Abstract
Objectives
To determine if older, diabetic women have a greater longitudinal decline in physical performance and if these changes differ by insulin sensitizer use.
Design
Prospective cohort study.
Setting
Baltimore, Minneapolis, Portland and the Monongahela valley in Pennsylvania, USA.
Participants
2864 community-dwelling women (mean age 78.5±3.6 years) enrolled in the Study of Osteoporotic Fractures in 1997–1998 and re-studied 4.9 ± 0.6 years later.
Measurements
Women were categorized as having no diabetes (n=2680) or having diabetes (n=184). The prescription medication inventory was used to determine use of insulin sensitizers (metformin/thiazolidinedione). The outcomes were longitudinal changes in physical performance measures, including grip strength, usual walk speed, and rapid walk speed.
Results
Estimates from fully adjusted models showed that diabetic women had greater declines in usual walk speed (−0.16 m/s [95%CI −0.19, −0.14]) and rapid walk speed (−0.21 m/s [95%CI −0.24, −0.17]) compared to non-diabetic women (usual: −0.11 m/s [95%CI −0.12, −0.11], rapid: −0.15 m/s [95% CI −0.16, −0.14]), p<0.01 for both comparisons. Diabetic women on insulin sensitizers had an attenuated decline in the loss of usual walk speed compared to those not on insulin sensitizers, p<0.05. Declines in grip strength did not differ significantly by diabetes status or insulin sensitizer use.
Conclusion
Older women with diabetes have a greater decline in walk speed, but not grip strength compared to older women without diabetes. Clinical studies in older adults to determine whether diabetes treatments like insulin sensitizers can prevent the loss in walk speed and mobility are needed.
Keywords: elderly, diabetes, insulin sensitizer, physical performance, walk speed
INTRODUCTION
The prevalence of diabetes in adults over the age of 65 years exceeds 30%.1 While studies of diabetes outcomes in younger adults have primarily focused on mortality, macrovascular complications, and microvascular disease, older adults with diabetes appear to have a higher risk of other adverse functional outcomes like disability and falls.2, 3 The American Diabetes Association and American Geriatrics Society released a recent joint consensus statement to highlight a growing body of evidence for a higher frequency of geriatric conditions in older adults with diabetes and a need for clinical studies to determine how falls and functional decline may be prevented in this population.4 Identifying specific contributors to functional decline in older adults with diabetes is critical, so that these factors can be targets in clinical studies of disability prevention and treatment for older adults with diabetes. Contributors to these risks may be the loss of muscle mass and lower extremity strength with aging which are accelerated in older adults with diabetes compared to non-diabetic, older adults.5, 6 However, little information is available on whether other measures of physical performance decline in older adults with diabetes. Therefore, we examined the longitudinal change in grip strength and usual and rapid walk speed by diabetes status in a cohort of older women.
Insulin resistance is an underlying feature of type 2 diabetes and is also associated with lower physical performance in cross-sectional studies. Among older adults without diabetes, greater insulin resistance was associated with lower muscle strength and gait speed.7, 8 Two of the most commonly prescribed medication types used to treat type 2 diabetes, metformin and thiazolidinediones, decrease peripheral insulin resistance and are considered insulin sensitizers.9, 10 Insulin sensitizers have been shown to improve running performance and endurance in sedentary mice.11 To examine whether these basic findings translate to humans, we also performed a secondary analysis to determine if the decline in physical performance tests differed by insulin sensitizer use.
METHODS
Study Population
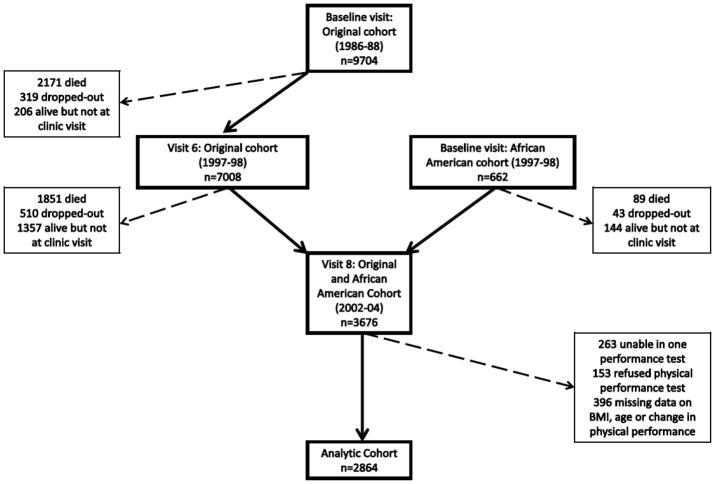
Between 1986 and 1988, 9704 Caucasian women aged 65 years and greater were recruited from 4 clinic sites in the US (Baltimore County, MD; Minneapolis, MN, the Monongahela valley, PA; and Portland, OR) to participate in the prospective Study of Osteoporotic Fractures (SOF).12 After this baseline visit, participants returned approximately every 2 years for follow-up visits. Between January 1997 and December 1998, 7008 of the original cohort were studied a sixth time and an additional 662 African American women were studied for the first time. Of this latter group of women, 3676 participated in a study visit on average 4.9 years later 2002–2004. The cohort for this longitudinal analysis comprised 2864 women who completed physical performance exams at study visits 1997–1998 and 2002–2004 (Fig. 1). All clinic site institutional review boards approved the study and written informed consent was provided by all study participants.
Figure 1.
Study Population
Diabetes Categories
At each clinic visit, self-reported questionnaire data on physician diagnosis of diabetes was ascertained. Current medications were brought to the clinic visit and recorded by study coordinators, and a computerized dictionary was used to categorize the medication ingredients from generic and brand names obtained from drug container labels.13 Women with a self-reported physician diagnosis of diabetes or use of diabetes medications were categorized as having diabetes, and those lacking diabetes diagnosis and medications were categorized as having no diabetes. Women using metformin and/or thiazolidinediones at clinic visit 6 in 1997–98 were considered to have diabetes treated with insulin sensitizers. Women were categorized as having diabetes treated without insulin sensitizers if they were not receiving metformin or thiazolidinediones, but had either a diagnosis of diabetes and/or use of other diabetes medications.
Physical performance
At each visit, participants had two trials to walk 6 meters at their usual speed timed in seconds with a stopwatch, and the usual walk speed (m/s) was calculated as the average of these two trials. Rapid speed (m/s) was calculated as the time to complete a 6 meter walk trial at a fast pace. The average grip strength (Kg) was calculated using two grip strength measurements taken with a Jamar Dynamometer in each hand.
Other measurements
Questionnaires were used to assess education (≥high school vs. <high school), current smoking (Y/N), walking for exercise (Y/N), self-rated health (good/excellent vs. very poor/poor/fair), and self-reported physician diagnosis of medical conditions including hypertension, prior myocardial infarction, angina, congestive heart failure, arthritis, depression, and chronic obstructive pulmonary disease. The medication inventory described above was also used to assess other medications including oral estrogen, steroids, and statins. Weight was measured with a balance beam scale and height was measured using a wall-mounted Harpendenstadiometer (Holtain Ltd, Crymych, Wales). Body mass index (BMI) was calculated from these measurements as weight (Kg) divided by height (m2). Participants with extreme high and low BMI and age were assigned to the race-specific lowest and highest values in the cohort so that they could not be identified by their unique values.
Statistical Methods
Differences in baseline characteristics by diabetes status were assessed using Χ2 tests for categorical variables and ANOVA for continuous variables. Separate linear regression models were used to compare the change in usual walk speed, rapid walk speed and grip strength for women with diabetes compared to the referent group of women without diabetes. All initial models were adjusted for age, race and clinic site. Multivariate regression models included adjustments for age, race, clinic site, baseline performance measure, body mass index, self-rated health, hypertension and estrogen use. These covariates were selected for inclusion in multivariable models because they differed by diabetes status and were selected for inclusion by backward stepwise regression with covariate retention threshold of p<0.10. Least squares mean changes in usual walk speed, rapid walk speed and grip strength were estimated from the multivariable regression models. Effect modification by exercise status was examined by testing the interaction between diabetes categories and self-reported walking for exercise in the above models.
For the secondary analysis to determine if physical performance differed for women with and without insulin sensitizer use, the above multivariable linear regression models were used to compare diabetic women with and without insulin sensitizer use to women without diabetes. Post-estimation testing with nonlinear constraints was used to compare diabetic women on insulin sensitizers to those who were not using insulin sensitizers. To account for potential misclassification of non-diabetic women at baseline who became diabetic at follow-up and changes in insulin sensitizer use between baseline and follow-up visits, these analyses were repeated after restricting to participants who remained in consistent diabetes categories and treatment groups at baseline and follow-up visits. To ensure the examination of insulin-resistant type 2 diabetes rather than insulin-sensitive type 1 diabetes, the above analyses were repeated after excluding women taking insulin at any visit. To determine whether the effect of insulin sensitizers could be due to metformin alone, the analyses were repeated after further excluding 2 women on thiazolidinediones. Statistical analyses were performed using STATA/IC 11.0 (STATACorp LP, College Station, TX).
RESULTS
Women in this cohort had a mean age of 78.5±3.6 years. In contrast to women with no diabetes, those with diabetes were younger, had higher BMI and grip strength, and had lower usual and rapid walk speed (Table 1). They were also less likely to be Caucasian, have good/excellent self-rated health, and use estrogen, but were more likely to have hypertension, a history of a myocardial infarction, congestive heart failure and use of statin. Among women with diabetes, 28 women were using insulin sensitizers and 156 women were not taking insulin sensitizers. No additional baseline characteristics differed significantly for diabetic women by insulin sensitizer use (results not shown). However, diabetic women on insulin sensitizers used a greater number of diabetes medications; whereas insulin use was more prevalent for diabetic women not on insulin sensitizers (Table 2).
Table 1.
Baseline Characteristics by Diabetes Categories. Data are means (standard deviation) or proportions
| No Diabetes n=2680 | Diabetes n=184 | p-value Χ2 or ANOVA | |
|---|---|---|---|
| Age (Years) | 78.60 (3.63) | 77.31 (3.77) | <0.01 |
| Caucasian (%) | 90.6 | 67.9 | <0.01 |
| Education ≥ High School (%) | 41.6 | 35.9 | 0.13 |
| Smoking (%)† | 4.1 | 4.1 | 0.98 |
| Body mass index (Kg/m2) | 26.99 (4.73) | 29.80 (5.48) | <0.01 |
| Usual walk speed (m/s) | 0.96 (0.18) | 0.90 (0.18) | <0.01 |
| Rapid walk speed (m/s) | 1.24 (0.25) | 1.15 (0.25) | <0.01 |
| Grip strength (Kg) | 17.89 (4.17) | 18.46 (4.82) | 0.08 |
| Walking exercise (%)‡ | 46.1 | 41.8 | 0.26 |
| Good/Excellent self-rated health (%) | 86.6 | 77.2 | <0.01 |
| Depression (%) | 5.8 | 3.8* | 0.27 |
| Osteoarthritis (%) | 18.7* | 16.9 | 0.54 |
| Chronic obstructive pulmonary disease (%) | 7.7* | 8.7 | 0.62 |
| Hypertension (%) | 34.0 | 53.8 | <0.01 |
| Myocardial Infarction (%) | 2.7 | 6.0 | 0.01 |
| Congestive heart failure (%) | 3.6 | 6.0 | 0.10 |
| Angina (%) | 6.9 | 8.2 | 0.52 |
| Statin Use (%) | 10.4 | 15.2 | 0.04 |
| Estrogen Use (%) | 22.9 | 13.6 | <0.01 |
| Steroid Use (%) | 2.1 | 3.8 | 0.11 |
Missing for 1 participant
Missing for 159 participants with no diabetes and 37 participants with diabetes
Missing for 5 participants with no diabetes and 2 participants with diabetes
Table 2.
Baseline Treatment Characteristics of Diabetic Women by Insulin Sensitizer Use
| No insulin sensitizers n=156 | Insulin sensitizers n=28 | |
|---|---|---|
| Number of diabetes medications, mean(SE) | 0.9 (0.5) | 1.9 (0.4) |
| Acarbose, n (%) | 2 (1.3) | 0 (0.0) |
| Sulfonylureas, n (%) | 100 (64.1) | 21 (78.6) |
| Insulin, n (%) | 29 (18.6) | 2 (7.1) |
| Metformin, n (%) | 0 (0.0) | 27 (96.4) |
| Thiazolidinedione, n (%) | 0 (0.0) | 2 (7.1) |
Over a period of 4.9 years, diabetic women had greater losses in usual walk speed compared to non-diabetic women in age-, race- and clinic site-adjusted models. The loss in usual walk speed for women with diabetes remained significantly greater than in women without diabetes even after accounting for baseline differences in BMI, usual walk speed, self-rated health, hypertension and estrogen use in the fully adjusted model (Table 3). The loss in rapid walk speed was significantly greater in women with diabetes compared to women without diabetes in the fully adjusted model. While the loss in average grip strength for diabetic women appeared to be slightly greater compared to non-diabetic women, the difference was not significant in minimally or fully adjusted models. When restricting the analysis to women in consistent diabetes categories at baseline and follow-up visits (177 women with diabetes and 2537 women without diabetes), the results were unchanged (data not shown). There was no interaction between diabetes status and self-reported walking for exercise on the change in physical performance measures, interaction p-values >0.1.
Table 3.
Adjusted Least Squares Mean Change (95% Confidence Interval) in Physical Performance by Diabetes Status
| No Diabetes n=2680 | Diabetes n=184 | p-value | |
|---|---|---|---|
| ΔWalk speed (m/s) | |||
| Model 1 | −0.12 (−0.12, −0.11) | −0.15 (−0.18, −0.13) | <0.01 |
| Model 2 | −0.11 (−0.12, −0.11) | −0.16 (−0.19, −0.14) | <0.01 |
| ΔRapid speed (m/s) | |||
| Model 1 | −0.15 (−0.16, −0.14) | −0.18 (−0.22, −0.15) | 0.13 |
| Model 2 | −0.15 (−0.16, −0.14) | −0.21 (−0.24, −0.17) | <0.01 |
| ΔGrip strength (Kg) | |||
| Model 1 | −1.26 (−1.40, −1.13) | −1.40 (−1.91, −0.88) | 0.61 |
| Model 2 | −1.26 (−1.38, −1.14) | −1.44 (−1.90, −0.98) | 0.47 |
Model 1 adjusted for age, race and clinic site
Model 2 adjusted for Model 1 covariates, baseline performance measure, body mass index, self-rated health, hypertension and estrogen use.
Compared to women with no diabetes, diabetic women not using insulin sensitizers had greater losses in usual and rapid walk speed, whereas diabetic women using insulin sensitizers had comparable losses in usual and rapid walk speed to women with no diabetes (Table 4). The post-estimation comparison of diabetic women by insulin sensitizer use revealed a significantly slower decline in usual walk speed for diabetic women on insulin sensitizers compared to diabetic women not using insulin sensitizers. Although diabetic women on insulin sensitizers appeared to have the greatest loss in grip strength, the loss did not differ significantly compared to diabetic women who were not on insulin sensitizers or to women with no diabetes. There was no interaction between exercise and categories of diabetes treatment on the change in physical performance measures, interaction p-values >0.1.
Table 4.
Adjusted Least Squares Mean Change (95% Conficence Interval) in Physical Performance by Diabetes Categories in the Entire Cohort, After Restricting to Consistent Diabetes Categories at Both Visits, After Further Excluding Any Insulin Use, and After Additional Exclusion of Any Thiazolidinedione (TZD)Use
| No diabetes | Diabetes not on insulin sensitizers | Diabetes on insulin sensitizers | |
|---|---|---|---|
| Entire Cohort | (n=2680) | (n=156) | (n=28) |
| ΔWalk speed (m/s) | −0.11 (−0.12, −0.11) | −0.17 (−0.20, −0.15)* | −0.10 (−0.16, −0.03)† |
| ΔRapid speed (m/s) | −0.15 (−0.16, −0.14) | −0.22 (−0.25, −0.18)* | −0.14 (−0.23, −0.06) |
| ΔGrip strength (Kg) | −1.26 (−1.38, −1.14) | −1.34 (−1.83, −0.84) | −2.00 (−3.16, −0.84) |
| Consistent at 2 visits | (n=2537) | (n=99) | (n=21) |
| ΔWalk speed (m/s) | −0.11 (−0.12, −0.11) | −0.18 (−0.21, −0.14)* | −0.08 (−0.16, −0.00)† |
| ΔRapid speed (m/s) | −0.15 (−0.16, −0.14) | −0.22 (−0.27, −0.18)* | −0.12 (−0.22, −0.02) |
| ΔGrip strength (Kg) | −1.23 (−1.35, −1.11) | −1.09 (−1.71, −0.48) | −2.02 (−3.34, −0.70) |
| Exclude Insulin Use | (n=2537) | (n=70) | (n=20) |
| ΔWalk speed (m/s) | −0.11 (−0.12, −0.11) | −0.16 (−0.21, −0.12)* | −0.07 (−0.15, 0.01)† |
| ΔRapid speed (m/s) | −0.15 (−0.16, −0.14) | −0.21 (−0.27, −0.16)* | −0.10 (−0.21, −0.00) |
| ΔGrip strength (Kg) | −1.23 (−1.35, −1.11) | −0.59 (−1.31, 0.14) | −1.91 (−3.26, −0.55) |
| Exclude TZD Use | (n=2537) | (n=70) | (n=13) |
| ΔWalk speed (m/s) | −0.11 (−0.12, −0.11) | −0.16 (−0.21, −0.12)* | −0.04 (−0.14, 0.05)† |
| ΔRapid speed (m/s) | −0.15 (−0.16, −0.14) | −0.21 (−0.27, −0.16)* | −0.09 (−0.22, 0.04) |
| ΔGrip strength (Kg) | −1.23 (−1.35, −1.11) | −0.59 (−1.31, 0.14) | −1.66 (−3.33, 0.01) |
Estimates are adjusted for age, race, clinic site, baseline physical performance measure, body mass index, self-rated health, hypertension and estrogen use.
p<0.05 vs. women with no diabetes
p<0.05 vs. women with diabetes not on insulin sensitizers
To address the effects of changing diabetes categories and use of other diabetes medications during this period, these analyses were repeated after first restricting to women who remained in consistent diabetes categories at the follow-up visit, then further excluding those on insulin at either visit, and further excluding women using thiazolidinediones at either visit. Overall, no significant alterations in the study results were found after these restrictions (Table 4). Additional adjustment for the number of diabetes medications in a model comparing just diabetics with and without insulin sensitizer use did not alter the significant attenuation in walk speed decline for diabetics on insulin sensitizers (results not shown).
DISCUSSION
All older women in this cohort had a decline in physical performance over 4.9 years. While there was no difference in the loss of grip strength between women with and without diabetes, women with diabetes had a greater loss in walk speed compared to women without diabetes. Exploratory secondary analyses suggest that diabetic women using insulin sensitizers, particularly metformin, had a lesser decline in usual walk speed compared to diabetic women who are not using insulin sensitizers.
To our knowledge, this is the first longitudinal study to report a more rapid decline in walk speed in older women with diabetes. Several factors may contribute to the decline in walk speed, including the loss in lower extremity strength, balance, and cognitive function. Studies have shown a greater decline in cognitive function and lower extremity strength in older adults with diabetes.5, 14, 15 Previous cross-sectional studies have shown poorer performance on balance tests with diabetes, but longitudinal results are not available.3, 16 While older adults with diabetes have a greater decline in lower extremity strength, we found a lack of difference in grip strength decline for older women with diabetes compared to those without diabetes that corroborate results reported by Park and colleagues.5 Recognizing that older adults with diabetes have a greater decline in lower extremity function rather than upper extremity performance has serious implications given evidence of decreased lower extremity performance, particularly gait speed, being more predictive of progressive and catastrophic activities of daily living disability and mobility disability than upper extremity performance measures.17 Furthermore, identification of specific impairments in physical performance will allow future clinical studies to target preventive therapies for the decline of these lower extremity performance measures in older adults with diabetes.
Exercise is an intervention that can lower insulin resistance.18 In this study, exercise did not appear to alter the associations between physical performance and diabetes categories. However, the ability to detect effect modification by exercise may have been limited by small sample sizes in the diabetes categories and the use of self-reported walking for exercise, a subjective measure of physical activity. Thus, the influence of physical activity on the loss of physical performance in women with and without diabetes should be examined further in clinical studies and cohorts with more objective assessments of physical activity and larger sample sizes.
This analysis added novel preliminary findings that the loss in usual walk speed was attenuated for diabetic women using insulin sensitizers compared to diabetic women not using insulin sensitizers. To ensure that the greater loss in walk speed for women who were not on insulin sensitizers was not due to a longer duration of or more severe form of diabetes, the analysis was repeated and results confirmed after excluding participants using insulin, since the use of insulin is related to a longer duration of diabetes with treatment of type 1 diabetes starting at younger ages or with treatment of more advanced type 2 diabetes with insulin insufficiency.19,20 To account for effects of potential misclassification of non-diabetic women at baseline who became diabetic at follow-up or changes in insulin sensitizer use prior to the follow-up visit, the analysis was repeated after restriction to women who remained in consistent diabetes and diabetes treatment categories on follow-up with no significant alteration in the study results. Because we previously found that metformin use in older diabetic men was associated with a significant attenuation in the loss of appendicular lean mass,21 we looked only at metformin use for older women on insulin sensitizers and found even further attenuation in the loss of usual walk speed. While there were statistically significant differences for the change in usual walk speed by insulin sensitizer use, we recognize that the number of women on insulin sensitizers was low in this analytic cohort (studied initially 1997–1998) because metformin was not released in the US until 1995 and the first thiaziolidinedione was not released until 1997.22 Moreover, the difference in magnitude of change between the groups is small. Given that these are observational findings, causality cannot be inferred, and additional studies are needed to confirm this preliminary finding and to explore how existing or new diabetes medications may prevent the decline in walk speed, mobility impairments and functional disability.
Potential explanations for the attenuation in walk speed decline with insulin sensitizer use may be due to either a reduction of insulin resistance or a direct effect on muscle. Insulin resistance is also associated with impaired cognitive function and vascular function.4, 23–25 Treatment of insulin resistance has been shown to prevent cognitive decline and micro- and macrovascular disease in diabetics.26, 27 Both intact cognitive and vascular function are likely important for the maintenance of physical performance. A less studied area, but possible alternative mechanism of insulin sensitizers could be through a direct effect on muscle. As noted above, metformin use in older diabetic men was associated with a significant attenuation in the loss of appendicular lean mass.21 Metformin has similar effects on muscle to aerobic exercise, and both can activate 5′ adenosine monophosphate-activated protein kinase (AMPK) which is associated with the development of an oxidative muscle phenotype.18, 28–32 Therefore, the attenuated loss in walk speed with no impact on grip strength for older women on metformin may be due to a preservation of oxidative muscle fibers. Further clinical studies to more directly test this hypothesis and also potential effects on endurance are needed.
While our findings are provocative and supported by potential biological explanations, there are study limitations to consider. The analysis was restricted to older women with longitudinal ascertainment of physical performance. In comparison to women who were studied 1997–1998 and did not follow-up, women who followed-up were younger, had better self-rated health, a lower prevalence of diabetes, hypertension and heart disease, and higher grip strength and walk speed. Therefore, the analytic cohort comprised a healthy and functional group of older women, and study results may not generalize to frail older women. Women with diabetes in this cohort were younger than women with no diabetes. This age difference may be explained by a lack of follow-up due to mortality or disability for older diabetic women. However, even without inclusion of these women, a greater loss in walk speed for older women with diabetes was still detected. The low prevalence of diabetes in this cohort of older women does not match the U.S. population-based estimate of 12.3% for older women from this time period and may also reflect a healthier cohort selection.33 However, the low prevalence may also be due to undiagnosed diabetes since this study did not ascertain fasting glucose levels or administer oral glucose tolerance tests. Misclassification of undiagnosed diabetic women as having no diabetes would bias the estimates for non-diabetic women towards greater loss. It is also possible that 28 of the 156 women categorized as having diabetes by self-report with no concurrent use of diabetes medications may have misreported a diagnosis of diabetes. This miscategorization would have attenuated losses in physical performance for women with diabetes. Despite these potential misclassifications we were still able to detect a significant difference in walk speed decline for women with diabetes compared to non-diabetic women in this cohort of women. There was also the potential for confounding by disease severity in the secondary analysis of insulin sensitizer use. We did not have a direct measure of diabetes control, like glycated hemoglobin levels. Although we tried to account for disease severity by adjusting for number of diabetes medications and excluding women using insulin who likely had type 1 diabetes or longer-standing type 2 diabetes with no alteration in our findings, residual confounding by glycemic control may have remained. While we adjusted for self-rated health, a more rigorous and objective measurement of overall health like the Charlson Comorbidity Index was not available,34 and residual confounding by overall health status also may exist.
In this cohort of older women, diabetes was associated with a more rapid decline in walk speed, but not grip strength. Moreover, older diabetic women using insulin sensitizers, particularly metformin, had an attenuated loss in walk speed. These findings add to the growing body of evidence for the development of functional impairments in older adults with diabetes.2, 5, 35–37 These results also highlight the importance of measuring functional outcomes, especially walk speed, in clinical studies of diabetes so that therapies to prevent disability in older adults with diabetes can be identified.
Acknowledgments
The Study of Osteoporotic Fractures is supported by the National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: R01AG005407, R01AR35582, R01AR35583, R01AR35584, R01AG005394, R01AG027574, and R01AG027576.
Sponsor’s Role: The sponsor played no role in the study concept, design, analysis, interpretation of data, or preparation of manuscript.
Footnotes
These findings have been presented orally at the 7th International Academy on Nutrition and Aging, July 2012 and at the 9th Annual Interdisciplinary Women’s Health Research Symposium, November 2012.
Author Contributions: Christine G. Lee: study concept and design, data analysis, interpretation of data, and manuscript preparation. Ann V. Schwartz: collection of data, interpretation of data, and critical review of the manuscript. Kristine Yaffe, Teresa A. Hillier and Erin S. LeBlanc: interpretation of data and critical review of the manuscript. Peggy M. Cawthon: study design, collection of data, interpretation of data, and critical review of the manuscript.
Conflict of Interest: The Study of Osteoporotic Fractures is supported by the National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: R01AG005407, R01AR35582, R01AR35583, R01AR35584, R01AG005394, R01AG027574, and R01AG027576. Christine G. Lee was supported by the Office of Research on Women’s Health and the National Institute of Child Health and Human Development, Building Interdisciplinary Research Careers in Women’s Health grant number HD043488-08. Ann V. Schwartz has served as a consultant for GlaxoSmithKline and has received research support from GlaxoSmithKline, Amgen and Merck. Kristine Yaffe has served as a consultant for Novartis and on DSMBs for Pfizer, Medivation, and Takeda Pharmaceuticals. Peggy M. Cawthon has received research support from Amgen and Merck.
References
- 1.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–67. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: A prospective study. Diabetes Care. 2002;25:1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 4.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 6.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilay JI, Cotsonis GA, Walston J, et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged > or = 70 years. Diabetes Care. 2009;32:736–738. doi: 10.2337/dc08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CK, Lin LY, Yu YH, et al. Inverse association between insulin resistance and gait speed in nondiabetic older men: Results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2002. BMC Geriatr. 2009;9:49. doi: 10.1186/1471-2318-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widen EI, Eriksson JG, Groop LC. Metformin normalizes nonoxidative glucose metabolism in insulin-resistant normoglycemic first-degree relatives of patients with NIDDM. Diabetes. 1992;41:354–358. doi: 10.2337/diab.41.3.354. [DOI] [PubMed] [Google Scholar]
- 10.Maggs DG, Buchanan TA, Burant CF, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 13.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 14.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160:174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Blackwell T, Kanaya AM, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 16.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: The Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onder G, Penninx BW, Ferrucci L, et al. Measures of physical performance and risk for progressive and catastrophic disability: Results from the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 18.Dube JJ, Amati F, Stefanovic-Racic M, et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donner T, Munoz M. Update on insulin therapy for type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1405–1413. doi: 10.1210/jc.2011-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleegler FM, Rogers KD, Drash A, et al. Age, sex, and season of onset of juvenile diabetes in different geographic areas. Pediatrics. 1979;63:374–379. [PubMed] [Google Scholar]
- 21.Lee CG, Boyko EJ, Barrett-Connor E, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 23.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: Potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 24.Clerk LH, Vincent MA, Jahn LA, et al. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 25.Bruehl H, Sweat V, Hassenstab J, et al. Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J Clin Exp Neuropsychol. 2010;32:487–493. doi: 10.1080/13803390903224928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbatecola AM, Lattanzio F, Molinari AM, et al. Rosiglitazone and cognitive stability in older individuals with type 2 diabetes and mild cognitive impairment. Diabetes Care. 2010;33:1706–1711. doi: 10.2337/dc09-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holman RR, Paul SK, Bethel MA, et al. Ten-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 28.Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 29.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 30.Suwa M, Egashira T, Nakano H, et al. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–1692. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- 31.Smith AC, Bruce CR, Dyck DJ. AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat sole us muscle. J Physiol. 2005;565(Pt 2):537–546. doi: 10.1113/jphysiol.2004.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J Appl Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- 33.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. 2010;6:134–143. doi: 10.2174/157339910791162961. [DOI] [PubMed] [Google Scholar]
- 36.Chiu CJ, Wray LA. Physical disability trajectories in older Americans with and without diabetes: The role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51:51–63. doi: 10.1093/geront/gnq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]