Abstract
Biliary atresia (BA) is an infantile obstructive cholangiopathy of unknown etiology with suboptimal therapy, which is responsible for 40 to 50% of all pediatric liver transplants. Although the etiology of bile duct injury in BA in unknown, it is postulated that a pre- or perinatal viral infection initiates cholangiocyte apoptosis and release of antigens that trigger a Th1 immune response that leads to further bile duct injury, inflammation, and obstructive fibrosis. Humoral immunity and activation of the innate immune system may also play key roles in this process. Moreover, recent investigations from the murine BA model and human data suggest that regulatory T cells and genetic susceptibility factors may orchestrate autoimmune mechanisms. What controls the coordination of these events, why the disease only occurs in the first few months of life, and why a minority of infants with perinatal viral infections develop BA are remaining questions to be answered.
Keywords: neonatal cholestasis, adaptive immunity, innate immunity, humoral immunity
Biliary atresia (BA) is the most common cause of neonatal cholestasis that progresses to cirrhosis and end-stage liver disease; hence, it is the leading indication for liver transplantation in children. It has a varying incidence, from in 1 in 5,000 live births in Taiwan to 1 in 18,000 in Europe and 1 in 12,000 in the United States. Clinical presentation is characterized by persistent jaundice and progressive cholestasis developing within weeks of birth, caused by a progressive fibroobliterative obstruction of extra- and intrahepatic bile ducts.1–5 Early diagnosis is important because hepatoportoenterostomy (HPE: the Kasai procedure) can restore bile flow and help prevent worsening of the liver disease if performed in the first 2 to 3 months, with best results before 30 to 45 days of life. Unfortunately, more than 70% of children eventually develop cirrhosis and require liver transplantation before adulthood, even if the HPE had been considered successful.2,3 Establishing the diagnosis before 45 days of life is not common in Western countries, thus the diagnosis is often delayed and treatment inadequate. Although there are no convenient means of screening newborns, use of a stool color card has been a promising way to identify BA in the first month of life.6
There are two major forms of BA: the acquired/perinatal form and the embryonic/congenital form. The rarer embryonic form is associated with other congenital anomalies including, but not limited to, the biliary atresia splenic malformation syndrome, and is believed to be caused by genetic or somatic mutations in genes that determine bile duct development, although none have been specifically identified in BA. Approximately 80% of patients with BA are of the acquired/perinatal form, which is the subject of this review. In this form, a progressive inflammatory injury of extrahepatic and intrahepatic bile ducts leads to fibrosis and obliteration of the lumen.1–3 Because treatment is currently inadequate, it is imperative that a better understanding of the pathogenesis of BA be achieved to identify new targets for therapeutic intervention. A leading hypothesis in the pathogenesis of BA is that bile duct injury is initiated by a virus infection (possibly prenatal) followed by an exaggerated inflammatory or autoimmune response targeting bile duct epithelia, resulting in progressive bile duct injury and obliteration and secondary biliary cirrhosis.1,2,5 This review will examine current evidence that provides clues to the pathogenesis of bile duct injury in BA, emphasizing a viral trigger and a proposed interplay of innate and adaptive immune responses in the pathogenesis of disease (Fig. 1).
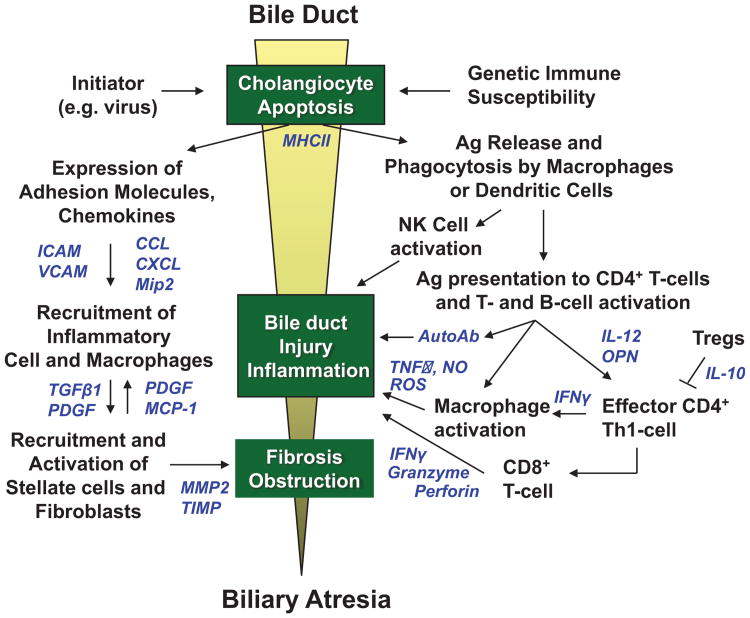
Figure 1.
Proposed etiology of bile duct injury in perinatal/acquired biliary atresia. A pre- or perinatal initiating event, such as a viral infection (rotavirus, reovirus, cytomegalovirus [CMV]) induces apoptosis of bile duct epithelia and aberrant MHC class II expression in extrahepatic and intrahepatic bile ducts in a genetically susceptible host. Viral, native, or altered bile duct antigens are phagocytosed by macrophages or dendritic cells and presented to naïve T cells in local lymph nodes in which CD4+ T cells are activated and proliferate (right side of figure). These activated CD4+ T cells (which may be autoreactive) home back to the original site of antigen exposure and elicit IFN-γ-induced macrophage stimulation and activation of cytotoxic CD8+ T cells and B cells. Release of TNF-α, nitric oxide (NO) and reactive oxygen species (ROS) by macrophages, autoantibodies by plasma cells, and granzyme, perforin, and interferon-γ (IFN-γ) by CD8+ T cells produce further cholangiocyte injury through apoptotic or necrotic pathways. Simultaneously, cholangiocytes and vascular endothelial cells upregulate expression of adhesion molecules and secrete chemokines to recruit neutrophils and macrophages to the site of bile duct injury (left side of figure). These cells then recruit and activate hepatic stellate cells (myofibroblasts) and fibroblasts, which secrete extracellular matrix causing fibrosis of injured bile ducts. The resulting cholangiocyte injury, inflammation, and fibrosis lead to complete bile duct obstruction and the phenotype of biliary atresia. Soluble inflammatory mediators in these pathways are shown in blue.
Triggers for Initiation of Bile Duct Injury in Biliary Atresia
The “trigger” for BA remains unknown. Theories on pathogenesis include genetic predisposition, abnormal morphogenesis,7,8 vascular abnormalities, exposure to environmental toxins, viral infection,9–11 and autoimmune mediated bile duct destruction.12,13 Initially, the inciting insult was thought to occur during the first 3 months of life in otherwise healthy infants; however, recent work by Harpavat et al suggests that BA may already be present in the immediate newborn period.14 In their study, all 34 infants with BA had significantly elevated serum direct or conjugated bilirubin levels in the first 4 days of life suggesting that at least in some patients, bile duct injury begins antenatally.14 Most attention has been directed to a perinatal viral infection as a key trigger in human BA and the mouse model of BA.
Viruses and Biliary Atresia
In 1974, Benjamin Landing first proposed that BA and other infantile obstructive cholangiopathies were caused by viral infection of the liver and hepatobiliary tree.15 This theory was initially supported by the presence on liver biopsy of a portal tract mononuclear cell infiltrate suggesting an inflammatory process leading to bile duct obstruction. More recently, this theory has been supported by the development of a virally induced mouse model of BA and by the observation that the immune response in BA is similar to that seen with viral infection (high expression of Mx protein, induction of Th1 response, and upregulation of Toll-like receptors [TLR] 3 and 7).16–18 Multiple viruses including hepatitis B,15 human herpes viruses,19 human papillomavirus,20 Epstein-Barr virus,21 cytomegalovirus (CMV),19,22 rotavirus,9 and reovirus23 have been proposed in the etiology of BA. Attempts to identify a virus in serum and liver tissue from infants with BA have yielded conflicting results. Attention in recent years has focused on reovirus, rotavirus, and CMV, which will be detailed below.
Reovirus
Reovirus is a double-stranded RNA virus from the Reoviridae family. Interest in reovirus arose from the observation that young laboratory mice infected with reovirus developed pathologic features in the intrahepatic and extrahepatic bile ducts and liver parenchyma similar to those seen in infants with BA, even after viral antigens had been cleared.24–26 Infection with reovirus type 3 in mice resulted in “oily hair syndrome” with bile duct obstruction, fat malabsorption, failure to thrive, diarrhea, and jaundice in nonfurbearing regions.27,28 After one week, mice had a tortuous and edematous extrahepatic bile duct and intrahepatic portal tracts with marked periportal inflammation.26,29 Specific changes in the amino acid sequence in the viral cell attachment protein, sigma, in the T3 Abney strain of reovirus type 3, are important in the tropism of the virus for bile duct epithelia.28 Furthermore, tropism of reovirus to bile duct epithelial cells is dependent on sialic acid binding.30 Most recently, Harada et al demonstrated that bile epithelial cells from patients with BA express TLR 3, which recognizes viral double-stranded RNA (dsRNA). When bile epithelial cells are stimulated by a synthetic analog of viral dsRNA, poly (I:C), they produce interferon-β1 and MxA, upregulate the expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), and induce cholangiocyte apoptosis. Thus, bile duct epithelial cells, in response to viral dsRNA, appear to undergo apoptosis that induces the processes leading to obstructive cholangiopathy.31
Human BA studies that examined serologic, immunohistochemical, and reverse-transcription polymerase chain reaction (RT-PCR) evidence of reovirus yielded conflicting results. Three studies reported an increased prevalence of anti reovirus IgG or IgM antibodies in BA patients compared with control infants10,32,33; however, two other studies found no difference.34,35 It should be noted that circulating IgG during the first 6 months of life often reflects transplacental passive acquisition of antibody as opposed to primary neonatal infection. Other groups have examined liver and hepatobiliary tissue for reovirus nucleic acids using RT-PCR. Using nested primers specific to the M3 gene segment of reovirus serotype 3, Steele et al did not detect reovirus RNA in formalin-fixed liver tissue from 14 BA patients.36 However, Tyler et al found reovirus L1 gene amplification products in liver and biliary tissues from 11 out of 20 (55%) patients with BA.23 Confirming the latter, Rauschenfels et al utilized nested primers specific to the L3 gene segment of reovirus and found evidence of reovirus in 21 out of 64 (33%) BA samples.18
Rotavirus
Rotavirus is also a double-stranded RNA virus from the Reoviridae family. In 1993, Riepenhoff-Talty et al first described two strains of group A rotaviruses that replicated in the liver and bile ducts of infant mice causing cholestasis similar to that seen in infants with BA.37 Thereafter, biliary obstruction was shown to be dependent on time of viral inoculation, being highest with inoculation at 12 hours of life and decreasing with later timing of infection.38 Mack et al further demonstrated that mice infected with Rhesus rotavirus (RRV) on day-of-life 1 became jaundiced by one week, developed biliary obstruction and portal inflammation by 2 weeks despite evidence of viral clearance, and died by 1 month of age.37,39 In the murine model, the cholangiocyte is one target for RRV infection.40 Biliary tropism appears to be strain specific, among five rotavirus strains only RRV and SA11-FM directly infect cholangiocytes and cause extrahepatic bile duct inflammation and obstruction.40 Studies utilizing single-gene reassortments have identified that RRV gene segment 4, which codes for the viral spike protein VP4, plays a significant role in cholangiocyte tropism and viral entry.41,42 Moreover, cholangiocyte expression of α2β1-integrin plays a critical role in rotavirus attachment and infection. When an anti-α2 monoclonal antibody was administered to newborn pups before infection with rotavirus, the mice were less susceptible to BA.43 In addition, nonstructural protein 1 (NSP1) allows viral replication due to its ability to suppress the host innate immune response by blocking type I interferon production.42 The RRV-induced BA mouse model has proved to be exceptionally helpful in investigating the role of the immune system and inflammation in the pathogenesis of bile duct injury in BA.
Evidence of rotavirus in human BA has been conflicting. Riepenhoff-Talty et al reported that 50% of BA liver specimens were positive for group C rotavirus RNA by RT-PCR compared with zero controls.9 However, Bobo et al did not detect rotavirus RNA (group A, B, or C) in any infant BA or control samples.44
Cytomegalovirus
CMV is a double-stranded DNA virus of the Herpesviridae family. Similar to reovirus and rotavirus, CMV can infect biliary epithelia as demonstrated by CMV inclusion bodies seen within bile duct epithelia.45–47 CMV has been implicated in neonatal hepatitis,48 ischemic vasculopathy,49 and intrahepatic bile duct paucity.50 In a recent study in China, CMV DNA was identified in 51 out of 85 (60%) BA patients51; however, other groups have found conflicting results.52 Fischler's group from Sweden has shown higher prevalence of CMV antibodies in the mothers of BA infants, higher serum CMV-IgM levels in infants with BA, and greater amounts of immunoglobulin deposits on the canalicular membrane of the hepatocytes in infants with BA with ongoing CMV infection.22,53 Brindley et al recently identified a liver-based T cell response to CMV in 56% of BA patients compared with minimal response in disease controls. In addition, a deficit of regulatory T cells was present in those BA patients who were positive for CMV, suggesting a possible mechanism for decreased inhibition of inflammation and autoreactivity allowing for ongoing bile duct injury.54 A group in China recently demonstrated a lower rate of jaundice disappearance, a higher incidence of cholangitis, and a more severe degree of liver fibrosis and inflammation after HPE in BA infants with CMV infection suggesting that CMV infection may correlate with a worse prognosis in BA.55 In contrast, Fischler et al did not detect a difference in long-term survival with regard to early CMV infection; however, this study was limited by a small sample size.56
Role of B Cells and Humoral Immunity in Biliary Atresia
In recent years, attention has turned to the possible role of B cells and humoral immunity in the pathogenesis of BA. Many experimental models of autoimmune diseases have shown the importance of B cells in disease pathogenesis, including type 1 diabetes,57 lupus,58 arthritis,59 and autoimmune thyroiditis.60 However, to date published work on the role of B cells and humoral immunity in BA has been limited. Initial studies found periductal immunoglobulin deposits along the basement membrane of bile duct epithelia within extrahepatic bile duct remnants of human BA patients.61 Mack et al then demonstrated the presence of periductal immunoglobulin deposits and serum autoantibodies reactive to bile duct epithelial proteins in RRV-diseased mice.62 Recently, Lu et al identified α -enolase as an autoantibody reactive to cytosolic proteins within bile duct epithelia that is present in mice with RRV induced biliary obstruction and in serum samples from human infants with BA. This 48-kD enzyme shares amino acid sequence similarities with rotavirus-encoded proteins, suggesting a role for molecular mimicry.63 Of note, the presence of α-enolase antibodies are also found in serum from patients with other autoimmune diseases including rheumatoid arthritis,64 inflammatory bowel disease,65 multiple sclerosis,66 autoimmune hepatitis,67 and primary biliary cirrhosis.68 In our laboratory, we have recently investigated the role of B cells in murine BA through the use of B cell knockout (Ig-α-/-) mice; preliminary data reveal that RRV-infected Ig-α-/- mice are protected from the development of BA.69 Thus, further investigation of humoral immunity and its role as a potential target in BA treatment needs to be explored.
Cellular Immune Response and Autoimmunity in Biliary Atresia
Adaptive immunity entails immune responses that are stimulated by repeat exposure to a pathogen or non-microbial proteins (antigens). The defining characteristics of adaptive immunity include the fine specificity for distinct molecules and memory that evokes the ability to respond to repeat exposures. Effector T cells in adaptive immunity produce cytokines that can directly damage cells or indirectly cause damage through activation of other immune cells (such as IFN-γ -producing CD4+ T cells that activate macrophages with subsequent generation of cytotoxic molecules such as nitric oxide and TNF-α). T cell responses have been categorized based on the type of cytokines that are generated: Th1 responses involve IL-2, IFN-γ, and TNF- α, and Th2 responses include IL-4, IL-5 and IL-10. In the past decade, much attention has focused on the role of Th1 cellular immunity in bile duct injury in BA. The RRV-induced murine model of BA recapitulates the immune response found in the human disease. In murine BA, the portal tract CD4+ T cells produce IFN-γ and TNF-α one week after RRV infection, followed by CD8+ T cell and macrophage infiltration by 2 weeks of age.39 Coinciding with the Th1 cellular cytokines identified, Leonhardt et al70 found that many chemokines associated with a Th1 response were upregulated in the murine model, including CCL2, CCL5, and CXCL10 (IFN-γ inducible protein [IP]-10 chemokine). Similarly, Carvalho et al71 reported increased gene expression of IFN-γ-induced chemokines CXCL9 and CXCL10 at one week post-RRV infection. With regard to the important role of IFN-γ in bile duct injury, Shivakumar et al72 demonstrated that RRV-infected IFN-γ knockout mice developed jaundice in a similar manner as the wild-type controls; however, the cholestasis resolved by 3 weeks of age in 77% of the knockout mice compared with progression of disease in 75% of the wild-type controls. This study strengthened the contention that the immune response, and not the initial viral infection, was responsible for the progression of bile duct injury. The role of IL-1273and TNF-α74 in the progression of murine BA has also been analyzed through the use of cytokine knockout mice. In contrast to the IFN-γ studies, the down-regulation of these cytokines failed to alter the progression of the bile duct injury and obstruction.
The potential role of Th2 cytokines in bile duct injury has been recently reported in murine BA.75 In this study, Stat1 knockout mice that lack the ability to generate Th1 responses developed a cystic biliary form of BA after RRV infection that was associated with excess Th2 cytokines. This interpretation is questioned by the fact the Stat1 knockout mice were unable to clear infection and had persistent high levels of replicating RRV that most certainly contributed to bile duct injury. Further assessment of the role of Th2 was assessed utilizing a Stat1/Stat6 double knockout mouse that cannot produce either Th1 (STAT1-dependent) or Th2 (STAT6-dependent) cytokines. Stat1/Stat6 double knockout mice developed BA similar to wild-type mice. An important study that was not included in this work, which would more precisely address the importance of Th2 cytokines to disease pathology, would be to analyze the incidence of BA in RRV-infected Stat6 single knockout mice that are unable to generate Th2 cytokines.
In human BA, the predominant cellular immune response at diagnosis encompasses activated CD4+ and CD8+ T cells within portal tracts that produce Th1 cytokines (IL-2, IFN- γ) and macrophages that generate TNF-α.76–79 These lymphocytes have been found invading between bile duct epithelia, leading to degeneration of intrahepatic bile ducts.80 The T cells are highly activated, expressing the proliferation cell surface marker CD71 and activation markers CD25 and LFA-176 In addition, the T cells display increased expression of the chemokine receptor CXCR3, which binds ligands that support Th1 cellular differentiation.79 Bezerra et al,81 utilizing gene expression microarray techniques to analyze BA liver biopsies, observed upregulation of proinflammatory genes including IFN- γ and osteopontin. Osteopontin is a Th1 cytokine that is secreted by a variety of cell types and plays a key role in immune cell recruitment to sites of inflammation as well as extracellular matrix formation and fibrosis. Two groups recently demonstrated increased levels of osteopontin in BA liver tissue that localized to bile duct epithelia and correlated with degree of fibrosis.17,82 In addition, a positive correlation was identified between osteopontin and both NF-κB (upstream transcription factor activating osteopontin) and TGFβ (downstream effector molecule associated with fibrosis).17 The upregulation of osteopontin persisted in BA, as older BA patients (mean age 8.2 years) had significantly increased levels of plasma osteopontin compared with controls, with the BA patients with persistent jaundice or portal hypertension expressing the highest levels of osteopontin.83
Cytokine production and activity is usually localized with in the target organ; however, with increased severity of inflammation, the cytokines may be detected in blood. To identify plasma biomarkers of disease, Narayanaswamy et al analyzed plasma Th1 (IL-2, IFN- γ), Th2 (IL-4, IL-10), and macrophage (IL-18, TNF-α) cytokines and adhesion molecules ICAM-1 and VCAM-1.84 All cytokines (except IL-10) and adhesion molecules increased within the first 6 months post-HPE, suggesting that the inflammatory process is progressive and not ameliorated by HPE. Importantly, sICAM-1 was identified as a probable biomarker of disease severity as plasma sICAM-1 correlated positively with serum bilirubin and a high sICAM-1 level predicted need for transplantation in the first year of life. ICAM-1 is an adhesion molecule that is expressed on cells of multiple lineages at sites of inflammation and mediates leukocyte and granulocyte migration through endothelial cells as well as promotes antigen-specific T-cell proliferation. Analysis of polymorphisms of the ICAM-1 gene in BA revealed that the ICAM G241R polymorphism was significantly associated with BA, suggesting that ICAMs play a role in disease pathogenesis.85
The inciting event that triggers the Th1 inflammatory response is unknown and theories include virus infection and bile duct-targeted autoinflammatory or autoimmune responses. The strongest evidence for autoimmunity has been gained from murine studies, where autoreactive T cells targeting bile duct epithelia have been identified. Two groups have demonstrated that autoreactive T cells specific to bile duct epithelia are present in murine BA and are associated with bile duct inflammation and injury.62,86 In vitro analyses demonstrated significant increases in liver T cells from BA mice that generated IFN-γ in response to either RRV or self-bile duct epithelial antigens.62 In addition, adoptive transfer of liver T cells from BA mice into immunodeficient recipients led to bile duct-specific inflammation and injury. This induction of bile duct pathology occurred in the absence of detectable transferred virus, suggesting that bile duct antigens were the target of the T cells. Further investigations aimed at determining the mechanisms (molecular mimicry or bystander activation) of the apparent virus-induced autoimmunity are necessary.
In humans, only circumstantial evidence exists for the role of autoimmunity in BA pathogenesis.13 Circumstantial evidence established by Rose and Witebsky87 for categorizing a disease as autoimmune in nature include the following. (1) Familial increased incidence of autoimmunity—this question will be answered shortly through data presently being collected within the nationwide Childhood Liver Disease Research and Education Network (ChiLDREN), a multicentered NIH-funded research consortium investigating many aspects of the pathogenesis and treatment of BA. (2) Lymphocytic infiltrate of the target organ, especially if there is restricted TCR-Vβ usage—multiple studies have identified lymphocytic infiltrates surrounding and invading the intrahepatic ducts and extrahepatic biliary remnant.76–80 Furthermore, antigen-specific T cell immunity involves oligoclonal expansion of T cells expressing similar T cell receptor variable regions of the β chain (TCR Vβ). Analysis of the TCR Vβ within BA liver and extrahepatic bile duct remnants revealed that the T cells are oligoclonal in nature, with a limited TCR Vβ repertoire, suggesting antigen-specific activation.88 The CD4+ TCR expansions were limited to Vβ3, 5, 9, and 12 T cell subsets and the CD8+ TCR Vβ expansions were predominantly Vβ20. Nucleotide sequencing of the expansions confirmed that each identified Vβ subset was composed of oligoclonal populations of T cells, suggesting proliferation in response to specific antigenic stimulation. Future studies will be required to identify the specific antigen(s) responsible for T cell activation and bile duct injury. (3) Statistical association with human leukocyte antigen genotype or aberrant expression of HLA class II antigens on the affected organ—normal bile duct epithelium express HLA class I but not class II, which is usually present on professional antigen presenting cells (i. e., B cells, macrophages, dendritic cells) and vascular endothelium. Bile duct epithelium from BA patients aberrantly expresses MHC class II, with strong expression of class II HLA-DR on liver bile duct epithelia.89,90 One of the strongest genetic associations with autoimmunity is with the HLA genes. HLA associations with BA have been reported with conflicting results. A European study analyzed the HLA genotypes in 101 BA children and found no significant differences compared with controls.91 In contrast, a Japanese study of 392 BA patients found significant association between BA and HLA-DR2 as well as a linkage disequilibrium with a high frequency of HLA-A24-B52-DR2.92 In the United States, through support from ChiLDREN, high resolution genotyping of all HLA class I and class II alleles is nearing completion on 180 BA patients and 360 controls to determine if there are any HLA associations with BA. (4) Favorable response to immunosuppression—at the time of HPE, immunosuppressive therapy has been investigated, with the goal of establishing long-term bile flow. In England, a randomized prospective, blinded, placebo-controlled trial of post-HPE corticosteroids in 71 BA patients was performed.93 The dose of oral prednisolone was 2 mg/kg/day from days 7 to 21 post-HPE and 1 mg/kg/d from days 22 to 28. There was no difference in bilirubin levels at 6 and 12 months post-HPE and both groups had similar rates of transplantation. A study from Germany assessed the use of high dose steroids post-HPE and found that overall survival with the native liver was 63% at 6 months and 31% at 2 year, with no statistical difference between the steroid and control groups.94 A recent meta-analysis of randomized trials and observational studies pertaining to steroids in BA encompassed all studies between 1969 and 2010 and included 233 BA patients.95 There was no significant difference in the effect of steroids on serum bilirubin levels at 6 months post-HPE or in delaying the need for early liver transplantation. A prospective, randomized, double-blinded, placebo-controlled trial of high dose post-HPE corticosteroid therapy (intravenous followed by oral administration) will be completed in late 2012-2013 from the United States.96
Contributors to Dysregulated Immunity in Biliary Atresia
Regulatory T-Cell (Treg) Deficits in Biliary Atresia
The Treg subset of CD4+ T cells is responsible for controlling T-cell-mediated immune responses to pathogens to prevent “bystander damage” of healthy cells. Furthermore, Tregs are necessary to prevent activation of autoreactive T cells. In neonates, the percentage of Tregs increases significantly in the first 5 days of life, reaching adult levels at that time.97 In BA, deficits in the number or function of Tregs would allow for inflammation to flourish unchecked. In the above-mentioned viral immunity study by Brindley et al,54 they determined if the presence of virus infection in BA was associated with quantitative changes in Tregs. Peripheral blood Treg quantification revealed significant deficits in Treg frequencies in BA patients compared with controls, with the most marked deficits in those BA patients who were positive for CMV. The authors speculated that loss of adequate numbers of Tregs in BA could be responsible for decreased inhibition of inflammation or autoreactivity, thereby allowing for exaggerated bile duct injury.
The importance of Tregs to the pathogenesis of BA has also been assessed in murine BA. It is known that mice in Tregs exit the thymus on day 3 of life and populate the peripheral lymph nodes and spleen. Miethke et al98 reported that RRV-infected neonatal mice had decreased numbers of Tregs 3 days after infection compared with mice that were 7 days old at the time of infection, suggesting that infection at birth was associated with the inability to produce a prompt Treg response. Adoptive transfer of total CD4+ T cells, but not Treg-depleted CD4+ T cells, into neonatal RRV-infected mice was associated with increased survival and decreased bile-duct targeted inflammation.99 Treg deficits were associated with upregulation of costimulatory molecules on dendritic cells and associated activation of CD8+ T cells, resulting in bile duct injury. Novel therapies aimed at expanding Treg populations in BA patients may prove to be beneficial.
DNA Hypomethylation: A Potential Mechanism Propagating the Inflammatory Response in Biliary Atresia
Changes in DNA methylation can be elicited by drugs, environmental factors, viruses, or genetic factors, leading to repression of gene expression.100 DNA hypomethylation has been implicated in playing a role in autoimmune diseases101 and in inhibiting lymphocyte differentiation.102 Importantly, a previous array study identified a set of genes, known to be regulated by DNA methylation, that were significantly increased in BA patients.103 Matthews et al104 hypothesized that DNA hypomethylation may be involved in the pathogenesis of BA. A novel model of BA was utilized to test this hypothesis: the zebrafish model of biliary obstruction. The effects of complete inhibition of DNA methylation on the biliary system was assessed using the zebrafish mutant duct-trip (dtp) which contains a mutation in the gene for Sadenosyl homocysteine hydrolase, resulting in global reduction of DNA methylation. Second, wild-type larvae were treated with the DNA methylation inhibitor, azaC. Both models showed diminished biliary excretion and poorly defined intrahepatic bile ducts on histology, suggesting a role of DNA hypomethylation in bile duct obstruction. Analysis of hepatic gene expression from the larvae that had received azaC revealed upregulation of genes involved in IFN-γ and NF-κB signaling, antigen processing, and presentation. Administration of glucocorticoid to azaC-treated zebrafish resulted in improved biliary flow associated with attenuation of IFN-γ target genes. Analysis of human BA liver tissue revealed significant decreases in DNA methylation based on bile duct cell nuclear methylcytosine levels, providing further evidence for the potential role of DNA hypomethylation in the pathogenesis of BA.
A complementary study performed by Dong et al105 assessed the DNA methylation patterns within CD4+ T cells from BA patients and found that certain genes were hypomethylated, including DNA methyltransferase (DNMT1), DNMT3a, and methyl-DNA-binding domain (MBD1). Importantly, the IFN-γ gene promoter region was also hypomethylated in BA CD4+ T cells and IFN-γ mRNA expression levels were significantly increased. The authors concluded that methylation changes in CD4+ T cells results in unchecked regulation of IFN-γ expression and contributes to bile duct injury in BA.
The Relationship of Single-Nucleotide Polymorphisms (SNPs) and Susceptibility Genes in the Pathogenesis of Biliary Atresia
Using the advanced technology of genome-wide association studies (GWAS), a group from China genotyped nearly half a million SNPs in 200 BA patients and 481 controls.106 The 10 SNPs most highly associated with BA were then analyzed with an independent set of 124 BA patients and 90 controls. The validation study revealed a strong association of BA with the SNP rs17095355 on chromosome 10q24. Two genes in the region of this SNP include X-prolyl aminopeptidase P1 (XPNPEP1) and adducin 3 (ADD3). XPNPEP1 is expressed in biliary epithelia and is involved in the metabolism of inflammatory mediators. Genetic defects of XPNPEP1 could result in dysregulation of control of the inflammatory response present in BA. ADD3 is expressed in hepatocytes and biliary epithelia and is involved in the assembly of spectrin-actin membrane protein networks at sites of cell to cell contact. Defective ADD3 could result in excessive deposition of actin and myosin, contributing to biliary fibrosis. The SNP rs17095355 identified in this Chinese study should be investigated in BA patients in the United States and Europe to determine if the gene defect spans all ethnicities. A thorough GWAS should also be performed in the United States and Europe to identify novel SNP associations with BA.
Activation of Innate Immunity and Biliary Atresia Pathogenesis
The innate immune system responds to infection or danger signals by producing a rapid, nonspecific inflammatory response with the release of proinflammatory cytokines such as TNF-α, IL-1, and IL-6. Cells of the innate immune system, including macrophages, neutrophils, dendritic cells, natural killer (NK) cells, mast cells, and others, possess two major classes of receptors: membrane-bound TLRs and cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLR), collectively known as pattern-recognition receptors (PRR).107 Importantly, bile duct epithelial cells also express PRRs.108 PRRs recognize pathogen-associated molecular patterns (PAMPs) on or released by infected cells, which are conserved molecular patterns that are invariant among an entire class of pathogens. Examples of PAMPs include bacterial lipopolysaccharide (LPS) and lipoproteins, dsRNA and single-stranded viral RNA (ssRNA). Each TLR subtype recognizes and binds to a particular set of PAMPs. For example LPS is detected by TLR4, dsRNA by TLR3, and ssRNA by TLR7/TLR8.109 It has also been shown that endogenous ligands (danger signals) from necrotic cells, in addition to pathogens, can activate TLR signaling, of potential importance as a link between TLR activation and the development of autoimmunity.110 PRR-PAMP interactions result in the synthesis and release a variety of inflammatory mediators (including cytokines and chemokines), culminating in pathogen (and sometimes host cell) death. In several disease models, the failure to regulate TLR signaling is associated with a chronic inflammatory disease.
The state of innate immunity activation has recently been investigated in BA. Saito et al111 reported upregulation of TLR8 in livers from BA patients at diagnosis compared with disease controls. In addition, there were significantly increased levels of hepatic TLR3 and TLR7 at diagnosis of BA in those patients who eventually required transplant (vs those who did not require transplant). Interestingly, these TLRs are receptors for either dsRNA or ssRNA present in viruses. Huang et al17 also analyzed liver specimens obtained at time of diagnosis of BA and found increased levels of TLR7 mRNA compared with choledochal cyst controls. Strong expression of TLR7 was noted by immunohistochemistry in bile duct epithelia, Kupffer cells, and neutrophils. Because TLR7 ligation by ssRNA viruses subsequently activates type 1 interferons through the signaling molecule MxA, this path way was interrogated further. Significantly increased levels of MxA were found in BA samples compared with controls, suggesting stimulation of type 1 interferons.17 Most recently, Harada et al31 demonstrated TLR3 expression in bile duct epithelial cells from patients with BA, which binds viral dsRNA, such as present in reovirus. When the bile epithelial cells were stimulated by a synthetic analog of viral dsRNA that activates TLR3 signaling, poly(I:C), they produced MxA and interferon-β1, upregulated the expression of TRAIL, and induced biliary apoptosis. Thus, bile duct epithelial cells have the capacity to play an active role in activating innate immunity through the dsRNA virus-TLR3 signaling pathway, generating cholangiocyte apoptosis that could lead to obstructive cholangiopathy.
Macrophages function in both the innate and adaptive immune responses. Increased numbers of macrophages/Kupffer cells have been identified in the portal tracts in BA at the time of diagnosis.76,77,112–114 Urushihara et al113 identified significantly increased number and size of Kupffer cells in the liver and increased serum IL-18. IL-18 (IFN-γ-inducing factor) is a macrophage-derived cytokine that works in concert with IL-12 to promote Th1 cell differentiation in the inflammatory setting. Our group also described dramatic increases in the number and size of macrophages infiltrating the portal tracts in BA and showed that these cells were producing high levels of TNF-β.77 In addition, marked CD68-positive macrophage infiltrate in the portal tracts correlated with a worse outcome in BA patients.114 Other investigators have focused on identifying polymorphisms of macrophage-associated genes that could result in altered macrophage function. Genomic DNA analysis from 90 BA patients identified significantly increased frequencies of T allele and T/T homozygosity of the CD14/-159 promoter polymorphism. The T/T homozygote has been associated with increased expression of CD14 in monocytes. CD14 is a macrophage cell-surface glycoprotein that recognizes endotoxin (LPS) and activates TNF-α. CD14 has also been found to be important in controlling virus infections.115 This study also found that the promoter polymorphism correlated with depressed levels of plasma soluble CD14 (sCD14) and poorer outcome. Circulating sCD14 is an important mediator in neutralizing LPS. The authors concluded that exaggerated activation of macro phages through CD14 promoter polymorphisms resulted in excess stimulation of innate immunity and contributed to bile duct damage. A study from Turkey demonstrated an increased frequency of the macrophage migration inhibitory factor (MIF)-173C allele in BA patients compared with controls.116 MIF is a pleiotropic lymphocyte and macrophage cytokine that plays an important role in innate immunity. Promoter polymorphisms of the MIF gene have been associated with overproduction of MIF and increased susceptibility to chronic inflammatory diseases.117,118
Work in the RRV mouse model of BA has demonstrated a role for NK cells in bile duct epithelial injury. Depletion of NK cells or antibody blockade of their Nkg2d receptor immediately after birth prevented jaundice in newborn mice infected with RRV, who subsequently survived into adulthood.72 This work has not been replicated in human infants with BA, but may identify a new cellular target for therapeutic development. These data suggest that a sustained induction of the innate response, without the development of tolerance, resulting in chronic inflammation and injury to bile duct epithelia119 may play a role in BA. There is a paucity of research on the role of dendritic cells, NK cells, and neutrophils in human BA and should be the focus of future research efforts.
Summary
Advances in molecular biology, immunology, genetics, microbiology, and biotechnology have provided the scientific environment needed to better understand the etiology of bile duct injury in BA. Over the past decade, a clearer picture of the role of the immune system in the initiation and progression of biliary injury, inflammation, and fibrosis in the aquired perinatal form of BA has emerged. The roles of autoimmunity, dysregulated cellular, humoral and innate immunity, and T-regulatory cell function will certainly be teased out in coming years, with the hope of developing biomarkers of disease susceptibility, progression and outcomes, and ultimately new therapeutics aimed at defined disease causing targets. Multiinstitutional and multinational collaborations will continue to be critical to amass patient populations, datasets, and specimens of sufficient quantity (and quality) to allow for large-scale analyses. What controls the coordination of these pathogenic events, why the disease only occurs in the first few months of life, and why a minority of infants with perinatal viral infections develop BA are remaining questions to be answered.
Acknowledgments
Supported in part by grants from the National Institutes of Health: NIDDK R01 DK078195 (Mack), NCRR Colorado CTSA Grant UL1RR025780 (Sokol), NIDDK T32 DK 067009 (Sokol), and NIDDK U01DK062453 (Sokol). Its contents are the authors' sole responsibility and do not necessarily represent official NIH views.
References
- 1.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57(5 Pt 2):87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 2.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37(1):4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374(9702):1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 4.Petersen C. Pathogenesis and treatment opportunities for biliary atresia. Clin Liver Dis. 2006;10(1):73–88,vivi. doi: 10.1016/j.cld.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bessho K, Bezerra JA. Biliary atresia: will blocking inflammation tame the disease? Annu Rev Med. 2011;62:171–185. doi: 10.1146/annurev-med-042909-093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lien TH, Chang MH, Wu JF, et al. Taiwan Infant Stool Color Card Study Group. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology. 2011;53(1):202–208. doi: 10.1002/hep.24023. [DOI] [PubMed] [Google Scholar]
- 7.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16(4):1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 8.Tan CE, Driver M, Howard ER, Moscoso GJ. Extrahepatic biliary atresia: a first-trimester event? Clues from light microscopy and immunohistochemistry. J Pediatr Surg. 1994;29(6):808–814. doi: 10.1016/0022-3468(94)90377-8. [DOI] [PubMed] [Google Scholar]
- 9.Riepenhoff-Talty M, Gouvea V, Evans MJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174(1):8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Morecki R, Glaser JH, Cho S, Balistreri WF, Horwitz MS. Biliary atresia and reovirus type 3 infection. N Engl J Med. 1982;307(8):481–484. doi: 10.1056/NEJM198208193070806. [DOI] [PubMed] [Google Scholar]
- 11.Jevon GP, Dimmick JE. Biliary atresia and cytomegalovirus infection: a DNA study. Pediatr Dev Pathol. 1999;2(1):11–14. doi: 10.1007/s100249900083. [DOI] [PubMed] [Google Scholar]
- 12.Sokol RJ, Mack C. Etiopathogenesis of biliary atresia. Semin Liver Dis. 2001;21(4):517–524. doi: 10.1055/s-2001-19032. [DOI] [PubMed] [Google Scholar]
- 13.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27(3):233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics. 2011;128(6):e1428–e1433. doi: 10.1542/peds.2011-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst—the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg. 1974;6:113–139. [PubMed] [Google Scholar]
- 16.al-Masri AN, Werfel T, Jakschies D, von Wussow P. Intracellular staining of Mx proteins in cells from peripheral blood, bone marrow and skin. Mol Pathol. 1997;50(1):9–14. doi: 10.1136/mp.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YH, Chou MH, Du YY, et al. Expression of toll-like receptors and type 1 interferon specific protein MxA in biliary atresia. Lab Invest. 2007;87(1):66–74. doi: 10.1038/labinvest.3700490. [DOI] [PubMed] [Google Scholar]
- 18.Rauschenfels S, Krassmann M, Al-Masri AN, et al. Incidence of hepatotropic viruses in biliary atresia. Eur J Pediatr. 2009;168(4):469–476. doi: 10.1007/s00431-008-0774-2. [DOI] [PubMed] [Google Scholar]
- 19.Domiati-Saad R, Dawson DB, Margraf LR, Finegold MJ, Weinberg AG, Rogers BB. Cytomegalovirus and human herpesvirus 6, but not human papillomavirus, are present in neonatal giant cell hepatitis and extrahepatic biliary atresia. Pediatr Dev Pathol. 2000;3(4):367–373. doi: 10.1007/s100240010045. [DOI] [PubMed] [Google Scholar]
- 20.Drut R, Drut RM, Gómez MA, Cueto Rúa E, Lojo MM. Presence of human papillomavirus in extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1998;27(5):530–535. doi: 10.1097/00005176-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Mahjoub F, Shahsiah R, Ardalan FA, et al. Detection of Epstein Barr virus by chromogenic in situ hybridization in cases of extra hepatic biliary atresia. Diagn Pathol. 2008;3:19. doi: 10.1186/1746-1596-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischler B, Ehrnst A, Forsgren M, Orvell C, Nemeth A. The viral association of neonatal cholestasis in Sweden: a possible link between cytomegalovirus infection and extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1998;27(1):57–64. doi: 10.1097/00005176-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Tyler KL, Sokol RJ, Oberhaus SM, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27(6):1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 24.Stanley NF, Dorman DC, Ponsford J. Studies on the pathogenesis of a hitherto undescribed virus (hepato-encephalomyelitis) producing unusual symptoms in suckling mice. Aust J Exp Biol Med Sci. 1953;31(2):147–159. doi: 10.1038/icb.1953.18. [DOI] [PubMed] [Google Scholar]
- 25.Papadimitriou JM. The biliary tract in acute murine reovirus 3 infection. Light and electron microscopic study. Am J Pathol. 1968;52(3):595–611. [PMC free article] [PubMed] [Google Scholar]
- 26.Bangaru B, Morecki R, Glaser JH, Gartner LM, Horwitz MS. Comparative studies of biliary atresia in the human newborn and reovirus-induced cholangitis in weanling mice. Lab Invest. 1980;43(5):456–462. [PubMed] [Google Scholar]
- 27.Phillips PA, Keast D, Papadimitriou JM, Walters MN, Stanley NF. Chronic obstructive jaundice induced by reovirus type 3 in weanling mice. Pathology. 1969;1(3):193–203. doi: 10.3109/00313026909071296. [DOI] [PubMed] [Google Scholar]
- 28.Wilson GA, Morrison LA, Fields BN. Association of the reovirus S1 gene with serotype 3-induced biliary atresia in mice. J Virol. 1994;68(10):6458–6465. doi: 10.1128/jvi.68.10.6458-6465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parashar K, Tarlow MJ, McCrae MA. Experimentalreovirus type3-induced murine biliary tract disease. J Pediatr Surg. 1992;27(7):843–847. doi: 10.1016/0022-3468(92)90380-p. [DOI] [PubMed] [Google Scholar]
- 30.Barton ES, Youree BE, Ebert DH, et al. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J Clin Invest. 2003;111(12):1823–1833. doi: 10.1172/JCI16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada K, Sato Y, Itatsu K, et al. Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology. 2007;46(4):1146–1154. doi: 10.1002/hep.21797. [DOI] [PubMed] [Google Scholar]
- 32.Glaser JH, Balistreri WF, Morecki R. Role of reovirus type 3 in persistent infantile cholestasis. J Pediatr. 1984;105(6):912–915. doi: 10.1016/s0022-3476(84)80076-1. [DOI] [PubMed] [Google Scholar]
- 33.Richardson SC, Bishop RF, Smith AL. Reovirus serotype 3 infection in infants with extrahepatic biliary atresia or neonatal hepatitis. J Gastroenterol Hepatol. 1994;9(3):264–268. doi: 10.1111/j.1440-1746.1994.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown WR, Sokol RJ, Levin MJ, et al. Lack of correlation between infection with reovirus 3 and extrahepatic biliary atresia or neonatal hepatitis. J Pediatr. 1988;113(4):670–676. doi: 10.1016/s0022-3476(88)80376-7. [DOI] [PubMed] [Google Scholar]
- 35.Dussaix E, Hadchouel M, Tardieu M, Alagille D. Biliary atresia and reovirus type 3 infection. N Engl J Med. 1984;310(10):658. doi: 10.1056/NEJM198403083101016. [DOI] [PubMed] [Google Scholar]
- 36.Steele MI, Marshall CM, Lloyd RE, Randolph VE. Reovirus 3 not detected by reverse transcriptase-mediated polymerase chain reaction analysis of preserved tissue from infants with cholestatic liver disease. Hepatology. 1995;21(3):697–702. [PubMed] [Google Scholar]
- 37.Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33(4 Pt 1):394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Czech-Schmidt G, Verhagen W, Szavay P, Leonhardt J, Petersen C. Immunological gap in the infectious animal model for biliary atresia. J Surg Res. 2001;101(1):62–67. doi: 10.1006/jsre.2001.6234. [DOI] [PubMed] [Google Scholar]
- 39.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115(2):200–209. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen SR, Jafri M, Donnelly B, et al. Effect of rotavirus strain on the murine model of biliary atresia. J Virol. 2007;81(4):1671–1679. doi: 10.1128/JVI.02094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Donnelly B, Bondoc A, et al. The rhesus rotavirus gene encoding VP4 is a major determinant in the pathogenesis of biliary atresia in newborn mice. J Virol. 2011;85(17):9069–9077. doi: 10.1128/JVI.02436-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng N, Sen A, Wolf M, Vo P, Hoshino Y, Greenberg HB. Roles of VP4 and NSP1 in determining the distinctive replication capacities of simian rotavirus RRV and bovine rotavirus UK in the mouse biliary tract. J Virol. 2011;85(6):2686–2694. doi: 10.1128/JVI.02408-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafri M, Donnelly B, Allen S, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobo L, Ojeh C, Chiu D, Machado A, Colombani P, Schwarz K. Lack of evidence for rotavirus by polymerase chain reaction/enzyme immunoassay of hepatobiliary samples from children with biliary atresia. Pediatr Res. 1997;41(2):229–234. doi: 10.1203/00006450-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Ko HM, Kim KS, Park JW, et al. Congenital cytomegalovirus infection: three autopsy case reports. J Korean Med Sci. 2000;15(3):337–342. doi: 10.3346/jkms.2000.15.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martelius T, Krogerus L, Höckerstedt K, Bruggeman C, Lautenschlager I. Cytomegalovirus infection is associated with increased inflammation and severe bile duct damage in rat liver allografts. Hepatology. 1998;27(4):996–1002. doi: 10.1002/hep.510270415. [DOI] [PubMed] [Google Scholar]
- 47.Evans PC, Coleman N, Wreghitt TG, Wight DG, Alexander GJ. Cytomegalovirus infection of bile duct epithelial cells, hepatic artery and portal venous endothelium in relation to chronic rejection of liver grafts. J Hepatol. 1999;31(5):913–920. doi: 10.1016/s0168-8278(99)80294-3. [DOI] [PubMed] [Google Scholar]
- 48.Chang MH, Huang HH, Huang ES, Kao CL, Hsu HY, Lee CY. Polymerase chain reaction to detect human cytomegalovirus in livers of infants with neonatal hepatitis. Gastroenterology. 1992;103(3):1022–1025. doi: 10.1016/0016-5085(92)90038-z. [DOI] [PubMed] [Google Scholar]
- 49.Potena L, Valantine HA. Cytomegalovirus-associated allograft rejection in heart transplant patients. Curr Opin Infect Dis. 2007;20(4):425–431. doi: 10.1097/QCO.0b013e328259c33b. [DOI] [PubMed] [Google Scholar]
- 50.Dimmick JE. Intrahepatic bile duct paucity and cytomegalovirus infection. Pediatr Pathol. 1993;13(6):847–852. doi: 10.3109/15513819309048271. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Yu J, Zhang R, et al. The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China. Clin Pediatr (Phila) 2012;51(2):109–113. doi: 10.1177/0009922811406264. [DOI] [PubMed] [Google Scholar]
- 52.Jevon GP, Dimmick JE. Biliary atresia and cytomegalovirus infection: a DNA study. Pediatr Dev Pathol. 1999;2(1):11–14. doi: 10.1007/s100249900083. [DOI] [PubMed] [Google Scholar]
- 53.Fischler B, Woxenius S, Nemeth A, Papadogiannakis N. Immuno globulin deposits in liver tissue from infants with biliary atresia and the correlation to cytomegalovirus infection. J Pediatr Surg. 2005;40(3):541–546. doi: 10.1016/j.jpedsurg.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 54.Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology. 2012;55(4):1130–1138. doi: 10.1002/hep.24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen C, Zheng S, Wang W, Xiao XM. Relationship between prognosis of biliary atresia and infection of cytomegalovirus. World J Pediatr. 2008;4(2):123–126. doi: 10.1007/s12519-008-0024-8. [DOI] [PubMed] [Google Scholar]
- 56.Fischler B, Svensson JF, Nemeth A. Early cytomegalovirus infection and the long-term outcome of biliary atresia. Acta Paediatr. 2009;98(10):1600–1602. doi: 10.1111/j.1651-2227.2009.01416.x. [DOI] [PubMed] [Google Scholar]
- 57.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ig mu null mice. J Exp Med. 1996;184(5):2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160(1):51–59. [PubMed] [Google Scholar]
- 59.O'Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174(6):3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 60.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD.H-2h4 mice have CD4 + CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203(2):349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadchouel M, Hugon RN, Odievre M. Immunoglobulin deposits in the biliary remnants of extrahepatic biliary atresia: a study by immunoperoxidase staining in 128 infants. Histopathology. 1981;5(2):217–221. doi: 10.1111/j.1365-2559.1981.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 62.Mack CL, Tucker RM, Lu BR, et al. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology. 2006;44(5):1231–1239. doi: 10.1002/hep.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. α-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139(5):1753–1761. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saulot V, Vittecoq O, Charlionet R, et al. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 2002;46(5):1196–1201. doi: 10.1002/art.10252. [DOI] [PubMed] [Google Scholar]
- 65.Vermeulen N, de Béeck KO, Vermeire S, et al. Identification of a novel autoantigen in inflammatory bowel disease by protein microarray. Inflamm Bowel Dis. 2011;17(6):1291–1300. doi: 10.1002/ibd.21508. [DOI] [PubMed] [Google Scholar]
- 66.Forooghian F, Cheung RK, Smith WC, O'Connor P, Dosch HM. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol. 2007;27(4):388–396. doi: 10.1007/s10875-007-9091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda Y, Miyazawa Y, Imoto M, et al. In situ distribution of enolase isozymes in chronic liver disease. Am J Gastroenterol. 1989;84(6):601–605. [PubMed] [Google Scholar]
- 68.Akisawa N, Maeda T, Iwasaki S, Onishi S. Identification of an autoantibody against alpha-enolase in primary biliary cirrhosis. J Hepatol. 1997;26(4):845–851. doi: 10.1016/s0168-8278(97)80251-6. [DOI] [PubMed] [Google Scholar]
- 69.Feldman A, Tucker R, Pelanda R, Mack C. Critical role of B cells in the development of bile duct injury and obstruction in murine biliary atresia; Paper presented at: American Association for the Study of Liver Diseases Annual Meeting; Nov. 4–8 2011; San Francisco, CA. [Google Scholar]
- 70.Leonhardt J, Stanulla M, von Wasielewski R, et al. Gene expression profile of the infective murine model for biliary atresia. Pediatr Surg Int. 2006;22(1):84–89. doi: 10.1007/s00383-005-1589-0. [DOI] [PubMed] [Google Scholar]
- 71.Carvalho E, Liu C, Shivakumar P, Sabla G, Aronow B, Bezerra JA. Analysis of the biliary transcriptome in experimental biliary atresia. Gastroenterology. 2005;129(2):713–717. doi: 10.1016/j.gastro.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 72.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114(3):322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohanty SK, Shivakumar P, Sabla G, Bezerra JA. Loss of interleukin-12 modifies the pro-inflammatory response but does not prevent duct obstruction in experimental biliary atresia. BMC Gastroenterol. 2006;6:14. doi: 10.1186/1471-230X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tucker RM, Hendrickson RJ, Mukaida N, Gill RG, Mack CL. Progressive biliary destruction is independent of a functional tumor necrosis factor-alpha pathway in a rhesus rotavirus-induced murine model of biliary atresia. Viral Immunol. 2007;20(1):34–43. doi: 10.1089/vim.2006.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Bessho K, Shivakumar P, et al. Th2 signals induce epithelial injury in mice and are compatible with the biliary atresia phenotype. J Clin Invest. 2011;121(11):4244–4256. doi: 10.1172/JCI57728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davenport M, Gonde C, Redkar R, et al. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36(7):1017–1025. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 77.Mack CL, Tucker RM, Sokol RJ, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56(1):79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed AF, Ohtani H, Nio M, et al. CD8+T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J Pathol. 2001;193(3):383–389. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path793>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 79.Shinkai M, Shinkai T, Puri P, Stringer MD. Increased CXCR3 expression associated with CD3-positive lymphocytes in the liver and biliary remnant in biliary atresia. J Pediatr Surg. 2006;41(5):950–954. doi: 10.1016/j.jpedsurg.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 80.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg. 1995;30(4):515–518. doi: 10.1016/0022-3468(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 81.Bezerra JA, Tiao G, Ryckman FC, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360(9346):1653–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 82.Whitington PF, Malladi P, Melin-Aldana H, Azzam R, Mack CL, Sahai A. Expression of osteopontin correlates with portal biliary proliferation and fibrosis in biliary atresia. Pediatr Res. 2005;57(6):837–844. doi: 10.1203/01.PDR.0000161414.99181.61. [DOI] [PubMed] [Google Scholar]
- 83.Honsawek S, Vejchapipat P, Chongsrisawat V, Thawornsuk N, Poovorawan Y. Association of circulating osteopontin levels with clinical outcomes in postoperative biliary atresia. Pediatr Surg Int. 2011;27(3):283–288. doi: 10.1007/s00383-010-2799-7. [DOI] [PubMed] [Google Scholar]
- 84.Narayanaswamy B, Gonde C, Tredger JM, Hussain M, Vergani D, Davenport M. Serial circulating markers of inflammation in biliary atresia—evolution of the post-operative inflammatory process. Hepatology. 2007;46(1):180–187. doi: 10.1002/hep.21701. [DOI] [PubMed] [Google Scholar]
- 85.Arikan C, Berdeli A, Kilic M, Tumgor G, Yagci RV, Aydogdu S. Polymorphisms of the ICAM-1 gene are associated with biliary atresia. Dig Dis Sci. 2008;53(7):2000–2004. doi: 10.1007/s10620-007-9914-1. [DOI] [PubMed] [Google Scholar]
- 86.Shivakumar P, Sabla G, Mohanty S, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology. 2007;133(1):268–277. doi: 10.1053/j.gastro.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited) Immunol Today. 1993;14(9):426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 88.Mack CL, Falta MT, Sullivan AK, et al. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology. 2007;133(1):278–287. doi: 10.1053/j.gastro.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Broomé U, Nemeth A, Hultcrantz R, Scheynius A. Different expression of HLA-DR and ICAM-1 in livers from patients with biliary atresia and Byler's disease. J Hepatol. 1997;26(4):857–862. doi: 10.1016/s0168-8278(97)80253-x. [DOI] [PubMed] [Google Scholar]
- 90.Feng J, Li M, Gu W, Tang H, Yu S. The aberrant expression of HLA-DR in intrahepatic bile ducts in patients with biliary atresia: an immunohistochemistry and immune electron microscopy study. J Pediatr Surg. 2004;39(11):1658–1662. doi: 10.1016/j.jpedsurg.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 91.Donaldson PT, Clare M, Constantini PK, et al. HLA and cytokine gene polymorphisms in biliaryatresia. Liver. 2002;22(3):213–219. doi: 10.1046/j.0106-9543.2002.01647.x. [DOI] [PubMed] [Google Scholar]
- 92.Yuasa T, Tsuji H, Kimura S, et al. Human leukocyte antigens in Japanese patients with biliary atresia: retrospective analysis of patients who underwent living donor liver transplantation. Hum Immunol. 2005;66(3):295–300. doi: 10.1016/j.humimm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Davenport M, Stringer MD, Tizzard SA, McClean P, Mieli-Vergani G, Hadzic N. Randomized, double-blind, placebo-controlled trial of corticosteroids after Kasai portoenterostomy for biliaryatresia. Hepatology. 2007;46(6):1821–1827. doi: 10.1002/hep.21873. [DOI] [PubMed] [Google Scholar]
- 94.Petersen C, Harder D, Melter M, et al. Postoperative high-dose steroids do not improve mid-term survival with native liver in biliary atresia. Am J Gastroenterol. 2008;103(3):712–719. doi: 10.1111/j.1572-0241.2007.01721.x. [DOI] [PubMed] [Google Scholar]
- 95.Sarkhy A, Schreiber RA, Milner RA, Barker CC. Does adjuvant steroid therapy post-Kasai portoenterostomy improve outcome of biliary atresia? Systematic review and meta-analysis. Can J Gastroenterol. 2011;25(8):440–444. doi: 10.1155/2011/125610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sokol RJ. New North American research network focuses on biliary atresia and neonatal liver disease. J Pediatr Gastroenterol Nutr. 2003;36(1):1. doi: 10.1097/00005176-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 97.Grindebacke H, Stenstad H, Quiding-Järbrink M, et al. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4 + CD25 high regulatory T cells. J Immunol. 2009;183(7):4360–4370. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- 98.Miethke AG, Saxena V, Shivakumar P, Sabla GE, Simmons J, Chougnet CA. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol. 2010;52(5):718–726. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lages CS, Simmons J, Chougnet CA, Miethke AG. Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology. 2012;56(1):219–227. doi: 10.1002/hep.25662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krüger DH, Schroeder C, Santibanez-Koref M, Reuter M. Avoidance of DNA methylation. A virus-encoded methylase inhibitor and evidence for counter selection of methylase recognition sites in viral genomes. Cell Biophys. 1989;15(1-2):87–95. doi: 10.1007/BF02991582. [DOI] [PubMed] [Google Scholar]
- 101.Ogasawara H, Okada M, Kaneko H, Hishikawa T, Sekigawa I, Hashimoto H. Possible role of DNA hypomethylation in the induction of SLE: relationship to the transcription of human endogenous retroviruses. Clin Exp Rheumatol. 2003;21(6):733–738. [PubMed] [Google Scholar]
- 102.Lee PP, Fitzpatrick DR, Beard C, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15(5):763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 103.Zhang DY, Sabla G, Shivakumar P, et al. Coordinate expression of regulatory genes differentiates embryonic and perinatal forms of biliary atresia. Hepatology. 2004;39(4):954–962. doi: 10.1002/hep.20135. [DOI] [PubMed] [Google Scholar]
- 104.Matthews RP, Eauclaire SF, Mugnier M, et al. DNA hypomethylation causes bile duct defects in zebrafish and is a distinguishing feature of infantile biliary atresia. Hepatology. 2011;53(3):905–914. doi: 10.1002/hep.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dong R, Zhao R, Zheng S. Changes in epigenetic regulation of CD4+ T lymphocytesin biliary atresia. Pediatr Res. 2011;70(6):555–559. doi: 10.1203/PDR.0b013e318232a949. [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Barceló MM, Yeung MY, Miao XP, et al. Genome-wide association study identifies a susceptibility locus for biliary atresia on 10q24. 2 Hum Mol Genet. 2010;19(14):2917–2925. doi: 10.1093/hmg/ddq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lane T, Lachmann HJ. The emerging role of interleukin-1β in autoinflammatory diseases. Curr Allergy Asthma Rep. 2011;11(5):361–368. doi: 10.1007/s11882-011-0207-6. [DOI] [PubMed] [Google Scholar]
- 108.Harada K, Nakanuma Y. Cholangiopathy with respect to biliary innate immunity. Int J Hepatol. 2012;2012(793569):793569. doi: 10.1155/2012/793569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chuang JH, Chou MH, Wu CL, Du YY. Implication of innate immunity in the pathogenesis of biliary atresia. Chang Gung Med J. 2006;29(3):240–250. [PubMed] [Google Scholar]
- 110.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50(6):1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 111.Saito T, Hishiki T, Terui K, et al. Toll-like receptor mRNA expression in liver tissue from patients with biliary atresia. J Pediatr Gastroenterol Nutr. 2011;53(6):620–626. doi: 10.1097/MPG.0b013e3182307c9c. [DOI] [PubMed] [Google Scholar]
- 112.Tracy TF, Jr, Dillon P, Fox ES, Minnick K, Vogler C. The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg. 1996;31(1):121–125. doi: 10.1016/s0022-3468(96)90333-4. discussion 125–126. [DOI] [PubMed] [Google Scholar]
- 113.Urushihara N, Iwagaki H, Yagi T, et al. Elevation of serum interleukin-18 levels and activation of Kupffer cells in biliary atresia. J Pediatr Surg. 2000;35(3):446–449. doi: 10.1016/s0022-3468(00)90211-2. [DOI] [PubMed] [Google Scholar]
- 114.Kobayashi H, Puri P, O'Briain DS, Surana R, Miyano T. Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg. 1997;32(4):590–593. doi: 10.1016/s0022-3468(97)90714-4. [DOI] [PubMed] [Google Scholar]
- 115.Shih HH, Lin TM, Chuang JH, et al. Promoter polymorphism of the CD14 endotoxin receptor gene is associated with biliary atresia and idiopathic neonatal cholestasis. Pediatrics. 2005;116(2):437–441. doi: 10.1542/peds.2004-1900. [DOI] [PubMed] [Google Scholar]
- 116.Arikan C, Berdeli A, Ozgenc F, Tumgor G, Yagci RV, Aydogdu S. Positive association of macrophage migration inhibitory factor gene-173G/C polymorphism with biliary atresia. J Pediatr Gastroenterol Nutr. 2006;42(1):77–82. doi: 10.1097/01.mpg.0000192247.55583.fa. [DOI] [PubMed] [Google Scholar]
- 117.Donn R, Alourfi Z, Zeggini E, et al. British Paediatric Rheumatology Study Group. A functional promoter haplotype of macrophage migration inhibitory factor is linked and associated with juvenile idiopathic arthritis. Arthritis Rheum. 2004;50(5):1604–1610. doi: 10.1002/art.20178. [DOI] [PubMed] [Google Scholar]
- 118.Nohara H, Okayama N, Inoue N, et al. Association of the -173 G/C polymorphism of the macrophage migration inhibitory factor gene with ulcerative colitis. J Gastroenterol. 2004;39(3):242–246. doi: 10.1007/s00535-003-1284-7. [DOI] [PubMed] [Google Scholar]
- 119.Glaser SS. Biliary atresia: is lack of innate immune response tolerance key to pathogenesis? Liver Int. 2008;28(5):587–588. doi: 10.1111/j.1478-3231.2008.01752.x. [DOI] [PubMed] [Google Scholar]