Abstract
In the last 20 years there has been increased focus on gender differences in health and disease. The earliest studies of lung cancer enrolled mainly men, as the incidence of lung cancer among women was exceedingly low. As social patterns changed around World War II and women began to smoke more, the epidemiology of lung cancer has changed. The higher percentage of lung cancer in non-smoking women as compared to non-smoking men suggests that lung cancer in women behaves differently. Studies of lung cancer in women indicate that there are differences in risk factors, histology, pathophysiology, treatment outcomes and prognosis as compared to men. The purpose of this review is to provide a concise summary of the literature on lung cancer as it pertains to women, with an emphasis on new areas of research and treatment options.
Keywords: Lung cancer, women, risk factors, genetic mutations
Epidemiology
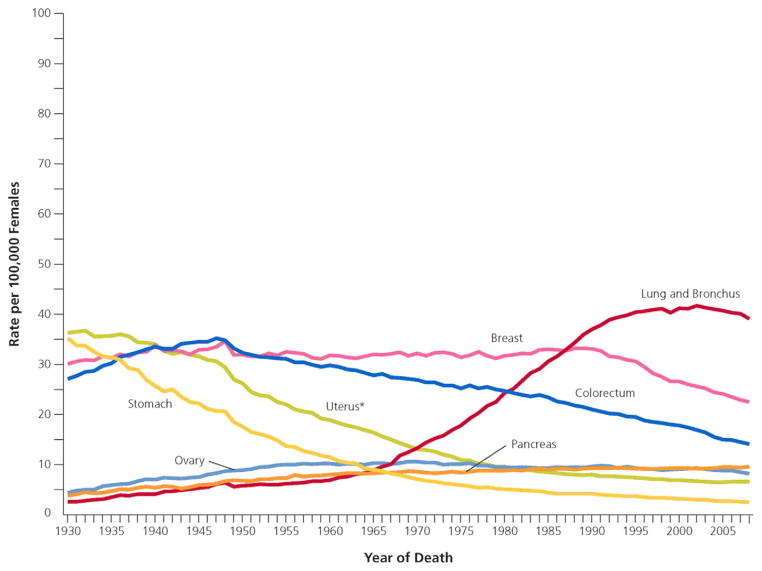
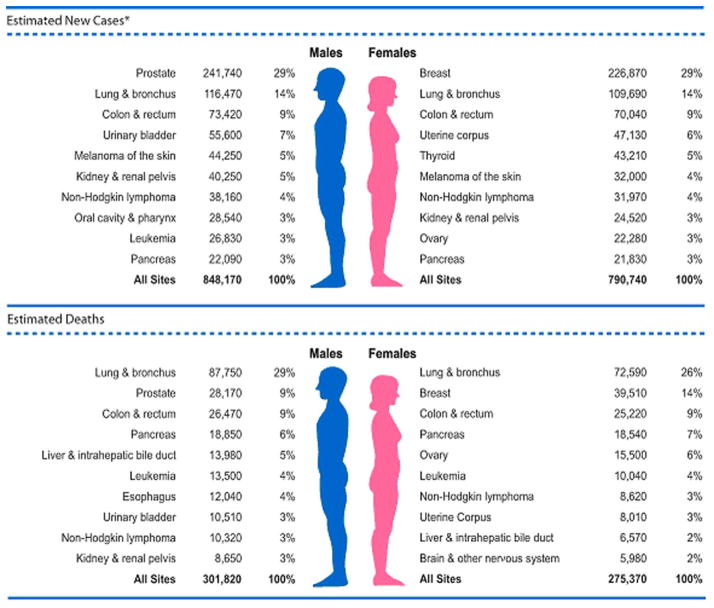
In the United States, lung cancer is the second most common cancer diagnosed in women and the leading cause of cancer-related mortality, with an estimated 109,690 new diagnoses and 72,590 deaths in 2012 (1,2). Female lung cancer comprised 26% of estimated cancer deaths in 2012, greater than the combined mortality from breast and colon/rectum cancer (1,3). Women have a 1 in 16 lifetime risk of developing lung cancer regardless of smoking status, 47% of diagnoses are in women over 70 years of age and 50% are diagnosed at advanced stages (2,4). Lung cancer incidence rates began significantly increasing in 1973 and reached a plateau in the late 1990s, over a decade later than men (1,2). Lung cancer mortality stabilized for the first time in 2003, two decades later than men, and has yet to decline (2,3). Five-year survival remains poor, but is higher in women as compared to men (less than 18% versus less than 14%) (4).(FIGURES 1 AND 2)
Figure 1.
Lung cancer deaths in women. Reprinted with permission 5
Figure 2.
Ten Leading Cancer Types for the Estimated New Cancer Cases and Deaths by Sex, United States, 2012. Reprinted with permission 1
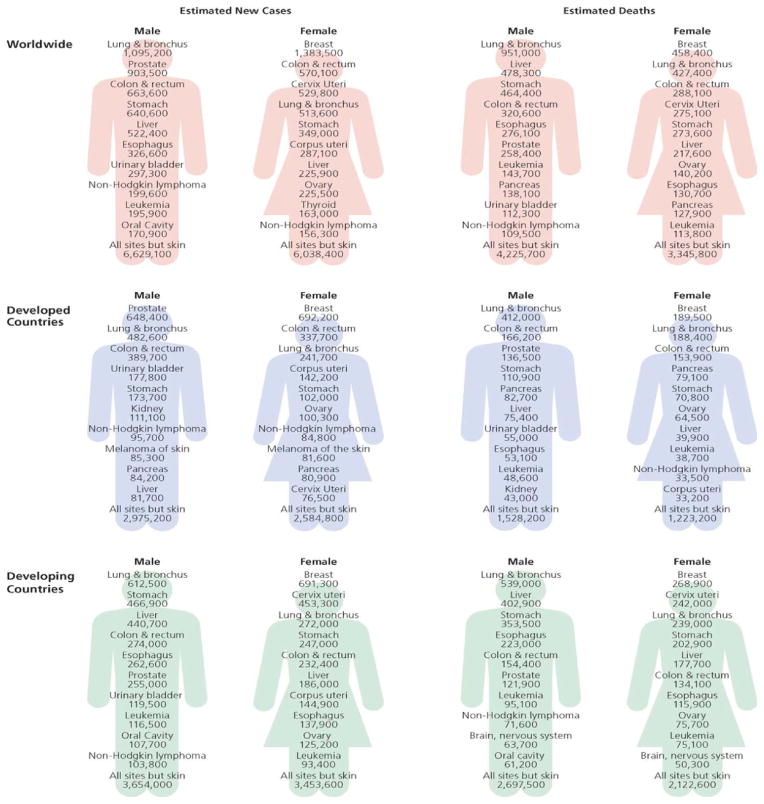
The global burden of lung cancer is increasing due to a more recent steep increase in tobacco smoking in the developing world. In 2008, 56% of new cancer diagnoses and 64% of cancer deaths occurred in low and medium resource countries. In these countries, lung cancer in women was the 3rd most commonly diagnosed cancer and 3rd most common cause of cancer-related death (6). The incidence of female lung cancer is highest in North America, Northern Europe and Australia/New Zealand. While incidence rates have reached a plateau in the above regions, both incidence and mortality continues to increase worldwide (6). (FIGURE 3)
Figure 3.
Estimated New Cancer Cases and Deaths Worldwide for Leading Cancer Sits by Level of Economic Development, 2008. Source: GLOBOCAN 2008. Reprinted with permission 6
Smoking
Tobacco remains the largest risk factor for lung cancer in women, responsible for 80–90% of cancer-related deaths and estimated to be responsible for at least 50% of the worldwide lung cancer burden (2,4,6). Smoking prevalence is higher among those who are less educated and less affluent (7,8). According to the World Health Organization (WHO), 80% of the world’s 1 billion smokers live in low- and middle-income countries (9), and the incidence among women in low income countries continue to rise (3). Trends in lung cancer incidence and mortality among women reflect changing trends in cigarette smoking, the prevalence of which peaked among women in the U.S. almost 20 years later than men (1,2,6,7). In the 1930s, 50% of U.S. men smoked versus only 20% of women. During World War II smoking became accepted among women and prevalence peaked in the 1960s, when 30% of women smoked (7). Daily cigarette consumption peaked in the 1970s for men and 1980s for women (10). According to the most recent data available through the CDC, 21.5% of men and 17.3% of women were smoking in 2010 (8). Lung cancer is disproportionately diagnosed in women over 60 years of age and women over 70 years of age have the highest mortality rates because they went through adolescence at the peak of the U.S. smoking epidemic (11). Women have greater difficulty with smoking cessation (10) and may have increased risk of lung cancer for a given level of tobacco exposure (2,3).
Histology
The four major types of lung cancer are broadly categorized as small cell lung cancer originating from neuroendocrine cells versus non small cell lung cancer (NSCLC) originating from bronchial epithelial cell precursors. NSCLC is further sub-divided into squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Large cell carcinoma is thought to be poorly or undifferentiated versions of the other cancers and criteria for diagnosis vary widely (12). NSCLC represents the majority of all lung cancer. All histologic subtypes of lung cancer have been associated with smoking, the strongest association being with small cell and squamous cell carcinoma, and less robust for adenocarcinoma (12). Adenocarcinoma is the most common histologic subtype of lung cancer in both smoking and nonsmoking men and women; 41.4% of lung cancer in women is adenocarcinoma compared to 34.1% in men (3, 12,13). In women, the incidence of adenocarcinoma is slowly increasing, while that of squamous cell carcinoma is slowly decreasing. The incidence of small cell carcinoma has remained relatively stable (2). (TABLE 1)
Table 1.
| Lung Adenocarcinoma Risk Factors in Women |
| Exposure to second hand smoke |
| Cigarette smoking |
| Occupational exposure (radon, asbestos, etc.) |
| Exposure to air pollution |
| Nutritional status |
| Genetic susceptibility |
| Immunologic factors |
| Disease history (tuberculosis, asthma, etc.) |
| Exposure to cooking fumes |
| Hormonal factors |
| Menstrual cycle/pregnancy history |
| HPV infection |
Other Risks
Among never smokers with lung cancer, women are disproportionately represented. Worldwide, lung cancer in nonsmokers is the 7th leading cause of cancer-related mortality (2). In the U.S. and Europe, approximately 20% of women with lung cancer have never smoked versus 2–6% of nonsmoking men. This trend is further accentuated in Asian populations, where 60–80% of women with lung cancer have never smoked, in contrast to 10–15% of nonsmoking men. (2,12). This discrepancy is thought to indicate that lung cancer in nonsmokers, and specifically nonsmoking women, is different with regard to risks and pathophysiology (3,12). Some studies indicate that nonsmokers are diagnosed at later stages of disease, possibly due to a higher threshold to evaluate symptoms, but this has not been confirmed (12). It is unclear whether incidence and mortality rates are changing over time, owing to the historically unreliable reporting of tobacco exposure (2,12). Proposed explanations for the gender differences in lung cancer incidence include environmental exposures, genetic differences, molecular abnormalities, hormonal differences, and oncogenic viral infection.
Environmental Exposures
Environmental exposures known to be carcinogenic to the lung include second hand smoke (SHS), asbestos, arsenic, radon, polycyclic aromatic hydrocarbons, cadmium, nickel, metal dusts and vinyl chloride (3,6,7). SHS was classified as a human carcinogen by the EPA in 1993; globally, women and children are disproportionately affected (3,7). Women married to men who smoke have been shown to have a 25–29% increased risk of developing lung cancer (7,12). Urinary metabolites of tobacco specific carcinogens are present in nonsmokers exposed to SHS, indicating a role in the increased lung cancer risk (12). Radon, a radioactive gas produced by the decay of uranium in rocks and soil, is inhaled and can be carcinogenic at higher concentrations (2,12). Indoor burning of cooking oil and other biomass fuels in poorly ventilated areas produces polycyclic aromatic hydrocarbons, which are associated with lung cancer. This effect is seen especially in East and South Asian women, but is significant in all developing countries (3,7, 12).
Genetic Factors
Family history is an independent risk factor for the development of lung cancer regardless of smoking status, with women at higher risk than men, indicating a role for heritable factors (3,12,14).
Germline Gene Variants and Risk
Candidate Genes
CYP1A1 encodes a phase I enzyme involved in the metabolism of polycyclic aromatic hydrocarbons in tobacco smoke that results in activation of the pre-carcinogen. Over-expression leads to increased formation of DNA adducts, thought to represent the first step in carcinogenesis, and has been shown in female lung cancer independent of smoking status (3,12,19). Glutathione S-transferase M1 is responsible for phase II detoxification of carcinogenic reactive intermediates. Decreased expression leads to increased levels of reactive oxygen species, known to be damaging to DNA. While 40–60% of the population lacks GSTM1, its absence has been associated with an increased risk for lung cancer solely in women, accentuated in the presence of CYP1A1 over-expression (3,14,19). Women with lung cancer are known to have impaired DNA repair mechanisms regardless of smoking status, which have been linked to many cancers (3), and are more frequent in younger women (<60 years old) and those with a family history (14). Decreased DNA repair capacity is thought to lead to increased response to platinum based chemotherapy, the effect of which is based on the creation of DNA adducts (14). Candidate genes include: XRCC1, OGG1, ERCC1, ERCC2, XPA, XRCC3, MLH1 and MSH2 (12). Gastrin releasing peptide receptor is located on the X-chromosome and escapes X-inactivation in women. It leads to bronchial cell proliferation and over-expression has been linked to both tobacco use and the development of lung cancer. Smoking women have the highest frequency of expression followed by nonsmoking women (3,14).
Genome Wide Association Studies
Germline mutations differentially represented in lung cancer populations have been identified through genome wide association studies. SNPs at 6p21.33, 15p15.33, 15q15.2, 15q25.1, and 22q12 are associated with lung cancer in European populations, while SNPs at 3q28, 5p15.33, 13q12.12, 17q24.3 and 22q12.2 are associated with lung cancer in Asian populations. One group identified variants at 6p21.32, 6q22.2 and 10q25.2 associated with lung cancer in never-smoking Asian women. SNPs at 12p13.33 are associated with the development of squamous cell carcinoma in smokers, and those at 15q25.1 with lung cancer in smokers independent of histology. Variants at 5p15 and 15q25 are independent of histology, while those at 6p21-22 and 9p21.3 are associated with squamous cell carcinoma. Two SNPs at 13q31.3 regulate GPC5 expression, reported to be associated with adenocarcinoma in never smokers. A SNP in TERT at 15p15.33 is associated with adenocarcinoma, with a stronger association in females, those with earlier onset disease, and never smokers (20–22). Mutations in the tumor genome inform prognosis due to influence on invasiveness and transformative characteristics of tumor cells. One group identified five SNPs associated with decreased survival in early stage NSCLC (STK39, PCDH7, A2BP1 and EYA), decreased further with increasing numbers of SNPs (23).
Somatic Gene Mutations and Treatment Response
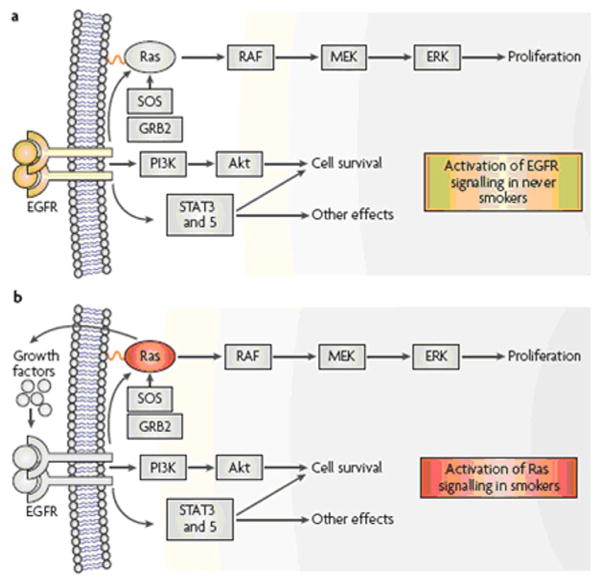
Epidermal Growth Factor Receptor (EGFR) mutations (FIGURE 4)
Figure 4. Two pathways to adenocarcinoma.
Ligand binding to epidermal growth factor receptor (EGFR) induces homo-and hetero-dimerization of the receptor, resulting in activation of downstream effectors including the Ras-MAPK (mitogen activated protein kinase), PI3K (phosphatidylinositol 3 kinase)-Akt, and signal transducer and activator of transcription (STAT) pathways that lead to cell proliferation, survival and many other effects associated with carcinogenesis. The EGFR pathway is frequently activated in never smokers by mutations in the EGFR gene. In smokers, mutations of the KRAS gene often occur, resulting in the release of growth factors, including transforming growth factor-α (TGF-α), which is a ligand for EGFR. In addition, Ras directly activates the PI3K-Akt pathway. Thus, the end result of KRAS or EGFR mutations are virtually identical, and mutations of both genes in adenocarcinomas of the lung are rarely seen. Other methods of activation of these pathways include gene amplification and mutations in BRAF, PIK3CA (a subunit of PI3K), and ERBB2 (also known as HER2). Reprinted with permission 12.
EGFR is a member of the human epidermal growth factor receptor family, which also includes HER2, HER3 and HER4. It is a transmembrane tyrosine kinase whose activation leads to signal transduction down intracellular pathways involved in cell proliferation and inhibition of apoptosis, pathways known to be involved in tumorigenesis and targeted by tyrosine kinase inhibitors (TKI) (12,15). Mutations in the EGFR receptor gene have been found in 40–80% of NSCLC, most frequently in adenocarcinoma, and are predictive of clinical response to tyrosine kinase inhibitors in the range of 65–90% depending on the study (15,16,17). Bronchioloalveolar carcinoma (BAC), a subtype of adenocarcinoma, has a particularly robust response to TKIs (19). Mutations are more prevalent in women, patients of Asian ethnicity, and are found almost exclusively in nonsmokers (16,18, 19). They have been associated with reduced survival, frequent lymph node metastases and poor response to chemotherapy (15). Over 90% of mutations are either an in-frame deletion on exon 19 or a point mutation on exon 21, leading to a constitutively activated EGFR receptor (15,18). Less commonly, other mutations lead to increased gene copy numbers that result in over-expression of the EGFR receptor (15). Specific mutations are thought to be associated with different levels of response to TKI therapy and different predicted survivals (15,19).
Erlotinib (Tarveca, OSI Pharmaceuticals, Inc., Melville, NY and Genentech, Inc., South San Francisco, CA) and gefitinib (Iressa, AstraZeneca Pharmaceuticals LP, Wilmington, DE) are tyrosine kinase inhibitors that have been FDA approved as second and third line agents for the treatment of advanced NSCLC (15,17). Gefitinib was FDA approved in May 2003 and erlotinib was FDA approved in November 2004. Subsequently, although there has been evidence that certain patient populations have a significant reduction in tumor burden in response to gefitinib, there has yet to be evidence for improved life expectancy (15). Other molecules targeting the EGFR pathway are under investigation including lapatinib (an EGFR TKI currently used in breast cancer), cetuximab, panitumumab and matuzumab (human monoclonal antibodies against EGFR), ZD6474 (an EGFR TKI currently used in the treatment of thyroid cancer), AEE788 (a TKI active against EGFR, HER2 and VEGF), canertinib and HK1272 (irreversible TKIs against EGFR and HER2) (19).
HER2 is another member of the human epidermal growth factor receptor family that is over-expressed in up to 63% of NSCLC, predominantly in adenocarcinomas (14,19,24). HER2 over-expression seems to confer poorer prognosis in women with lung cancer, but so far no studies have shown a survival advantage for targeted HER2 therapy (14,24). VEGF, a platelet-derived growth factor involved in angiogenesis, is up-regulated in some NSCLC and indicates a poor prognosis. Bevacizumab (Avastin, Genentech/Roche), a monoclonal antibody against the VEGF receptor, has been shown to improve response rate, progression free survival and overall survival when added to first line chemotherapy for advanced NSCLC, and was FDA approved in 2006 (19). Vandtinib (ZD 6476) is a VEGF TKI currently under investigation (19).
EML4-ALK
The EML4-ALK fusion tyrosine kinase is found in 3–11% of patients with NSCLC, usually adenocarcinomas, and typically in younger nonsmoking patients. It is a chromosomal rearrangement that results in a constitutively active tyrosine kinase, leading to unopposed cell proliferation. EGFR and KRAS mutations are mutually exclusive with EML4-ALK mutations, and the EML4-ALK mutation has not been demonstrated to be differentially present in women versus men. Crizotinib (Xalkori, Pfizer, New York, NY) is a small molecule inhibitor of the tyrosine kinase that was FDA approved in August 2011 for patients with advanced NSCLC who have the EML4-ALK translocation (24).
KRAS
KRAS proteins mediate cell proliferation, and mutations lead to unchecked cell proliferation. Mutations are found predominantly in cancers associated with smoking and adenocarcinoma, and portend a poor prognosis. KRAS mutations are mutually exclusive with EGFR mutations, as they occur at the same locus, and there is debate as to whether women have a higher frequency of mutations. (3,12,14).
Hormone Effects
Estrogen receptors (ERα and ERβ) are expressed on lung cancer cells of men and women, ERβ being the more common haplotype (3,19). In vitro, estrogen promotes the growth of healthy and malignant lung tissue and anti-estrogen treatments have suppressed tumor growth, suggesting a hormonal role for tumorigenesis (25). Hydroxylated estrogen metabolites may be carcinogenic through the production of free radicals that lead to DNA damage (4,26). Polymorphisms in genes related to estrogen synthesis and metabolism have been associated with the presence of EGFR mutations, suggesting a role for estrogen metabolism in DNA mutations (18).
Factors affecting lifetime exposure to estrogen may affect lung cancer risk. Both increased and decreased risk related to parity, age at menarche and age at menopause has been described (2,3,19,26). Data regarding hormone replacement therapy has been similarly conflicting (2,14,19,28). A meta-analysis in 2012 found that only longer menstrual cycles and older age at menarche were protective (26). Associations between ESR2 expression (the gene for the ERβ receptor) and either lung cancer risk or mortality have not been found (13,25,27). Efforts to find efficacious estrogen-based therapies are ongoing. Newer formulations of paclitaxel that are activated in an estrogen dependent manner are under development (19).
Human Papilloma Virus
Studies in East Asian women have shown increased expression of high-risk HPV haplotypes in pulmonary squamous cells and association with subsequent lung cancer development. HPV was found in 43–49% of adenocarcinomas as compared to 24–29% of squamous cell carcinomas in one study out of Taiwan. Fifty-six percent of Taiwanese women with adenocarcinoma had HPV 16/18 in their serum in another study. Forty-nine percent of women with lung cancer expressing HPV in one population also had a history of carcinoma-in-situ III (CIN III) by pap smear. There are two proposed mechanisms for HPV presence in lung tissue. HPV serum positivity suggests that cervical infection leads to circulating virus, which then leads to systemic dissemination including pulmonary tissue. A second theory states that high-risk oral-genital sexual behavior leads to oral HPV squamocolumnar junction infections and subsequently lung squamous cell infection. The pathogenesis of HPV in squamous cell carcinoma is well known based on cervical cancer research, but its role in the pathogenesis of adenocarcinoma is unclear (14,29).
Treatment
With the exception of the above advances in molecularly targeted therapy, the backbone of lung cancer treatment remains unchanged. Detailed descriptions of lung cancer staging as well as stage-and histology-based treatment approaches have been described elsewhere and are not the purpose of this review. In general, small cell lung cancer is divided into limited versus extensive disease. Limited disease is typically treated with platinum and etoposide combined with radiation therapy, while extensive disease is treated with platinum and etoposide alone. NSCLC is divided into 4 stages. Stage IA requires surgical resection alone. Stages IB through IIIA require surgical resection followed by adjuvant chemotherapy typically of a cisplatin-based doublet. Stage IV is treated with chemotherapy involving a platinum-based doublet plus bevacizumab. If an EGFR mutation is present, monotherapy with either erlotinib or gefitinib is used. If an EML4-ALK mutation is present, monotherapy with crizotinib is used. Surgical resection in Stage IV disease is undertaken in the presence of a solitary, surgically-resectable metastasis or for symptom control. Radiation therapy is similarly employed for symptom control.
Outcomes
Women exhibit greater survival rates regardless of stage, histology, treatment modality or smoking status, even after adjusting for gender-specific life expectancy (13,14,30). Nonsmoking status predicts better survival and may possibly predict better response to therapy (12). The etiology behind these observations is theorized to be related to the above detailed genetic and molecular differences (19). Better survival in older women may be related to higher prevalence of co-morbidities among men of the same age (13). The decreased DNA repair capacity previously described may allow women to respond better to systemic chemotherapy (14,19).
Areas of Future Research
Prospective trials with large numbers of patients designed to specifically address outcomes differences between men and women are needed, as the above data comes from retrospective studies (19). Further research into the role of estrogen exposure, both endogenous and exogenous, is needed to clarify its role. Prospective studies designed to assess causality between HPV infection and lung cancer risk are needed, as there has been known success with HPV vaccinations in cervical cancer (29). The differential expression of specific genetic mutations provides opportunity for molecularly-targeted therapy, which may decrease side effects and improve overall survival (19). Lastly, trials assessing lung cancer screening must incorporate risk factors other than smoking in order to better estimate the at-risk population (3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. Ca Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Egleston BL, Meireles SI, Flieder DB, et al. Population-based trends in lung cancer incidence in women. Semin Oncol. 2009;36:506–515. doi: 10.1053/j.seminoncol.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR. 2011;196:287–295. doi: 10.2214/AJR.10.5412. [DOI] [PubMed] [Google Scholar]
- 4.Marshall AL, Christiani DC. Genetic susceptibility to lung cancer – light at the end of the tunnel? Carcinogenesis. 2013;34:487–502. doi: 10.1093/carcin/bgt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Tiwari RC, Mirray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Ca Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Paulus JK, Christiani DC. Women and Health. 2. Waltham, MA: Academic Press; 2012. Environmental exposures and cancer; pp. 641–677. [Google Scholar]
- 8.www.who/int/mediacentre/factsheets/fs339/en/index.html
- 9.www.cdc/gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/
- 10.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking related mortality in the United States. NEJM. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers – a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 13.Fu JB, Kau Y, Severson RK, et al. Lung cancer in women: analysis of the national surveillance, epidemiology, and end results database. Chest. 2005;127:768–777. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 14.Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest. 2005;128:370–381. doi: 10.1378/chest.128.1.370. [DOI] [PubMed] [Google Scholar]
- 15.Bethune G, Bethune D, Ridgway N, et al. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2:48–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh R, Lim KH, Kuo HT, et al. Female sex and broncioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest. 2005;128:317–321. doi: 10.1378/chest.128.1.317. [DOI] [PubMed] [Google Scholar]
- 17.Van Dyke AL, Cote ML, Prysak GM, et al. COX 2/EGFR expression and survival among women with adenocarcinoma of the lung. Carcinogenesis. 2008;29:1781–1787. doi: 10.1093/carcin/bgn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, Yang TY, Chen KC, et al. EGFR L858R mutation and polymorphisms of genes related to estrogen biosynthesis and metabolism in never-smoking female lung adenocarcinoma patients. Clin Cancer Res. 2011;17:2149–2158. doi: 10.1158/1078-0432.CCR-10-2045. [DOI] [PubMed] [Google Scholar]
- 19.Berardi R, Verdecchi L, Di Pietro Paolo M, et al. Women and lung cancer: clinical and molecular profiling as a determinate for treatment decisions: a literature review. Crit Rev Oncol Hematol. 2009;69:223–236. doi: 10.1016/j.critrevonc.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Timofeeva MN, Hung RJ, Rafnar T, et al. Influence of common genetic variation on lung cancer risk: meta-analysis of 14,900 cases and 24,485 controls. Hum Mol Genet. 2012;21:4980–4995. doi: 10.1093/hmg/dds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Sheu CC, Ye Y, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010;11:321–330. doi: 10.1016/S1470-2045(10)70042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan G, Hsiung CA, Matsuo K, et al. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nature Genetics. 2012;44:1330–1335. doi: 10.1038/ng.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YT, Heist RS, Chirieac LR, et al. Genome-Wide Analysis of Survival in Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol. 2009;27:2660–2667. doi: 10.1200/JCO.2008.18.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Saad S, Al Shibli K, Donnem T, et al. Clinical significance of epidermal growth factor receptors in non-small cell lung cancer and a prognostic role for HER2 gene copy number in female patients. J Thorac Oncol. 2010;5:1536–1543. doi: 10.1097/JTO.0b013e3181ea510a. [DOI] [PubMed] [Google Scholar]
- 25.Paulus JK, Zhou W, Kraft P, et al. Haplotypes of estrogen receptor beta and risk of non-small cell lung cancer in women. Lung Cancer. 2011;71:258–2563. doi: 10.1016/j.lungcan.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Yin Z, Shen L, et al. Menstrual factors, reproductive factors and lung cancer risk: a meta analysis. Chin J Lung Cancer. 2012;15:701–719. doi: 10.3779/j.issn.1009-3419.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toh C, Ahmad B, Soong R, et al. Correlation between epidermal growth factor receptor mutations and expression of female hormone receptors in east-Asian lung adenocarcinomas. J Thorac Oncol. 2010;5:17–22. doi: 10.1097/JTO.0b013e3181c0a602. [DOI] [PubMed] [Google Scholar]
- 28.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Tsai YC, Chen YC, et al. Human papilloma virus and female lung adenocarcinoma. Semin Oncol. 2009;36:542–552. doi: 10.1053/j.seminoncol.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura H, Ando K, Shinmyo T, et al. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg. 2011;17:469–480. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]