Abstract
Purpose
After curative treatment for colorectal cancer (CRC), routine colonoscopies are recommended. We aimed to identify all studies of ethnic disparities in CRC surveillance and examine any association between race/ethnicity and colonoscopy use.
Methods
We conducted a systematic review to address the association between race/ethnicity and colonoscopy use among CRC survivors. We searched Medline for relevant articles. Two authors reviewed titles, abstracts, and articles based on pre-determined inclusion/exclusion criteria.
Results
Of the 1544 titles reviewed, eight studies published since 2001 investigated racial/ethnic disparities in colonoscopy use. Four articles showed a small significant ethnic disparity in the receipt of timely colonoscopy, and the remaining four articles showed a nonsignificant trend in the same direction. The effect did not vary by time of diagnosis or proportion of minorities in each study, though studies with larger samples showed somewhat greater racial/ethnic disparities in colonoscopy use.
Conclusions
We found at least a small disparity in the use of colonoscopy among CRC survivors, suggesting that ethnic disparities continue beyond prevention, detection, and treatment of CRC. It is important to identify areas of unequal care in CRC survivorship and to promote timely surveillance among CRC survivors who belong to racial/ethnic minorities to decrease disparities in mortality.
Keywords: survivorship, colorectal cancer, surveillance, colonoscopy, ethnic disparity
Introduction
After curative treatment for colorectal cancer (CRC), routine surveillance using colonoscopy may improve patient outcomes by detecting local recurrences and second CRCs early enough to treat. Although the magnitude of benefit has not been shown in trials to date, and the optimal surveillance frequency has yet to be determined, multiple clinical practice guidelines have long reflected the general consensus that surveillance colonoscopy should be the standard of care [1-6]. However, CRC survivors appear to be receiving fewer timely surveillance colonoscopies than recommended, even accounting for differing guidelines [7-17]. This literature review focuses on the underuse of surveillance colonoscopy, specifically among racial and ethnic minorities. The incidence of CRC is higher among some racial/ethnic minorities (although not Latinos) compared to whites, as is mortality [18]. These poor outcomes among minorities may result from documented disparities in prevention (e.g., diet and exercise) and early detection (screening) [19, 20]. Further, racial/ethnic minorities with CRC receive less aggressive treatment, which may also contribute to differences in mortality [21]. We hypothesize that racial disparities in CRC persist beyond treatment completion, with minorities receiving fewer timely post-treatment surveillance colonoscopies than whites, contributing to poorer outcomes for racial/ethnic minorities.
Although some studies have directly addressed ethnic disparities in receipt of surveillance colonoscopy, the results are mixed and inconclusive. Further, some studies may have indirectly addressed race/ethnicity among many predictors of colonoscopy surveillance, and these studies may not be readily identifiable in the literature. By systematically reviewing the current literature on the receipt of colonoscopy among CRC survivors, our goal is to identify all relevant studies of ethnic disparities in CRC surveillance and enhance our understanding of how a modifiable factor (colonoscopy use) contributes to increased mortality rates among minority CRC populations.
Methods
We searched for articles assessing CRC surveillance among diverse ethnic CRC survivors. We searched PubMed for articles published through December 2010 using the following search string derived from keywords relevant to our topic: ((colonoscopy OR endoscopy OR surveillance) AND (utilization OR guideline OR compliance OR adherence OR receipt)) AND ((colorectal OR colon OR rectal) AND (neoplasm OR cancer)) AND (resection OR surgery OR curative). We did not include any terms relating to ethnic disparity in the search strategy, because we did not want to exclude relevant studies that assessed race/ethnicity as a predictor of surveillance but did not report this in the title or abstract. We sequentially screened titles, abstracts, and full texts of articles for inclusion, using the following criteria for each step in the screening process: if the author(s) reported original data, if surveillance practices among CRC survivors were assessed, and if race or ethnicity was assessed in relation to colonoscopy use. We excluded articles that were not in English. We documented the number of articles screened at each step based on a modified PRISMA flowchart [22]. When full-text articles were screened, we listed the primary reason for exclusion. Four authors participated in the screening process, with two authors reviewing each article at each step. When authors disagreed on the relevance based on title or abstract review, the article was included in the next screening step. Disagreement at the level full-text screening was resolved in discussion among all authors. Only at the stage of full-text screening did we eliminate studies for not assessing race/ethnicity as a predictor of colonoscopy adherence, in order to ensure that we did not inappropriately exclude studies that included race/ethnicity but did not mention this predictor in the title or abstract.
We reviewed the relationship between race/ethnicity and colonoscopy use in each study. Guidelines for the timing of colonoscopy have often conflicted, and they have changed over time [1-6]. For this reason, we did not assess guideline adherence, but simply assessed racial/ethnic variations in colonoscopy use as measured in each study. For each study, we recorded sample characteristics including dates of diagnosis, race/ethnicity distribution, and sample size for each study. We reviewed these variables to assess whether they moderated any racial/ethnic disparities in screening rates across studies.
Results
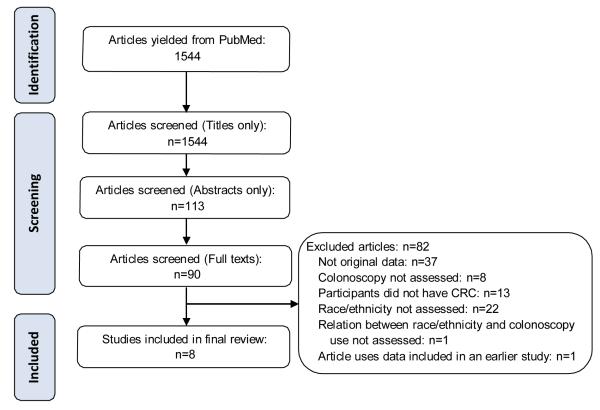
The PubMed search yielded 1,544 unique articles. After screening titles and abstracts for eligibility, 90 articles remained that were eligible for full-text screening. Nine articles ultimately met our criteria for review. Of these nine articles, eight were unique studies and formed the final sample of articles for our review (Figure). The eight studies were published between 2001 and 2010, with data from regional clinical populations, national registries, and population-based studies [7, 10, 11, 15-17, 19, 23]. Typically, less than 15% of each of the study samples was non-White; however, four studies included large national samples that were powered to detect differences in use of surveillance among smaller minority populations [10, 17, 19, 23].
Figure.
Modified PRISMA flowchart to illustrate steps involved in article inclusion
Because of our inclusion criteria, all studies assessed the use of colonoscopy among CRC patients. In four studies the receipt of colonoscopy could not be distinguished from the receipt other colon exams [10, 11, 15, 16]. In these four studies, colonoscopy represented between 70% and 90% of all colon exams received. Our review thus summarized colonoscopy use (when possible) or the use of all colon exams when it was not possible to single out colonoscopies.
There were statistically significant ethnic disparities in four of the eight studies, with blacks less likely to receive timely endoscopic surveillance than their white counterparts in all four studies [7, 10, 15, 19] Cooper at al.[19] used Medicare claims to assess surveillance colonoscopies for multiple indications, including Crohn’s disease, personal history of colorectal polyps, ulcerative colitis, and a personal history of CRC [19]. Among this mixed cohort, the annualized rate of surveillance colonoscopies occurring in 1999 was higher for whites than blacks (1.28% vs. 0.62%, respectively, of all Medicare beneficiaries). Because of the cross-sectional nature of this study, even if the analysis were limited to CRC survivors, the timing of surveillance with regard to diagnosis or treatment is unknown. A later study by Cooper and colleagues [7] using linked data from Surveillance Epidemiology and End Results (SEER) and Medicare investigated adherence to existing surveillance guidelines among CRC survivors, which was conservatively defined as at least one colonoscopy within 3 years after diagnosis [7]. Fewer blacks were adherent to colonoscopy guidelines than whites (65.4% vs. 74.6% respectively), and in a multivariate logistic regression model controlling for clinical, sociodemographic, and geographic factors, blacks were still significantly less likely to undergo colonoscopy than whites (OR=0.57, P=0.001). Ellison et al.[10] also used SEER-Medicare data to investigate colon exams among CRC survivors diagnosed between 1986 and 1996 and found that blacks were less likely than whites to have both their first and second colon exams, and that the racial difference in surveillance persisted at 18 months, 3 years, and 5 years after diagnosis [10].
Among CRC survivors within 11 health maintenance organizations (HMOs), Rolnick et al. [15] investigated receipt of colon exams, limiting the analysis to patients who were either black or white [15]. The median time to the first colon exam among these patients was significantly longer for blacks (18.7 months) than whites (14.3 months). Racial differences in receiving at least one colon exam rates persisted over time, as measured at 1 year, 3 years, and 5 years. Without respect to time, blacks were 38% less likely to receive a colonoscopy than whites.
The remaining four studies did not show statistically significant ethnic disparities, but they still showed nonsignificant trends of ethnic minorities being less likely to receive timely colonoscopies than whites. An early study by Cooper and colleagues [9] of patients diagnosed in 1991 using SEER-Medicare data showed no statistical differences between racial/ethnic groups, with 51% of white and 47% of black patients undergoing at least one colonoscopy by 3 years after diagnosis [9]. Elston Lafata et al. [11] investigated colonoscopy use among CRC survivors within an HMO in Michigan, and found that whites were 1.43 times as likely as non-whites to get colonoscopy, though this relationship was not statistically significant [11]. Rulyak et al. [16] measured colonoscopy use among CRC survivors among members of an HMO in Washington [16]. The authors found that there was a trend of decreased use of colon exams (mostly colonoscopies) among blacks compared to whites (RR=0.70, n.s.). The opposite was true among those of non-black and non-white race (predominantly those of Asian descent), with a trend toward increased use of colon exams compared to whites (RR=1.47, n.s.). However, only 7% of the sample of 1,002 patients was non-white, limiting the ability to generalize of these findings. More recently, Salz et al. [17] used data from the Cancer Care Outcomes and Surveillance (CanCORS) study, whose sample is a combination of patients in HMO, population-based, and Veterans Affairs settings, to assess colonoscopy use among CRC survivors [17]. The authors found a trend toward less receipt of colonoscopies within 14 months of surgery for blacks than for non-Hispanic whites (47% vs. 49%), which was not statistically significant in multivariate analysis (OR=0.89 for blacks compared to whites, n.s.). In multivariate analysis, non-white Hispanics were less likely than whites to receive a colonoscopy (OR=0.95) and participants of other races were more likely receive a colonoscopy (OR=1.50), but neither relationship was statistically significant, and each of these groups formed a small portion of the study sample.
Results were more mixed for non-white and non-black CRC patients, possibly due to small sample sizes and heterogeneity within this group [7, 10, 11, 16, 17, 19, 23].
Two studies that found racial disparities in the timeliness of the first post-treatment colonoscopy examined whether racial disparities change over time since diagnosis. Rolnick et al. [15] found that racial differences were statistically significant at each time point of 1, 3, and 5 years after diagnosis [15]. Further, Ellison et al. [10] found that racial differences increased at each time point of 1.5 years, 3 years, and 5 years after diagnosis, even when accounting for sociodemographic, hospital, and clinical covariates [10].
Only one study investigated racial differences in receipt of subsequent colon examinations beyond the first examination. Controlling for sociodemographic, hospital, and clinical characteristics, Ellison et al. [10] found that black patients who received one colon exam were 9% less likely than their white counterparts to receive any subsequent colon examinations (p<0.05) [10]. Racial differences did not persist at the third colon exam.
Among CRC survivors in all eight studies, the dates of diagnosis ranged from 1986 through 2005, and the dates overlapped substantially between studies. There was no apparent pattern of the presence or absence of statistically significant disparities in colonoscopy use based on the year of patient diagnosis.
Sample size and percentage of minorities may affect the ability to detect a difference in colonoscopy rates within studies. Studies ranged from 251 to 52,105 participants with CRC, and one study included more than 33 million people[19], but was not limited to colorectal cancer survivors. Four studies included fewer than 2,000 participants, and only one of these smaller studies [15] showed a statistically significant disparity in surveillance, compared with three of the four larger studies [7, 10, 19] showing a statistically significant disparity. The percentage of minorities ranged from 6% to 37% across studies, with three studies [11, 15, 17] consisting of at least 25% minorities. Two of the three studies with the highest proportion of minority participants did not find statistically significant disparities [11, 17].
Discussion
Surveillance colonoscopies are recommended for CRC survivors, with the intent of detecting treatable recurrences, metachronous cancers, and precancerous polyps. Prior research has shown that colonoscopies are underused among CRC survivors [10-17, 24]. Despite evidence of widespread disparities in CRC prevention, detection, treatment, and outcomes, few studies have investigated racial and ethnic disparities in the context of survivorship care. Our systematic review of the literature yielded only eight studies that addressed racial and ethnic disparities in colonoscopy use among CRC survivors. In all eight studies, a trend showed decreased colonoscopy uptake among ethnic minorities, although this relationship was only statistically significant in four of the studies. The three largest studies showed statistically significant ethnic disparities, suggesting a small effect of race/ethnicity that might only be detected in a large study [7, 10, 24]. However, across studies, the inclusion of a larger proportion of minorities, which could facilitate the detection of a small effect, seemed unrelated to surveillance disparities.
Surveillance colonoscopy among CRC survivors may be analogous to CRC screening in the general population, in that both are routine tests on asymptomatic adults. However, after a diagnosis of CRC and subsequent curative treatment, a patient may be “in the system.” Ideally, post-treatment care for this elevated-risk population is the same across all racial and ethnic groups. Further, post-treatment care for CRC survivors is relatively simple The only elements of post-treatment care specifically recommended by clinical practice guidelines are periodic testing for recurrence or second CRCs: carcinogenic embryonic antigen (CEA) testing, computed tomography (CT) scans, colonoscopy, and proctosigmoidoscopy for rectal cancers [1-6]. This review demonstrates that CRC survivorship care, despite its simplicity and limited focus on recurrence or second cancers, is of somewhat lower quality for minorities.
There is other evidence that ethnic disparities are present even after diagnosis. Blacks are less likely than whites to receive curative surgery and adjuvant chemotherapy for CRC [21, 25-28]. There is also evidence that blacks receive less pain management than their white counterparts [29]. The current study shows that the disparities continue in the realm of surveillance, even after treatment is complete. Disparities may not be limited to frequency of surveillance; one study found that black patients were more likely to receive alternative types of colon exams (such as barium enema) than their white counterparts [10].
The reasons for ethnic disparities in CRC surveillance are unclear. One possibility is that general health plays a role. Minorities are more likely to have comorbidities than their white counterparts, and it may be that after the completion of curative treatment, the focus of care for some survivors is the management of comorbidities rather than surveillance [19]. In fact, sicker survivors may not be appropriate candidates for colonoscopy if the benefits do not outweigh the risks of the procedure.
Access to care may play a role, although even in studies of managed care populations, where patients presumably have equal access to services, disparities in surveillance colonoscopy use persist [7, 10, 11, 15, 16, 23, 24]. Similarly, studies of Medicare beneficiaries show evidence of disparities, although copayments may present a barrier, especially for those without Medigap insurance [7, 10, 23, 24]. Minorities may be disproportionately faced with issues of access to care, which may extend beyond the availability of affordable services; transportation, the ability to miss work for a colonoscopy or its preparation, and other factors may be difficult for minorities with lower socioeconomic status. These factors have been shown to affect screening colonoscopy among asymptomatic adults, and they may still play a role in the survivorship setting.
Colonoscopy use likely depends on receiving an appropriate referral from the provider that manages the survivor’s post-treatment care. The management of care after the completion of curative treatment remains poorly defined, and survivors often have gaps in ongoing care [30]. Receipt of surveillance colonoscopy has been shown to be higher among CRC survivors who continue to see an oncologist after treatment completion compared to CRC survivors who do not [31]. It is possible that the underuse of colonoscopy is a result of poorly coordinated care, and that minorities are less likely to have participatory relationships with their physicians during their visits compared to their white counterparts [32]. This is an area for further study, particularly in understanding follow-up patient care and how to increase the knowledge of physicians about the importance of colonoscopy.
Conclusion
This review highlights the need to ensure equal access to and use of colonoscopy among all survivors of CRC. Although there are a limited number of studies, most of which only looked at differences between black and white survivors, at least a small disparity exists in the uptake of colonoscopy among CRC survivors. This disparity may be typical of other disparities in the receipt of post-treatment care. It is important to identify other areas of unequal care in CRC survivorship and to promote timely surveillance among CRC survivors who belong to racial and ethnic minorities.
Table.
Summary of Studies examining the relationship between race/ethnicity and colorectal cancer surveillance.
| First author |
Year | Sample (N) |
Race/Ethnicity Detail |
Dates of Diagnosis (follow-up time) |
Findings |
|---|---|---|---|---|---|
| Cooper, G.S. |
2000 | SEER- Medicare cohort (N=5,716) |
94% Non-black 6% Black |
1991 (followed through 1994) |
- 46.9% of blacks obtained a colonoscopy vs. 51.4% among whites and 45% in patients of other races in 3 years (n.s.). |
| Elston Lafata, J. |
2001 | Multispeciality HMO, Michigan (N=251) |
63% White 37% Minority - 98% Black - 2% Other |
1990 – 1995 (median follow- up 4 yrs.) |
- White participants more likely to receive colon exam (colonoscopy, barium enema, or sigmoidoscopy) than blacks (RR = 1.43,n.s.). |
| Cooper, G.S. |
2004 | SEER- Medicare cohort (N=33.8 million, not limited to cancer survivors) |
87% White 7.81% Black 5.77% Other |
Unknown diagnosis dates, Colonoscopy data from 1999 |
- Annualized rates of colonoscopy surveillance among males was 0.62% among blacks vs. 1.28% among whites (P<0.0001) - Annualized rates of colonoscopy surveillance among females was 0.57% among blacks vs. 0.88% among whites (P<0.0001). |
| Ellison, G.L. |
2003 | SEER- Medicare cohort (N=52,105) |
86% White 6% Black 9% Other |
1986-1996 (followed through 1998) |
- Blacks were less likely than whites to have a first colon exam (colonoscopy, endoscopy, barium enema) (RR=0.87, P<0.05 among those diagnosed before 1991 and RR=0.75, P<0.05 among those diagnosed 1991-1996). - Blacks were 9% less likely to have a second colon exam than Whites. - At 18 months post-treatment, colon exams were conducted among 57% of whites, 48% of blacks, and 45% of people of other races. - At 3 years, colon exams were conducted among 67% of whites, 61% of blacks, and 56% of people of other races. - At 5 years, colon exams were conducted among 74% of whites, 70% of blacks, and 63% of people of other races. |
| Rulyak, S.J. |
2004 | Group Health Cooperative HMO, Washington (N=1,002) |
93% White 4% Black 4% Other |
1993-1999 (median follow- up 3.6 years) |
- Blacks less likely to receive colon exams (colonoscopy, barium enema, or sigmoidoscopy) than whites (RR=0.70, n.s.). - Asians more likely to receive colon exams compared to white patients (RR=1.37, n.s.). |
| Rolnick, S. | 2005 | 11 HMOs in Cancer Research Network, U.S. (N=881) |
75% White 25% Black |
1990-2000 (5 years follow- up) |
- Median time to exam (colonoscopy, barium enema, or sigmoidoscopy) was 14.3 months for whites, 18.7 months for blacks (P=0.0002). - Blacks were less likely to receive first surveillance exam at 1 year (HR=0.47, P=.0008), 3 years (HR=0.55, P<.0001), and 5 years (HR=0.59, P=.0009) post-treatment compared to whites (HR = 0.62, P<0.05). |
| Cooper, G.S. |
2008 | SEER- Medicare cohort (N=9,426) |
87% White 6% Black 3% Hispanic 1% Asian 2% Other |
2000-2001 (3.5 years of follow-up) |
- 3-year follow-up guidelines for colonoscopy were met by 65.4% black, 72.9% Hispanic, 74.6% white and 68.7% of patients of other races (P= .001). - Blacks were significantly less likely than whites to undergo colonoscopy within 3 yrs. (OR=0.57, P=0.001) |
| Salz, T. | 2010 | Multiregional population- based cohort study in U.S. (N=1,423) |
67% White 18% Black 6% Hispanic 9% Other |
2003-2005 (14 months of follow-up) |
- Blacks and Hispanics were less likely to undergo colonoscopy 14 months post-surgery (47% and45%, respectively) than whites (49%). - Blacks were less likely than whites to receive a colonoscopy in 14 months (OR=0.89, n.s.). - Hispanics were less likely than whites to receive a colonoscopy (OR=0.95, n.s.), and participants of other races were more likely than whites to receive a colonoscopy (OR=1.50, n.s.). |
Acknowledgment
NIH site grant number 5R21CA137434-01A1, PI DuHamel
References
- 1.NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, Inc.; 2003. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, Inc.; 2005. [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, Inc.; 2006. [Google Scholar]
- 4.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47(6):807–17. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 5.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23(33):8512–9. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130(6):1865–71. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Cooper GS, Kou TD, Reynolds HL., Jr. Receipt of guideline-recommended follow-up in older colorectal cancer survivors : a population-based analysis. Cancer. 2008;113(8):2029–37. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 8.Cooper GS, Payes JD. Temporal trends in colorectal procedure use after colorectal cancer resection. Gastrointest Endosc. 2006;64(6):933–40. doi: 10.1016/j.gie.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Cooper GS, Yuan Z, Chak A, et al. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer. 1999;85(10):2124–31. [PubMed] [Google Scholar]
- 10.Ellison GL, Warren JL, Knopf KB, et al. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38(6 Pt 2):1885–903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elston Lafata J, Johnson C. Cole, Ben-Menachem T, et al. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39(4):361–72. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43(6):592–9. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 13.Hilsden RJ, Bryant HE, Sutherland LR, et al. A retrospective study on the use of post-operative colonoscopy following potentially curative surgery for colorectal cancer in a Canadian province. BMC Cancer. 2004;4:14. doi: 10.1186/1471-2407-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knopf KB, Warren JL, Feuer EJ, et al. Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries. Gastrointest Endosc. 2001;54(5):563–71. doi: 10.1067/mge.2001.118949. [DOI] [PubMed] [Google Scholar]
- 15.Rolnick S, Alford S. Hensley, Kucera GP, et al. Racial and age differences in colon examination surveillance following a diagnosis of colorectal cancer. J Natl Cancer Inst Monogr. 2005;(35):96–101. doi: 10.1093/jncimonographs/lgi045. [DOI] [PubMed] [Google Scholar]
- 16.Rulyak SJ, Mandelson MT, Brentnall TA, et al. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc. 2004;59(2):239–47. doi: 10.1016/s0016-5107(03)02531-8. [DOI] [PubMed] [Google Scholar]
- 17.Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R.L. Horner MJ, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2006. National Cancer Institute; Bethesda, MD: 2009. [Google Scholar]
- 19.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100(2):418–24. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 20.Doubeni CA, Laiyemo AO, Klabunde CN, et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010;38(2):184–91. doi: 10.1016/j.amepre.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball JK, Elixhauser A. Treatment differences between blacks and whites with colorectal cancer. Med Care. 1996;34(9):970–84. doi: 10.1097/00005650-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper GS, Yuan Z, Chak A, et al. Patterns of endoscopic follow-up after surgery for nonmetastatic colorectal cancer. Gastrointest Endosc. 2000;52(1):33–8. doi: 10.1067/mge.2000.106685. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Koroukian SM. Geographic variation among Medicare beneficiaries in the use of colorectal carcinoma screening procedures. Am J Gastroenterol. 2004;99(8):1544–50. doi: 10.1111/j.1572-0241.2004.30902.x. [DOI] [PubMed] [Google Scholar]
- 25.Cooper GS, Yuan Z, Landefeld CS, et al. Surgery for colorectal cancer: Race-related differences in rates and survival among Medicare beneficiaries. Am J Public Health. 1996;86(4):582–6. doi: 10.2105/ajph.86.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao Y, Landrine H, Jemal A, et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health. 2011;65(3):211–7. doi: 10.1136/jech.2009.096008. [DOI] [PubMed] [Google Scholar]
- 27.Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87(22):1686–93. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 28.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 29.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Translation. Institute of Medicine and National Research Council; Washington, D.C.: 2005. [Google Scholar]
- 31.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–9. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 32.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583–9. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]