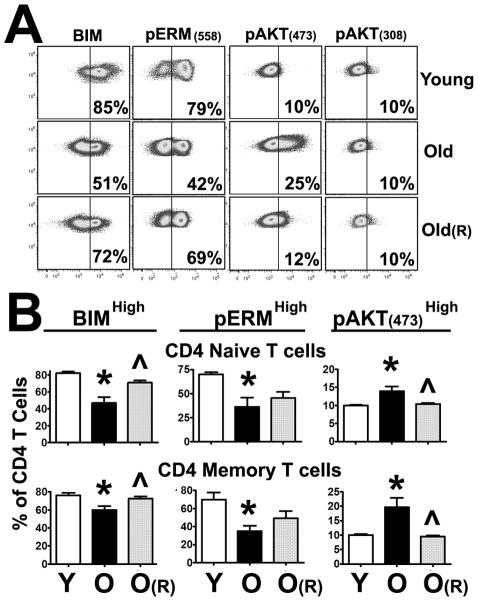
Rapamycin treatment can prolong the lifespan of lower organisms and mice (3, 37). While the underlying mechanism is not well understood, it has been suggested that inhibition of mTORC1 may be important (38). However, long-term rapamycin treatment can also inhibit TORC2 function (2, 39), and its effects on immune function during aging remain unknown. With increased age, CD4 T cells show decreased pERM(558) expression, which is associated with declines in TCR signaling, immune synapse formation, and activation (23, 30). Other laboratories have documented age-related declines in the expression of the pro-apoptotic protein BIM (33). Because these proteins are regulated by TOR, we hypothesized that long-term rapamycin-induced TOR inhibition could prevent some of the age-related changes in CD4 T cells. To test this hypothesis, we treated a genetically heterogeneous population of mice (UM-HET) with rapamycin starting at 9 months of age. The UM-HET mice were given either control chow (Purina 5LG6) or chow containing 3 different doses of rapamycin for 12 months (High: 42 ppm; Middle: 14.7 ppm; Low: 4.7 ppm) as described previously (34). Then, we harvested the spleens from 9-month-old (Young, Y), untreated 22-month-old (Old, O), and rapamycin-treated 22-month-old (OR) mice and evaluated the effects of rapamycin on the age-related alterations of CD4 T cells using intracellular staining and flow cytometry. Flow cytometric methods were selected because they enable the analysis of the expression of multiple age-related changes (pERM, BIM, pAKT(473), and pAKT(308)) in both naïve and memory CD4 T cells using CD44 and CD62L differentiation markers (40). A typical analysis is shown in Figure 1A. We found that aging decreases the number of CD4 T cells expressing high levels of BIM and pERM(558) when compared to CD4 T cells from young mice (first 2 columns of Figure 1A). Interestingly, untreated CD4 T cells from old mice showed an increased number of cells with higher levels of pAKT(473), but aging had no effect on pAKT(308) (Y vs. O in Figure 1A). All doses of rapamycin led to a decline in the CD4/CD8 T cell ratio and diminished the age-related increases in the proportion of memory cells as indicated by the CD44 and CD62L markers (see Supplemental Figure 1A). These results correspond well with those in the literature demonstrating that rapamycin affects CD4 T cell differentiation (41). However, as shown in Figure 1A, rapamycin treatment led to partial, though not complete, prevention of the declines in BIM and pERM expression and increased pAKT(473) in both naive and memory CD4 T cells, with no effects on pAKT(308). To test for statistical significance, we analyzed the data from at least 12 independent experiments (with a minimum of 6 females and 6 males from each group) As shown in Figure 1B, 12-month rapamycin treatment, at 14.7 ppm, seem to prevent some of the BIM declines in both naïve (p = 0.003) and memory (p = 0.02) CD4 T cells. These effects could also be observed with the lower and higher rapamycin doses (see Supplemental Figure 1). Furthermore, rapamycin also appeared to prevent declines in pERM expression in both naïve and memory CD4 T cells. However, statistical significance was achieved only at the high dose (see Supplemental Figure 1). Furthermore, the age-related increase in pAKT(473) was significant in both naïve (p = 0.005) and memory (p = 0.04) CD4 T cells (Y vs. O in Figure 1B). Long-term rapamycin treatment can inhibit TORC2 function (2); as expected, we observed that rapamycin treatment prevented the age-related increase in pAKT(437) (naïve, p = 0.007; memory, p = 0.001) to near to the levels observed in CD4 T cells from young donors (O vs. OR in Figure 1B). On the other hand, we found no significant age- or rapamycin treatment-associated changes (example in Figure 1A, full data set not shown) in pAKT(308), as predicted by the models showing that pAKT(308) is upstream of TOR (42).
Figure 1. Long-term in vivo rapamycin treatment can prevent age-related changes in CD4 T cells.
A) Spleen cells from young (Y), old (O), or rapamycin-treated (for 12 months) old mice (OR) were stained for CD4 and CD44 surface markers and intracellular staining for pERM, BIM, pAKT(473), pAKT(308) was performed. Then, gated resting naïve CD4+CD44-Low and memory CD4+CD44-High cells were used to evaluate the effects of age and rapamycin. The vertical line shows the threshold used to distinguish high expression of BIM, pERM, and pAKT(473)cells for the calculations illustrated in the panel. B) Quantification of the age-related changes in at least 6 experiments, including a total of 12 young, 12 old, and 12 rapamycin-treated mice. The values represent the mean ± SEM. The (*) indicates statistically significant age-related changes relative to the CD4 T cells controls from young mice, while the (^) represents statistically significant effects of rapamycin preventing the age-related changes relative to CD4 T cells from old mice.
The age-related increase of pAKT(473) is not the result of changes in AKT1 expression (example in Figure 2, full data set not shown), instead suggesting enhanced downstream TORC2 signaling. To further test this hypothesis, we measured the phosphorylation levels of downstream targets of TORC2, including pNDRG1(346), pAKT(473), and pSGK1(442) using western blotting in highly purified CD4 T cells from the spleens of young (6–8 months) and old (22–24 months) CB6F1 mice. Figure 2 shows a typical western blot comparing the phosphorylation status of these proteins and their relative total expression in CD4 T cells from young and old donor mice. In addition, Figure 2 shows the statistical analysis of the relative ratios of at least 6 independent experiments (with a total of 12 young and 6 old mice). We found that aging significantly increases the relative level of pAKT(473) (p = 0.001) and pNDRG1(346) (p = 0.0003). We also analyzed pSGK1(422) and found a similar 2-fold age-related increase (p = 0.0002); however, since this antibody may recognize other substrates in the same molecular weight range (43), we cannot conclude that pSGK1 is affected by aging (data not shown). The age-related increases in TORC2 activity are not the results of changes in the expression levels of TORC2 associated proteins Rictor and Sin1 in purified CD4 T cells from young and old donors (See Supplemental Figure 3). Nevertheless, the results in rapamycin-treated mice, in addition to the flow cytometric analysis of untreated naïve and memory CD4 T cells with regard to pAKT(473), suggest that aging enhances TORC2 signaling and function.
Figure 2. Phosphorylation of downstream TORC2 signaling molecules increases with age in CD4 T cells.
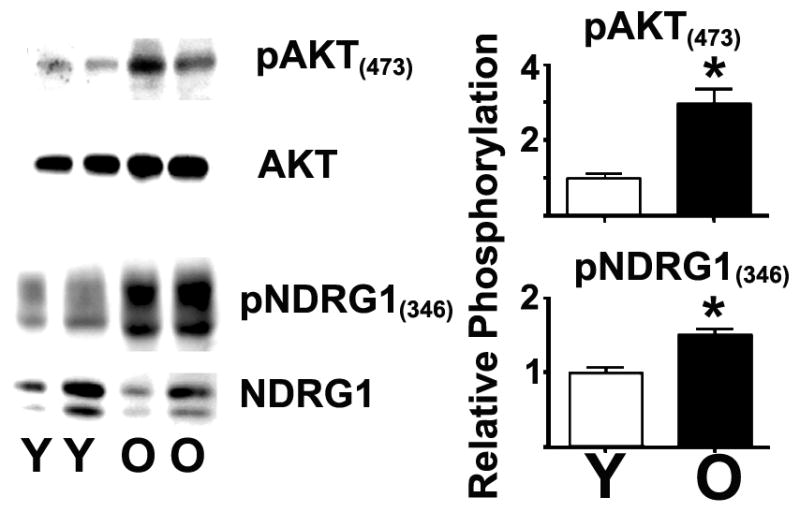
A) Representative western blot analysis of the pAKT(473) and pNDRG1(345) levels and the expression levels of AKT and NDRG1 in resting CD4 T cells from the spleens of young (Y) or old (O) CB6F1 mice. B) Quantification of the age-related changes from 8 independent experiments, including a total of 12 young and 8 old mice. The values represent the mean ± SEM, and (*) indicates statistically significant age-related changes relative to CD4 T cell controls from young mice (Y).
In vitro functional consequences of enhanced TORC1 or TORC2 signaling in naïve CD4 T cells
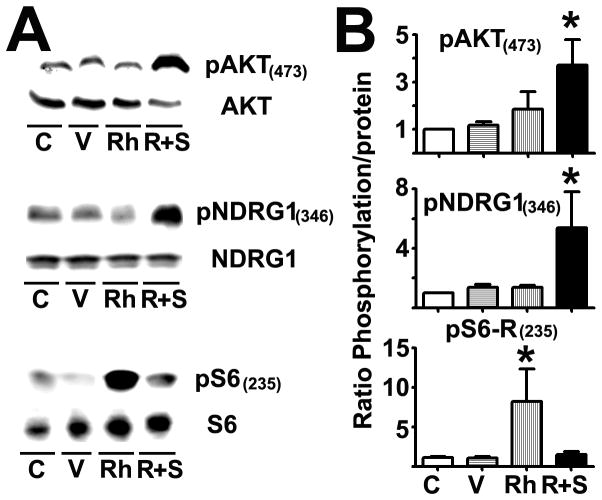
Data from Figure 1 and 2 suggest that aging may increase TORC2 activity; however, these results do not exclude the possibility that TORC1 plays a role in T cell immunosenescence. To differentiate between the roles of TORC2 and TORC1, we purified CD4 T cells from the spleens of young CB6F1 donors (>90% naïve cells). Then, we transfected the cells with constructs capable of activating TORC1 or TORC2 function. We enhanced TORC1 activity using Rheb overexpression (Rh), while we enhanced TORC2 activity by cotransfecting CD4 T cells with Rictor and Sin1 proteins. The latter method was based on data suggesting that using Rictor plus Sin1 can stabilize the TORC2 complex, leading to its activatio (21, 44). We selected electroporation as a method of transfection because it results in consistent efficiency between different constructs and multiple experiments (see below). After electroporation, untransfected and transfected CD4 T cells from young donors were incubated for 24 hours. Then, cells were stained for CD4 and a live/dead fixable red stain to identify live cells, followed by intracellular staining against the corresponding protein-tags: a) Flag for Rheb, b) HA for Rictor, and c) Myc for Sin1; analysis was performed using intracellular flow cytometry. A parallel transfection of CD4 T cells with GFP was performed for measuring the daily transfection efficiency as recommended by the electroporation kit manufacturer. We observed a 30–50% transfection efficiency of CD4 T cells for all experiments with approximately 20–30% cell death (Supplemental Figure 2). After removing the dead cells, around 10 × 106 CD4 T cells from each group were lysed and analyzed by western blotting using specific antibodies for pAKT(473) and pNDRG(346), which are downstream targets of TORC2, and pS6(235), which is a downstream target of TORC1. As shown in Figure 3A, compared to control (C) or vector alone (V), Rheb (Rh) overexpression significantly enhanced pS6(235) levels (p = 0.03) without affecting mTORC2 targets. On the other hand, Rictor plus Sin1 (R+S) overexpression significantly enhanced pAKT(473) (p = 0.01) and pNDRG1(346) (p = 0.02) levels without affecting pS6(235). These results suggest that we can independently enhance TORC1 and TORC2 activity in naïve CD4 T cells (see Supplemental Figure 3 for all of the western blot results obtained from a typical experiment).
Figure 3. Transfection with Rheb and cotransfection with Rictor plus Sin1 can upregulate mTORC1 and mTORC2 signaling, respectively.
A) CD4 T cells from young CB6F1 mice remained untransfected (C) or were transfected with vector (V), Rheb (Rh), or Rictor and Sin1 (R+S) as described in the Methods section. Live cells were lysed and the aliquots were analyzed by western blotting using pAKT(473), pNDRG1(346), and pS6-R(235) antibodies or the respective total proteins. B) Quantification of the relative phosphorylation levels from 4 independent experiments. The values represent the mean ± SEM, and (*) indicates statistically significant changes with respect to controls (C).
To analyze whether enhanced TORC1 and TORC2 signaling affects the levels of BIM and pERM, we performed similar experiments as described in Figure 3. However, to facilitate the measurement of multiple TORC downstream targets in the same samples, we performed intracellular staining as described in Figure 1. A typical flow cytometric analysis of untransfected (C) and transfected groups (V, Rh, and R+S) is shown in Figure 4A. As expected, untransfected or vector-alone CD4 T cells from young mice expressed high levels of BIM and pERM and had relatively low levels of pNDRG1(346) and pS6(235). Furthermore, Rheb overexpression (Rh) increased pS6(235) levels but did not affect those of BIM or pERM (Figure 4A, middle panels). In contrast, overexpression of Rictor plus Sin1 decreased the number of CD4 T cells expressing BIM and pERM while enhancing pNDRG1(346) levels (Figure 4A, lower panels). In addition, none of the constructs appeared to affect pAKT(308) (data not shown). To test whether these changes were statistically significant, we performed a series of 6 independent experiments as shown in Figure 4B. The data confirmed that vector and Rheb overexpression did not affect BIM or pERM levels. However, enhanced mTORC2 significantly reduced BIM (p < 0.0001) and pERM (p < 0.0001) levels and increased pNDGR1(346) (p = 0.03) levels without affecting pS6(235). In this data set, we found that Rheb or Rictor plus Sin1 overexpression has no significant effect on the expression of endogenous ERM, NDRG1, and S6 proteins (data not shown), suggesting that the above changes are the result of alterations in the phosphorylation pattern. These overall findings suggest that age-related increases in TORC2 signaling lead to declines in pERM and BIM expression, as is found with aging, and support a model in which inhibition of TORC2 (shown in Figure 1) is responsible for preventing age-related declines in CD4 T cell function in rapamycin-treated mice.
Figure 4. Upregulation of TORC2, but not TORC1, signaling leads to declines in BIM and pERM expression.
A) Representative flow cytometric analysis measuring pERM(558), BIM, pNDRG1(346), and pSR-R(236) levels in gated live CD4 T cells either untransfected (C) or transfected with vector, Rheb, or Rictor plus Sin1. The vertical line represents the threshold used to distinguish between high or low levels of the respective phosphoproteins and protein expression used for quantification. B) Quantification of the relative expression levels from 8 independent experiments. The values represent the mean ± SEM, and (*) indicates statistically significant changes with respect to controls.
In vivo functional consequences of enhanced TORC1 or TORC2 signaling in the activation and proliferation of naïve CD4 T cells
To test the hypothesis that enhanced TORC2 or TORC1 signaling affects in vivo CD4 T cell from young mice function in a manner that is consistent with the response of CD4 T cells from old mice (31, 45, 46), we used an in vivo model of immune function based on adoptive transfer of transgenic naïve CD4 T cells that recognize a specific sequence on the PCC protein (AND mice) into syngeneic CD4KO mice as previously described (31). In this model, in vivo CD4 T cell expansion is dependent on PCC priming, and the rate of CD4 T cell proliferation significantly declines with aging as measured by cell division trackers and flow cytometric analysis. To test the hypothesis that elevated TORC2 activity may be responsible for declines in CD4 T cell function, we purified naïve CD4 T cells from the spleens of young AND mice (4 months), and aliquots of 5 × 106 CD4 T cells were labeled with a CFSE cell division tracker. In addition, aliquots of 20 × 106 CD4 T cells were labeled with a violet cell division tracer. After labeling, approximately 6 × 106 violet-labeled CD4 T cells were electroporated with each of the constructs (vector, Rh, and R+S). Then, 2 × 106 live CD4 T cells from each transfection group were mixed with 1 × 106 untransfected cells (CFSE controls, C) and adoptively transferred to CD4KO young hosts (2–4 months). This dual labeling of untransfected (CFSE) and transfected (Violet) CD4 T cells transferred to a single host minimizes the effects of host variation and enables analysis between multiple hosts. Twenty-four hours after adoptive transfer, each host mouse was primed with 10 μg of PCC in PBS. Forty-eight hours later, the spleen and lymph nodes were harvested and the proliferation of the adoptively transferred CD4 T cells was analyzed by flow cytometry as previously described (31). Figure 5A shows a typical profile of CD4 T cell division for the untransfected or vector-alone groups, where approximately 25% of the CD4 T cells in the lymph nodes and 55% in the spleen have not yet divided. These cell division profiles correspond well with previous literature (31, 32). Rheb overexpression does not appear to affect proliferation relative to untransfected or vector-alone groups. In contrast, overexpression of Rictor plus Sin1 increased the number of non-dividing CD4 T cells (50% in the lymph nodes; 75% in the spleen). To test whether these declines were statistically significant, we performed a series of 3 independent experiments with at least 2 host mice receiving cells from each of the transfection groups (control/vector, control/Rh, control/R+S) per experiment. As shown in Figure 5B, we found no effect of Rheb overexpression. These results suggest that enhanced TORC1 signaling does not seem to affect cell proliferation under our experimental conditions. However, overexpression of Rictor plus Sin1 leads to a significant (30–40%) increase in the number of non-dividing CD4 T cells in the lymph nodes (p = 0.004) and spleen (p = 0.002) relative to controls in the same host. These reductions in proliferation are almost identical to the age-related declines in proliferation that we previously reported using the same in vivo model (31). These results suggest a model in which an age-related increase in TORC2 signaling leads to declines in CD4 T cell function. Furthermore, we previously demonstrated that aging decreases the expression of ICOS on CD4 T cells (32), a surface protein involved in the interactions with B cells and dendritic cells (DCs) during immune responses (47). As shown in Figure 5B, Rictor plus Sin1 overexpression leads to significant decline in total ICOS expression in the lymph nodes (p < 0.001) and spleen (p = 0.002) (for a typical flow cytometric analysis see Supplemental Figure 4A). We also found that most of the decreased ICOS expression occurred in the non-dividing population, suggesting that enhanced TORC2 signaling blocked CD4 T cell activation from young mice, as we previously described in CD4 T cells from old mice (32). It is possible that overexpression of Rictor and Sin1 may result in an unresponsive or anergic state in CD4 T cells. To test this possibility, we performed similar experiments as shown in Figure 5 while increasing the PCC priming dose to 100 μg. Under this strong stimulus, the control CD4 T cells underwent numerous rounds of cell division (See Supplemental Figure 4B). The proliferation differences between the control and Rictor plus Sin1-overexpressing cells did not reach statistical significance, suggesting that the CD4 T cells were not anergic, but rather that increased TORC2 signaling led to TCR signaling alterations that required a higher threshold of TCR activation, thus causing declines in TCR or CD28 signals observed during aging (48). Analysis of cotransfection with GFP or intracellular stains and the HA or Myc tag (Supplemental Figure 4C and D) suggested no differences in the efficiency of transfection and tag expression compared to that described for our in vitro experiments using the CB6F1 model (Supplemental Figure 2), indicating that similar alterations of TORC1 and TORC2 activity are likely to have occurred in the in vivo transfer system using AND mice.
Figure 5. Upregulation of TORC2, but not TORC1, signaling leads to in vivo declines in CD4 T cell proliferation and ICOS expression in response to antigen priming.
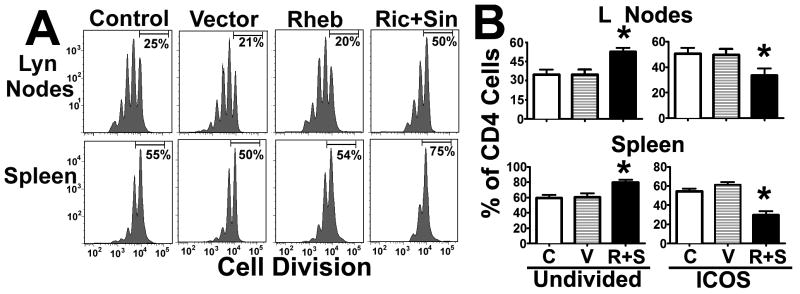
A) CD4 T cells from young AND mice were stained with CFSE or violet cell division tracker, either untransfected (Control, C) or transfected with vector (V), Rheb (Rh), or Rictor plus Sin1 (R+S), and were adoptively transferred into CD4KO host mice as described in the Methods section. Forty-eight hours after priming, the spleens and lymph nodes were collected and cellular proliferation and ICOS expression were analyzed by flow cytometry. The histogram shows a representative experiment of each group with the percentages of non-dividing CD4 T cells. B) Quantification of the relative proliferation and expression of ICOS from 3 independent experiments. The values represent the mean ± SEM, and (*) indicates statistically significant changes with respect to controls.
Enhanced TORC2 function decreases TCR signaling, leading to declines in CD4 T cell activation
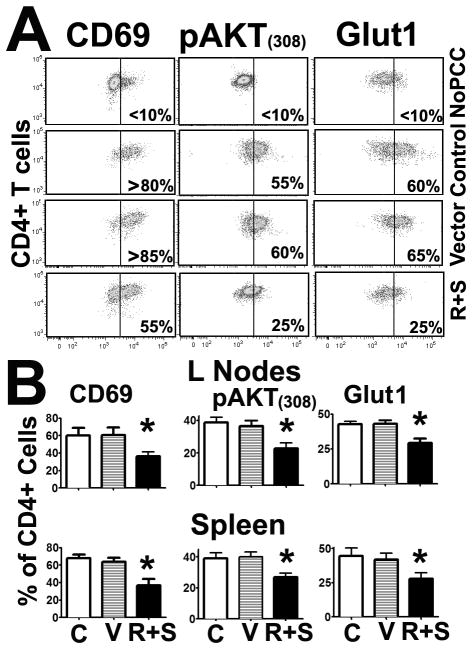
We have reported that CD4 T cells from old mice exhibit defects in CD28 signaling that include the inability to enhance pAKT(308) levels and the de novo expression of Glut1 and CD69 (32). The decreased ICOS expression shown in Supplemental Figure 4 suggest that enhanced TORC2 signaling may lead to declines in CD4 T cell activation and CD28 signaling from young mice similar to those observed in CD4 T cells from old mice. To test this hypothesis, we performed experiments similar to those of Figure 5, except that the analysis of the CD4 T cells was performed at 24 hours after priming and thus before cell division had occurred. Control, vector alone-, or R+S-transfected CD4 T cells from the spleen and lymph nodes were analyzed for pAKT(308), Glut1, and CD69 levels as previously described (32). We excluded Rheb from this analysis because Rheb overexpression does not affect ICOS expression or proliferation, suggesting no effect on CD4 T cell activation (Figure 5). Figure 6A shows a typical analysis in which host mouse priming led to enhanced pAKT(308) levels and the de novo expression of Glut1 and CD69 in CD4 T cells, as we previously reported (32). In contrast, adoptively transferred CD4 T cells transfected with Rictor and Sin1 (R+S) showed defects in the antigen-dependent changes in pAKT(308), Glut1, and CD69 levels. To test whether these differences were statistically significant, we performed a series of 3 experiments. As shown in Figure 6B, we found that enhancing TORC2 signaling could significantly inhibit the expression of CD69 (lymph nodes, p = 0.006; spleen, p = 0.01), Glut1 (lymph nodes, p = 0.002; spleen, p < 0.001), and pAKT(308) (lymph nodes, p = 0.03; spleen, p < 0.001). These results suggest that enhanced TORC2 signaling leads to defects in the CD28 signaling pathways that mimic the effects of aging (32) and support a model in which TORC2 is a key mediator in the age-related decline of CD4 T cell function in mice.
Figure 6. Upregulation of TORC2 signaling leads to declines in early CD28 activation signaling.
A) CD4 T cells from young AND mice were transfected and adoptively transferred into young CD4KO hosts as described in Figure 5, and 24 hours after priming the gated CD4 T cells were analyzed for CD69, Glut1, and pAKT(308) expression. The dot-plots represent a typical experiment showing untransfected (Control) or vector- (V) or Rictor plus Sin1- (R+S) transfected CD4 T cells. A further control with non-primed host mice is also shown (No PCC). The vertical line represents the threshold used to distinguish between high and low levels of expression in each group used for quantification. B) Quantification of the relative expression levels from 3 independent experiments. The values represent the mean ± SEM, and (*) indicates statistically significant changes relative to primed controls.
Discussion
In lymphocytes, tissue-specific TORC1 and TORC2 knockout mice show defects in CD4 T cell differentiation (11). Elimination of TORC2 function in cell lines has indicated that TORC2 regulates several important signaling pathways involved in cytoskeletal organization and apoptosis, including PKC function, transcription factor localization (49), and CD28-PI3K-AKT signaling pathways (perhaps by regulating phosphatase expression) (50, 51). However, the functional consequences of elevated TORC1 and TORC2 activity and their roles in aging remain unknown (52), with the exception of the association with cancer (53). We have previously shown that CD4 T lymphocytes exhibit alterations in the cytoskeleton, in TCR signaling, and in immune synapse formation that are associated with decreased pERM expression (29) and defects in CD28 signaling (32). Other investigators have reported declines in BIM expression (33). We hypothesized that some of these changes are modulated by central mechanism(s) such as TOR signaling. We tested this hypothesis by treating a genetically heterogeneous population of mice (UM-HET) with different doses of rapamycin, including a dose (14.7 ppm) already known to extend life (4). As expected, rapamycin prevented CD4 T cell differentiation (Supplemental Figure 1; (54), and these effects may be the result of TORC1 and/or TORC2 inhibition. There is evidence that rapamycin inhibition of TORC2 may be detrimental to some tissues and may impair glucose homeostasis (39). In contrast, our rapamycin data suggest that TOR inhibition can prevent key age-related changes in naïve and memory CD4 T cells, including increased pAKT(437) and pNDRG levels as well as declines in pERM and BIM expression (Figures 1 and 2). However, because mice were treated systematically with rapamycin it is still unclear whether these changes are due to direct effects on CD4 T cells or on other cells types such as APCs. Materials from rapamycin-treated and control mice were taken from mice included in a separate study designed to evaluate potential effects of this drug on mouse lifespan (34). One weakness of the protocol was the lack of control mice fed with microencapsulated mock alone. We think it is highly unlikely the material used for encapsulation (for composition see www.patent.ipexl.com/20120064143) could, without incorporated rapamycin, either extend life span or prevent age-related changes in lymphocytes, but we cannot at this point exclude the hypothesis that some of the effects we attribute to rapamycin might be caused by the encapsulation vehicle itself. We also note a recent report, using C57BL/6J mice, which suggested that rapamycin treatment could reverse some of the age-related changes in the immune system, including increases in CD44 high CD4 T cells from old mice (55), and also documenting age-independent effects in male C57BL/6 mice. Our data (see Figure S1) suggest similar conclusions: rapamycin prevented some of the age-related changes in CD4 T cells (specifically changes in CD44 high cells), but also produced effects on outcomes not usually altered by aging, such as the significant decline in CD4 T cell number.
In the other hand, our findings suggest that selective inhibition of TORC2 may have beneficial effects on preventing the age-related decline of immune function. This model could be tested once a specific inhibitor of TORC2 function becomes available that can be administrated in vivo to old mice to reverse the age-related decline of immune function.
The rapamycin data and enhancement of TORC2 signaling in old mice (Figures 1 and 2) do not directly address the question of which TOR complex is involved in the age-associated decline of immune function. To test which TOR complex is involved, we transfected primary naïve CD4 T cells from young mice with Rheb to activate TORC1 signaling, or with Rictor plus Sin1 to activate TORC2 signaling (Figures 3 and 4 and Supplemental Figures 2 and 3). Then, we measured the effects on pERM and BIM expression. As shown in Figure 4, enhancing TORC2, but not TORC1, signaling leads to significant declines in BIM and pERM. Single transfections with Sin1 or Rictor do not alter pERM or BIM expression in naïve CD4 T cells from young donors (data not shown). In addition, Sin1 transfection alone does not increase the phosphorylation of downstream targets of TORC2 (data not shown). On the other hand, transfection with Rictor alone induces some changes in pAKT and pNDRG, but these are inconsistent and do not reach statically significance (data not shown). The results suggest that activation of TORC2 may require the overexpression of both proteins. This is consistent with the literature suggesting that Rictor and Sin1 form a complex that associates with and stabilizes TORC2 leading to its activation (21, 44). The overall results suggest that some aspects of the age-related changes in CD4 T cells are regulated by TORC2, and support a model in which aging increases TORC2 activity, leading to changes in the function of CD4 T cells from old mice. This model may help to explain and expand our knowledge of how inhibition of each of these TOR complexes by rapamycin prevents some aspects of the age-related decline of immune function in CD4 T cells and other cell types (56–58) and extend life. Furthermore, it would be interesting to establish which specific TORC2 pathways are involved in the age-related changes in CD4 T cells, including alterations in PKC localization (59, 60), Rac and Rho-A signaling (30), phosphatases (61), as well as the regulation of transcription factors that may affect apoptosis and proliferation (62). However, the complexity of TORC2 signaling would require the use of complementary approaches, including microarray analysis and signaling pathway knockouts to test which specific mechanism(s) is involved. Such results would provide important clues as to how aging affects T lymphocytes.
In addition, we tested the biological significance of enhanced TORC2 signaling by studying in vivo CD4 T cell activation and proliferation. In this regard, using an in vivo model of adoptive transfer, we demonstrated that specific aspects of CD28 signaling in naïve CD4 T cells from old mice are defective, including defects in enhancing pAKT(308), Glut1, CD69, and ICOS expression (32). These defects lead to decreased CD4 T cell proliferation (31) that has been linked to declines in immune function (24) such as CD4 T cell-dependent antibody production (32, 63). We used this in vivo model of CD4 T cell function to test whether enhancement of TORC1 or TORC2 signaling may lead to similar defects in proliferation, CD28 signaling, and activation. As is shown in Figure 5, we found that enhancing TORC1 signaling does not seem to decrease the proliferation of naïve CD4 T cells from young mice. In contrast, as shown in Figures 5 and 6, enhancing TORC2 signaling leads to significant (30–40%) declines in the proliferation, CD28 signaling, and activation of naïve CD4 T cells. These decreases resemble those that we described in CD4 T cells of old mice, suggesting the involvement of TORC2 signaling in the decline of CD4 T cell function. A more extensive analysis of other markers of CD4 T cell activation will be needed to confirm this observation. In the other hand, because TOR is involved in lymphocyte differentiation and cytokine production (11), it is likely that enhancing TORC2 signaling may lead to changes in cytokine production. It would be interesting to further pursue this hypothesis by analyzing how enhancing TORC2 signaling might result in a pattern of cytokine expression that also resembles the aging phenotype of CD4 T cells (24), i.e., declines in IL-2 and defective memory formation (64). Prolonged activation of TOR can lead to a feedback loop which inactivates upstream activators of TOR (65). These may include elements of CD28 signaling pathways that affect TOR function in CD4 T cells. In principle, these might involve upstream TORC2-dependent regulation of PDK1/PI3K pathways or PTEN phosphatases (65), or other mechanisms still to be investigated. Unfortunately, the transient nature of Rictor and Sin1 expression in CD4 T cells after proliferation may preclude some of these experiments and analyses.
It would be interesting to evaluate whether TORC2 function is also altered in humans, particularly in CD4 T cells that show declines or defects in CD28 expression (66). We have shown that defects in CD28 signaling of murine CD4 T cells are similar to those observed in human CD4 T cells (32); thus, it would be interesting to test this hypothesis in CD28-deficient human CD4 T cells using our model. Furthermore, most of our work made use of CD4 T cells from young donors, and it would therefore be important to test the hypothesis that modulating TORC2 activity would also improve function of aged T cells. It is also possible, however, that manipulation of Rictor or Sin1 levels could alter aspects of T cell development and function (67–69) in addition to acute effects on T cell activation. Work along these lines to see if manipulation of TORC2 activity can prevent, or reverse, immune dysfunction is a major goal of our future studies. Nevertheless, the overall results presented herein suggest a novel model in which age-associated enhanced TORC2 signaling leads to defects of lymphocyte function in mice and likely humans as well.
Supplementary Material
Acknowledgments
Support: This work was supported by an NIH grant (AG019619) and the University of Michigan Nathan Shock Center for the Biology of Aging (AG024824).
We wish to thank Lynn Winkleman, Jessica Sewald, Lisa Burmeister, Sabrina van Roekel, Jacob Schaeffer, Alvarado Malaga, and Graham Dominick for technical assistance.
Abbreviations
- TOR
mammalian target of rapamycin
- TORC1
mammalian target of rapamycin complex 1
- TORC2
mammalian target of rapamycin complex 2
- Rh
Rheb
- R+S
Rictor plus Sin-1
- Glut-1
glucose transporter type I
Footnotes
Conflict of Interest: None of the authors have any financial or commercial conflicts of interest to declare.
References
- 1.Evans DS, Kapahi P, Hsueh WC, Kockel L. TOR signaling never gets old: aging, longevity and TORC1 activity. Ageing research reviews. 2011;10:225–237. doi: 10.1016/j.arr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR Signaling and Rapamycin Influence Longevity by Regulating SKN-1/Nrf and DAF-16/FoxO. Cell metabolism. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proud CG. Amino acids and mTOR signalling in anabolic function. Biochemical Society transactions. 2007;35:1187–1190. doi: 10.1042/BST0351187. [DOI] [PubMed] [Google Scholar]
- 6.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends in biochemical sciences. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nature cell biology. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 8.Garami A, Zwartkruis FJT, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Molecular cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 9.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. The Biochemical journal. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, He YW, Zhong XP. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. European journal of immunology. 2011;41:3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunological reviews. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waickman AT, Powell JD. Mammalian target of rapamycin integrates diverse inputs to guide the outcome of antigen recognition in T cells. Journal of immunology (Baltimore, Md : 1950) 2012;188:4721–4729. doi: 10.4049/jimmunol.1103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadri M, Daquinag A, Wang S, Xue T, Kunz J. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Molecular biology of the cell. 2005;16:1883–1900. doi: 10.1091/mbc.E04-07-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60:827–837. doi: 10.2337/db10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saci A, Cantley LC, Carpenter CL. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Molecular cell. 2011;42:50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Developmental cell. 2010;19:845–857. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. The EMBO journal. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VLJ, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nature immunology. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 19.Gupta M, Hendrickson AEW, Yun SS, Han JJ, Schneider PA, Koh BD, Stenson MJ, Wellik LE, Shing JC, Peterson KL, Flatten KS, Hess AD, Smith BD, Karp JE, Barr S, Witzig TE, Kaufmann SH. Dual mTORC1/mTORC2 inhibition diminishes Akt activation and induces Puma-dependent apoptosis in lymphoid malignancies. Blood. 2012;119:476–487. doi: 10.1182/blood-2011-04-346601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncharova EA, Goncharov DA, Li H, Pimtong W, Lu S, Khavin I, Krymskaya VP. mTORC2 is required for proliferation and survival of TSC2-null cells. Molecular and cellular biology. 2011;31:2484–2498. doi: 10.1128/MCB.01061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 22.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends in biochemical sciences. 2009;34:620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Miller RA, Berger SB, Burke DT, Galecki A, Garcia GG, Harper JM, Sadighi Akha AA. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunological reviews. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 24.Haynes L, Maue AC. Effects of aging on T cell function. Current opinion in immunology. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cellular immunology. 1997;175:51–57. doi: 10.1006/cimm.1996.1040. [DOI] [PubMed] [Google Scholar]
- 26.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. Journal of immunology (Baltimore, Md : 1950) 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 27.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. Journal of immunology (Baltimore, Md : 1950) 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 28.Garcia GG, Miller RA. Age-related changes in lck-Vav signaling pathways in mouse CD4 T cells. Cellular immunology. 2009;259:100–104. doi: 10.1016/j.cellimm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia GG, Miller RA. Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing research reviews. 2011;10:26–34. doi: 10.1016/j.arr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia GG, Sadighi Akha AA, Miller RA. Age-related defects in moesin/ezrin cytoskeletal signals in mouse CD4 T cells. Journal of immunology (Baltimore, Md : 1950) 2007;179:6403–6409. doi: 10.4049/jimmunol.179.10.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia GG, Miller RA. Ex vivo enzymatic treatment of aged CD4 T cells restores antigen-driven CD69 expression and proliferation in mice. Immunobiology. 2011;216:66–71. doi: 10.1016/j.imbio.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkey E, Miller RA, Garcia GG. Ex vivo enzymatic treatment of aged CD4 T cells restores cognate T cell helper function and enhances antibody production in mice. Journal of immunology (Baltimore, Md : 1950) 2012;189:5582–5589. doi: 10.4049/jimmunol.1200487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. Journal of immunology (Baltimore, Md : 1950) 2010;185:4535–4544. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin J, Ma Z, Selliah N, Shivers DK, Cron RQ, Finkel TH. Effective gene suppression using small interfering RNA in hard-to-transfect human T cells. Journal of immunological methods. 2006;312:1–11. doi: 10.1016/j.jim.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Haag J, Voigt R, Soeder S, Aigner T. Efficient non-viral transfection of primary human adult chondrocytes in a high-throughput format. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:813–817. doi: 10.1016/j.joca.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Experimental gerontology. 2011;46:382–390. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 39.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science (New York, N Y) 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson ARO, Lee WT. Differences in signaling molecule organization between naive and memory CD4+ T lymphocytes. Journal of immunology (Baltimore, Md : 1950) 2004;173:33–41. doi: 10.4049/jimmunol.173.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Current opinion in cell biology. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochemical Society transactions. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) The Biochemical journal. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 44.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Current opinion in immunology. 2005;17:486–491. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Current opinion in immunology. 2010;22:326–332. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJY, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing research reviews. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Gough NR. Focus issue: TOR signaling, a tale of two complexes. Science signaling. 2012;5:eg4. doi: 10.1126/scisignal.2003044. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Readinger JA, DuBois W, Janka-Junttila M, Robinson R, Pruitt M, Bliskovsky V, Wu JZ, Sakakibara K, Patel J, Parent CA, Tessarollo L, Schwartzberg PL, Mock BA. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. 2011;117:1228–1238. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, Goronzy JJ. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E879–888. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohne Y, Takahara T, Maeda T. Evaluation of mTOR function by a gain-of-function approach. Cell cycle (Georgetown, Tex) 2009;8:573–579. doi: 10.4161/cc.8.4.7660. [DOI] [PubMed] [Google Scholar]
- 53.Lionello M, Blandamura S, Loreggian L, Ottaviano G, Giacomelli L, Marchese-Ragona R, Velardita C, Staffieri A, Marioni G. High mTOR expression is associated with a worse oncological outcome in laryngeal carcinoma treated with postoperative radiotherapy: a pilot study. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2012;41:136–140. doi: 10.1111/j.1600-0714.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 54.O’Brien TF, Zhong XP. The role and regulation of mTOR in T-lymphocyte function. Archivum immunologiae et therapiae experimentalis. 2012;60:173–181. doi: 10.1007/s00005-012-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Holter SM, Moreth K, Prehn C, Puk O, Racz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Hofler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. Journal of immunology (Baltimore, Md : 1950) 2010;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 58.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nature medicine. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 59.Ohkusu K, Du J, Isobe KI, Yi H, Akhand AA, Kato M, Suzuki H, Hidaka H, Nakashima I. Protein kinase C alpha-mediated chronic signal transduction for immunosenescence. Journal of immunology (Baltimore, Md : 1950) 1997;159:2082–2084. [PubMed] [Google Scholar]
- 60.Fernandez-Gutierrez B, Jover JA, De Miguel S, Hernandez-Garcia C, Vidan MT, Ribera JM, Banares A, Serra JA. Early lymphocyte activation in elderly humans: impaired T and T-dependent B cell responses. Experimental gerontology. 1999;34:217–229. doi: 10.1016/s0531-5565(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 61.Das F, Ghosh-Choudhury N, Dey N, Mandal CC, Mahimainathan L, Kasinath BS, Abboud HE, Choudhury GG. Unrestrained mammalian target of rapamycin complexes 1 and 2 increase expression of phosphatase and tensin homolog deleted on chromosome 10 to regulate phosphorylation of Akt kinase. The Journal of biological chemistry. 2012;287:3808–3822. doi: 10.1074/jbc.M111.246397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Naggar S, Liu Y, Dean DC. Mutation of the Rb1 pathway leads to overexpression of mTor, constitutive phosphorylation of Akt on serine 473, resistance to anoikis, and a block in c-Raf activation. Molecular and cellular biology. 2009;29:5710–5717. doi: 10.1128/MCB.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunological reviews. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasaki AT, Firtel RA. Regulation of chemotaxis by the orchestrated activation of Ras, PI3K, and TOR. Eur J Cell Biol. 2006;85:873–895. doi: 10.1016/j.ejcb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends in immunology. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boulbes D, Chen CH, Shaikenov T, Agarwal NK, Peterson TR, Addona TA, Keshishian H, Carr SA, Magnuson MA, Sabatini DM, Sarbassov dos D. Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2. Mol Cancer Res. 2010;8:896–906. doi: 10.1158/1541-7786.MCR-09-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwal NK, Chen CH, Cho H, Boulbes DR, Spooner E, Sarbassov DD. Rictor regulates cell migration by suppressing RhoGDI2. Oncogene. 2013;32:2521–2526. doi: 10.1038/onc.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schroder WA, Buck M, Cloonan N, Hancock JF, Suhrbier A, Sculley T, Bushell G. Human Sin1 contains Ras-binding and pleckstrin homology domains and suppresses Ras signalling. Cell Signal. 2007;19:1279–1289. doi: 10.1016/j.cellsig.2007.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.