Abstract
Alternative anticoagulants to warfarin (dabigatran, rivaroxaban and apixaban) are becoming available for the prevention of thromboembolic stroke in atrial fibrillation, but there is a lack of information on their comparative effectiveness. We evaluated this using a discrete event simulation with a lifetime horizon of analysis, based on an indirect comparison of the RE-LY, ROCKET-AF and ARISTOTLE trial results for patients with the characteristics of the US atrial fibrillation population. Over a lifetime, apixaban, dabigatran and rivaroxaban accrued 0.130 (95% central range [CR] −0.030 to 0.264), 0.106 (95% CR −0.048 to 0.248) and 0.095 (95% CR −0.052 to 0.242) more quality-adjusted life-years than warfarin, respectively, with apixaban having a 55% probability of accruing the highest total lifetime QALYs. In the absence of a definitive trial, and acknowledging the limitations of an indirect comparison, the available evidence suggests apixaban to be the more effective anticoagulant.
Keywords: Anticoagulants, atrial fibrillation, meta-analysis, comparative effectiveness, stroke
Introduction
Atrial fibrillation (AF) is estimated to affect 2.5 million people in the United States, and results in a fivefold increase in the risk of ischemic stroke1,2. Associated costs exceed $7 billion annually3. Antithrombotic agents have proven benefits in preventing stroke in patients with AF. Until recently, vitamin K antagonists, such as warfarin, were the only form of oral thromboprophylactic anticoagulation treatment4. Although clinically effective and inexpensive, warfarin increases the risk of hemorrhage, interacts with many drugs and, because of the considerable variability in patient response, requires careful monitoring5,6.
Newer oral anticoagulants, which include the direct thrombin inhibitor dabigatran etexilate (hereafter referred to as dabigatran), and the direct factor Xa inhibitors rivaroxaban and apixaban, have recently been developed. The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study evaluated two doses (110mg and 150mg twice daily) of dabigatran as an alternative to warfarin in 18,113 patients with at least one risk factor for stroke7. The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) compared rivaroxaban with warfarin in 14,264 patients at elevated risk of stroke8. The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial compared apixaban with warfarin in 18,201 patients with at least one risk factor for stroke9.
After median follow up periods of approximately two years, the primary outcome of stroke or systemic embolism showed a potential improvement in the intention-to-treat population with all three alternatives, demonstrating superiority of the licensed 150mg dose of dabigatran (1.11% v 1.71% per year; p<0.001) and apixaban (1.27% v 1.60% per year; p=0.01) and non-inferiority of rivaroxaban (2.1% v 2.4% per year; p=0.12), compared with warfarin. Rates of major bleeding were not significantly different between dabigatran 150mg and warfarin or between rivaroxaban and warfarin, but apixaban was associated with a lower risk of major bleeding (2.13% v 3.09% per year; p<0.001).
Dabigatran, rivaroxaban and apixaban have all been approved by the US Food and Drug Administration but whilst their efficacies in relation to warfarin have been demonstrated, their comparative effectiveness remains unknown. With no prospect of a head-to-head trial, we describe an adjusted, indirect comparison to help guide treatment selection. The analysis assesses the trade-offs in thrombotic and bleeding risks for all four anticoagulants, and incorporates a preference-based, patient-centred outcome, the quality-adjusted life-year (QALY), to combine health-related quality of life with survival. Our analysis acknowledges differences in trial designs and populations, and their potential impacts on estimated comparative effectiveness, by adopting a probabilistic approach for a range of plausible scenario analyses.
Results
The results of the simulations at 2 years matched the results of each trial. No value deviated by more than 3.2% (data not presented), a level of variability that would be expected given the stochastic nature of the simulation. At 2 years, apixaban accrued 0.15 more quality-adjusted life-weeks than dabigatran, 0.26 more than rivaroxaban, and 0.78 more than warfarin.
Clinical outcomes and net health benefit
In the base-case analysis, apixaban, dabigatran and rivaroxaban extended life by 2.05, 1.51 and 1.10 months respectively, compared with warfarin (table 1). The corresponding incremental net health benefits were 0.130 (95% central range [CR] −0.030 to 0.264), 0.106 (95% CR −0.048 to 0.248) and 0.095 (95% CR −0.052 to 0.242) QALYs. In pairwise comparisons, using warfarin as the comparator, apixaban, dabigatran and rivaroxaban were associated with a positive incremental net health benefit in 90%, 84% and 82% of simulations, respectively. Using rivaroxaban as a comparator, apixaban and dabigatran were associated with an incremental net health benefit in 71% and 61% of simulations, and finally apixaban was associated with an incremental net health benefit against dabigatran in 65% of simulations.
Table 1.
Lifetime estimates of event rates, net health benefits, and incremental differences versus comparator, derived from probabilistic sensitivity analysis
| Referent | Mean estimate (95% central range) |
Mean difference (95% central range) |
Comparator |
|---|---|---|---|
| Quality-adjusted life-years (QALYs) | |||
| Warfarin | 5.636 (5.546, 5.733) | −0.095 (−0.242, 0.052) | Rivaroxaban |
| Rivaroxaban | 5.731 (5.631, 5.834) | −0.011 (−0.164, 0.144) | Dabigatran |
| Dabigatran | 5.742 (5.652, 5.854) | −0.024 (−0.174, 0.130) | Apixaban |
| Apixaban | 5.766 (5.652, 5.881) | 0.130 (−0.029, 0.265) | Warfarin |
| Life years | |||
| Warfarin | 9.638 (9.498, 9.737) | −0.092 (−0.286, 0.120) | Rivaroxaban |
| Rivaroxaban | 9.729 (9.579, 9.865) | −0.034 (−0.241, 0.172) | Dabigatran |
| Dabigatran | 9.763 (9.604, 9.893) | −0.045 (−0.254, 0.147) | Apixaban |
| Apixaban | 9.808 (9.655, 9.946) | 0.171 (−0.031, 0.362) | Warfarin |
| Stroke or systemic embolism | |||
| Warfarin | 0.303 (0.264, 0.339) | 0.020 (−0.033, 0.074) | Rivaroxaban |
| Rivaroxaban | 0.283 (0.238, 0.319) | 0.031 (−0.029, 0.083) | Dabigatran |
| Dabigatran | 0.251 (0.213, 0.301) | 0.050 (−0.001, 0.099) | Apixaban |
| Apixaban | 0.201 (0.169, 0.254) | −0.102 (−0.154, −0.050) | Warfarin |
| Transient ischemic attack | |||
| Warfarin | 0.123 (0.091, 0.158) | 0.031 (−0.019, 0.084) | Rivaroxaban |
| Rivaroxaban | 0.092 (0.070, 0.123) | −0.006 (−0.057, 0.046) | Dabigatran |
| Dabigatran | 0.097 (0.069, 0.128) | 0.020 (−0.034, 0.069) | Apixaban |
| Apixaban | 0.077 (0.055, 0.104) | −0.046 (−0.093, 0.008) | Warfarin |
| Intracranial hemorrhage | |||
| Warfarin | 0.073 (0.064, 0.081) | 0.014 (−0.002, 0.026) | Rivaroxaban |
| Rivaroxaban | 0.059 (0.052, 0.066) | 0.018 (0.000, 0.025) | Dabigatran |
| Dabigatran | 0.040 (0.035, 0.047) | −0.002 (−0.015, 0.014) | Apixaban |
| Apixaban | 0.042 (0.033, 0.047) | −0.031 (−0.046, −0.013) | Warfarin |
| Major bleed (including intracranial hemorrhage) | |||
| Warfarin | 0.307 (0.262, 0.347) | −0.021 (−0.076, 0.036) | Rivaroxaban |
| Rivaroxaban | 0.328 (0.283, 0.374) | 0.017 (−0.037, 0.069) | Dabigatran |
| Dabigatran | 0.311 (0.274, 0.363) | 0.069 (0.014, 0.115) | Apixaban |
| Apixaban | 0.242 (0.205, 0.278) | −0.065 (−0.116, −0.010) | Warfarin |
| Non-fatal myocardial infarction | |||
| Warfarin | 0.067 (0.047, 0.086) | 0.007 (−0.013, 0.029) | Rivaroxaban |
| Rivaroxaban | 0.059 (0.043, 0.080) | −0.022 (−0.043, −0.002) | Dabigatran |
| Dabigatran | 0.081 (0.063, 0.099) | 0.019 (−0.002, 0.033) | Apixaban |
| Apixaban | 0.062 (0.044, 0.086) | −0.004 (−0.022, 0.019) | Warfarin |
Lifetime incidences of stroke or systemic embolism were 33.6% lower with apixaban, 17.0% lower with dabigatran and 6.7% lower with rivaroxaban, when compared to warfarin. Lifetime incidences of major hemorrhagic events were 21.3% lower with apixaban, but 7.0% and 1.3% higher with rivaroxaban and dabigatran, respectively. Incidences of myocardial infarction were 10.7% lower with rivaroxaban and 6.5% lower with apixaban, but 22.3% higher with dabigatran.
The relative effects of each treatment on the two constructs of the QALY, health-related quality of life and life-years gained, is illustrated in efigure 1.
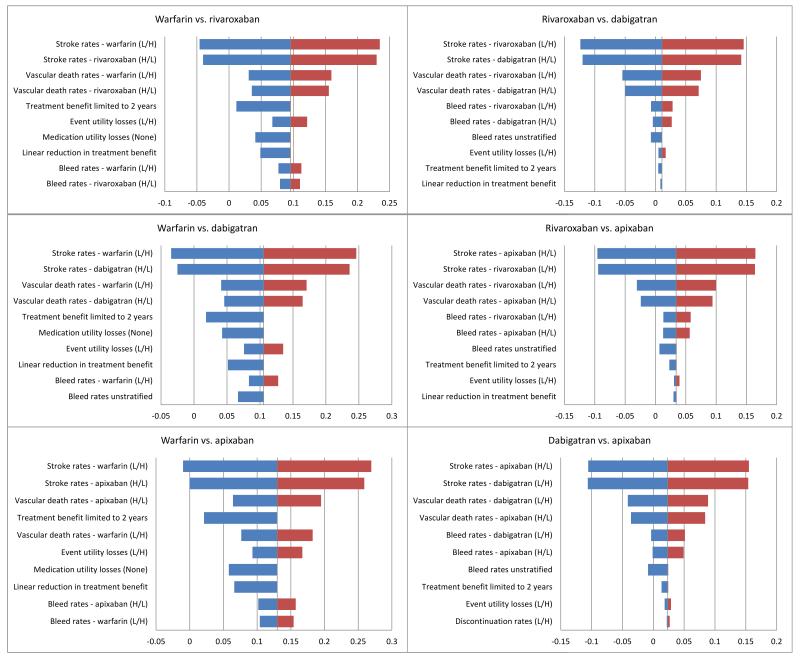
Univariate parameter sensitivity analysis results are presented in the form of tornado plots (figure 1).
Figure 1.
Each figure presents the ten parameters which led to the greatest change in overall QALYs. L/H refer to lower and higher limits of parameter estimates.
Structural sensitivity analysis
In the probabilistic sensitivity analysis of structural uncertainty, the base-case ordering of QALYs (apixaban, dabigatran, rivaroxaban, warfarin, in descending order) was replicated in 65.1% of the simulations. The alternative ordering (dabigatran, apixaban, rivaroxaban, warfarin) occurred in 17.2% of simulations, and the ordering (apixaban, rivaroxaban, dabigatran, warfarin) occurred in 13.4% of simulations. No other ordering occurred in more than 1% of simulations. Overall, apixaban accrued the highest number of QALYs in 79.9% of the simulations, dabigatran in 18.0% and rivaroxaban in 2.1%, with warfarin never accruing the highest number.
Using a Markov model, instead of a discrete event simulation, led to changes in both event rates and the numbers of QALYs and life-years accrued. However, the same ordering was maintained, with apixaban the most effective with 8.49 QALYs, then dabigatran with 8.35, rivaroxaban with 8.28 and warfarin with 8.08 QALYs.
Subgroup analyses
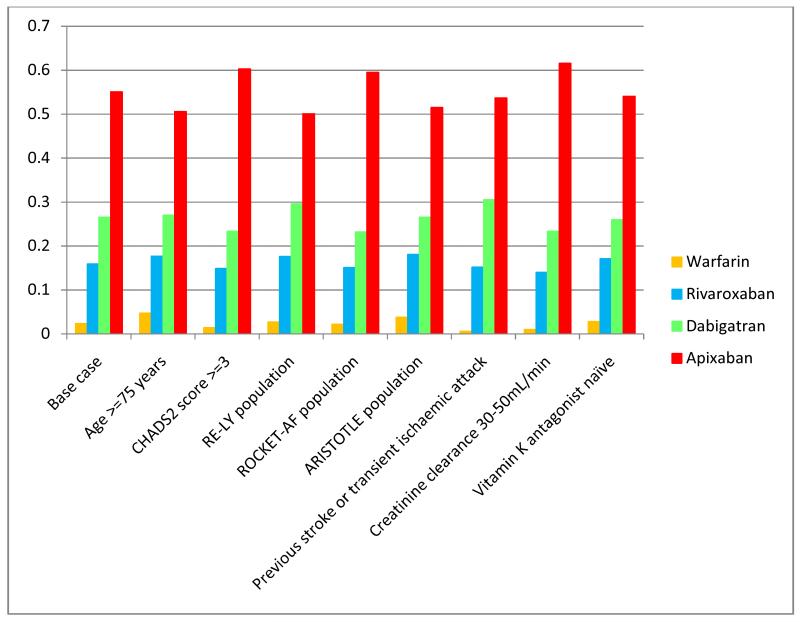
Among the subgroups analysed, the ordering of medicines according to mean QALYs and probability of being most effective was consistent with the base-case analysis (table 2, figure 2). Apixaban had the highest probability of being the most effective in patients with impaired renal function, and the lowest in older populations (≥75 years), but these probabilities were over a very narrow range (50.1% to 61.6%).
Table 2.
Net benefit results for subgroups, based on probabilistic sensitivity analysis
| QALYs (Probability most effective) | ||||
|---|---|---|---|---|
| Subgroup | Warfarin | Rivaroxaban | Dabigatran | Apixaban |
| Base case | 5.637 (0.024) | 5.735 (0.159) | 5.745 (0.266) | 5.767 (0.551) |
| Age ≥75 years | 3.848 (0.047) | 3.940 (0.177) | 3.948 (0.270) | 3.972 (0.506) |
| CHADS2 score ≥3 | 5.482 (0.014) | 5.590 (0.149) | 5.617 (0.234) | 5.649 (0.603) |
| RE-LY population | 5.658 (0.027) | 5.746 (0.176) | 5.769 (0.296) | 5.784 (0.501) |
| ROCKET-AF population | 5.582 (0.022) | 5.666 (0.151) | 5.678 (0.232) | 5.710 (0.595) |
| ARISTOTLE population | 5.651 (0.038) | 5.747 (0.181) | 5.761 (0.266) | 5.786 (0.515) |
| Previous stroke or transient ischemic attack |
5.460 (0.006) | 5.545 (0.152) | 5.562 (0.305) | 5.582 (0.537) |
| Creatinine clearance 30-50mL/min | 5.561 (0.010) | 5.664 (0.140) | 5.677 (0.234) | 5.701 (0.616) |
| Vitamin K antagonist naïve | 5.641 (0.028) | 5.729 (0.171) | 5.742 (0.260) | 5.766 (0.541) |
Figure 2.
Probability of each treatment is the most effective, based on accrual of most lifetime QALYs, for each identified patient subgroup.
Discussion
Based on an accepted method of comparative effectiveness research (CER) that preserves the randomisation of treatment allocation, and which results in an adjusted, indirect comparison15, apixaban appears as the most effective oral anticoagulant, followed by dabigatran, rivaroxaban then warfarin. Differences were driven principally by differential stroke rates and the risks of intracranial hemorrhage, which were lower for all newer agents compared with warfarin. This ordering remained consistent across patient subgroups, though the differences in net health benefits changed, with groups having lower risks of stroke associated with smaller differential QALYs.
There is no subgroup in which the probability of apixaban being the most effective is below 50%, and none where the probability of warfarin being the most effective is above 5%. The sensitivity analyses indicate that the parameters to which the outcome was most sensitive were stroke rates and vascular death rates.
We are aware of two other adjusted,27,28 and one unadjusted29 indirect treatment comparisons. Lip et al.28 concluded that there were no discernable differences among treatments, while the analysis by Mantha et al,27 despite being based on the same clinical trial data, indicated that apixaban was equally effective to dabigatran 150mg, more effective than rivaroxaban, and associated with less major bleeding than both. The crude estimates of net clinical benefit calculated by Banerjee et al.29 for a Danish population are subject to bias, as the odds ratios derived from the trials were not adjusted. None of the analyses modelled patients representative of the US atrial fibrillation population, used a preference-based, patient-centred outcome measure, used an appropriate time horizon of analysis or considered alternative scenarios of analysis.
Our analysis, by contrast, adopts a lifetime horizon, uses QALYs to synthesise the differential impacts of benefits and harms on health, and considers both structural and parameter uncertainty. QALYs may reveal differences among treatments which might be less apparent when considering individual clinical events. Adjusted, indirect treatment comparisons are accepted by healthcare decision-makers across several jurisdictions as the method of choice in situations where data from head-to-head trials are unavailable30. This Bayesian approach results in a meaningful outcome for prescribers, that is, the probability of a treatment being the best option. Judgements based on confidence intervals and hypothesis tests, based on the frequentist notion of assuming the null hypothesis until sufficient evidence indicates otherwise, can be criticised as inappropriate in this context, as decisions regarding treatment alternatives cannot be deferred as such evidence is unlikely to become available. Moreover, a lack of statistical significance does not necessarily imply equivalence in outcome31.
There are potential caveats to our CER methodology, however. First, there are many important differences across trials in terms of their design (e.g. RE-LY being open-label and the use of sham INR testing only in ROCKET-AF), patient populations (e.g. higher risk of stroke, greater experience of previous stroke or TIA, and less time in INR range in ROCKET-AF) and reporting (e.g. difference in the definitions of some clinical events). These have been discussed extensively elsewhere14,28,32 and, collectively, potentially undermine the assumption necessary for indirect comparison methodology, that any such differences do not affect the comparative effectiveness of the treatments being assessed. Although there is no method of testing the validity of this assumption, there is no prospect of a head-to-head comparison among newer anticoagulants and prescribers will in any case make qualitative judgements or naïve, unadjusted comparisons of competing treatment options. Our analysis is a more valid approach than simply comparing individual trials or trial arms in an unadjusted way33.
Second, the modelled extrapolation to a lifetime horizon of analysis is necessary to reflect differential impacts of treatments on health and survival that extend beyond the protocol-defined trial follow-up period34. The analysis used a discrete event simulation method, given there are no obvious discrete disease states into which patients can be classified11. However, this required an assumption that risk equations derived from 2-year data apply beyond that time. Relaxing this assumption by analysis at 2-years resulted in the same rank ordering of net health benefits, as did the use of an alternative, Markov model structure.
Despite these limitations there was a high level of consistency in the ranking of treatment effectiveness across all the different simulations performed. Had the results been dependent on any specific modelling assumption, then this would be apparent in the sensitivity analysis. We can reasonably conclude that any biases in our analysis, if any are present, are inherent in the data and therefore impossible to correct under any modelling framework.
It is important to note that the analysis did not consider additional factors that impinge on treatment choice. These include cost-effectiveness10, patient convenience, preference (or aversion) to individual treatments35, the relative forgiveness of treatments to missed doses36, the lack of antidotes to over-anticoagulation with the newer oral anticoagulants37, the merits or otherwise of patients being monitored regularly when prescribed warfarin, and longer term and rarer adverse events that might only become apparent with more extensive experience of use in routine practice38,39,40. Furthermore, the newer agents also interact with other drugs and there are specific safety considerations in certain vulnerable populations (e.g. the very elderly and those with severe renal impairment). There may also be sub-group(s) of patients, which were not explored in the present analysis – such as those with genetic polymorphisms of CYP2C9 or VKORC1 – in which the balance of harms and benefits differ significantly from the mean41.
There is no doubt of the efficacy of the newer oral anticoagulants and the favourable risk-benefit profile when compared to warfarin in the pivotal trials; however there are important differences among the agents and our analysis currently points to the likely superiority of apixaban over others. We recommend, however, that analyses of population databases of real-life user populations are performed to test hypotheses derived from our model. Certainly our results, and those from any observational studies, would not be expected to supplant evidence from randomised controlled trials, but should to be kept under review as the evidence matures.
Methods
Comparative effectiveness was assessed using an indirect analysis that extrapolated benefits and harms to a lifetime horizon, consistent with AF being a lifelong condition requiring indefinite treatment.
The analysis is based on a discrete event simulation model which we have described previously10, and which allows for explicit incorporation of both structural and parameter uncertainty11. The model simulates the clinical events and outcomes experienced by individual patients. The risks of their occurrence are determined from patients’ characteristics which are updated according to time and event history. Comparative effectiveness was determined from incremental net health benefits, measured as the differences between treatments in QALYs, and from modelled clinical event rates10,12.
Model population
In the base-case analysis, patients’ baseline characteristics, which were assumed to be uncorrelated, were representative of the stroke risk profile of the US atrial fibrillation population13. Patients had a mean age of 73.0 years, with 38.8%, 36.8%, 18.0%, 6.4% having CHADS2 (Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, prior Stroke/transient ischemic attack) scores of 1, 2, 3 and ≥4 respectively13.
For each treatment, identical cohorts of 100,000 patients were generated. Each patient was given a simulated set of characteristics consisting of the presence or absence (at the start of the simulation) of the following: hypertension, diabetes mellitus, congestive heart failure, prior stroke, prior transient ischemic attack, prior myocardial infarction and prior intracranial hemorrhage, drawn from binomial distributions based on the probability of having each condition at baseline (table 3).
Table 3.
Patients’ baseline characteristics
| Baseline characteristics* | RE-LY | ROCKET-AF | ARISTOTLE |
|---|---|---|---|
| Number of patients | 18113 | 14264 | 18201 |
| Hypertension7,8,9 | 78.9% | 90.5% | 87.4% |
| Diabetes7,8,9 | 23.3% | 39.9% | 25.0% |
| Heart failure7,8,9 | 32.0% | 62.5% | 35.4% |
| Prior stroke7 | 12.5% | 34.4%† | 11.9%† |
| Prior transient ischemic attack7 | 9.2% | 25.3%† | 8.7%† |
| Prior myocardial infarction7,8,9 | 16.6% | 17.3% | 14.2% |
| Prior intracranial haemorrhage7 | 3.9% | 10.7%† | 3.7%† |
Percentage in initial population.
These values were imputed from the data available in the RE-LY study and the distribution of CHADS2 scores at the start of the trial, which was known for all three studies, under the assumption that the ratio of strokes to transient ischemic attacks and intracranial haemorrhages would be consistent between trials. Probability of prior stroke or TIA in ROCKET-AF was 55%, and in ARISTOTLE was 19%.
Interventions
The analysis considered a dose of 5mg twice daily of apixaban, and the licensed doses of dabigatran 150mg twice daily, rivaroxaban 20mg once daily, and dose-adjusted warfarin.
Clinical parameters
Annualised clinical event rates were extracted from the RE-LY, ROCKET-AF and ARISTOTLE trials7,8,9 identified from a systematic review of the literature14. Based on the method of Bucher et al,15 indirect comparisons were adjusted according to the results of their direct comparisons with warfarin. This adjustment accounts for differing baseline risks between trials by assuming a constant relative treatment effect e.g. for two trials comparing A and B, and B and C, with relative risks for a given event of RRAB and RRBC respectively, the indirect, relative effect of C versus A is estimated as:
Event rates for dabigatran, apixaban and rivaroxaban were calculated by multiplying relative treatment effects by warfarin event data, calculated from a meta-analysis of the warfarin arms of the three trials (table 4). Hypertension and diabetes incidence rates were taken from US general population data16,17, as were age-specific non-vascular mortality data18, all with the assumption these accurately reflect the atrial fibrillation population.
Table 4.
Clinical event rates
| Parameter | Warfarin | Dabigatran | Rivaroxaban | Apixaban | Aspirin |
|---|---|---|---|---|---|
| Stroke (CHADS2 score ≤ 1)* | 0.092 | 0.054 | 0.075† | 0.068 | 0.149 |
| Stroke (CHADS2 score 2)* | 0.141 | 0.082 | 0.126 | 0.121 | 0.227 |
| Stroke (CHADS2 score 3)* | 0.196 | 0.116† | 0.134 | 0.113† | 0.316 |
| Stroke (CHADS2 score 4)* | 0.312 | 0.215† | 0.244 | 0.210† | 0.503 |
| Stroke (CHADS2 score 5)* | 0.290 | 0.240† | 0.279 | 0.233† | 0.468 |
| Stroke (CHADS2 score 6)* | 0.364 | 0.310† | 0.351 | 0.302† | 0.587 |
| Systemic embolism* | 0.014 | 0.011 | 0.003 | 0.012 | 0.022 |
| Pulmonary embolism* | 0.008 | 0.011 | 0.009‡ | 0.006‡ | 0.013 |
| Transient ischemic attack* | 0.084 | 0.072 | 0.066‡ | 0.062‡ | 0.135 |
| Myocardial infarction* | 0.076 | 0.101 | 0.062 | 0.067 | 0.076 |
| Congestive heart failure* | 0.062 | 0.048 | 0.049‡ | 0.045‡ | 0.062 |
| Vascular death (excluding stroke and systemic and pulmonary embolism)* |
0.228 | 0.208 | 0.216 | 0.212 | 0.228 |
| Probability of death from stroke or systemic embolism |
2.546 | 2.546 | 2.546 | 2.546 | 2.546 |
| Probability of death from pulmonary embolism | 1.591 | 1.591 | 1.591 | 1.591 | 1.591 |
| Major bleed (CHADS2 score ≤ 1)* | 0.261 | 0.198 | 0.225† | 0.159 | 0.115 |
| Major bleed (CHADS2 score 2)* | 0.318 | 0.292 | 0.338† | 0.243 | 0.140 |
| Major bleed (CHADS2 score ≥ 3)* | 0.443 | 0.467 | 0.480† | 0.306 | 0.194 |
| Probability that major bleed is intracranial hemorrhage |
2.336 | 1.023 | 1.495 | 1.407 | 2.336 |
| Minor bleed* | 1.656 | 1.502 | 1.714 | 1.178 | 0.726 |
| Diabetes* | 0.141 | 0.141 | 0.141 | 0.141 | 0.141 |
| Hypertension* | 0.323 | 0.323 | 0.323 | 0.323 | 0.323 |
| Probability of discontinuation (year 1)* | 1.447 | 2.205 | 1.448 | 1.423 | N/A |
| Probability of discontinuation (year 2 onwards)* | 0.676 | 0.670 | 0.722 | 0.498 | N/A |
Presented as rates per 1000 person years.
Where stratified event rates were not available, unknown stratified risks were imputed based on the assumption that the relative risks of events for patients with different CHADS2 scores would be independent of treatment.
Imputed, based on the relative risks of different events from the RE-LY study, on the assumption that the relative risks of different thromboembolic events would be independent of treatment.
Whenever the necessary data were not available (e.g. a particular parameter was not reported for a given trial), values were imputed, based on data from the other trials and US population data (tables 4,5). All assumptions were assessed through sensitivity analysis (see structural sensitivity analysis, below).
Table 5.
Health state utilities assigned to treatments and clinical events, and the corresponding duration of acute events.
| Parameter | Value | Probabilistic sensitivity analysis distribution |
|---|---|---|
| Atrial fibrillation (age 67)22 | 0.774 | 1-Gamma (43.06,0.005) |
| Stroke/systemic embolism (permanent disutility)24 | 0.235 | Normal (0.235,0.003) |
| Stroke/systemic embolism (temporary disutility)22,23 | 0.139 | Normal (0.139,0.01) |
| Stroke/systemic embolism (temporary disutility, years)23 | 0.083 | Uniform (0,0.183) |
| Myocardial infarction (permanent disutility)22 | 0.041 | Normal (0.041,0.002) |
| Myocardial infarction (temporary disutility)22,23 | 0.125 | Normal (0.125,0.01) |
| Myocardial infarction (temporary duration, years)23 | 0.083 | Uniform (0,0.183) |
| Intracranial hemorrhage (permanent disutility)22 | 0.052 | Normal (0.052,0.001) |
| Pulmonary embolism (temporary disutility)22,23 | 0.139 | Normal (0.139,0.01) |
| Pulmonary embolism (temporary duration, years)23 | 0.083 | Uniform (0,0.183) |
| Transient ischemic attack (temporary disutility)22,23 | 0.103 | Normal (0.103,0.01) |
| Transient ischemic attack (temporary duration, years)23 | 0.014 | Uniform (0,0.027) |
| Major bleed (temporary disutility)22,23 | 0.139 | Normal (0.139,0.01) |
| Major bleed (temporary duration, years)23 | 0.083 | Uniform (0,0.183) |
| Minor bleed (temporary disutility)23 | 0.060 | Normal (0.06,0.01) |
| Minor bleed (temporary duration, years)23 | 0.014 | Uniform (0.0.027) |
| Warfarin disutility24 | 0.013 | Gamma (1.3,0.01) |
| Dabigatran/rivaroxaban/apixaban disutility | 0.002 | Gamma (0.2,0.01) |
| Aspirin disutility24 | 0.002 | Gamma (0.2,0.01) |
In order to better reflect the use of oral anticoagulants in routine care, the analysis also includes the trial-derived probabilities of (and reasons for) discontinuation of treatment. Patients who discontinued dabigatran, rivaroxaban or apixaban because of a bleed or who discontinued warfarin for any reason were assumed to be switched to aspirin, whilst other discontinuing patients were switched to warfarin. This assumption was tested in a sensitivity analysis. Relative risks of events for aspirin came from a published meta-analysis of trials comparing warfarin and aspirin19, and the AVERROES trial comparing apixaban with aspirin in patients deemed unsuitable for vitamin K antagonist therapy20.
QALYs were discounted at 3% per annum to reflect time preference21, but no discounting was applied to discrete clinical events.
Utility estimates
The age-adjusted baseline health utility for a person with atrial fibrillation, together with the utility decrements associated with cardiovascular sequelae (excluding stroke) and hemorrhagic events, were taken from the EQ-5D scores in a US Medical Expenditure Panel Survey of several thousand patients22,23. The permanent utility decrement associated with stroke was derived from the European Stroke Prevention Study, based on the proportions of disabling to non-disabling strokes (43% of non-fatal strokes were non-disabling across RE-LY, ROCKET-AF and ARISTOTLE). The analysis incorporated utility losses inherent to warfarin (e.g. as a result of monitoring), and aspirin (assumed to be the same for dabigatran, rivaroxaban and apixaban)24. Multiple utility decrements were assumed to be additive and utility values are given in table 5.
In order to assess the sensitivity of the model to the choice of utility parameters, a sensitivity analysis was conducted, replacing base-case utility values with those from an alternative cost-effectiveness study in atrial fibrillation25.
Parameter sensitivity analysis
Univariate sensitivity analyses of each parameter in the model were conducted to assess the effect of varying assumptions on the stability of the results. 95% confidence intervals were used as the upper and lower limits for parameters or, where these were not available, plausible percentage ranges.
A probabilistic parameter sensitivity analysis was also conducted as a Monte Carlo simulation of 2,000 sets of parameters sampled from appropriate distributions. This provided estimates of the 95% central ranges (2.5th to 97.5th percentile) for clinical event rates and net health benefits, and the probability of each treatment option resulting in the highest net health benefit.
Structural sensitivity analysis
The model necessitated a large number of assumptions, either for simplification purposes or because the desired data were not available in the necessary format. The robustness of the results in relation to different assumptions was assessed quantitatively.
Univariate analyses considered the different options presented in etable 1. A probabilistic analysis was performed by sampling 10,000 times, at random, from the subspace of possible structural assumptions, with each assumption being equally likely to be selected. As previously, the outputs are presented as the probabilities with which each treatment option results in the highest net health benefit.
In order to assess whether the choice of a discrete event simulation framework had a significant impact on the results, a secondary analysis was performed, replacing our simulation model with a published Markov model26.
Scenario analyses
Subgroup analyses were performed to calculate the net health benefits (and associated 95% central ranges) in the following pre-specified populations. Analyses for patients aged 75 or older; patients with a CHADS2 score of 3 or more; patients with the baseline characteristics of those in each of the three studies; and patients who have previously had a stroke or transient ischemic attack were performed by altering the baseline patient characteristics in the model. Separate, indirect comparisons were made for patients with impaired renal function (30-50mL/min creatinine clearance); and patients who were naïve to vitamin K antagonist treatment.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Dabigatran, rivaroxaban and apixaban are effective alternatives to warfarin for the prevention of thromboembolic stroke in patients with atrial fibrillation. However there is no evidence on their direct comparative effectiveness.
What question did this study address?
The study addressed the question of which oral anticoagulant is most effective, in terms of modelled quality-adjusted life-years. An indirect treatment comparison was made in patients who are representative of the US atrial fibrillation population.
What this study adds to our knowledge
Compared with the alternatives, apixaban was ranked highest both in terms of the absolute number of QALYs accrued over a lifetime, and the probability of achieving the greatest QALY gains.
How this might change clinical pharmacology and therapeutics
This evidence can help inform treatment selection, particularly as there is no prospect of a definitive trial to compare each treatment directly.
Acknowledgments
We thank Catrin Tudor-Smith, PhD, and Steven Lane, PhD, University of Liverpool, for statistical advice. No one received financial compensation for his or her contributions.
Funding/Support: The study was funded by the Medical Research Council, as part of the North West Hub for Trial Methodological Research (NWHTMR). The funding sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: None
References
- [1].Lip GYH, Tse HF. Management of atrial fibrillation. Lancet. 2007;370(9587):604–18. doi: 10.1016/S0140-6736(07)61300-2. [DOI] [PubMed] [Google Scholar]
- [2].Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- [3].Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds MR, Zimetbaum P. Assesing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–56. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- [4].Fuster V, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- [5].Gage BF, Fihn SD, White RH. Warfarin therapy for an octogenarian who has atrial fibrillation. Ann Intern Med. 2001;134(6):465–74. doi: 10.7326/0003-4819-134-6-200103200-00011. [DOI] [PubMed] [Google Scholar]
- [6].Connolly SJ, et al. Benefit of oral anticoagulation over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):1029–37. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- [7].Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- [8].Patel MR, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- [9].Granger CB, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- [10].Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ. 2011;343 doi: 10.1136/bmj.d6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caro JJ, Möller J, Getsios D. Discrete event simulation: the preferred technique for health economic evaluations. Value in Health. 2010;13(8):1056–60. doi: 10.1111/j.1524-4733.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- [12].Hughes DA, Bayoumi AM, Pirmohamed M. Current assessment of risk-benefit by regulators: is it time to introduce decision analyses? Clin Pharmacol Ther. 2007;82(2):123–7. doi: 10.1038/sj.clpt.6100240. [DOI] [PubMed] [Google Scholar]
- [13].Singer DE, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-Analysis of Efficacy and Safety of New Oral Anticoagulants (Dabigatran, Rivaroxaban, Apixaban) Versus Warfarin in Patients With Atrial Fibrillation. Am J Cardiol. 2012 doi: 10.1016/j.amjcard.2012.03.049. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- [15].Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomised controlled trials. J Clin Epidemiol. 1997;50(6):683–921. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- [16].U.S. Department of Health and Human services [Accessed 5th July 2012];National Diabetes Statistics. 2011 http://diabetes.niddk.nih.gov/dm/pubs/statistics.
- [17].Hajjar I, Morley Kotchen J, Kotchen TA. Hypertension: trends in prevalence, incidence and control. Ann Rev Public Health. 2006;27:465–90. doi: 10.1146/annurev.publhealth.27.021405.102132. [DOI] [PubMed] [Google Scholar]
- [18].Wilmoth JR, Shkolnikov V. [Accessed 5th July 2012];The human mortality database. 2006 http://www.mortality.org.
- [19].Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- [20].Connolly SJ, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- [21].Lipscomb J, Weinstein MC, Torrance GW. Time Preference (Chapter 7) In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- [22].Sullivan PW, Ghushcyan V. Preference based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks and costs of warfarin pharmacogenomic testing. Pharmacoeconomics. 2010;28(1):61–74. doi: 10.2165/11318240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [24].Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–36. [PubMed] [Google Scholar]
- [25].Freeman JV, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- [26].Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123(22):2562–70. doi: 10.1161/CIRCULATIONAHA.110.985655. [DOI] [PubMed] [Google Scholar]
- [27].Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012;108(3) doi: 10.1160/TH12-02-0093. doi:10.1160/TH12-02-0093. [DOI] [PubMed] [Google Scholar]
- [28].Lip GY, Larsen TB, Skjøth F, Rasmussen LH. Indirect Comparisons of New Oral Anticoagulant Drugs for Efficacy and Safety When Used for Stroke Prevention in Atrial Fibrillation. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.03.019. doi:10.1016/j.jacc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- [29].Banerjee A, Lane DA, Torp-Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107(3):584–9. doi: 10.1160/TH11-11-0784. [DOI] [PubMed] [Google Scholar]
- [30].Jansen JP, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value in Health. 2011;14(4):417–28. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- [31].Altman DG, Bland JM. Statistics notes: Absence of evidence is not evidence of absence. BMJ. 1995;311:485. doi: 10.1136/bmj.311.7003.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O’Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012;34(4):894–901. doi: 10.1016/j.clinthera.2012.01.019. [DOI] [PubMed] [Google Scholar]
- [33].Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guzauskas GF, Hughes DA, Bradley SM, Veenstra DL. A risk-benefit assessment of prasugrel, clopidogrel, and genotype-guided therapy in patients undergoing percutaneous coronary intervention. Clin Pharmacol Ther. 2012;91(5):829–37. doi: 10.1038/clpt.2011.303. [DOI] [PubMed] [Google Scholar]
- [35].Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. doi: 10.1186/1471-2296-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88(4):457–9. doi: 10.1038/clpt.2010.171. [DOI] [PubMed] [Google Scholar]
- [37].Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- [38].Radecki RP. Dabigatran: uncharted waters and potential harms. Ann Intern Med. 2012;157(1):66–8. doi: 10.7326/0003-4819-157-1-201207030-00467. [DOI] [PubMed] [Google Scholar]
- [39].Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172(5):397–402. doi: 10.1001/archinternmed.2011.1666. [DOI] [PubMed] [Google Scholar]
- [40].Cairns J. Review: Dabigatran increases MI and reduces mortality compared with warfarin, enoxaparin, or placebo. Ann Intern Med. 2012;156(12):JC6–11. doi: 10.7326/0003-4819-156-12-201206190-02011. [DOI] [PubMed] [Google Scholar]
- [41].International Warfarin Pharmacogenetics Consortium et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360(8):753–64. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.