Abstract
Background
Osteochondral autografts and allografts require mechanical force for proper graft placement into the defect site; however, impaction compromises the tissue. This study aimed to determine the effect of impaction force and number of hits to seat the graft on cartilage integrity.
Hypothesis
Under constant impulse conditions, higher impaction load magnitudes are more detrimental to cell viability, matrix integrity and collagen network organization and will result in proteoglycan loss and nitric oxide release.
Study Design
Controlled laboratory study
Methods
Osteochondral explants, harvested from fresh bovine trochleas, were exposed to a series of consistent impact loads delivered by a pneumatically driven device. Each plug received the same overall impulse of 7 Ns, reflecting the mean of 23 clinically inserted plugs. Impaction loads of 37.5N, 75N, 150N, and 300N were matched with 74, 37, 21, and 11 hits respectively. Following impaction, the plugs were harvested and cartilage was analyzed for cell viability, histology by safranin-o and picosirius red, and release of sulfated glycosaminoglycans and nitric oxide. Data were compared with non-impacted control.
Results
Impacted plugs had significantly lower cell viability than non-impacted plugs. A dose response relationship in loss of cell viability with respect to load magnitude was seen immediately and after 4 days but lost after 8 days. Histologic analysis revealed intact cartilage surface in all samples (loaded or control), with loaded samples showing alterations in birefringence. While the sulfated GAG release was similar across varying impaction loads, release of nitric oxide increased with increasing impaction magnitudes and time.
Conclusions
Impaction loading parameters have a direct effect on the time course of the viability of the cartilage in the graft tissue.
Clinical Relevance
Optimal loading parameters for surgical impaction of osteochondral grafts are those with lower load magnitudes and a greater number of hits to ensure proper fit.
Keywords: impaction, loading, articular cartilage, viability, allograft, osteochondral defect
Introduction
Full-thickness articular cartilage lesions of the knee can cause significant pain and reductions in function in otherwise healthy individuals. Due to the avascular nature of cartilage, it has a limited ability to repair itself6, 29 and, such chondral defects may progress and lead to degenerative arthritis.16, 25, 35, 39 Because of these sequellae, various cartilage repair procedures have been developed.1, 2 Among those, osteochondral grafting (autograft and allograft) have garnered significant attention because of their ability to replace the lesion with true hyaline cartilage and allow for a relatively short recovery period.9, 14, 28
In the clinical setting, osteochondral plugs are impacted multiple times as they are seated into the defect area. Damage to the chondrocytes during impaction is a concern since excessive levels of mechanical load can generate chondrocyte death and matrix damage.11, 13, 15, 32, 33, 38 In a previous study,31 our laboratory demonstrated that surgical impaction of bovine osteochondral plugs with varying loads leads to significantly decreased cell viability compared to control plugs. Borazjani et al.4 reported similar data for adult human tissue. In their experiment a total of 24 grafts were implanted with standard impaction techniques causing cell death especially in the superficial zone. The authors characterized the biomechanics of the graft insertion on 10 grafts and found the applied loads and impaction taps quite variable: a nearly 50% variation in maximum load and hits were observed during insertion, requiring a total impulse of 6.2 Ns until the plugs were fully seated. In classical mechanics, the impulse defines the impact intensity, which is the integral of applied load over time. Hence, the same impulse can be reached by either increasing load and shortening time or, vice versa, by reducing load and increasing time. In other words, the surgeon can hit the plug harder with less hits or softer requiring more hits. It is unknown whether there is an advantage of one or the other in preventing osteochondral graft damage.
The purpose of this study is to use a controlled mechanical setting to determine the optimal loading parameters for satisfactory seating of an osteochondral plug in an in vitro model based on clinically relevant impulse, load, and number of impaction hits. We hypothesized that (under constant impulse conditions) higher load magnitudes will be detrimental to cell viability, matrix integrity, and collagen network organization and will result in proteoglycan loss and nitric oxide release.
Materials & Methods
Characterization of Plug Seating
Tissue Retrieval
Fresh bovine stifle joints were obtained from 6 to 8 month old steers at a local abattoir within 1 hour of sacrifice and wrapped in sterile wet gauze to prevent drying. Harvest of the osteochondral plugs from the trochlea occurred within 3 hours of obtaining the stifle joints. The samples were removed using a sterile technique, using the Arthrex OATS system (Naples, FL). Each plug was 8mm in diameter and the subchondral bone was cut so the entire depth of the plug was 10mm.
Plug Seating
To determine the necessary impulse for satisfactory seating of the 8 mm diameter osteochondral plug into the femoral condyle, 23 plugs were taken from 6 fresh bovine trochleas. A senior orthopedic surgeon (BJC) then inserted the plugs into medial femoral condyle recipient holes, separated by at least 5mm, by first press-fitting them by hand followed by impaction with a plastic tamp device and surgical mallet. Using donor plugs from the trochlea with subsequent implantation into the femoral condyle has been established in clinical practice2 as well as animal models.21 As in our earlier investigation,31 the tamp device was modified with a 1kN load cell (Entran Devices Inc., Fairfield, NJ; full scale error < 0.41%) to record the applied force profile during impaction for each plug at a sampling frequency of 1.5kHz. Subsequently, the overall impulse for each plug was calculated by taking the integral of the loading profile over time until the plug was completely seated.
Surgical Implantation Characterization
The overall impulse was 6.98 ± 5.10Ns (mean ± SD) to insert a single plug. During insertion the impaction force ranged from 49N to 154N, averaging 84N ± 33N. An earlier study using a similar experimental set-up found loads as high as 307N.31 Thus, for the controlled mechanical impaction study, we chose reduplicating load levels at 37.5N, 75N, 150N, and 300N as representative values for the impaction range used by a surgeon. These loads relate to nominal stress levels of 0.75MPa, 1.5MPa, 3.0MPa, and 6.0MPa, respectively, after dividing the loads by the surface area of the cartilage plug. In order to keep the determined overall impulse of 7Ns constant, the number of hits for each of the above load levels was adjusted to 74, 37, 21, and 11, respectively. As expected, the load magnitude was linked to the loading rate. The loading rates were 9.1kN/s, 25.3kN/s, 35.9kN/s, and 62.4kN/s, respectively.
Controlled Mechanical Impaction
Impaction
One hundred and fifty osteochondral plugs (from 10 pairs of bovine knees), harvested as described above, were used for controlled mechanical impaction. A block randomization code was used to distribute the 15 trochlear plugs removed per animal (left and right knee) into a load and time point matrix. A pneumatic impaction device (SmartImpactorTM, Chicago, IL) was used to deliver consistent loads and loading rates. Loading groups were formed as determined by the characterization of plug seating, described above. The load levels/hits in these four groups were as follows: 37.5N/#74; 75N/#37; 150N/#21; 300N/#11 The average coefficient of variation of the attempted load levels was 3%.19 In addition, a control group undergoing the same protocol but receiving no impaction (“0N”) was included.
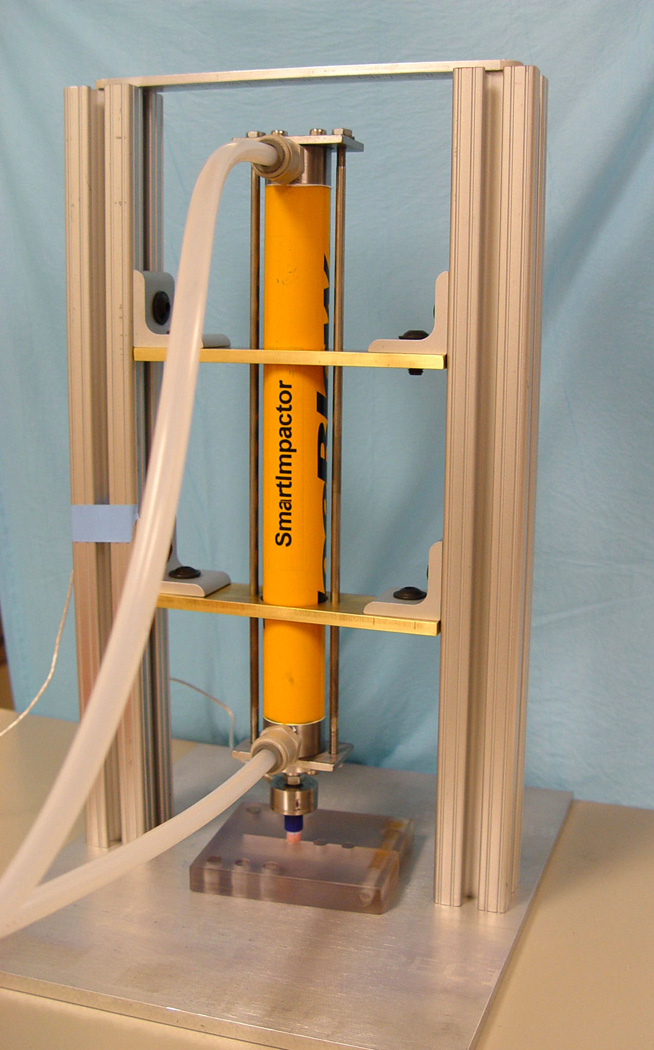
A custom-made plug clamp was used to hold the trochlear plugs in quasi-unconfined compression (Figure 1). Plugs were then impacted based upon preliminary data, applying the average number of hits at each impact magnitude necessary to seat the graft. The distal end of the impaction device was fitted with a load cell as well as the end-piece of a plastic surgical tamp, which allowed it to simulate the impaction interface in the clinical situation. The impaction device was held free-floating in a custom-built aluminum frame so that it could deliver impacts that were flush with the articular cartilage surface. Thirty plugs were assigned to each of the four loading groups as well as control (non-impacted) group and used for three endpoints (i.e. 10 plugs per Day 0, 4, and 8).
Figure 1.
Overview (left) and close-up (right) of the pneumatic impaction device (SmartImpactorTM, Chicago, IL) used to impact osteochondral plugs with consistent loads.
Tissue Culturing
Subsequent to impaction, bone was separated from the cartilage with a scalpel. Full-thickness plugs of articular cartilage were cultured in 1mL of minimal essential media (MEM) with 10% fetal bovine serum (FBS), glutamate, non-essential amino acids, anti-fungal, and anti-microbial agents at 37°C for 4 or 8 days. Media was changed every three days. For the Day 4 group, media was changed and analyzed at days three and four. For the Day 8 group, media was changed and analyzed at days three, six, and eight. Media removed from culture was immediately stored at −80°C until analysis.
Endpoint Analyses
Samples were analyzed, blinded to treatment group, immediately after impaction (Day 0), and at Day 4 and 8 of culture. Changes in cell viability, matrix integrity and collagen network organization, and release of nitric oxide and proteoglycan were studied using the following protocols:
Cell Viability
A 1-mm wide full-thickness section of cartilage taken near the center of the plug was stained for cell viability with placed in 4 µM calcein-AM and 8 µM ethidium homodimer (Molecular Probes Inc, Portland, OR) and imaged on a confocal laser-scanning microscope (Nikon E200, Melville, NY). Digital images were captured using Metamorph and Image J (Version 1.34, National Institutes of Health, USA), and the cell viability ratio, defined as live to total (live + dead) cell count, was calculated for the entire image. To identify potential zonal variations, each full-thickness cartilage specimen was further examined at different quartile depths from the articular surface as described previously for Live/Dead analyses.23 Thus, the four regions were Zone 1 (first quartile), Zone 2 (second quartile), Zone 3 (third quartile), and Zone 4 (fourth quartile). Adobe Photoshop 6.0 (Adobe Systems Inc., USA) was used to divide the cartilage into four zones of equal depth. As the average cartilage thickness was 2.7 ± 0.6mm, each region was approximately 0.7mm deep.
Histology
Cartilage samples were fixed in 10% neutral buffered formalin containing 0.5% cetylpyridinium chloride and processed for paraffin embedding. Full-thickness specimens were sectioned (8µm) and stained with Safranin-O.34 Samples were graded by the modified Mankin scale37 to evaluate structure, cellularity, and safranin-O staining, as a marker for proteoglycan content. In addition, cartilage specimens were also analyzed for changes in the collagen network using picrosirius red40. Sections were analyzed with a Nikon Microphot-FXA (Nikon Inc., Garden City, NY) microscope equipped with a polarization filter. The relative sign of induced birefringence was determined by turning the analyzer in two opposite directions. The optical properties of the tissue sample (i.e., the presence or absence of birefringence) indicating orientation of the collagen fibers8, 17 were recorded using Metamorph software. Sections from experimental and control groups were processed and stained concurrently to control for any variations in uptake of the stain.
Nitric Oxide (NO) Release
The NO present in each of the media samples was assayed using a Nitrate/Nitrite Colorimetric Assay Kit (LDH Method) by Cayman Chemical (Ann Arbor, MI). The absorbance of each sample was measured at 530nm. Once the Nitrate+Nitrite concentration (µM) of each of the media samples was determined, concentrations were cumulated such that the cumulative concentration for Day 4 equals day three plus day four and the cumulative concentration for Day 8 equals day three plus day six plus day eight. All samples were normalized to the percentage of live cells.
Sulfated Glycosaminoglycan Release
Sulfated GAG content was detected by a colorimetric method employing DMMB as previously described7 with nasal bovine serum D1 PG as the standard. Once the sGAG concentration (µg/ml) of each of the media samples was determined, concentrations were cumulated such that the cumulative concentration for Day 4 equals day three plus day four and the cumulative concentration for Day 8 equals day three plus day six plus day eight.
Data Analysis
If not stated differently, data are presented as mean ± standard deviation. Extreme outliers in cell viability as identified using Boxplots and confirmed using Grubbs’ test (90% confidence interval) were not included into statistical analysis. After confirming normality, the effects of varying load levels were assessed cross-sectionally (comparison of loads at one time point) and longitudinally (comparison of time points at one specified load) by a one-way ANOVA and post hoc with a Newman-Keuls Multiple Comparison test. Release of NO and sGAG was analyzed non-parametrically by Kruskal-Wallis with post hoc Dunn’s Multiple Comparison test. Modified Mankin scores were analyzed using Kruskal-Wallis test. Significance was set to p≤0.05. Statistical analyses were conducted using GraphPad Prism 5.0 (La Jolla, CA) and SPSS v.11.5 (SPSS, Inc., Chicago, IL).
Results
Chondrocyte Viability after controlled impaction
At each time point, control plugs showed a higher cell viability ratio compared with each of the four loaded plugs (p<0.05) (Table 1). Even though the same impulse was applied to every plug within the loaded groups, differences were obvious. In addition, Day 4 showed higher cell viability with 37.5N compared to 300N (p<0.05). Interestingly, at Day 4 and even more obviously at Day 8, these differences leveled out and all loaded plugs displayed a similar low viability ratio of approximately 20%. (Table 1)
Table 1.
Summary table of cell viability ratios (live cells/total cells) showing significant differences between load levels (shown at bottom of chart) and between time points (right side of table). Data are displayed as means ± standard deviation.
| Cell Viability Ratio (Live/Total) | Longitudinal Comparison |
Significance | |||
|---|---|---|---|---|---|
| Day 0 | Day 4 | Day 8 | |||
| 0N | 0.816 ± 0.102 | 0.633 ± 0.203 | 0.643 ± 0.218 | Day 0 vs Day 4 | ns |
| Day 0 vs Day 8 | ns | ||||
| Day 4 vs Day 8 | ns | ||||
| 37.5N | 0.592* ± 0.176 | 0.417* ± 0.135 | 0.201* ± 0.134 | Day 0 vs Day 4 | <0.05 |
| Day 0 vs Day 8 | <0.05 | ||||
| Day 4 vs Day 8 | <0.05 | ||||
| 75N | 0.504* ± 0.186 | 0.341* ± 0.185 | 0.198* ± 0.116 | Day 0 vs Day 4 | <0.05 |
| Day 0 vs Day 8 | <0.05 | ||||
| Day 4 vs Day 8 | ns | ||||
| 150N | 0.521* ± 0.167 | 0.264* ± 0.121 | 0.192* ± 0.115 | Day 0 vs Day 4 | <0.05 |
| Day 0 vs Day 8 | <0.05 | ||||
| Day 4 vs Day 8 | ns | ||||
| 300N | 0.402* ± 0.172 | 0.167*# ± 0.144 | 0.162* ± 0.084 | Day 0 vs Day 4 | <0.05 |
| Day 0 vs Day 8 | <0.05 | ||||
| Day 4 vs Day 8 | ns | ||||
| Cross-sectional Comparison | * p<0.05 vs 0N | * p<0.05 vs 0N | * p<0.05 vs 0N | ||
| # p<0/05 vs 37.5N | |||||
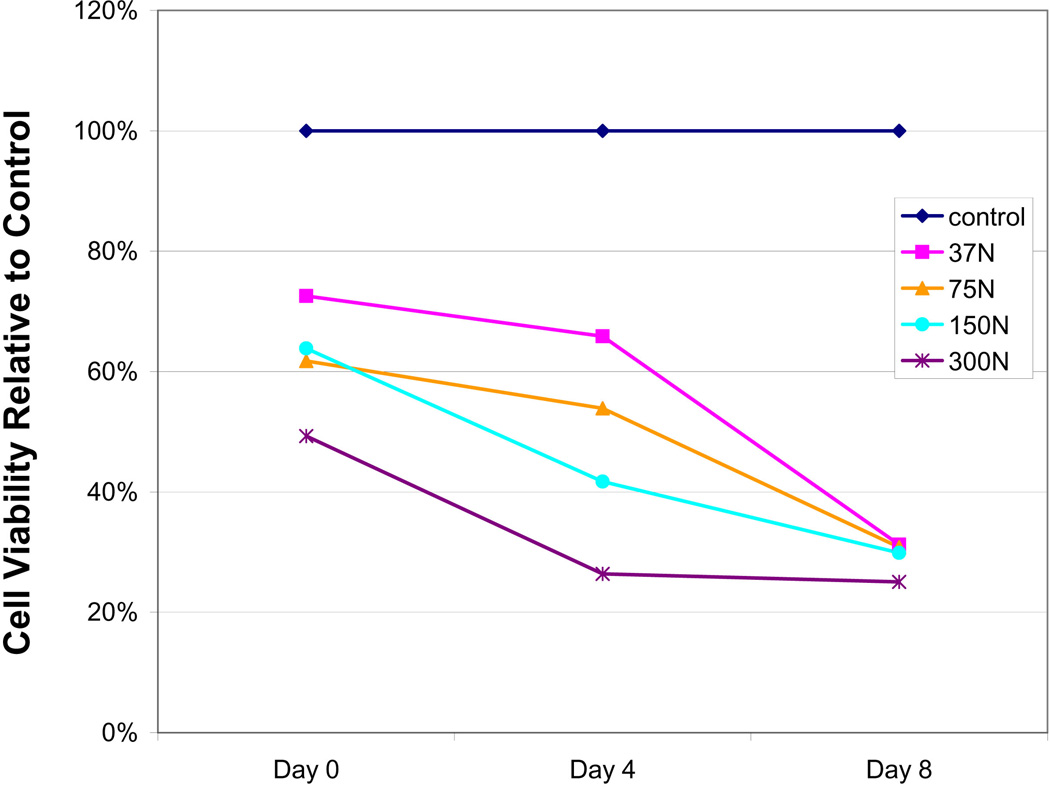
The control group showed no significant changes in cell viability for the investigated time period (p>0.05 for all time points), although average cell viability diminished to some extent over time. A longitudinal effect, however, was seen for all loaded groups revealing significant differences between time points. In particular, all Day 0 to Day 4 and/or Day 8 comparisons were significant (p<0.05). Day 4 to Day 8 comparisons were not significant except for the 37.5 N group. (Table 1). The longitudinal characteristics of impaction loading are graphically displayed in Figure 2, by normalizing the data of Table 1 to non-impacted control. Over time, irrespectively of the applied load level, cell viability aspired to a 25–30% threshold value.
Figure 2.
Graphical depiction of mean cell viability at different loading levels over time. To highlight the effects of load, cell viability ratios were normalized to control samples (Table 1).
Initially, cell death primarily manifested in superficial regions, and later spread into deeper zones as can be seen qualitatively in Figure 3. A more detailed quantitative analysis at different quartile depths from the articular surface confirmed the qualitative perspective on the zonal variation in cell viability (Table 2): At Day 0, Zone 1 displayed the lowest cell viability ratios of all impacted samples. Over time cell death spread and this difference became less prevalent. Table 2 summarizes the cell viability ratio within each region and details cross-sectional as well as longitudinal differences between zones. Interestingly, the highest rate of cell death was observed for Zone 3 between Days 0 and 4 for all load levels.
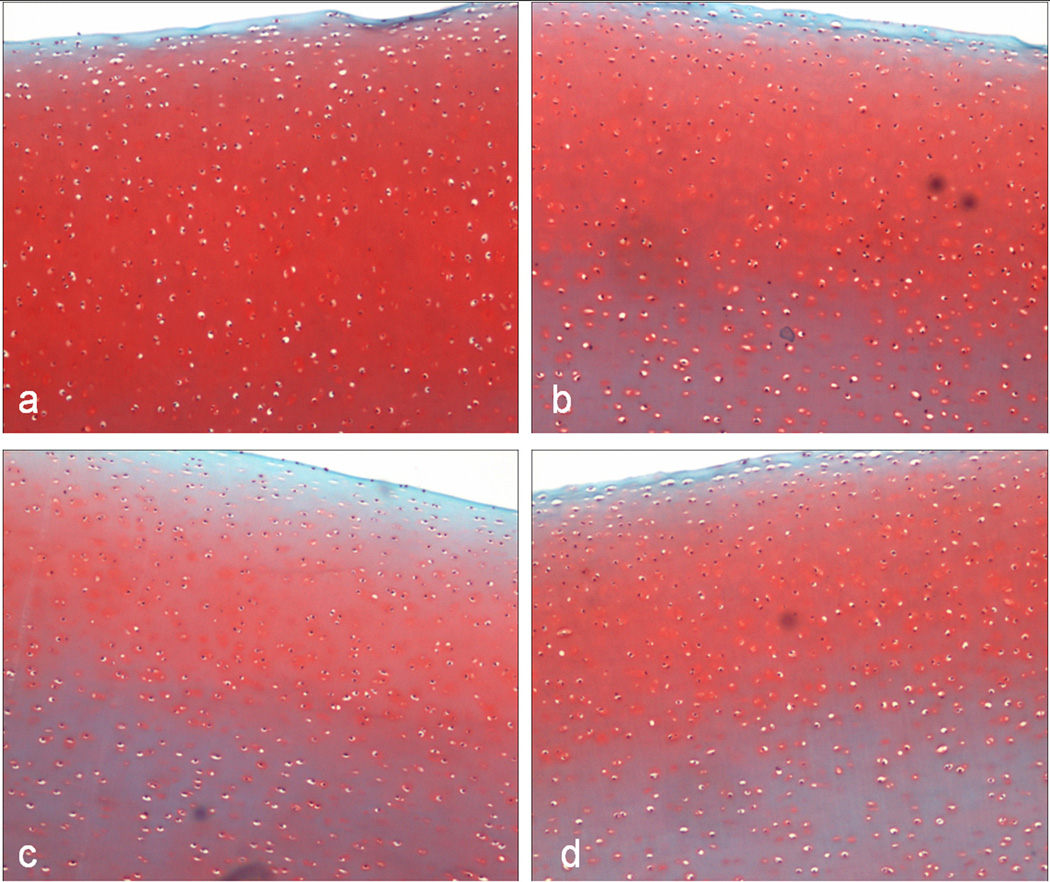
Figure 3.
(A) Cell viability at Day 0 for different load levels: (a) control; (b) 75N; (c) 150N; and (d) 300N. Note the increasing spread of cell death into deeper tissue levels.
Table 2.
Summary of zonal variation in cell viability ratio (Mean + SD).
| Day 0 | Day 4 | Day 8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 1 | Zone 2 | Zone 3 | Zone 4 |
| Control | 0.63 ± 0.22 | 0.83 ± 0.15 | 0.91a ± 0.05 | 0.86 ± 0.15 | 0.56 ± 0.20 | 0.76 ± 0.18 | 0.68 ± 0.26 | 0.65 ± 0.25 | 0.48 ± 0.27 | 0.61 ± 0.39 | 0.46c ± 0.23 | 0.45c ± 0.27 |
| 37.5 N | 0.51 ± 0.17 | 0.61*,a ± 0.25 | 0.71*,a ± 0.18 | 0.64* ± 0.23 | 0.39 ± 0.21 | 0.37* ± 0.23 | 0.36* ± 0.31 | 0.39 ± 0.32 | 0.26c ± 0.19 | 0.19*,c ± 0.19 | 0.28c ± 0.24 | 0.31c ± 0.24 |
| 75 N | 0.37* ± 0.10 | 0.48* ± 0.22 | 0.66*,a ± 0.26 | 0.63* ± 0.25 | 0.29* ± 0.19 | 0.39* ± 0.21 | 0.33* ± 0.23 | 0.48 ± 0.27 | 0.23 ± 0.19 | 0.24*,c ± 0.20 | 0.30c ± 0.24 | 0.35c ± 0.26 |
| 150 N | 0.44 ± 0.20 | 0.60*,a ± 0.25 | 0.73*,a ± 0.21 | 0.72a ± 0.25 | 0.32* ± 0.25 | 0.19* ± 0.19 | 0.21* ± 0.33 | 0.26* ± 0.33 | 0.16*,c ± 0.11 | 0.22*,c ± 0.14 | 0.24c ± 0.20 | 0.16*,c ± 0.20 |
| 300 N | 0.27* ± 0.14 | 0.46* ± 0.25 | 0.63*,a ± 0.24 | 0.61* ± 0.31 | 0.24* ± 0.17 | 0.35* ± 0.31 | 0.28* ± 0.32 | 0.35 ± 0.38 | 0.14* ± 0.13 | 0.18*,c ± 0.22 | 0.23c ± 0.17 | 0.31 ± 0.22 |
represents significant differences (p < 0.05) for a loaded sample versus control in a given day and zone.
a, b, and c represent significant differences (p < 0.05) for Day 0 versus Day 4, Day 4 versus Day 8, and Day 0 versus Day 8, respectively.
Histology – Safranin-O Staining
At both Day 4 and Day 8, the articular surface of all samples was smooth and intact with the superficial layer devoid of Safranin-O staining. Loaded samples had an area of reduced staining extended from the articular surface to the outer region of the radial layer. These effects appeared to be more pronounced with rising load levels (Figure 4), but proved insignificant when analyzed quantitatively. Modified Mankin scores37 ranged from 0 to 1.5 and showed no differences in structure, cellularity, and safranin-O staining at different loads or time points.
Figure 4.
Safranin-O stained sections of cartilage samples at Day 8. (a) Control; (b) 37.5N. Notice reduced staining down to the radial zone; (c) 150N. Notice reduced staining extending to the radial zone, as well as pericellular loss of staining throughout the entire depth of cartilage; (d) 300N. Note the cellularity in addition to reduced staining.
Picrosirius Red Staining
As expected for the control samples, a thin layer of the superficial tangential zone demonstrated a bright birefringence with an abrupt change to no birefringence in the middle layer of the cartilage plug. This is coherent with the orientation change of the collagen fibers in this transitional zone.36, 41 At 37.5N approximately half of the samples exhibited alterations towards birefringence of the normally non-birefringent middle layer indicating a disturbance of the collagen orientation. This was a consistent finding for all impacted samples and appeared to increase in proportion with higher load levels. Findings were similar for Day 4 and 8.
Nitric Oxide release
Nitric oxide (NO) release was affected by load level and time (Table 3). At Day 4 cumulative NO release was significantly higher (p<0.05) for the 300N group compared with control and all other load levels. The control group displayed the lowest value but was not significantly different from the 37.5, 75, or 150N. Similarly, at Day 8 cumulative NO release was significantly higher (p<0.05) for the 300N group compared to control and 75N. Although only significant at 37.5N and 150N, at Day 8, all loaded samples had an NO release increased by a factor 2 to 3 compared to Day 4.
Table 3.
Cumulative NO release in response to varying impaction loads. Presented as normalized mean (nitrate + nitrite (uM) / live/total cells) ± standard deviation.
| Total Nitrate+Nitrite (uM) / (live/total cells) | Longitudinal Comparison |
Significance | ||
|---|---|---|---|---|
| Day 4 | Day 8 | |||
| 0N | 72.6* ± 57.5 | 133.9* ± 69.5 | Day 4 vs Day 8 | ns |
| 37.5N | 120.8* ± 54.2 | 518.3 ± 349.3 | Day 4 vs Day 8 | <0.05 |
| 75N | 162.9* ± 110.0 | 306.2* ± 143.3 | Day 4 vs Day 8 | ns |
| 150N | 153.9* ± 68.4 | 500.7 ± 154.3 | Day 4 vs Day 8 | <0.05 |
| 300N | 454.5 ± 247.8 | 1050.3± 624.4 | Day 4 vs Day 8 | ns |
| Cross-sectional Comparison | * p<0.05 vs 300N | * p<0.05 vs 300N | ||
Sulfated Glycosaminoglycans
Sulfated GAG release was similar across varying impaction loads (including control) and ranged from 1.1 to 1.3 µg/ml for Day 4 and 1.4 to 1.7 µg/ml for Day 8. No differences were noted.
Discussion
This study analyzed the biologic effects of applying controlled mechanical loads, with a force and impulse range consistent with that of surgical implantation, to bovine osteochondral explants. Assuming safe and constant press-fit conditions the variables ‘load magnitude’ and ‘number of hits’ are dependent on each other and are related via the necessary impulse for plug insertion. While loading in general was detrimental to the tissue, only the highest load level (300N) demonstrated significant effects for most endpoints. However, load magnitude seemed to affect cell viability and the time course of tissue degradation, in particular cell death which showed a slightly different delineation of cell viability although ultimately (Day 8) all samples, irrespective of load, had similar cell viability. While higher loads appeared to accelerate this process, the final cell viability level could be governed by the applied impulse. A recent study by Patil et al,30 where osteochondral grafting was performed in 4 fresh-frozen cadaver knees, came to similar conclusions: as long as the load levels were held in typical limits, the decline in cell viability with load after 5 days was not significant.
Currently, the exact threshold for the minimum amount of chondrocyte viability needed to maintain the extracellular matrix over time is not known. Kandel et al18 examined fresh osteochondral grafts retrieved after clinical failure, years after implantation, and found viable chondrocytes along with intact extracellular matrix architecture in most specimens. However, a retrieval study by Enneking and Campanacci12 found that frozen osteochondral allografts (devoid of viable chondrocytes) were only able to maintain an intact cartilage matrix for 2–3 years. Beyond this period, degeneration of the matrix was apparent. Thus, although the extracellular matrix can maintain its integrity over the intermediate-term (2–3 years), long-term survival is dependent on the viability of the chondrocytes and their ability to sustain the matrix.
The bovine tissue model of this study exaggerates the loss in cell viability. It is known that bovine chondrocytes of young animals react sensitively to applied load level.22 Hence, as anticipated, we found a higher amount of cell death than in the study of Borazjani et al4 who used human tissue (despite of a similar impaction impulse). Clinically, the exact survival rate of transplanted chondrocytes is not well defined. Czitrom10 reported on 4 cases where viability of articular cartilage was assessed after clinical transplantation of fresh osteochondral allografts. Biopsies of these grafts at 12, 24, and 41 months after transplantation yielded cell viability that ranged from 69 to 99%, but the viability decreased to 37% for a graft 6 years post-transplantation. In a goat model, Lane et al21 found that cell viability in transplanted osteochondral grafts was approximately 85% after a 6 month survival period. Although these findings are encouraging, one must note that there were only 4 cases in the Czitrom study and 3 cases in the Lane study and the loads during impaction were not measured. Additionally, the fact that the viabilities were not closer to 100% implies that there was some damage incurred either in the implantation or procurement process.
Similar to previous studies showing load dependent chondrocyte death with impaction under unconfined compression.13, 20, 32, 33, 38 our study demonstrated a dose response relationship in loss of cell viability with respect to load magnitude. This association is seen to an extent on Days 0 and 4, but is lost on Day 8. The prevailing explanation for these events is that, in addition to the initial necrosis induced by impaction, there is an apoptotic response that begins immediately after impaction and takes several days to become significant. Such a reasoning is supported by the NO data.. D’Lima et al11 have found that a major mechanism of cell death in human cartilage impacted in unconfined compression with 14 MPa was apoptosis, which can be inhibited in part by caspase inhibitors. It has been shown that apoptosis is accelerated with higher loads,24 which may explain the load dependent rates of cell viability loss for the different loads in our study. Loening et al24 noted approximately 60% positive staining for apoptosis with stresses of 4.5 MPa. Protein and gene analyses may be warranted to understand the exact mechanism behind this phenomenon.
We also found that the loss of cell viability progressed into deeper zones of the cartilage with higher impaction loads. Our approach to studying cell viability by zones is similar to that used by Lewis et al,23 who studied the effect of single impactions and found that the cell viability was only statistically significant between impacted and control plugs in the most superficial zone (Zone 1), with the impacted viable cell density being 63% of the controls. In our study, we also demonstrated zonal variation with a significantly lower viability in the superficial region (Zone 1) than in the deeper regions. We also found that the loss of cell viability progressed into deeper zones of the cartilage with higher impaction loads. In a different study using trochlear bovine explants and stresses ranging from 10 to 60 MPa, Milentijevic et al26 described a linear association between depth of cell death and increasing peak stress.
The reduced viability of the superficial zone in control plugs, a finding also reported previously,3 is likely secondary to desiccation during the plug procurement process. This phenomenon can also be expected in the impacted plugs, but plays a minor role when compared to the contribution of load impaction. The large amounts of viability loss in Zones 3 and 4 for the control group with time are not fully understood, but may be secondary to the finite amount of nutrients available in culture. In the native joint, synovial fluid is continuously replenished to maintain a certain level of available nutrients to the cartilage. As the chondrocytes in the deeper zones of cartilage have a higher metabolic rate than the chondrocytes in the superficial zone, the nutritional needs of the deep zone chondrocytes are greater than the superficial zones. Thus, in a culture system with limited amounts of nutrients, we expect larger losses in the deeper zones than the more superficial zones.
Our histologic study did not reveal any apparent association between cartilage fibrillation and matrix disruption with the amount of load applied. These findings are supported by the sulfated GAG release, which were similar between all load levels. Nam et al28 also found that osteochondral transplants in rabbit femoral condyles had near normal histologic scores after 6 and 12 weeks of survival. Safranin-O staining for presence of matrix proteoglycans demonstrated a loss of staining with impacted plugs. The loss of staining was consistent, irrespective of load magnitude, and extended down to the radial zone. We also found decreased pericellular staining with load, which is indicative of chondrocytic chondrolysis. A reduction in cellularity was seen with increasing load, which does demonstrate some level of load magnitude dependency. These findings are consistent with Borrelli et al5 who found decreased staining intensity in cartilage impacted with 35 MPa of stress. Loening et al24 found increased glycosaminoglycan loss with stresses of at least 12.8 MPa. They have attributed this loss to collagen network damage secondary to injurious compression.
We conducted picrosirius red staining to evaluate for any alterations in the collagen architecture. We generally found an increase in the birefringence of the middle zones of the loaded specimens. This zone usually lacks birefringence under polarized light because of the oblique orientation of the collagen fibers in this zone. It is plausible that the impactions led to damage to the matrix including glycosaminoglycan loss as well as changes in the collagen architecture. The collagen architecture may also be altered indirectly as the interactions between the collagen and proteoglycans in the matrix help define the overall structure of the cartilage. Thus, loss of proteoglycans may also potentiate the changes seen in the collagen orientation. The changes we observed were more prevalent with increasing load magnitudes as well as with longer culture times.
Differences in NO release at Day 4 were insignificant except for the 300N load level, which showed a greater release of NO when compared to control. At Day 8, all load levels displayed increased NO levels compared to control. These findings may be explained by the fact that higher loads led to more immediate damage, while lower load levels initiated a slower process of cell death occurring over time, attributable to apoptosis.24
There were several limitations to our study. Because of the difficulty of using conventional material testing machines to deliver consistent maximal loads, we have developed a pneumatically controlled impaction device (SmartImpactorTM, Chicago, IL) for this purpose. The impaction device was not set up to measure displacement. Because of this limitation, we were not able to measure strain, and therefore conduct any formal stress-strain analyses within the tissue. We have found that load magnitude was linked to loading rate. Previous studies have shown that the loading rate can also determine the amount of matrix damage and cell death,13, 27 thus this may have confounded our results. Another potential limitation is the use of plugs 8-mm in diameter (approximately 0.5cm2 in area), which is more consistent with osteochondral autograft procedures. Osteochondral allograft procedures, on the other hand, are recommended for lesions with an area of > 2cm2.13, 27 For allografts, the loads imparted by the surgeon are distributed over a larger area, thereby decreasing the stress magnitude. Despite this difference, the general principal of the biologic effects of surgical impaction of osteochondral grafts is the same for both autografts as well as allografts.
Normalizing sGAGs to a live cell count may not provide an accurate assessment of GAG release. Small sample numbers resulted for the Day 8 groups due to contamination during culture. Therefore, day 8 samples have less reliable release averages with large deviations. In addition, as load increased, variability within the samples increased. For both NO and sGAGs, the unloaded (0N) plugs showed an increased release from Day 4 to 8, suggesting suboptimal media conditions. Finally, this was a controlled laboratory study conducted in vitro. The environment that each sample was exposed to was very different from the in vivo scenario as in the clinical situation. Thus, conclusions regarding the effects of surgical impaction on osteochondral grafts should not be solely based on this study alone and further in vivo studies are necessary.
Our goal was to determine loading parameters to minimize cartilaginous damage in osteochondral grafts placement. As we have found larger drops in cell viability with very high loads, most likely through an apoptotic mechanism, we recommend using the lowest load magnitude possible to insert the osteochondral grafts. An initial observation of our study that the rate of cell death is load magnitude dependent, might be important for future studies applying combined mechanical/biochemical approaches with known classes of pro-anabolic and anti-apoptotic interventions. There is also potential for the development of surgical tools that can deliver impaction loads at pre-set parameters.
Acknowledgements
The technical assistance of Toni Kressner and Thorsten Schwenke (impaction), Carol Pacione (cell culture), and David Karwo (histology) is greatly acknowledged. This study was supported in part by NIH grant T32 AR052272.
References
- 1.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 2.Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443–460. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 3.Ball ST, Amiel D, Williams SK, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418(418):246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 4.Borazjani BH, Chen AC, Bae WC, et al. Effect of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg Am. 2006;88(9):1934–1943. doi: 10.2106/JBJS.E.00992. [DOI] [PubMed] [Google Scholar]
- 5.Borrelli J, Jr, Torzilli PA, Grigiene R, et al. Effect of impact load on articular cartilage: development of an intra-articular fracture model. J Orthop Trauma. 1997;11(5):319–326. doi: 10.1097/00005131-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter JA, Rosenberg L, Coutts R, et al. Articular cartilage: injury and repair. In: Woo SL, Buckwalter JA, editors. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge: American Academy of Orthopaedic Surgeons; 1987. 465-466-492. [Google Scholar]
- 7.Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. 1987;161(1):103–108. doi: 10.1016/0003-2697(87)90658-0. [DOI] [PubMed] [Google Scholar]
- 8.Constantine VS, Mowry RW. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968;50(5):419–423. doi: 10.1038/jid.1968.68. [DOI] [PubMed] [Google Scholar]
- 9.Convery FR, Akeson WH, Keown GH. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972;82:253–262. [PubMed] [Google Scholar]
- 10.Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72(4):574–581. [PubMed] [Google Scholar]
- 11.D'Lima DD, Hashimoto S, Chen PC, et al. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9(8):712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 12.Enneking WF, Campanacci DA. Retrieved human allografts : a clinicopathological study. J Bone Joint Surg Am. 2001;83-A(7):971–986. [PubMed] [Google Scholar]
- 13.Ewers BJ, Dvoracek-Driksna D, Orth MW, et al. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19(5):779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 14.Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A(2):185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322(1):87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Nurse C, Dandy DJ. Fracture-separation of articular cartilage in the adult knee. J Bone Joint Surg Br. 1985;67(B):42–43. doi: 10.1302/0301-620X.67B1.3968141. [DOI] [PubMed] [Google Scholar]
- 17.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 18.Kandel RA, Gross AE, Ganel A, et al. Histopathology of failed osteoarticular shell allografts. Clin Orthop. 1985;(197):103–110. [PubMed] [Google Scholar]
- 19.Kressner T, Schwenke T, Goerke UJ, et al. Application of reproducible impulse loading to study osteochondral grafting procedures. J Biomech. 2006;39(Suppl 1):479. [Google Scholar]
- 20.Kurz B, Jin M, Patwari P, et al. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19(6):1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 21.Lane JG, Massie JB, Ball ST, et al. Follow-up of osteochondral plug transfers in a goat model: a 6-month study. Am J Sports Med. 2004;32(6):1440–1450. doi: 10.1177/0363546504263945. [DOI] [PubMed] [Google Scholar]
- 22.Levin AS, Chen CT, Torzilli PA. Effect of tissue maturity on cell viability in load-injured articular cartilage explants. Osteoarthritis Cartilage. 2005;13(6):488–496. doi: 10.1016/j.joca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JL, Deloria LB, Oyen-Tiesma M, et al. Cell death after cartilage impact occurs around matrix cracks. J Orthop Res. 2003;21(5):881–887. doi: 10.1016/S0736-0266(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 24.Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 25.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996;67(2):165–168. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 26.Milentijevic D, Helfet DL, Torzilli PA. Influence of stress magnitude on water loss and chondrocyte viability in impacted articular cartilage. J Biomech Eng. 2003;125(5):594–601. doi: 10.1115/1.1610021. [DOI] [PubMed] [Google Scholar]
- 27.Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech. 2005;38(3):493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Nam EK, Makhsous M, Koh J, et al. Biomechanical and histological evaluation of osteochondral transplantation in a rabbit model. Am J Sports Med. 2004;32(2):308–316. doi: 10.1177/0363546503259616. [DOI] [PubMed] [Google Scholar]
- 29.Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26(2):309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 30.Patil S, Butcher W, D'Lima DD, et al. Effect of osteochondral graft insertion forces on chondrocyte viability. Am J Sports Med. 2008;36(9):1726–1732. doi: 10.1177/0363546508316765. [DOI] [PubMed] [Google Scholar]
- 31.Pylawka TK, Wimmer M, Cole BJ, et al. Impaction affects cell viability in osteochondral tissues during transplantation. J Knee Surg. 2007;20(2):105–110. doi: 10.1055/s-0030-1248028. [DOI] [PubMed] [Google Scholar]
- 32.Quinn TM, Allen RG, Schalet BJ, et al. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19(2):242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 33.Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am. 1977;59(8):1068–1076. [PubMed] [Google Scholar]
- 34.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69–82. [PubMed] [Google Scholar]
- 35.Sahlstrom A, Johnell O, Redlund-Johnell I. The natural course of arthrosis of the knee. Clin Orthop Relat Res. 1997;340(340):152–157. doi: 10.1097/00003086-199707000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Speer DP, Dahners L. The collagenous architecture of articular cartilage. Correlation of scanning electron microscopy and polarized light microscopy observations. Clin Orthop Relat Res. 1979;139(139):267–275. [PubMed] [Google Scholar]
- 37.Thomas CM, Fuller CJ, Whittles CE, et al. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Torzilli PA, Grigiene R, Borrelli J, Jr, et al. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121(5):433–441. doi: 10.1115/1.2835070. [DOI] [PubMed] [Google Scholar]
- 39.Vellet AD, Marks PH, Fowler PJ, et al. Occult posttraumatic osteochondral lesions of the knee: prevalence, classification, and short-term sequelae evaluated with MR imaging. Radiology. 1991;178(1):271–276. doi: 10.1148/radiology.178.1.1984319. [DOI] [PubMed] [Google Scholar]
- 40.Williams JM, Uebelhart D, Thonar EJ, et al. Alteration and recovery of the spatial orientation of the collagen network of articular cartilage in adolescent rabbits following intra-articular chymopapain injection. Connect Tissue Res. 1996;34(2):105–117. doi: 10.3109/03008209609021496. [DOI] [PubMed] [Google Scholar]
- 41.Xia Y, Moody JB, Burton-Wurster N, et al. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9(5):393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]