Summary
Background
Innate immunity contributes to the pathogenesis of autoimmune diseases, such as type 1 diabetes, but until now no randomised, controlled trials of blockade of the key innate immune mediator interleukin-1 have been done. We aimed to assess whether canakinumab, a human monoclonal anti-interleukin-1 antibody, or anakinra, a human interleukin-1 receptor antagonist, improved β-cell function in recent-onset type 1 diabetes.
Methods
We did two randomised, placebo-controlled trials in two groups of patients with recent-onset type 1 diabetes and mixed-meal-tolerance-test-stimulated C peptide of at least 0·2 nM. Patients in the canakinumab trial were aged 6–45 years and those in the anakinra trial were aged 18–35 years. Patients in the canakinumab trial were enrolled at 12 sites in the USA and Canada and those in the anakinra trial were enrolled at 14 sites across Europe. Participants were randomly assigned by computer-generated blocked randomisation to subcutaneous injection of either 2 mg/kg (maximum 300 mg) canakinumab or placebo monthly for 12 months or 100 mg anakinra or placebo daily for 9 months. Participants and carers were masked to treatment assignment. The primary endpoint was baseline-adjusted 2-h area under curve C-peptide response to the mixed meal tolerance test at 12 months (canakinumab trial) and 9 months (anakinra trial). Analyses were by intention to treat. These studies are registered with ClinicalTrials.gov, numbers NCT00947427 and NCT00711503, and EudraCT number 2007-007146-34.
Findings
Patients were enrolled in the canakinumab trial between Nov 12, 2010, and April 11, 2011, and in the anakinra trial between Jan 26, 2009, and May 25, 2011. 69 patients were randomly assigned to canakinumab (n=47) or placebo (n=22) monthly for 12 months and 69 were randomly assigned to anakinra (n=35) or placebo (n=34) daily for 9 months. No interim analyses were done. 45 canakinumab-treated and 21 placebo-treated patients in the canakinumab trial and 25 anakinra-treated and 26 placebo-treated patients in the anakinra trial were included in the primary analyses. The difference in C peptide area under curve between the canakinumab and placebo groups at 12 months was 0·01 nmol/L (95% CI −0·11 to 0·14; p=0·86), and between the anakinra and the placebo groups at 9 months was 0·02 nmol/L (−0·09 to 0·15; p=0·71). The number and severity of adverse events did not differ between groups in the canakinumab trial. In the anakinra trial, patients in the anakinra group had significantly higher grades of adverse events than the placebo group (p=0·018), which was mainly because of a higher number of injection site reactions in the anakinra group.
Interpretation
Canakinumab and anakinra were safe but were not effective as single immunomodulatory drugs in recent-onset type 1 diabetes. Interleukin-1 blockade might be more effective in combination with treatments that target adaptive immunity in organ-specific autoimmune disorders.
Funding
National Institutes of Health and Juvenile Diabetes Research Foundation.
Introduction
Type 1 diabetes mellitus is characterised by progressive autoimmune destruction of pancreatic β cells, resulting in lifelong dependence on exogenous insulin administration and risk of acute and late complications. At initial diagnosis, substantial β-cell function remains.1 Persistent endogenous insulin secretion, defined as stimulated C-peptide concentration greater than 0·2 nmol/L during a mixed meal tolerance test (MMTT), is associated with reduced occurrence of severe hypoglycaemia and microvascular complications.2,3 Thus, interventions that stop or delay decline of β-cell function are desirable.
Clinical trials to preserve β-cell function in new-onset type 1 diabetes have focused on the adaptive immune system. Treatment with anti-CD34–7 or abatacept,8 which target T lymphocytes, or anti-CD20,9 which targets B lymphocytes, temporarily arrested the auto-immune process, stabilising β-cell function for about 6–12 months. However, the disease recurred in all of these cases, consistent with transient suppression of the adaptive immune system rather than durable immuno modulation.
Recent research has focused on the role of the innate immune system in type 1 diabetes. Findings from a pilot clinical trial suggested that inhibition of tumour necrosis factor-α might have a beneficial effect in type 1 diabetes.10 However, particular attention has focused on the role of the proinflammatory cytokine interleukin-1β, which is secreted by several cell types in response to tissue insult. By binding to pancreatic β-cell interleukin-1 type 1 receptors, interleukin-1 signals β-cell secretory dys function and apoptosis via the nuclear factor κB and mitogen-activated protein kinase pathways, leading to endoplasmic reticulum and mitochondrial stress.11 Hyperglycaemia also induces production and release of interleukin-1β by pancreatic β cells;12 interleukin-1β seems to act locally to inhibit insulin biosynthesis and release13,14 and induce β-cell apoptosis via activation of the death receptor Fas.12,15 Because of its direct β-cell proapoptotic action and mediatory effects on pancreatic β-cell glucotoxicity, interleukin-1β has been implicated in the pathogenesis of both type 1 and type 2 diabetes.
In addition to its effects in the innate immune system, interleukin-1β might be important in the pathogenesis of type 1 diabetes via its role as a potent amplifier of the adaptive immune response. Interleukin-1β enhances expansion and survival of naive and memory T lymphocytes, promotes differentiation of T lymphocytes towards pathological phenotypes including T-helper-1 (Th1) and Th17, and enables effector T lymphocytes to proliferate despite the modulating presence of regulatory T lymphocytes.16 Interleukin-1β might also have an important role in monocyte trafficking.17
Thus, there is a strong preclinical rationale to implicate interleukin-1 as an immune mediator of pancreatic β-cell destruction leading to type 1 diabetes.11 Furthermore, in an open-label pilot trial, interleukin-1 receptor blockade for 28 days in 15 children with new-onset diabetes was well tolerated and lowered insulin needs and insulin-dose-adjusted glycated haemoglobin concentrations compared with historical controls.18 So far, there have been no randomised, placebo-controlled trials of interleukin-1 antagonism in patients with recent-onset type 1 diabetes. Therefore, we hypothesised that inter leukin-1β inhibition in new-onset type 1 diabetes would preserve β-cell function by blocking direct and hyper glycaemia-mediated β-cell toxicity, reducing overall inflammation and favouring regulatory T lymphocyte development and function. To assess this theory, in two trials we tested whether canakinumab, a human mono clonal anti-interleukin-1 antibody, or anakinra, a human interleukin-1 receptor antagonist, maintained or enhanced β-cell function in recent-onset type 1 diabetes. These studies also assessed the feasibility and safety and tolerability of anti-interleukin-1 treatment.
Methods
Participants
Both studies were investigator-initiated, parallel-group, randomised, placebo-controlled phase 2a clinical trials and conformed to all applicable regulatory requirements. Full details of the TrialNet canakinumab study protocol are available on the Type 1 Diabetes TrialNet website and details of the Anti-Interleukin-1 in Diabetes Action (AIDA) trial are on the AIDA website. Eligible participants for the canakinumab trial were patients aged 6–45 years at onset of diabetes diagnosed within the past 100 days before enrolment, who were positive for at least one diabetes-associated autoantibody (microassayed insulin if duration of insulin therapy was <7 days; glutamic acid decarboxylase-65 [GAD-65]; islet-cell antigen-512 [ICA-512]; or islet-cell autoantibodies), and who had a peak C-peptide concentration of at least 0·2 nmol/L after a standardised MMTT undertaken at least 21 days after diagnosis of diabetes and within 37 days of randomisation. Exclusion criteria were serological or clinical evidence of present infection; past infection with hepatitis B, hepatitis C, or HIV; a positive PPD test; a history of malignancies or complicated medical issues; immunodeficiency; or clinically significant blood count abnormalities.
Eligible participants for the anakinra (AIDA) trial were patients aged 18–35 years at onset of diabetes with first symptoms of diabetes reported within the past 12 weeks before enrolment, who were positive for GAD-65 auto-antibodies and had a peak C peptide of at least 0·2 nM after a standardised MMTT undertaken after resolution of any polyuria, polydipsia, or keto acidosis. Exclusion criteria were severe renal disease (creatinine concentration >100 μmol/L) or liver disease (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase over twice the local laboratory upper reference limit); history of heart disease, signs of cardiac failure, or abnormal electrocardiograph; present or previous malignancy; pregnancy or breastfeeding; failure of fertile women to comply with contraception planning (birth control pills, intrauterine device, or gestagen implants); participation in other clinical intervention studies; anti-inflammatory treatment (except aspirin ≤100 mg/day); active infections, history of recurrent infection, or predisposition to infections; neutropenia (neutrophil count <1·5 × 109 cells/L) or anaemia (haemoglobin <80 g/L); immune-suppressive treatment or immune deficiency; presence at diagnosis of late diabetic complications; concurrent vaccination with live vaccine; use of etanercept within 4 weeks before screening or during the study period; and hypersensitivity to Escherichia coli-derived proteins, anakinra, or any components of the product.
The protocols and consent documents were approved by independent ethics committees or institutional review boards. All participants or parents provided written informed consent before enrolment, and participants younger than 18 years provided assent. Independent data and safety reviews were undertaken regularly.
Randomisation and masking
Patients were individually randomised by the data monitoring unit common for both trials by a centralised computer-generated random allocation sequence with locked computer file concealment. The TrialNet canakinumab study used 2:1 randomisation in permuted blocks of six, and the AIDA trial used 1:1 randomisation in blocks of four within recruiting centres. All participants and investigators were masked to treatment assignment.
Procedures
Screening, enrolment, and study visits took place at 12 TrialNet sites in the USA and Canada and at 14 AIDA sites across Europe. All patients received intensive diabetes management with a goal of maintaining American Diabetes Association glycated haemoglobin A1c (HbA1c) targets for their age. In the TrialNet canakinumab study, the participants’ primary physicians were responsible for their diabetes manage ment, with support from the site research team. In the AIDA study, diabetes was managed by the site research teams. Patients were treated with several daily insulin injections or by insulin pump, with frequent daily self-monitoring of blood glucose concentrations. Non-insulin drugs that affect glycaemic control were not allowed.
Participants in the canakinumab trial received monthly subcutaneous injections of 2 mg/kg (maximum 300 mg) canakinumab (Novartis, Basel, Switzerland) or an identically appearing placebo for 12 doses. The next dose was rescheduled in participants with signs of active infection within the previous 48 h. If the patient remained ill during the study window, that dose was skipped and not made up. Participants who were seronegative for Epstein-Barr virus at baseline underwent assessment of Epstein-Barr viral load at each visit; if laboratory evidence of active Epstein-Barr virus infection developed, treatment with the study drug was stopped until resolution. All participants were followed up for at least 12 months.
On Aug 17, 2011, Novartis reported neutropenia as a potential risk of canakinumab treatment. As a result, monthly pre-injection white blood cell counts were added to the protocol. Before that, white blood cell counts were only obtained at 0, 6, and 12 months. The dose was withheld if the absolute neutrophil count (ANC) was less than 1500 per mm3. At the time this amendment was introduced, no participant had completed the 12-month visit.
Participants in the AIDA trial received the recombinant human interleukin-1 receptor antagonist anakinra at the US Food and Drug Administration approved daily dose of 100 mg or placebo as a single, self-administered, subcutaneous injection every morning for 9 months. Anakinra and placebo were provided and distributed by Amgen (Thousand Oaks, CA, USA). Compliance was assessed by counting returned empty vials and by measuring plasma interleukin-1 receptor antagonist concentration at follow-up visits. Suspension of treat ment for up to 2 weeks was allowed to resolve intercurrent disease or adverse events.
For both studies, β-cell function was assessed by standard mixed-meal stimulated C-peptide secretion,19 a validated surrogate marker for subsequent hard clinical outcomes in patients with type 1 diabetes.2,3 Participants were given 6 mL/kg of Boost High Protein (Nestlé Nutrition, Vervey, Switzerland) to a maximum of 360 mL ingested within 5 min, and blood samples were taken at −10, 0, 15, 30, 60, 90, and 120 min after start of ingestion for analysis of C peptide and glucose.
Routine clinical laboratory tests were done locally at participating centres for both trials. Outcome variables were analysed centrally by core laboratories. C peptide was measured with a two-site immunoenzymometric assay (Tosoh Bioscience, South San Francisco, CA, USA; TrialNet canakinumab trial) or a time-resolved fluoroimmunoassay (AutoDELFIA C-peptide kit, Perkin Elmer–Wallac, Waltham, MA, USA; AIDA trial). HbA1c was measured with ion-exchange high-performance liquid chromatography (Variant II, Bio-Rad Diagnostics, Hercules, CA, USA; TrialNet canakinumab trial) or with a high-performance liquid chromatography assay (Tosoh G7, Tosoh Bioscience, Tokyo, Japan; AIDA trial). Auto antibodies (microassayed insulin, GAD-65, and ICA-512) were measured with radioimmunobinding assays and islet-cell autoantibodies with indirect immunofluorescence. In the TrialNet canakinumab trial, HLA class 2 alleles were measured with PCR amplification and sequence-specific hybridisation. In the AIDA trial, high-sensitivity C-reactive protein (CRP) and interleukin-6 concentrations were measured by chemiluminescence enzyme immunometric assays on the Immulite auto-analyser (Siemens Healthcare Diagnostics, Erlangen, Germany), and interleukin-1 receptor antagonist concentrations were measured by an enzyme-linked immunosorbent assay (human interleukin-1 receptor antagonist/interleukin-1F3 immuno assay SRA00B, R&D Systems, Abingdon, UK). Overall specimen completeness was over 95%.
The prespecified primary outcome in both trials was comparison of the area under the curve (AUC) of stimulated C-peptide response over a 2-h MMTT at the 12 month (TrialNet canakinumab) or 9 month (AIDA) visit. MMTTs were done at baseline and 1, 3, 6, and 9 months (participants in the TrialNet canakinumab trial had additionally the 12-month visit and 18-month and 24-month off-treatment follow-up visits). Prespecified secondary outcomes consisted of C-peptide slope over time (TrialNet canakinumab); peak and time to peak of MMTT stimulated C peptide (AIDA); the time in trial until peak C peptide is less than 0·2 nmol/L (TrialNet canakinumab); fasting glucose concentration (AIDA); HbA1c and insulin dose over time (both trials); and change in circulating interleukin-6 and CRP concentrations (AIDA). Pre specified subgroup analyses were by age, sex, ethnic origin, body-mass index (AIDA only), baseline C peptide, baseline insulin use, baseline HbA1c and HLA type, and duration of symptoms (AIDA only).
Adverse events were coded using the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, with relation to study drug assessed by the investigators.
Statistical analysis
For the canakinumab trial, a sample size of 66 patients was needed to provide 85% power to detect a 54% increase in the transformed mean of C peptide in the canakinumab treatment group relative to the placebo group, with a one-sided test at α=0·05 and 10% missing values. Screening of new patients was closed before this target sample size was reached but patients who had already begun screening were allowed to proceed. For the anakinra trial, a sample size of 80 patients in each group (160 patients in total) was needed to provide 80% power to detect a 30% difference in the C-peptide AUC between treatment groups, with a two-sided test at α=0·05 and 10% missing values.
All analyses were based on the prespecified intention-to-treat cohorts with known measurements (complete case analysis); missing values were assumed to be missing at random. The p values are two-sided unless otherwise specified. The significance levels associated with the treatment effect were from the Wald test (from the fitted model). Treatment comparisons of adverse event grades were analysed with the Wilcoxon rank sum test and the occurrence of each adverse effect type was analysed with the Fisher’s exact test (one-sided tests). Spotfire S+ (version 8.1) was used for all analyses.
We used an ANCOVA model adjusted for age, sex, baseline value of the dependent variable, and treatment assignment to analyse the mean AUC of C peptide, percentage of HbA1c, and total daily insulin dose. The predicted means and associated 95% CIs for each treatment group were calculated at the means of the other covariates.
A normalising transformation of log (Xc peptide + 1) was prespecified for the mean AUC of C peptide, and normal plots of the residuals suggested that this was adequate (data not shown). The unadjusted geometric-like mean was calculated using the same transformation and was then averaged and the inverse transformation was applied. The mean AUC of C peptide is equal to the AUC divided by the 2-h interval. The AUC was computed by the trapezoidal rule from the first 2 h of the timed measurement of C peptide during the MMTT. The time to first stimulated peak C peptide of less than 0·2 nmol/L was analysed with the Cox model with covariates for testing and the Kaplan-Meier method for plotting the results. The rate of change of the mean AUC of C peptide was estimated by use of a mixed effects model, with random intercept and slope adjusted for age, sex, mean AUC of C peptide at baseline, and treatment assignment. The initial fit included a fixed interaction effect of treatment and time but was removed because there was no statistical evidence to suggest that value was anything other than zero. The 95% CIs for the difference in the population means of C peptide and the percent decrease of C peptide from baseline were determined using a bootstrap technique.
The TrialNet canakinumab and AIDA trials are registered with ClinicalTrials.gov, numbers NCT00947427 and 00711503, respectively, and the AIDA trial is also registered as EudraCT number 2007-007146-34.
Role of the funding source
The TrialNet canakinumab trial was designed by the Type 1 Diabetes TrialNet Study Group. Novartis provided unpublished clinical data from other canakinumab trials, provided input regarding the drug dose, and supplied canakinumab and placebo. Novartis had no other involvement in study design, undertaking, or manage ment; data collection, analysis, or interpretation; or writing of the report. A Cooperative Research and Development Agreement between Novartis and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (sponsor of the Type 1 Diabetes TrialNet canakinumab study), specified that both the NIDDK and Novartis would keep study data confidential until published. The authors provided Novartis with a copy of the original report before sub mission; Novartis made no amendments but did make suggestions in terms of readability of language. The TrialNet canakinumab writing committee had full access to all the data in the canakinumab study and had the final responsibility for the decision to submit for publication. JSS assumes responsibility for the overall content and integrity of the canakinumab portion of the manuscript.
The Juvenile Diabetes Research Foundation (JDRF) approved the AIDA study design and protocol and decided to prematurely stop enrolment, but was not involved in data collection, data analysis, data interpretation, or writing of the report. Amgen supplied anakinra and placebo, prepared drug labelling, and distributed the drug according to good laboratory practice principles, including temperature monitoring until delivery to centres. Amgen had no other involvement in study design, undertaking, or management; data collection, analysis, or interpretation; or writing of the report. TMP had full access to all the data in the AIDA study and had the final responsibility for the decision to submit for publication.
Results
Canakinumab trial
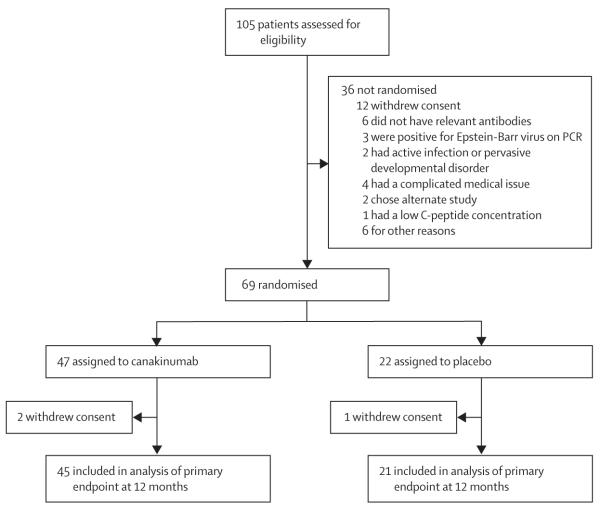
Between Nov 12, 2010, and April 11, 2011, 105 participants were assessed for eligibility and 69 were randomly assigned; 47 to canakinumab and 22 to placebo (figure 1; appendix). The last patient completed the 12-month follow-up on April 27, 2012.
Figure 1.
Canakinumab trial profile
756 (91%) of 828 potential injections were given. Of the 72 missed injections (39 in the canakinumab group and 33 in the placebo group), 48 (67%) were held (ie, skipped) by the investigators as per the study protocol (eg, because of active infection or low ANC at time of injection). Of the 69 participants, one (in the canakinumab group) did not have the 6-month MMTT and three (two in the canakinumab group and one in the placebo group) did not have the 1-year MMTT. Clinical and demographic characteristics were well balanced between treatment groups (table 1).
Table 1.
Baseline demographics and laboratory characteristics of participants in the canakinumab trial
| Canakinumab (n=47) |
Placebo (n=22) |
|
|---|---|---|
| Age (years) | ||
| Mean (SD) | 11·7 (4·0) | 12·5 (6·4) |
| Median (range) | 11 (6–25) | 10·5 (6–31) |
| Male | 24 (51%) | 14 (64%) |
| White* | 42 (93%) | 22 (100%) |
| Non-Hispanic† | 47 (100%) | 22 (100%) |
| Numbers of positive autoantibodies | ||
| 1 | 4 (9%) | 0 (0%) |
| 2 | 8 (17%) | 4 (18%) |
| 3 | 17 (36%) | 8 (36%) |
| 4 | 18 (38%) | 10 (45%) |
| Days from diagnosis to first randomisation | ||
| Mean (SD) | 75·8 (17·9) | 75·6 (21·8) |
| Median (range) | 76 (36–104) | 80 (21–99) |
| Weight (kg) | 49·1 (21·7) | 46·3 (18·5) |
| Body-mass index (kg/m2) | 20·7 (5·53) | 19·8 (3·73) |
| Area under the curve for C peptide (nmol/L) |
0·65 (0·36) | 0·62 (0·29) |
| Glycated haemoglobin (%) | 7·09 (1·16) | 6·81 (0·95) |
| Total daily insulin dose (units/kg per day) | 0·37 (0·26) | 0·35 (0·15) |
| Diabetes-associated HLA alleles present‡ | ||
| DR3 and DR4 | 13 (28%) | 6 (29%) |
| DR3 only | 12 (26%) | 4 (19%) |
| DR4 only | 13 (28%) | 7 (33%) |
| Neither | 9 (19%) | 4 (19%) |
Data are number (%) or mean (SD), unless otherwise specified. Some percentages do not total 100 because of rounding.
Excludes two participants in the canakinumab group who did not report ethnic origin.
Recorded on case report form.
Excludes one participant in the placebo group who did not have a genetic test.
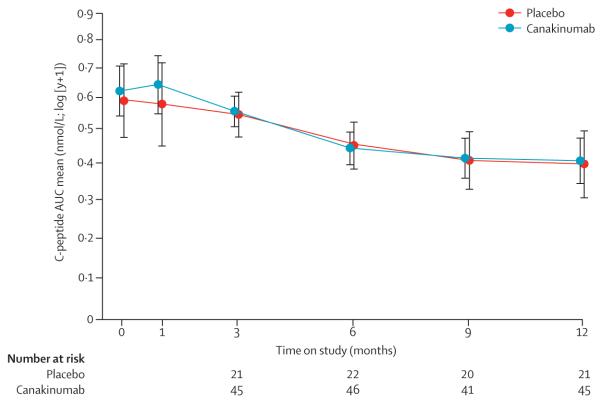
Population means for the 2 h AUC of stimulated C peptide adjusted for age, sex, and baseline C-peptide value were similar between the groups at 1 year (canakinumab 0·41 nmol/L, 95% CI 0·34–0·47; and placebo 0·40 nmol/L, 0·30–0·49; figure 2). The difference between groups was not significant (0·01 nmol/L, 95% CI −0·11 to 0·14; p=0·86). Between baseline and 1 year, there was a 35% (95% CI 21·8–45·6) and 33% (3·1–51·6) reduction in the population means of C peptide in the canakinumab and placebo groups, respectively. When the population means were assumed to be linear over time in a mixed model, the decay rates (ie, slopes) were not different between the groups (appendix). Time to stimulated peak C peptide less than 0·2 nmol/L also did not differ between the two groups (appendix). Percentages of HbA1c increased gradually over time and were similar between canakinumab-treated and placebo-treated participants at 1 year (p=0·76; appendix). Similarly, there was no difference in insulin dose at 1 year between groups (p=0·53; appendix).
Figure 2. Canakinumab trial primary outcome.
Population means of stimulated C-peptide 2 h mean AUC over time for each treatment group. The estimates are from the analysis of covariance model adjusted for age, sex, baseline value of C peptide, and treatment assignment. AUC=area under the curve. Bars=95% CI.
In a predefined subgroup analysis, a homogeneity test of treatment effect on age, sex, ethnic origin, baseline C peptide, insulin use, percentage of HbA1c, and HLA type was done (appendix). The canakinumab-treated group had significantly (homogeneity test) lower C-peptide concentrations at 1 year in participants with C peptide in the lower tertile at baseline (p=0·036).
There were two serious adverse events during the canakinumab trial: a case of appendicitis in a participant in the placebo group and suicidal ideation in a participant in the canakinumab group (table 2). Both were deemed unrelated to study drugs. The number and severity of adverse events did not differ between groups (table 3). Despite its potential anti-inflammatory effects, canakinumab did not result in more frequent or more severe infections.
Table 2.
Adverse events by worst grade experienced in the canakinumab trial
| Canakinumab (n=47) | Placebo (n=22) | |
|---|---|---|
| No adverse event | 13 (28%) | 6 (27%) |
| Grade 1 | 2 (4%) | 0 (0%) |
| Grade 2 | 28 (60%) | 12 (55%) |
| Grade 3 | 3 (6%) | 3 (14%) |
| Grade 4 | 1 (2%) | 1 (5%) |
| Grade 5 | 0 (0%) | 0 (0%) |
Data are number of participants (%). Worst grade by treatment group was not statistically different by Wilcoxon rank sum test. Some percentages do not total 100 because of rounding.
Table 3.
The number of events and participants by adverse event type in the canakinumab trial
| Canakinumab (n=47) |
Placebo (n=22) |
|||
|---|---|---|---|---|
| Number of events |
Number of participants (%) |
Number of events |
Number of participants (%) |
|
| Pain | 12 | 6 (13%) | 2 | 1 (5%) |
| Flu-like symptoms | 1 | 1 (2%) | 1 | 1 (5%) |
| Infection | 22 | 13 (28%) | 16 | 8 (36%) |
| Gastrointestinal | 7 | 5 (11%) | 6 | 5 (23%) |
| Pulmonary or upper respiratory | 2 | 2 (4%) | 2 | 2 (9%) |
| Constitutional symptoms | 5 | 4 (9%) | 1 | 1 (5%) |
| Blood or bone: ANC or AGC | 12 | 8 (17%) | 6 | 3 (14%) |
| Blood or bone: other | 0 | 0 (0%) | 0 | 0 (0%) |
| Surgery or intraoperative injury | 0 | 0 (0%) | 1 | 1 (5%) |
| Neurological | 4 | 4 (9%) | 1 | 1 (5%) |
| Dermatological or skin | 5 | 5 (11%) | 3 | 3 (14%) |
| Musculoskeletal or soft tissue | 4 | 4 (9%) | 0 | 0 (0%) |
| Auditory or ear | 1 | 1 (2%) | 0 | 0 (0%) |
| Endocrine | 3 | 3 (6%) | 0 | 0 (0%) |
| Blood or bone marrow | 3 | 1 (2%) | 0 | 0 (0%) |
| Ocular or visual | 0 | 0 (0%) | 1 | 1 (5%) |
| Total | 81 | · · | 40 | · · |
Each adverse effect category by treatment group was tested using a one-sided (alternative of higher frequency in canakinumab group) Fisher’s exact test; none reached statistical significance. ANC=absolute neutrophil count. AGC=absolute granulocyte count.
Neutropenia was common in both groups and did not differ significantly by treatment arm (p=0·11, one-sided). Eight (17%) canakinumab-treated patients had 12 episodes of neutropenia compared with three (14%) placebo-treated patients who had six episodes of neutropenia. Neutropenia was grade 2 (ANC 1000–1499 per mm3) in most cases, but there were four instances of grade 3 neutropenia (ANC 500–999 per mm3): one instance each in two canakinumab-treated patients and two instances in a patient on placebo. In all but one of these cases (the placebo-treated patient), several doses of study drug were not associated with subsequent neutropenia.
Anakinra trial
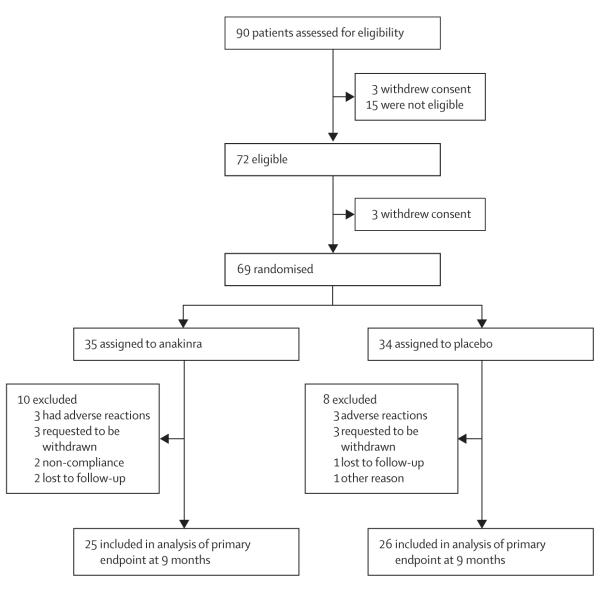
Between Jan 26, 2009, and May 25, 2011, 90 participants were assessed for eligibility and 72 were eligible, 69 of whom were randomly assigned to anakinra (n=35) or placebo (n=34; figure 3; appendix). The study was stopped on July 31, 2011 owing to slow recruitment. A similar number of participants withdrew in the two groups, all within 4 months of the start of treatment. 25 participants assigned to anakinra and 26 assigned to placebo completed the 9-month follow-up visit. The last patient completed follow-up on Jan 17, 2012.
Figure 3.
Anakinra trial profile
The mean ratio of used vials to elapsed days was lower in the anakinra group than in the placebo group (61·8% vs 67·9%, respectively, Wilcoxon test p<0·0001) Nevertheless, the mean plasma interleukin-1 receptor antagonist concentrations in the anakinra group at follow-up visits were increased 226–815 times over the corresponding means in the placebo group throughout the study (all p<0·001; appendix).
The groups were well balanced for clinical and demographic characteristics with the exception of inter-leukin-6 and interleukin-1 receptor antagonist concentrations (table 4). For interleukin-6 concentration, the reported difference was attributable to one extreme value that distorted the mean and standard deviation for the placebo group. For interleukin-1 receptor antagonist concentration, there were two extreme values that distorted the mean and standard deviation for the anakinra group.
Table 4.
Baseline demographics and laboratory characteristics of participants in the anakinra trial
| Anakinra (n=35) | Placebo (n=34) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 26·6 (5·3) | 25·0 (4·5) |
| Median (range) | 27 (18–34) | 25 (18–34) |
| Men | 26 (74%) | 22 (65%) |
| White* | 32 (97%) | 33 (100%) |
| Duration from diagnosis to treatment start (days) | ||
| Mean (SD) | 64·2 (18·0) | 59·8 (17·1) |
| Median (range) | 65·0 (29–95) | 62·5 (32–85) |
| Weight (kg)† | 72·0 (10·9) | 72·0 (12·7) |
| Body-mass index (kg/m2)‡ | 22·9 (2·7) | 22·8 (2·8) |
| Area under the curve for C peptide (nmol/L) | 0·62 (0·26) | 0·73 (0·37) |
| Glycated haemoglobin (%)§ | 7·63 (1·43) | 7·30 (1·15) |
| Fasting plasma glucose (mmol/L) | 6·9 (1·9) | 7·3 (1·8) |
| Area under the curve for glucose (mmol/L) | 10·4 (2·3) | 10·9 (2·4) |
| Total daily insulin dose (units/kg per day)¶ | 0·42 (0·34) | 0·38 (0·35) |
| Interleukin-6 (ng/L)∥ | 2·32 (1·38) | 2·53 (15·6) |
| Interleukin-1 receptor antagonist (μg/L)∥ | 31·1 (125·0) | 0·31 (0·20) |
| C-reactive protein (mg/L)** | 1·47 (3·04) | 1·43 (2·31) |
Data are number (%) or mean (SD) unless otherwise specified.
Excludes two participants in the anakinra group and one in the placebo group who did not report ethnic origin.
Data missing for two patients in the anakinra group and two in the placebo group.
Data missing for three patients in the anakinra group and two in the placebo group.
Data missing for three patients in the anakinra group.
Data missing for one patient in the anakinra group and three in the placebo group.
Data missing for one patient in the anakinra group.
Data missing for one patient in the placebo group.
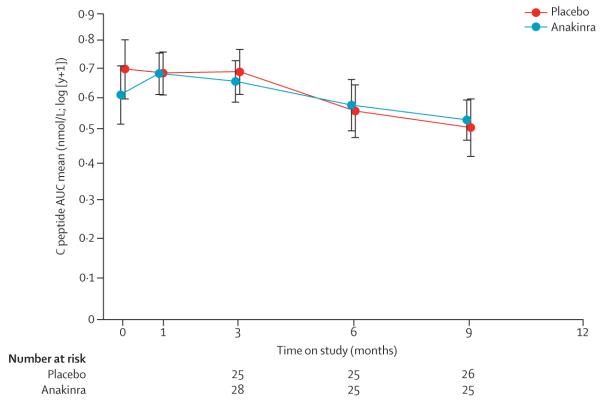
Population means for the 2 h AUC of stimulated C peptide adjusted for age, sex, and baseline C-peptide value were similar between the groups at 9 months (anakinra 0·53 nmol/L, 95% CI 0·44–0·62; and placebo 0·51 nmol/L, 0·42–0·60, figure 4). The difference was not significant (0·02 nmol/L, 95% CI −0·09 to 0·15; p=0·71).
Figure 4. Anakinra trial primary outcome.
Population means of stimulated C-peptide 2 h mean AUC over time for each treatment group. The estimates are from the analysis of covariance model adjusted for age, sex, baseline value of C peptide, and treatment assignment. AUC=area under the curve. Bars=95% CI.
Anakinra did not affect incremental or peak C-peptide response to a MMTT, percentage of HbA1c or fasting and AUC glucose concentration during an MMTT, plasma interleukin-6 or overall CRP concentrations (appendix), or time to peak C-peptide response to an MMTT (data not shown). No participant achieved an insulin-free state with maintenance of a HbA1c percentage less than 7·5%.
A predefined subgroup analysis of treatment effect was done within the following variables: age, sex, ethnic origin, baseline C peptide, baseline body-mass index, insulin use, HbA1c, HLA type, and symptom duration. Only the interaction between treatment and the three categorised levels of C peptide was statistically significant (p=0·006; appendix).
The anakinra group reported significantly higher grades of adverse events than the placebo group (p=0·018, one-sided Wilcoxon rank sum test), primarily as a result of the higher frequency of grade 2 events in the anakinra group (table 5). Analysis of adverse events by category revealed that dermatological and skin events was the only category that was significantly different between treatment groups (p=0·017, one-sided Fisher’s exact test; table 6). This difference was attributable to 17 and four participants with injection site reactions from the anakinra and placebo groups, respectively (p=0·0009).
Table 5.
Adverse events by worst adverse effect grade experienced in the anakinra trial
| Anakinra (n=35) | Placebo (n=34) | |
|---|---|---|
| No adverse event | 7 (20%) | 13 (38%) |
| Grade | 1 16 (46%) | 16 (47%) |
| Grade 2 | 10 (29%) | 4 (12%) |
| Grade 3 | 2 (6%) | 1 (3%) |
| Grade 4 | 0 (0%) | 0 (0%) |
| Grade 5 | 0 (0%) | 0 (0%) |
Data are number of participants (%). Worst grade was statistically significantly different between treatment groups by a one-sided (alternative of higher frequency in anakinra group) Wilcoxon rank sum test (p=0·018). Some percentages do not total 100 because of rounding.
Table 6.
The number of events and participants by adverse events type in the anakinra trial
| Anakinra (n=35) |
Placebo (n=34) |
|||
|---|---|---|---|---|
| Number of events |
Number of participants (%) |
Number of events |
Number of participants (%) |
|
| Allergic or immunological | 3 | 3 (9%) | 1 | 1 (3%) |
| Auditory or ear | 0 | 0 (0%) | 2 | 2 (6%) |
| Blood or bone marrow | 3 | 2 (6%) | 0 | 0 (0%) |
| Constitutional symptoms | 1 | 1 (3%) | 3 | 2 (6%) |
| Dermatological or skin | 24 | 19 (54%) | 11 | 9 (26%) |
| Endocrine | 1 | 1 (3%) | 1 | 1 (3%) |
| Gastrointestinal | 7 | 6 (17%) | 3 | 3 (9%) |
| Infection | 17 | 11 (31%) | 12 | 8 (24%) |
| Lymphatics | 1 | 1 (3%) | 0 | 0 (0%) |
| Metabolic or laboratory | 3 | 1 (3%) | 0 | 0 (0%) |
| Musculoskeletal or soft tissue | 5 | 4 (11%) | 2 | 2 (6%) |
| Neurological | 2 | 2 (6%) | 1 | 1 (3%) |
| Ocular or visual | 3 | 2 (6%) | 1 | 1 (3%) |
| Pain | 14 | 7 (20%) | 10 | 7 (21%) |
| Pulmonary or upper respiratory | 3 | 3 (9%) | 4 | 3 (9%) |
| Renal or genitourinary | 1 | 1 (3%) | 0 | 0 (0%) |
| Sexual or reproductive function | 1 | 1 (3%) | 0 | 0 (0%) |
| Vascular | 1 | 1 (3%) | 0 | 0 (0%) |
| Total events | 90 | · · | 51 | · · |
Each adverse event category by treatment group was tested using a one-sided Fisher’s exact test. Only the difference between groups in the adverse event category dermatological or skin reached statistical significance (p=0·017). Of the 28 participants who had dermatological or skin reactions, 21 had injection-site reactions, 17 of whom were from the anakinra group (p=0·0009).
Discussion
Our results show that inhibition of interleukin-1β with canakinumab or anakinra did not slow β-cell decline in recent-onset type 1 diabetes. At the end of the two trials, stimulated C-peptide concentrations and percentages of HbA1c did not differ between intervention-treated and placebo-treated patients. Although both trials studied individuals with recent-onset type 1 diabetes, their entry criteria—particularly regarding age—and their trial design were different. Enrolment proceeded rapidly in the canakinumab study, probably because of the enthusiasm of younger patients and their parents for participation in clinical trials. The anakinra study had slow enrolment and was stopped owing to slow recruitment. The protocol was more demanding for patients in the anakinra study because daily injections were needed by the participants, whereas in the canakinumab trial there were monthly injections of drug at the clinical sites.
Several approaches to preserve β-cell function in new-onset type 1 diabetes have targeted different aspects of the autoimmune process with partial success (panel). Anti-CD3 monoclonal antibody4–7 and abatacept,8 both of which affect T lymphocytes, prevented β-cell decline for 6–12 months. Anti-CD20, which is directed against B lymphocytes, had a comparable effect.9 These attempts to alter the immune course of type 1 diabetes relied on single-drug therapy. In the cancer specialty, the advent of protocols employing combination therapy has been associated with dramatically improved outcomes. Similarly, to halt β-cell destruction and prevent type 1 diabetes, a rational combination of synergistic drugs directed against different aspects of the autoimmune process might be needed.
Although anti-interleukin-1β single-drug therapy did not prevent decline of β-cell function in these two trials, this approach might still be attractive as a component of combination therapy, both because of the part inter-leukin-1β plays in local β-cell inflammation and apoptosis11–15 and because interleukin-1β inhibition might help promote regulatory T-lymphocyte responses.16 In preclinical studies, although interleukin-1β inhibition alone did not affect C-peptide secretion in NOD mice, the combination of interleukin-1β blockade and anti-CD3 monoclonal antibody resulted in significantly greater clinical remission of diabetes than anti-CD3 monoclonal antibody alone.23
Future plans to use interleukin-1β blockade in combination therapy must take into consideration the timing of drug initiation. Once clinical type 1 diabetes is apparent, it might be too late for interleukin-1β blockade to significantly affect the disease course. Interleukin-1β gene expression in peripheral blood monocytes is high at initial diabetes diagnosis but normalises within 1 month.24 Furthermore, diabetes autoantibody-positive first-degree relatives of individuals with type 1 diabetes, known to be at high risk for future development of diabetes, have increased monocyte and dendritic cell expression of interleukin-1β.25 Thus, interleukin-1β inhibition might be more effective earlier, during the prediabetes period. If a combination therapy approach is used, careful attention will need to be paid to the potential side-effects of any drugs used, both individually and in combination.
In summary, as a single-drug therapy for new-onset type 1 diabetes, neither canakinumab nor anakinra slowed the decline in β-cell function in the two clinical trials. The drugs were generally well tolerated with no major safety issues emerging. These data, combined with NOD mouse-model evidence of synergy with other immunomodulatory drugs,23 suggest that interleukin-1β blockade might be more suitable for combination therapy protocols in new-onset type 1 diabetes or in prevention trials in individuals with pre-type 1 diabetes.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed for articles published up to Sept 1, 2012, with the search terms “immune intervention” and “type 1 diabetes”, “canakinumab”, and “anakinra”. Four randomised trials with adequate sample size showed some preservation of β-cell function in type 1 diabetes mellitus as evidenced by stimulated C-peptide secretion. These trials used anti-CD3,4–7 anti-CD20,9 and abatacept.8 An additional study suggested some efficacy with early treatment using GAD-65 antigen,20 but this was not supported by two subsequent trials.21,22 So far, there have been no randomised, placebo-controlled trials of interleukin-1 antagonism in patients with recent-onset type 1 diabetes.
Interpretation
Our results showed that inhibition of interleukin-1β with either canakinumab or anakinra did not slow the reduction in β-cell function in new-onset type 1 diabetes. Further study is needed to establish whether such treatment might be more effective in combination with other drugs or earlier in the course of type 1 diabetes (ie, in the prediabetes period).
Acknowledgments
The canakinumab trial was sponsored by the Type 1 Diabetes TrialNet Study Group. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061016, U01 DK061034, U01 DK061036, U01 DK061040, U01 DK061041, U01 DK061042, U01 DK061055, U01 DK061058, U01 DK084565, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085505, U01 DK085509, and contract HHSN267200800019C; the National Center for Research Resources, through Clinical Translational Science Awards UL1 RR024131, UL1 RR024139, UL1 RR024153, UL1 RR024975, UL1 RR024982, UL1 RR025744, UL1 RR025761, UL1 RR025780, UL1 RR029890, UL1 RR031986, and General Clinical Research Center Award M01 RR00400; the JDRF; and the American Diabetes Association. The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, JDRF, or the American Diabetes Association. Novartis (Basel, Switzerland) provided canakinumab (Ilaris), input regarding dosage, and other suggestions but had no direct involvement with study design, conduct, or management; data collection, analysis or interpretation; or manuscript preparation. There are no agreements concerning confidentiality of the canakinumab trial data between the sponsor and the authors or the institutions named in the credit lines. Roche Diagnostics provided blood glucose monitoring meters and strips to research participants in the canakinumab trial. The anakinra trial was funded by the JDRF international grants 17-2008-1804 and 8-2008-175 in addition to local support provided by individual study centres. Amgen (Thousand Oaks, California, USA) provided and distributed anakinra study drugs but had no direct involvement in the study design, conduct, or management; data collection, analysis or interpretation; or manuscript preparation.
Contributors
AM served as canakinumab study chair and wrote the first draft of the canakinumab section of this manuscript. AM, BBu, DJB, LAD, SEG, RG, CJG, KCH, JBM, PR, SS, DS, DKW, DMW, JPK, and JSS were involved in the conduct of the canakinumab study and the collection and review of study data. TM-P served as anakinra study chair and wrote the first draft of the anakinra section of this manuscript. LP, EdK, A-GZ, BBö, KB, NS, JFB, PP, DM, MYD, LC, AW, HHL, HP, and TM-P were involved in the conduct of the ankinra study and the collection and review of study data. JPK and BBu were involved in both studies. The other authors reviewed and commented on various versions of the paper, and suggested revisions.
The Type 1 Diabetes TrialNet Canakinumab Study Group investigators
Writing Committee: A Moran, B Bundy, C J Greenbaum, J P Krischer, S Sanda, and J S Skyler. Authors: Canada D K Wherrett; USA A Moran, B Bundy, D J Becker, L A DiMeglio, S E Gitelman, R Goland, C J Greenbaum, K C Herold, J B Marks, P Raskin, S Sanda, D Schatz, D M Wilson, J P Krischer, J S Skyler, and the Type 1 Diabetes TrialNet Canakinumab Study Group. Steering committee present members: Australia P Colman, J Wentworth (Walter and Eliza Hall Institute of Medical Research, Parkville, VIC); Canada D Wherrett (University of Toronto Toronto, ON); Finland O Simell (Hospital District of Southwest Finland, Turku); Italy E Bosi (San Raffaele Hospital, Milan), M G Roncarolo (San Raffaele Scientific Institute, Milan); UK P Bingley (University of Bristol, Bristol), M Peakman (Guy’s, King’s, and St Thomas’ School of Medicine, London); USA J S Skyler (Chairman; University of Miami Diabetes Research Institute, Miami, FL); M Anderson, S Gitelman (University of California San Francisco, San Francisco, CA), M Atkinson, M Clare-Salzler, D Schatz, W Winter (University of Florida, Gainesville, FL), K Bourcier (National Institute of Allergy and Infectious Diseases, Bethesda, MD), D Becker, M Trucco (University of Pittsburgh, Pittsburgh, PA), J Blum, L DiMeglio (Indiana University, Indiana, PA), J Buckner (Benaroya Research Institute, Seattle, WA), H P Chase, G S Eisenbarth*, P Gottlieb (University of Colorado Barbara Davis Center for Childhood Diabetes, Aurora, CO), C G Fathman, D M Wilson (Stanford University, Stanford, CA), R Goland (Columbia University, New York, NY), G Grave (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, MD), C Greenbaum (Benaroya Research Institute, Seattle, WA), B Hering, A Moran (University of Minnesota, Minneapolis, MN), K Herold, R Sherwin (Yale University, New Haven, CT), R Insel (Juvenile Diabetes Research Foundation, New York, NY), J P Krischer (University of South Florida, Tampa, FL), J Marks, A Pugliese (University of Miami Diabetes Research Institute, Miami, FL), J P Palmer (University of Washington, Seattle, WA), P Raskin, M Siegelman (University of Texas Southwestern Medical School, Dallas, TX), W Russell, J Thomas (Vanderbilt University, Nashville, TN), J Fradkin (ex-officio), E Leschek (ex-officio), L Spain (ex-officio), P Savage (NIDDK, Bethesda, MD). Steering committee past members: Australia L Harrison (Walter and Eliza Hall Institute of Medical Research, Parkville, VIC); Canada J Mahon (University of Western Ontario, London, ON); Finland K Nanto-Salonen (Hospital District of Southwest Finland, Turku); Germany A Ziegler (Helmholtz Center Munich Diabetes Research Group at the Technical University of Munich, Munich); USA C Benoist, T Orban (Joslin Diabetes Center, Boston, MA), J Bluestone (University of California San Francisco, San Francisco, CA), D Brown, J Wagner (University of Minnesota, Minneapolis, MN), C Cowie (NIDDK, Bethesda, MD), S Jordan (Cedars-Sinai Medical Center, Los Angeles, CA), F R Kaufman, R Parkman (Childrens Hospital Los Angeles, Los Angeles, CA), J M Lachin (George Washington University, Washington, DC), G Nepom (Benaroya Research Institute, Seattle, WA), M Pescovitz†, H Rodriguez (Indiana University, Indiana, PA), J Peyman, J Ridge (National Institute of Allergy and Infectious Diseases, Bethesda, MD). Executive committee present members: USA J S Skyler (University of Miami Diabetes Research Institute, Miami, FL), K Bourcier (National Institute of Allergy and Infectious Diseases, Bethesda, MD), C J Greenbaum (Benaroya Research Institute, Seattle, WA), J P Krischer (University of South Florida, Tampa, FL), E Leschek, P Savage, L Spain (NIDDK, Bethesda, MD), L Rafkin (University of Miami Diabetes Research Institute, Miami, FL). Executive committee past members: USA C Cowie, S Malozowski (NIDDK, Bethesda, MD), M Foulkes, H Krause-Steinrauf, J M Lachin, S J Zafonte (George Washington University, Washington, DC), J Peyman, J Ridge (National Institute of Allergy and Infectious Diseases, Bethesda, MD). Chairman’s office (University of Miami Diabetes Research Institute, Miami, FL): J S Skyler, C J Greenbaum, N S Kenyon, L Rafkin, I Santiago, J M Sosenko. TrialNet coordinating center (University of South Florida, Tampa, FL, USA) present members: J P Krischer, B Bundy, A L Ritzie, D M Amado, M Abbondondolo, T Adams, P Alies, F Badias, C Beam, M Boonstra, D Boulware, D Cuthbertson, C Eberhard, J Ford, J Ginem, H Guillette, B Hays, M Henry, P Law, C Linton, S Liu, J Lloyd, S Muller, R O’Donnell, Y Parrimon, K Paulus, J Pilger, J Ramiro, A Roberts, K Sadler, A Terry, M Wootten, P Xu, K Young. TrialNet coordinating center (University of South Florida, Tampa, FL, USA) past members: M Bassi, D Freeman, M Granger, M Kieffer, L Nallamshetty, A Shor. Data safety and monitoring board present members: USA E Blumberg (Chair; University of Pennsylvania, Philadelphia, PA); G Beck (Cleveland Clinic, Cleveland, OH), J Braun (University of California Los Angeles, Los Angeles, CA), D Brillon (Cornell University, Ithaca, NY), R Gubitosi-Klug (Case Western Reserve University, Cleveland, OH), L Laffel (Joslin Diabetes Center, Boston, MA), R Veatch (Georgetown University, Washington, DC), D Wallace (Research Triangle Institute, Durham, NC). Data safety and monitoring board past members: Canada B Zinman (University of Toronto, Toronto, ON); Denmark J Nerup (University of Copenhagen, Copenhagen); Sweden A Lernmark (Lund University, Lund); USA B Lo (University of California San Francisco, San Francisco, CA), H Mitchell (Rho, Chapel Hill, NC), A Naji (University of Pennsylvania, Philadelphia, PA), T Orchard (University of Pittsburgh, Pittsburgh, PA), M Steffes (University of Minnesota, Minneapolis, MN), A Tsiatis (North Carolina State University, Raleigh, NC). Infectious disease safety committee: USA B Loechelt (Medical Monitor; Children’s National Medical Center, Washington, DC), L Baden (Harvard University, Cambridge, MA), M Green (University of Pittsburgh, Pittsburgh, PA), A Weinberg (University of Colorado, Boulder, CO). Laboratory directors: USA S Babu, L Yu, G S Eisenbarth (University of Colorado Barbara Davis Center for Childhood Diabetes, Aurora, CO), S Marcovina, J P Palmer (University of Washington, Seattle, WA), A Weinberg (University of Colorado, Boulder, CO), W Winter (University of Florida, Gainesville, FL). Protocol committee: UK M Peakman (King’s College London, London); USA A Moran (Chair; University of Minnesota, Minneapolis, MN), C Greenbaum, S Sanda (Benaroya Research Institute, Seattle, WA), E Leschek, L Spain (NIDDK, Bethesda, MD), D Matheson (University of Miami Diabetes Research Institute, Miami, FL), M Pescovitz† (Indiana University, Indiana, PA), A Pugliese, J S Skyler, L Rafkin (University of Miami Diabetes Research Institute, Miami, FL). Clinical Center Staff involved in this protocol: Benaroya Research Institute (Seattle, WA, USA) C Greenbaum, J Bollyky, S Sanda, D Tridgell, M McCulloch-Olson, H Vendettuoli, D Hefty, M Ramey, C Webber, K Kuhns, N Hilderman, A Dove, M Hammond, J Klein, E Batts; Columbia University (New York, NY, USA) R Goland, E Greenberg, M P Gallagher, J Trast, M Chan, K Smith; Indiana University (Indianapolis, IN, USA) M Pescovitz†, L DiMeglio, C Evans-Molina, M Spall, J Terrell, R Hufferd, L Ford; Stanford University (Stanford, CA, USA) D M Wilson, B A Buckingham, T Aye, T Esrey, A Soto, B Baker, B Berry; University of California San Francisco (San Francisco, CA, USA) S E Gitelman, S M Rosenthal, M Anderson, S Adi, K Breen, C Hamilton; University of Florida (Gainesville, FL, USA) D Schatz, M Haller, M Clare-Salzler, M Atkinson, R Cook, D Mancini, A Abraham, J Ferguson, G Cole; University of Miami Diabetes Research Institute (Miami, FL, USA) J B Marks, A Pugliese, D Matheson, C Blaschke, L Arazo, R Arce, M Cisneros, B Acosta; University of Minnesota (Minneapolis, MN, USA) A Moran, B Nathan, J Wagner, B Hering, J Smith, A Street, J Leschyshyn, C Gibson, C Kwong; University of Pittsburgh (Pittsburgh, PA, USA) D Becker, I Libman, K Riley, K Delallo, N Gurtunca, M Trucco, B Copemen, B Elnyczky; University of Texas Southwestern Medical School (Dallas, TX, USA) P Raskin, J Ricahrd, E Roe, S Mirfakhraee, P White, M Alford, S Fernandez, J Arthur, M Hutchins, R Davis; University of Toronto (Toronto, ON, Canada) D K Wherrett, R Kovalakovska, L A Eisel, B Ahenkorah, M Sriskandarajah, M-J Ricci, J Cevallos, R Steger; Yale University (New Haven, CT, USA) K C Herold, L Feldman, R Sherwin. *Dr Eisenbarth died in November, 2012. †Dr Pescovitz died in December, 2010.
The AIDA Study Group investigators
Steering and writing committee: Denmark T Mandrup-Poulsen (Principal Investigator and trial sponsor; University of Copenhagen, Copenhagen), F Pociot (University Hospital of Glostrup, Glostrup); Switzerland M Y Donath (University Hospital of Basel, Basel); USA C A Dinarello (University of Colorado Denver-Anschutz Medical Campus, Aurora, CO). Data management unit (University of South Florida, Tampla, FL): USA J P Krischer (Principal Investigator), B Bundy (Biostatistician), D Cuthbertson (Biostatistician), S Bollepalli (Endocrinologist; Pediatrics Epidemiology Center), L Shanker (Coordinator), F Badias (Senior Applications Developer). Data and safety monitoring board: Finland M Knip (University of Helsinki, Helsinki); Sweden G Dahlquist (Bioethicist; University of Umeå, Umeå); UK E A M Gale (Chair; University of Bristol, Bristol), M Peakman (King’s College London, London). Central Laboratories: Steno Diabetes Center Central Laboratory (Gentofte, Denmark) M Frandsen (Head), H Niebling (Coordinating Technician), C Leth (Technician); University Hospital of Zurich Central Laboratory (Zurich, Switzerland) A von Eckardstein (Head of Institute of Clinical Chemistry), S Regenass (Laboratory Director Clinical Immunology), T Hornemann (Laboratory IT Coordinator), I Peereboom (Coordinating Technician), M Seiler (Coordinating Technician). Investigators (numbers in parentheses are screened/randomised participants, respectively, followed by local funding sources, if any): Denmark—Aalborg Hospital, Aalborg (1/1) H-H Lervang (Principal Investigator), M P Rasmussen (Site Coordinator), L Holm Pedersen (Technician); Aarhus University Hospital, Aarhus (7/4) J B Nielsen (Principal Investigator), J F Bak (Principal Investigator), J Rungby (Investigator); Bispebjerg University Hospital, Bispebjerg (1/1) H Perrild (Principal Investigator), A Frederiksen (Study Nurse); Steno Diabetes Center, Gentofte (4/3, Øresund Diabetes Academy, Novo Nordisk) T Mandrup-Poulsen (Principal Investigator), L Pickersgill (Central Site Coordinator), L Tarnow (Head, Clinical Research Unit), H U Andersen (Investigator), U Bjerre-Christensen (Investigator), B Hemmingsen (Study Nurse), H Foght (Central Site Technician), A Thyde (Central Site Administrator). Germany—German Diabetes Center, Düsseldorf (7/6) N Schloot (Principal Investigator), B Nowotny (Investigator), C Herder (Investigator), D Krog (Investigator), S Kahl (Investigator), P Heidkamp (Study Nurse); University of Frankfurt-am-Main, Frankfurt (8/6) K Badenhoop (Principal Investigator), G Meyer (Investigator), M Sandler (Site Coordinator), E Althaus (Study Nurse); Munich University of Technology, Forschergruppe Diabetes e.V., and Helmholtz Center Munich, Munich (16/13), Charlotte Fievet Foundation, Forschergruppe Diabetes e.V., and The German Center for Diabetes Research, Helmholtz Center Munich A-G Ziegler (Principal Investigator), D Wiesenäcker (Site Coordinator), M Wallner (Site Coordinator), E Giannopoulou (Site Coordinator), M Walter (Investigator), M Bunk (Study Nurse), M Herbst (Study Nurse); Ulm University, Ulm (14/10) B Böehm (Country Coordinator and Principal Investigator), S Rosinger (Site Coordinator), J Aufschild (Investigator), S Merger (Investigator), R Blagieva (Investigator). Italy—University Campus Bio-Medico, Rome (5/4) P Pozzilli (Principal Investigator), C Guglielmi (Site Coordinator), N Onori (Investigator), G Sironi (Investigator), A Soare (Investigator), L Valente (Technician). Netherlands—Leiden University Medical Center, Leiden (16/14) E de Koning (Principal Investigator and Coordinator), B Roep (Investigator), F Kleijwegt (Investigator), M Dijk (Study Nurse). Spain—Hospital Universitario Cruces, UPV/EHU, Ciberdem, Bilbao (3/1) L Castaño (Principal Investigator), F Vázquez (Site Coordinator), S Gaztambide (Investigator), J González Mielgo (Investigator), R M Axpe Pascual (Study Nurse); Hospital Universitario Insular de Gran Canaria, Las Palmas de Gran Canaria (1/1) A M Wägner (Country Coordinator and Principal Investigator), M del Pino Alberiche (Investigator), M A García Núñez (Investigator), D Marrero (Investigator), J Nóvoa (Investigator), A Diez (Investigator), L Martin (Study Nurse), J Aide (Study Nurse); Hospital Arnau de Vilanova, Lleida (3/3) D Mauricio (Principal Investigator), M Hernández Garcia (Site Coordinator), L Carrasco (Study Nurse), S Núria Garcia (Study Nurse). Switzerland—University Hospital Basel, Basel (3/2) M Donath (Principal Investigator), P Zala (Site Coordinator), E Seelig (Investigator), K Timper (Investigator).
Footnotes
Conflicts of interest AM has served on an advisory board for Pfizer and has received grants from Tolerx, Merck, and Osiris Therapeutics. SEG has served on an advisory board for Genentech. RG has received grants from Diamyd and Tolerx. CJG has received grants from Bayhill Therapeutics, Diamyd, and Tolerx. JBM has served on an advisory board for Amgen and has received research grants from Amylin, Biogen, Bristol-Myers Squibb, Diamyd, Eli Lilly, Genentech, Macrogenics, Roche, and Sanofi. PR has served on advisory boards for Amgen, AstraZeneca, and Novo Nordisk, has served on speakers bureaus for Novo Nordisk, and has received grants from Aegera, Andromeda Biotech, Astra Zeneca, Boehringer Ingelheim, Calibra Medical, Eli Lilly, Halozyme, Hoffman-LaRoche, Osiris Therapeutics, Pfizer, and Reata. DKW has received lecture fees from Eli Lilly and Medtronic. DMW has served on advisory boards for DexCom and Genentech and has received grant support from Genentech, Diamyd, and Osiris Therapeutics. JSS was on the Board of Directors for Amylin Pharmaceuticals, DexCom, and Moerae Matrix; served on advisory boards for SanofiDiabetes and Viacyte; and has received grants from Bayhill Therapeutics, Halozyme, Intuity, Mesoblast, and Osiris Therapeutics. NS is employed by Lilly, Germany; has received grants from Andromeda Biotech, Tolerx, and Roche; has served as a speaker for Novo Nordisk and Sanofi-Aventis; and has served on advisory boards for Boehringer Ingelheim, GlaxoSmith Kline, and Amdromeda Biotech. BBu, DJB, LAD, KCH, SS, DS, JPK, JFB, PP, DM, MYD, LC, AW, HHL, HP, and TM-P declare that they have no conflicts of interest.
References
- 1.Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53:426–33. doi: 10.2337/diabetes.53.2.426. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial: a randomised, controlled trial. Ann Intern Med. 1998;128:517–23. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW, Sibley S, Jackson M, Thomas W. Beta cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–36. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 4.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–98. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 5.Herold KC, Gitelman S, Greenbaum C, et al. Treatment of patients with new onset type 1 diabetes with a single course of ant-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132:166–73. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 7.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–23. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 8.Orban T, Bundy B, Becker DJ, et al. for the Type 1 Diabetes TrialNet Abatacept Study Group Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–19. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. for the Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–49. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandrup-Poulsen T, Pickersgill LMS, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:158–66. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 12.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–60. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandler S, Andersson A, Hellerstrom C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology. 1987;121:1424–31. doi: 10.1210/endo-121-4-1424. [DOI] [PubMed] [Google Scholar]
- 14.Spinas GA, Hansen BS, Linde S, et al. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987;30:474–80. doi: 10.1007/BF00279615. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Takane-Gyotoku N, Yuan X, Ichikawa E, Inada C, Nonaka K. Mouse islet cell lysis mediated by interleukin-1-induced Fas. Diabetologia. 1996;39:1306–12. doi: 10.1007/s001250050574. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanda S, Bollyky J, Standifer N, Nepom G, Hamerman JA, Greenbaum C. Short-term IL-1beta blockade reduces monocyte CD11b integrin expression in an IL-8 dependent fashion in patients with type 1 diabetes. Clin Immunol. 2010;136:170–73. doi: 10.1016/j.clim.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Sumpter KM, Adhikari S, Grishman EK, White PC. Preliminary studies related to anti-interleukin-1β therapy in children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2011;7:656–67. doi: 10.1111/j.1399-5448.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum CJ, Mandrup-Poulsen T, Friedenberg-McGee P, et al. for the Type 1 Diabetes TrialNet Research Group and the European C-peptide Trial Study Group Randomized comparisons of the mixed meal tolerance test versus the glucagon stimulation test for the assessment of beta cell function in type 1 diabetes. Diabetes Care. 2008;31:1966–71. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludvigsson J, Faresjo M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–20. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 21.Wherrett DK, Bundy B, Becker DJ, et al. for the Type 1 Diabetes TrialNet GAD Study Group Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–27. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–42. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 23.Ablamunits V, Henegariu O, Bondo Hansen J, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade. Diabetes. 2012;61:145–54. doi: 10.2337/db11-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White P. Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab. 2007;92:3705–11. doi: 10.1210/jc.2007-0979. [DOI] [PubMed] [Google Scholar]
- 25.Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D. Dysregulated toll like receptor induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1. Diabetes. 2012;61:2525–33. doi: 10.2337/db12-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.