Abstract
Earlier research has demonstrated that hyperbaric oxygen (HBO2) can produce an antinociceptive effect in models of acute pain. Recent studies have revealed that HBO2 can produce pain relief in animal models of chronic pain as well. The purpose of the present investigation was to ascertain whether HBO2 treatment might suppress allodynia in rats with neuropathic pain and whether this effect might be blocked by the opioid antagonist naltrexone (NTX). Male Sprague Dawley rats were subjected to a sciatic nerve crush under anesthesia and mechanical thresholds were assessed using an electronic von Frey anesthesiometer. The time course of the HBO2-induced anti-allodynic effect in different treatment groups was plotted, and the area-under-the-curve (AUC) was determined for each group. Seven days after the nerve crush procedure, rats were treated with HBO2 at 3.5 atmospheres absolute (ATA) for 60 min and exhibited an anti-allodynic effect, compared to nerve crush-only control rats. Twenty-four hours before HBO2 treatment, another group of rats was implanted with Alzet® osmotic minipumps that continuously released NTX into the lateral cerebral ventricle for 7 days. These NTX-infused, HBO2-treated rats exhibited an allodynic response comparable to that exhibited by rats receiving nerve crush only. Analysis of the AUC data showed that HBO2 significantly reduced the nerve crush-induced allodynia; this anti-allodynic effect of HBO2 was reversed by NTX. These results implicate opioid receptors in the pain relief induced by HBO2.
Keywords: Hyperbaric oxygen, anti-allodynia, naltrexone, opioid receptors, neuropathic pain, sciatic nerve crush, rat
1. Introduction
Inadequate management of pain may result in poor clinical outcomes and reduced quality of life for the patient. Effective treatment of chronic pain, in particular, presents a challenge to modern medicine. Pain relief is not an approved clinical indication of HBO2 treatment (Gesell, 2008). Nonetheless, there is evidence that hyperbaric oxygen (HBO2) treatment can mitigate both acute and chronic pain. There are observations in the literature that HBO2 treatment can reduce pain in clinical patients who are afflicted with various chronic pains, including complex regional pain syndrome (CRPS) (Peach, 1995; Tuter et al., 1997; Kiralp et al., 2004), idiopathic trigeminal neuralgia (Gu et al., 2012), fibromyalgia (Yildiz et al., 2004), migraine (Wilson et al., 1998), cluster headache (Di Sabato et al., 1993) and other painful conditions (Dall’Era et al., 2006; Jones et al., 2006; Handschel et al., 2007).
Experimentally, HBO2 treatment can reduce allodynia in rats with peripheral nerve injuries (Thompson et al., 2010; Li et al., 2011; Gu et al., 2012; Zhang et al., 2012) and experimental arthritis (Warren et al., 1979; Wilson et al., 2006, 2007). Research from our laboratory demonstrated that HBO2 treatment effectively reduces pain in animal models of acute pain (Ohgami et al., 2009; Zelinski et al., 2009a; Chung et al., 2010; Quock et al., 2011). Our results from these experiments show that HBO2-induced antinociception is significantly attenuated by the opioid antagonist naltrexone (NTX). Hence, we concluded that HBO2 treatment might lead to activation of central opioid receptors that can modulate pain.
The purpose of the present investigation was to determine whether the pain-relieving effect of HBO2 treatment in chronic pain might also involve a central opioid pain-relieving mechanism.
2. Results
2.1. Time course of the mechanical threshold
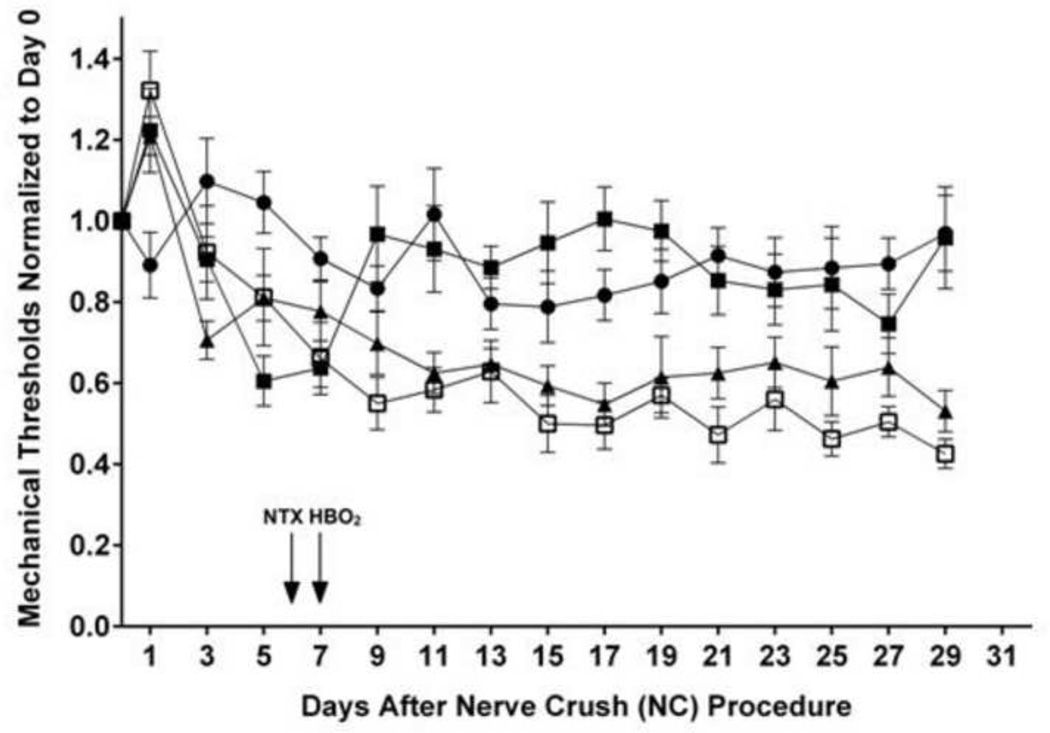
Fig.1 compares the time course of the normalized mechanical thresholds in different treatment groups: control; nerve crush (NC) alone; nerve crush followed seven days later by a 60-min HBO2 treatment at 3.5 atmospheres absolute (ATA) (NC + HBO2); nerve crush followed six days later by implantation of a continuously-releasing NTX osmotic minipump and, on the seventh day, by a 60-min HBO2 treatment at 3.5 ATA (NC + NTX + HBO2).
Figure 1.
Time course of normalized mechanical thresholds of rats following: ●, control; ▲, nerve crush (NC) alone; ■, nerve crush followed seven days later by a 60-min HBO2 treatment at 3.5 ATA (NC + HBO2); and □, nerve crush followed six days later by implantation of a continuously-releasing naltrexone osmotic minipump and, on the seventh day, by a 60-min HBO2 treatment at 3.5 ATA (NC + NTX + HBO2). All mechanical thresholds were normalized to averaged baseline thresholds on day 0. Each symbol represents the mean response of 8-9 rats per group.
Mechanical thresholds temporarily increased in all three NC groups following the nerve crush procedure but started to decrease by day 4 after NC. Compared to the control group, the thresholds of the NC group continued to fall until stabilizing at approximately 40% lower than the control threshold. Treatment with HBO2 on day 7 was followed by an increase in the mechanical threshold back to levels comparable to the control group. In rats implanted with NTX-releasing mini-pumps on day 6 followed by HBO2 treatment on day 7, the mechanical threshold approximated that of the NC only group.
Monitoring of the mechanical thresholds continued up to 29 days following NC. At that time, the thresholds of the control and NC + HBO2 groups were in one cluster within 10% of the starting threshold, and the thresholds of the NC only and NC + NTX + HBO2 groups were in another cluster 40–50% lower than the original threshold on day 0.
2.2. AUCs of changes in mechanical thresholds
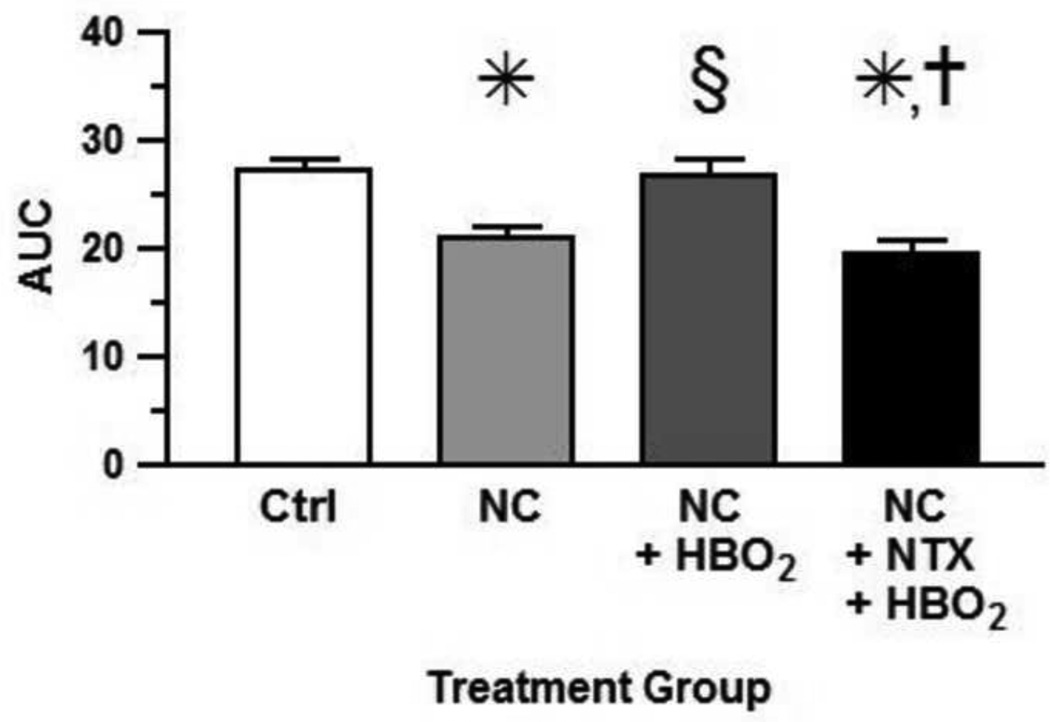
Fig. 2 compares the area under the curve (AUC) of the four different treatment groups. The AUC of the mechanical threshold to allodynia of the NC group was significantly lowered the as compared to that of the untreated control group (P < 0.05). Treatment with HBO2 significantly increased the AUC as compared to that of the NC control (P < 0.05) and returned the AUC of the mechanical threshold back to that of the untreated control level, i.e., an anti-allodynic effect.
Figure 2.
AUCs of changes in mechanical threshold. Each bar represents the mean AUC and each vertical line represents the SEM of 8-9 rats per group. Significance of difference: *, P < 0.05, compared to the control group; §, P < 0.05, compared to NC group; and †, P < 0.05, compared to NC + HBO2 (one-way ANOVA and post-hoc Bonferroni's multiple comparison test).
However, when NTX, a nonspecific opiate antagonist, was administered by continuous i.c.v. minipump infusion, the treatment completely blocked the anti-allodynic effect of HBO2 (F= 8.93, P = 0.0002).
3. Discussion
There are reports that HBO2 treatment effectively relieved neuropathic pain in rats with peripheral nerve injury. Thompson et al. (2010) demonstrated that HBO2 treatment could cause anti-nociception in two rat models of neuropathic pain, ligation of the L5 spinal nerve and chronic constriction injury (CCI) of the sciatic nerve. Rats were treated for 90 min with HBO2 at 2.4 ATA daily for 14 consecutive days. HBO2 treatment clearly improved mechanical thresholds (i.e., less hypersensitivity during the two weeks of treatment and for up to five days following the last HBO2 treatment). Based on evidence that HBO2 reduced carrageenan-induced inflammation (Sümen et al., 2001; Wilson et al., 2006), it was posited that HBO2 reduced inflammation-associated neuropathic pain through this anti-inflammatory action. Wilson et al. (2007) also demonstrated that HBO2 treatment was equieffective with aspirin in reducing both joint inflammation and hyperalgesia in rats following intraarticular injection of carrageenan.
Li et al. (2011) subjected rats to daily HBO2 treatment at 2.4 ATA for 60 min for seven days following CCI of the sciatic nerve. Mechanical and cold allodynia responses on days 4 and 7 after CCI were significantly reduced in rats that received HBO2 treatment. CCI-induced hyperalgesia and allodynia is generally associated with increased levels of tumor necrosis factor (TNF)-α (George et al., 2000) and interleukin (IL)-1β (George et al., 2004). Sciatic nerve homogenates from HBO2-treated rats contained significantly reduced levels of TNF-α but not IL-1β on days 4 and 7 after CCI, suggesting that reduced production of TNF-α might contribute to HBO2-induced relief of neuropathic pain (Li et al., 2011).
More recently,Gu et al. (2012) conducted similar experiments in rats with CCI of the sciatic nerve. Rats were treated with NBO2 (normobaric 100% oxygen), HBA (hyperbaric air) and HBO2 at 1.5, 2.0 or 3.0 ATA for daily 70-min treatments on seven consecutive days. HBO2 but not NBO2 or HBA treatment significantly lowered the severity and shortened the duration of thermal hyperalgesia as well as mechanical allodynia. In both situations, 3.0 ATA but not 1.5 or 2.0 ATA HBO2 produced significant pain relief. CCI caused induction of c-Fos and activation of astrocytes in the dorsal horn neurons of the spinal cord ipsilateral to the nerve injury. Both the early HBO2 treatment and the late HBO2 treatment attenuated the c-Fos induction as well as the astrocyte activation (Gu et al., 2012). CCI caused significant elevation in amounts of phosphorylated protein levels of NMDA receptor subtypes NR1 (pNR1) and NR2B (pNR2), extracellular signal-regulated kinases (pERK), calmodulin-dependent kinase II (pCaMKII) and cAMP response element-binding (pCREB) protein. HBO2 treatment starting early after the CCI procedure reversed all these changes except for pNR1.
In previous research from our laboratory, we reported that HBO2 treatment could produce relief of acute pain in a time-dependent manner in the glacial acetic acid-induced abdominal constriction test. Mice treated with an i.p. injection of glacial acetic acid then placed into a hyperbaric chamber exhibited a reduced number of abdominal constrictions during a 6-min observation period under HBO2 at 3.5 ATA (Ohgami et al., 2009; Quock et al., 2011).
When mice were subjected to HBO2 treatment for 60 min, the antinociceptive state persisted for at least 90 min after treatment before gradually subsiding (Zelinski et al., 2009a). Mice that received four daily 60-min HBO2 treatments exhibited an unusually long-lasting biphasic antinociceptive effect (Chung et al., 2010). There was an early-phase antinociceptive response that lasted approximately 6 h before dissipating by 12 h. However, a late-phase antinociceptive response reemerged at 24 h and peaked five days after the last HBO2 treatment which lasted for up to three weeks.
The present results show that a single 60-min HBO2 treatment caused a rapid increase in mechanical threshold that was maintained at the same level as the control group through day 29 after NC. This improvement in the mechanical threshold occurred despite the fact that the implanted minipump was depleted of NTX by day 13. This suggests a long term consequence of opioid receptor blockade during and immediately after the HBO2 treatment. This is reminiscent of a previous observation that naltrexone blockade of opioid receptors during the 4-day window of HBO2 treatment antagonized the late-phase of HBO2-induced antinociception two weeks after the last HBO2 treatment (Chung et al., 2010).
The findings of this study are also consistent with these earlier results that implicate an involvement of a central opioid mechanism in the antinociceptive effects of HBO2. The antinociceptive effect observed 90 min after a 60-min HBO2 treatment at 3.5 ATA was abolished by i.p. pretreatment with NTX (Zelinski et al., 2009a). The early-phase antinociception that occurs three h after the last of four daily 60-min HBO2 treatments was also significantly antagonized by i.c.v. pretreatment with NTX. Other results show that HBO2-induced antinociception is sensitive to antagonism by norbinaltorphimine, a κ opioid antagonist (Portoghese et al., 1987), and rabbit antiserum against dynorphin1–17, an endogenous κ opioid ligand (Chavkin et al., 1982). This suggested that HBO2-induced antinociception in the mouse abdominal constriction test involves stimulated neuronal release of dynorphin1–17, which then activates brain κ opioid receptors.
The results of the present study strongly implicate brain opioid receptors are involved but the specific subtype is unclear because of the nonselective nature of NTX blockade of opioid receptors. But it is clear that opioid receptors are probably implicated in the anti-allodynic effect of HBO2 on allodynia induced in rats by peripheral nerve injury. The continuous infusion of NTX into the lateral cerebral ventricle indicates that the site of these opioid receptors is central but does not identify a specific brain site. Within the brain, high densities of opioid receptors are located in the limbic system (septum and hippocampus), corpus striatum, hypothalamus, periaqueductal gray (PAG), nucleus raphe magnus (NRM), and dorsal raphe of the rostral ventral medulla (Mansour et al., 1987).
Earlier investigations have shown involvement of opioid receptors in the arcuate nucleus of the anterior hypothalamus and PAG in analgesia elicited by defeat in a social conflict model (Miczek et al., 1985). The arcuate nucleus of the anterior hypothalamus contains proopiomelanocortin (POMC) neurons that project to the PAG (Pilcher et al., 1988), which, in turn, plays a vital role in descending antinociceptive pathways (Heinricher et al., 2009). In a previous study, our laboratory has demonstrated that the anesthetic gas nitrous oxide may stimulate a neuronal release of β-endorphin from central sites (Zelinski et al., 2009b), including the arcuate nucleus and PAG (Ohgami et al., 2010) to produce an acute antinociceptive effect in the rat hot plate test (Hara et al., 1994). HBO2-induced antinociception may also involve this arcuate-PAG pathway.
In summary, the central infusion of an opioid receptor blocker disrupts the antiallodynic effect of HBO2 in rats with peripheral nerve injury. While not precluding the possible contribution of a peripheral anti-inflammatory action of HBO2 to the relief of neuropathic pain, these findings further support our contention that HBO2 also activates a centrally-mediated, pain-modulating pathway.
4. Experimental procedures
4.1. Animals
Male S/A albino rats, 160–180 g, were purchased from Simonsen Laboratories (Gilroy, CA) and used in this research. Experiments were approved by the Washington State University Institutional Animal Care and Use Committee (IACUC) with post-approval review and carried out in accordance with The Guide for the Care and Use of Laboratory Animals, 8th Edition (National Academies Press, Washington, DC, 2010). All measures to minimize pain or discomfort were taken by the investigators, including prophylaxis of post-operative pain (meloxicam) and infection (ampicillin).
Rats were individually housed in plastic cages in the Wegner Hall Vivarium at Washington State University with access to food and water ad libitum. The facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), was maintained on a 12-h light:dark cycle (lights on 07:00–19:00 h) under standard conditions (22 ± 1°C room temperature, 33% humidity). Rats were kept in the holding room for at least four days after arrival in the facility for acclimation prior to use.
4.2. Sciatic Nerve Crush Procedure
The sciatic nerve crush procedure was carried out under anesthesia with ketamine (100 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.). Prior to surgery, the skin of the right hind limb was shaved and sterilized with 10% povidone iodine solution. An incision was made through the skin on the posterior thigh, and the muscle was blunt-dissected to expose the right sciatic nerve. The nerve was crushed with a pair of forceps for 10 s then crushed again at a 180° angle for another 10 s. In control animals, the sciatic nerve was exposed in identical manner but was not subjected to crush. At the end of surgery, the incised skin was closed with two wound clips. Rats received i.m. injections of ampicillin and meloxicam for prophylaxis of post-surgical infection and pain. After seven days, rats were subjected to HBO2 treatment (3.5 ATA for 60 min) and allodynia was assessed for at least 25 days.
4.3. Assessment of Allodynia
Allodynia was assessed in Plexiglas® test chambers (dimensions 21 cm L × 21 cm W × 25 cm H) mounted atop a sheet of galvanized steel mesh (hardware cloth) mounted at a height of 24 cm to allow access to the rats’ paws from underneath. Rats were acclimated to the test chambers for 45 min before testing. An electronic von Frey anesthesiometer (Stoelting, Wood Dale, IL) was used to provoke a flexion reflex followed by a flinch response, and the mechanical threshold pressure was recorded. Assessments were carried out in triplicate at 5-min intervals. The mechanical threshold was measured every other day starting five days before nerve crush surgery; the three data were averaged to arrive at the baseline.
4.4. Exposure to Hyperbaric Oxygen (HBO2)
Rats were placed in individual standard mouse cages (dimensions 27 cm L × 17 cm W × 12 cm H), two of which were inserted inside a B-11 research hyperbaric chamber (Reimers Systems, Inc., Lorton, VA) as previously described (Ohgami et al., 2008). The chamber was ventilated with 100% O2, U.S.P. (A-L Compressed Gases Inc., Spokane, WA) at a flow rate of 20 L/min to minimize CO2 accumulation. The pressure within the cylindrical clear acrylic chamber (28 cm diameter × 56 cm L) was increased from 1.0–3.5 ATA over 2 min. The pressure was held at 3.5 ATA for 60 min. The animals breathed spontaneously during HBO2 treatment. After completion of the HBO2 exposure, the chamber was decompressed over 2–3 min.
Under the conditions of a small, sealed hyperbaric chamber, it was not possible to quantify the CO2 in the chamber. However, a Model 602–3 POET II anesthesia monitor (Criticare Systems, Waukesha, WI) failed to detect any CO2 when the sample probe was inserted into the outlet of the hyperbaric chamber.
Control groups of rats were exposed to room air at 1.0 ATA (absence of 100% O2 and 3.5 ATA). Preliminary experiments demonstrated that neither 100% O2 nor 3.5 ATA alone produced any antinociceptive effect; only the combination of 100% O2 and 3.5 ATA evoked an antinociceptive response (Liu et al., 2013; Liu et al., unpublished manuscript under review).
4.5. Surgery for continuous intracerebroventricular delivery of drug
On the day prior to HBO2 treatment, rats were anesthetized with ketamine (100 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.). Rats were mounted in a stereotaxic headholder (Cartesian Research, Inc., Sandy, OR), and brain infusion cannulae (Alza, Cupertino, CA) were implanted into the right lateral cerebral ventricle at stereotaxic coordinates –1.00 mm AP, 1.5 mm ML and –2.00 mm DV, relative to bregma (Paxinos and Watson, 2006). Each brain infusion cannula consisted of a 12.8-mm length of 28-G stainless steel tubing embedded in a polycarbonate pedestal that was secured to the skull with screws and dental cement. A side connector protruded from the pedestal and was connected to a dorsally subcutaneously implanted Alzet® osmotic minipump model 1007D (100-µl reservoir, 0.5 ± 0.1 µl/hr pumping rate, delivers for up to one week) with polyvinylchloride tubing. The concentration of NTX stored in each minipump was 2.0 mg/ml. The osmotic minipumps were connected to the brain infusion cannula 24 h prior to the first HBO2 treatment and allowed to deliver their content over the next seven days. The incision was closed with a wound clip.
4.6. Drugs
Naltrexone hydrochloride (NTX) was purchased from Tocris Bioscience (Ellisville, MO). NTX was freshly prepared in 0.9% physiological saline solution. Meloxicam (MetaCam®, Boehringer Ingelheim, Ridgefield, CT) and ampicillin (Apothecon®, Princeton, NJ) were used for postoperative prophylaxis of post-operative pain and infection. The doses were 2.0 mg/kg meloxicam and 100 mg/kg ampicillin administered i.m.
4.7. Statistical Analysis of Data
To assess differences in mechanical thresholds between treatment groups, all mechanical thresholds were normalized to average baseline thresholds on day 0. Areas under the curves (AUC) were calculated according to the linear trapezoidal rule and analyzed by analysis of variance (ANOVA) and post-hoc Bonferroni’s multiple comparison test.
5. Conclusion
HBO2 treatment causes an anti-allodynic effect in rats with sciatic nerve crush-induced neuropathic pain. The reversal of the anti-allodynic effect by centrally-administered NTX suggests that brain opioid mechanisms may be involved in the relief of neuropathic pain induced by HBO2.
Highlights.
Sciatic nerve crush produces a state of allodynia that lasts for up to 29 days after the procedure.
HBO2 treatment seven days after nerve crush produces an antiallodynic effect.
Implantation of an Alzet® osmotic minipump releasing 1.0 µg/hr of naltrexone reversed the HBO2-induced antiallodynia.
Acknowledgements
This research was supported by NIH Grants GM-77153 and AT-007222 and the Allen I. White Distinguished Professorship from Washington State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the κ-opiate receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chung E, Zielinski LM, Ohgami Y, Shirachi DY, Quock RM. Hyperbaric oxygen treatment induces a 2-phase antinociceptive response of unusually long duration in mice. J. Pain. 2010;11:847–853. doi: 10.1016/j.jpain.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Era MA, Hampson NB, His RA, Madsen B, Corman JM. Hyperbaric oxygen therapy for radiation induced proctopathy in men treated for prostate cancer. J. Urol. 2006;176:87–90. doi: 10.1016/S0022-5347(06)00491-5. [DOI] [PubMed] [Google Scholar]
- Di Sabato F, Fusco BM, Pelaia P, Giacovazzo M. Hyperbaric oxygen therapy in cluster headache. Pain. 1993;52:243–245. doi: 10.1016/0304-3959(93)90137-E. [DOI] [PubMed] [Google Scholar]
- George A, Buehl A, Sommer C. Wallerian degeneration after crush injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor alpha protein. Neurosci. Lett. 2004;372:215–219. doi: 10.1016/j.neulet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- George A, Marziniak M, Schafers M, Toyka KV, Sommer C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain. 2000;88:267–275. doi: 10.1016/S0304-3959(00)00333-X. [DOI] [PubMed] [Google Scholar]
- Gesell LB, editor. The Hyperbaric Oxygen Therapy Committee Report: Indications and Results. 12th Ed. Durham: Undersea and Hyperbaric Medical Society; 2008. [Google Scholar]
- Gu N, Niu JY, Liu WT, Sun YY, Liu S, Lv Y, Dong HL, Song XJ, Xiong LZ. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. Eur. J. Pain. 2012;16:1094–1105. doi: 10.1002/j.1532-2149.2012.00113.x. [DOI] [PubMed] [Google Scholar]
- Handschel J, Brüssermann S, Depprich R, Ommerborn M, Naujoks C, Kübler NR, Meyer U. Evaluation of hyperbaric oxygen therapy in treatment of patients with osteomyelitis of the mandible. Mund. Kiefer Geschtschir. 2007;11:285–290. doi: 10.1007/s10006-007-0073-5. [DOI] [PubMed] [Google Scholar]
- Hara S, Gagnon MJ, Quock RM, Shibuya T. Effect of opioid peptide antisera on nitrous oxide antinociception in rats. Pharmacol. Biochem. Behav. 1994;4:699–702. doi: 10.1016/0091-3057(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Evans AW, Bristow RG, Levin W. Treatment of radiation proctitis with hyperbaric oxygen. Radiother. Oncol. 2006;78:91–94. doi: 10.1016/j.radonc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kiralp M, Yildiz S, Vural D, Keskin I, Ay H, Dursun H. Effectiveness of hyperbaric oxygen therapy in the treatment of complex regional pain syndrome. J. Int. Med. 2004;Res. 32:258–262. doi: 10.1177/147323000403200304. [DOI] [PubMed] [Google Scholar]
- Li F, Fang L, Huang S, Yang Z, Nandi J, Thomas S, Chen C, Camporesi E. Hyperbaric oxygenation therapy alleviates chronic constrictive injury-induced neuropathic pain and reduces tumor necrosis factor-alpha production. Anesth. Analg. 2011;113:626–633. doi: 10.1213/ANE.0b013e31821f9544. [DOI] [PubMed] [Google Scholar]
- Liu S, Zylstra CC, Shirachi DY, Quock RM. The acute antinociceptive effect of hyperbaric oxygen (HBO2) is not accompanied by an increase in markers of oxidative stress. FASEB J. 2013;27 doi: 10.1016/j.lfs.2013.12.207. 890.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian HK, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology (Berl.) 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Chung E, Quock RM. Nitrous oxide-induced NO-dependent neuronal release of β-endorphin from the rat arcuate nucleus and periaqueductal gray. Brain Res. 2010;1366:38–43. doi: 10.1016/j.brainres.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami Y, Zylstra CC, Quock LP, Chung E, Shirachi DY, Quock RM. Nitric oxide in hyperbaric oxygen-induced acute antinociception in mice. NeuroReport. 2009;20:1325–1329. doi: 10.1097/WNR.0b013e3283305a49. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Burlington: Academic Press; 2007. [Google Scholar]
- Peach G. Hyperbaric oxygen and the reflex sympathetic dystrophy syndrome: a case report. Undersea Hyperb. Med. 1995;22:407–408. [PubMed] [Google Scholar]
- Pilcher WH, Joseph SA, McDonald JV. Immunocytochemical localization of pro-opiomelanocortin neurons in human brain areas subserving stimulation analgesia. J. Neurosurg. 1988;68:621–629. doi: 10.3171/jns.1988.68.4.0621. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and norbinaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Quock LP, Zhang Y, Chung E, Ohgami Y, Shirachi DY, Quock RM. The acute antinociceptive effect of HBO2 is mediated by a NO-cyclic GMP-PKG-KATP channel pathway in mice. Brain Res. 2011;1368:102–107. doi: 10.1016/j.brainres.2010.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sümen G, Çimşit M, Eroğlu L. Hyperbaric oxygen treatment reduces carrageenan-induced acute inflammation in rats. Eur. J. Pharmacol. 2001;431:265–268. doi: 10.1016/s0014-2999(01)01446-7. [DOI] [PubMed] [Google Scholar]
- Thompson CD, Uhelski ML, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases pain in two nerve injury models. Neurosci. Res. 2010;66:279–283. doi: 10.1016/j.neures.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Tuter NV, Danilov AB, Poliakova LV. The treatment of a complex regional pain syndrome. Zh. Nevrol. Psikhiatr. Im S. S. Korsakova. 1997;97:33–35. [PubMed] [Google Scholar]
- Warren J, Sacksteder MR, Thuning CA. Therapeutic effect of prolonged hyperbaric oxygen in adjuvant arthritis of the rat. Arthritis Rheum. 1979;22:334–339. doi: 10.1002/art.1780220404. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Toepfer VE, Senapati AK, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment is comparable to acetylsalicylic acid treatment in an animal model of arthritis. J. Pain. 2007;8:924–230. doi: 10.1016/j.jpain.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res. 2006;1098:126–128. doi: 10.1016/j.brainres.2006.04.088. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Foresman BH, Gamber RG, Wright T. Hyperbaric oxygen in the treatment of migraine with aura. Headache. 1998;38:112–115. doi: 10.1046/j.1526-4610.1998.3802112.x. [DOI] [PubMed] [Google Scholar]
- Yildiz S, Kiralp MZ, Akin A, Keskin I, Ay H, Dursun H, Cimsit M. A new treatment modality for fibromyalgia syndrome: hyperbaric oxygen therapy. J. Int. Med. Res. 2004;32:263–267. doi: 10.1177/147323000403200305. [DOI] [PubMed] [Google Scholar]
- Zelinski LM, Ohgami Y, Chung E, Shirachi DY, Quock RM. A prolonged NO-dependent, opioid-mediated antinociceptive effect of hyperbaric oxygen in mice. J. Pain. 2009a;10:167–172. doi: 10.1016/j.jpain.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski LM, Ohgami Y, Quock RM. Exposure to nitrous oxide stimulates a nitric oxide-dependent neuronal release of β-endorphin in ventricular cisternally-perfused rats. Brain Res. 2009b;1300:37–40. doi: 10.1016/j.brainres.2009.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gibbons CR, Howlader S, Son MC, Yeon J, Shirachi DY, Quock RM. Suppression of paclitaxel-induced neuropathic pain by hyperbaric oxygen. FASEB J. 2012;26 662.9. [Google Scholar]