Abstract
Background
Despite the publication and dissemination of the Advanced Cardiac Life Support guidelines, variability in the use of drugs during resuscitation from out-of-hospital cardiac arrest may exist between different Emergency Medical Services throughout North America. The purpose of this study was to characterize the use of such drugs and evaluate their relationship to cardiac arrest outcomes.
Methods and Results
The Resuscitation Outcomes Consortium Registry – Cardiac Arrest collects out-of-hospital cardiac arrest data from 264 Emergency Medical Services agencies in 11 geographical locations in the US and Canada. Multivariable logistic regression was used to assess the association between drug use, characteristics of the cardiac arrest and a pulse at emergency department arrival and survival to discharge. A total of 16,221 out-of-hospital cardiac arrests were attended by 74 Emergency Medical Services agencies. There was a considerable variability in the administration of amiodarone and lidocaine for the treatment of shock resistant ventricular tachycardia/ventricular fibrillation. For non-shockable rhythms, atropine use ranged from 29-95% and sodium bicarbonate use ranged from 0.2-73% across agencies in the 89% of agencies that used the drug. Epinephrine use ranged from 57-98% within agencies. Neither lidocaine nor amiodarone were associated with a survival benefit while there was an inverse relationship between the administration of epinephrine, atropine and sodium bicarbonate and survival to hospital discharge.
Conclusions
There is considerable variability among Emergency Medical Services agencies in their use of pharmacological therapy for out-of-hospital cardiac arrests which may be resolved by performing large randomized trials examining effects on survival.
Keywords: Antiarrhythmic Drugs, Cardiac Arrest, Advanced Cardiac Life Support, Emergency Medical Services
Introduction
Out-of-hospital cardiac arrest (OHCA) is a serious public health problem with a reported average incidence of 52 Emergency Medical Services (EMS) treated events per 100,000 of the population per year in North America. [1] Despite the publication and widespread application of international Advanced Cardiac Life Support (ALS) guidelines [2] survival rates remain extremely low. The aim of the ALS guidelines is to help standardise the provision of basic and advanced level care based on the available evidence and expert opinion. In order to help optimise cardiac and cerebral perfusion during cardiopulmonary resuscitation (CPR), increase defibrillation success and achieve a good neurological outcome, the ALS guidelines recommend the administration of specific anti-arrhythmic and vasoactive drugs under certain conditions. [3]
However, there are limited data regarding the beneficial effects of many of these agents. A recent study has suggested that there may be no benefit in the administration of intravenous (IV) medications over CPR and defibrillation and no IV drugs of any kind. [4] This lack of evidence is reflected in the recently published 2010 AHA/ILCOR guidelines which state that during cardiac arrest, provision of high-quality CPR and rapid defibrillation are of primary importance and drug administration is of secondary importance. [2] The 2010 guidelines have removed atropine from the current treatment guidelines for OHCA and the accompanying 2010 Consensus on Science document suggested that placebo trials are needed to evaluate antiarrhythmic and vasopressor use in OHCA. [5]
As a result of the paucity of evidence to support the administration of drugs during ALS there may be a significant difference in the utilisation of drugs during resuscitation between different EMS agencies. In order to assess the patterns of drug use in cardiac arrest and understand the relation between cardiac arrest characteristics and drug use it is useful to examine drug use across a wide range of EMS agencies and relate these to outcomes.
The aim of this study was to describe the variability of drug administration for OHCA between EMS agencies across North America in a large multicenter registry of cardiac arrests and examine whether there was an association between administration of individual drugs and the presence of a pulse at emergency department (ED) admission as well as survival to hospital discharge.
Methods
Setting and Design
Resuscitation Outcomes Consortium (ROC) is a North American consortium of research groups engaged in studies in cardiac arrest and severe trauma funded by the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, the Canadian Institutes of Health Research (CIHR), the Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada and the American Heart Association. A detailed registry of cardiac arrest, the ROC Epistry-Cardiac Arrest, was created in 2005 to prospectively gather data on OHCA. The consortium includes 264 EMS agencies across 11 geographically distinct sites. [6] This study complies with the Declaration of Helsinki and has been approved by locally appointed ethics committees.
Data related to out-of-hospital treatments and outcomes were collected by use of standardized operational definitions, including initial cardiac rhythms, response times, descriptions of responders, timing of CPR and defibrillation, response to interventions, return of spontaneous circulation (ROSC), the presence of a pulse at arrival to the ED and survival to hospital discharge. [7] All data were managed by a central Data Coordinating Center at the University of Washington. Site-specific quality assurance plans included education of EMS providers in coding certain variables and definitions.
Patient Population
Specific treatment protocols are developed by each EMS agency as a medical directive agency provided their prehospital treatment records, including dispatch records, Prehospital Care Reports, Ambulance Care Reports, and ECG recordings, when available, to site coordinators, who abstracted the data and entered it into a central database. All adults who experienced an OHCA across the 11 sites in ROC Epistry – Cardiac Arrest [7] between December 2005 and June 2007 who were treated by advanced level EMS personnel were eligible for the present study. EMS agencies were only included if there were more than 25 cases of OHCA treated by ALS providers. Only subjects who received treatment were included in the analysis.
The initial rhythm was determined to be ventricular tachycardia/ventricular fibrillation (VT/VF) if the initial automated external defibrillator analysis recommended a shock or if the EMS provider interpreted the initial rhythm as VT/VF, and rhythm diagnosis was subsequently confirmed by research staff. Shock resistant VT/VF was defined as the requirement for ≥ 3 shocks during the cardiac arrest. The pharmacological agents are recorded in the patient care report. [7] Drug dose data was available for epinephrine only. Data relating to the administration of vasopressin was not mandatory. Other information collected included patient and event demographics, clinical information, out-of-hospital interventions, disposition, hospital information and outcomes. [7]
Pharmacological Agents
The administration of pharmacological agents by the first ALS agency responding to the cardiac arrest was summarised by agency. Drugs examined included amiodarone, lidocaine, epinephrine, vasopressin, atropine and sodium bicarbonate. The reporting of vasopressin administration was optional.
Statistical Analysis
All statistical analyses were performed with an available statistical package (S+, version 8.0.4, Cary, NC and R version 2.9.0). Baseline characteristics and variables related to the cardiac arrest are presented as mean (± SD). A significance level of 0.05 was used.
Multivariable logistic regression was used to assess the association between drug use and outcomes. Variables accounted for were age, gender, EMS witnessed, bystander witnessed, bystander CPR, initial rhythm VT/VF, time from 911 call to EMS arrival on scene, and site. The relationship between outcome and time from 911 call to EMS arrival on scene was modelled using a natural cubic spline with three degrees of freedom. Outcomes were expressed in terms of odds ratio (OR). A cubic smoothing spline was used to explore the relationship between survival to hospital discharge and pre-hospital dose of epinephrine.
Results
A total of 16,221 OHCAs were attended by 74 EMS agencies with ALS capability and with greater than 25 cardiac arrests. Overall 83% of all patients received at least one pharmacological agent during CPR. Approximately 24% of patients had a first documented rhythm of VT/VF and 36% of patients received at least one shock during the cardiac arrest. The overall mean age was 66.1 (± 16.8) years and 63% were male. The baseline characteristics of all patients are summarised in Table 1.
Table 1.
Baseline Variables for Patients who received Pharmacological Agents during OHCA
| Baseline or Cardiac Arrest Associated Characteristics |
All Subjects | Amiodarone | Lidocaine | Epinephrine | Atropine | Bicarbonate | No Drug Therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Rhythms n=16221 |
Initial VT/VF n=3868 |
All Rhythms n=946 |
Initial VT/VF n=594 |
All Rhythms n=1905 |
Initial VT/VF n=1312 |
All Rhythms n=13053 |
Initial VT/VF n=3036 |
All Rhythms n=11378 |
Initial VT/VF n=2257 |
All Rhythms n=3078 |
Initial VT/VF n=741 |
All Rhythms n=2753 |
Initial VT/VF n=541 |
|
| Age (Mean ±SD) |
66.1
(16.8) |
64.2
(15.1) |
65.1
(15.1) |
64.5 (15.0) |
65.0 (14.9) |
63.7 (14.5) |
65.9 (16.7) |
64.2 (15.2) |
65.9 (16.8) |
64.2 (15.1) |
62.7 (17.0) |
62.6 (15.1) |
67.7 (17.0) |
65.4 (14.9) |
| Male (%) | 63 | 76 | 73 | 76 | 74 | 78 | 64 | 76 | 64 | 77 | 66 | 78 | 57 | 71 |
|
Bystander
Witnessed (%) |
38 | 60 | 58 | 66 | 57 | 64 | 39 | 60 | 37 | 58 | 39 | 61 | 32 | 55 |
|
EMS Witnessed
(%) |
9 | 8 | 7 | 5 | 8 | 7 | 8 | 5 | 7 | 5 | 6 | 5 | 13 | 21 |
|
Call to Response Time (Mean ±SD) 1 |
8.8 (6.8) |
8.5
(5.9) |
7.2 (4.2) | 6.7 (3.7) |
8.7 (5.4) | 8.5 (5.2) |
8.6 (6.7) | 8.3 (5.3) |
8.6 (6.9) | 8.4 (5.5) |
9.3 (5.9) | 9.3 (5.4) |
9.6 (7.0) | 9.9 (8.4) |
|
Patients
Receiving ≥ 1 Shock (%) 2 |
36 | 96 | 95 | 98 | 94 | 99 | 38 | 96% | 35 | 96 | 44 | 97 | 24 | 92% |
|
Number of Shocks Received (Mean ±SD) 3 |
3.3 (2.8) |
3.8
(3.1) |
4.6 (2.8) | 5.1 (2.9) |
4.9 (3.4) | 5.3 (3.6) |
3.6 (2.9) | 4.2 (3.2) |
3.3 (2.8) | 3.9 (3.1) |
4.1 (3.4) | 5.1 (3.7) |
1.9 (1.3) | 1.9 (1.3) |
|
Call to First Shock (Mean ±SD) 4 |
14.2
(9.4) |
10.0
(4.8) |
14.3
(8.4) |
10.4 (4.6) |
12.7 (8.1) | 9.5 (4.0) |
14.9 (9.6) | 10.2 (4.7) |
15.8 (10.1) |
10.4 (5.0) |
16.1 (10.5) |
10.1 (4.5) |
10.7 (6.9) | 9.4 (5.3) |
|
Arrest to First Shock (Mean ±SD) 5 |
6.2 (9.1) |
2.8
(3.7) |
9.9 (8.0) | 10.0 (11.3) |
4.7 (6.5) | 2.2 (3.8) |
10.411.2) | 4.6 (5.8) |
12.8 (12.6) |
4.5 (5.9) |
10.4 (12.5) |
4.3 (7.1) |
2.0 (1.3) | 2.0 (1.3) |
Percentages refer to the proportion of patients in each rhythm category who received the drug listed
Time from receiving the call at dispatch to arrival of EMS
Patients who received ≥ 1 shock throughout the duration of resuscitation
No of shocks among patients with > 1 shock
Time from receiving the call at dispatch to delivery of shock
Time from confirmation of cardiac arrest by EMS crew until deliver of shock
Anti-arrhythmic Drugs (Tables 1 and 2)
Table 2.
The Rates of Agency Use of Various Pharmacological Agents in OHCA cases
| Amiodarone | Lidocaine | Epinephrine | Atropine | Bicarbonate | ||
|---|---|---|---|---|---|---|
|
All Cardiac
Arrests |
Agencies using drug - % (n/N)
Mean Use % (±SD) Median % ( IQR) Range of Use (%) |
55% (41/74) 7% (8) 1% (0-14) 0.1-25 |
96% (71/74) 12% (8) 10% (6-16) 0.2-34 |
100% (74/74) 81% (8) 81% (77-85) 57-98 |
100% (74/74) 71% (14) 74% (66, 79) 27-91 |
91% (67/74) 18% (17) 13% (3-28) 0.3-71 |
|
Initial Rhythm
VT/VF |
Agencies using drug - % (n/N)
Mean % (±SD) Median % ( IQR) Range of Use (%) |
51% (38/74) 17% (21) 1% (0-38) 0.3-67 |
95% (70/74) 31% (20) 28% (17-43) 0.5-80 |
100% (74/74) 80% (11) 81% (76-88) 50-100 |
100% (74/74) 61% (18) 63% (50-73) 18-100 |
73% (54/74) 18% (19) 14% (0-28) 0.5-67 |
| Received Shock |
Agencies using drug - % (n/N)
Mean % (±SD) Median % (IQR) Range of Use (%) |
55% (41/74) 16% (20) 3% (0-34) 0.2-63 |
96% (71/74) 29% (19) 28% (14-38) 0.5-75 |
100% (74/74) 84% (8) 84% (80-89) 55-100 |
100% (74/74) 69% (16) 73% (64-78) 25-100 |
84% (62/74) 22% (20) 20% (3-35) 0.4-73 |
|
Received ≥ 3
Shocks |
Agencies using drug - % (n/N)
Mean % (±SD) Median % (IQR) Range of Use (%) |
56% (41/73) 26 (30) 5 (0, 57) 0.4-90 |
90% (66/73) 39 (25) 37 (20, 58) 0.8-100 |
100% (73/73) 92 (9) 94 (89, 100) 50-100 |
100% (73/73) 70(17) 71 (59, 82) 22-100 |
73% (53/73) 26(26) 20 (0, 43) 2-90 |
|
Non Shockable
Initial Rhythm |
Agencies using drug - % (n/N)
Mean % (±SD) Median % ( IQR) Range in % over all agencies |
50% (37/74) 3% (4) 0% (0-5) 0.1-18 |
84% (62/74) 5% (4) 5% (2-7) 0.2-16 |
100% (74/74) 81% (9) 82% (76-87) 48-97 |
100% (74/74) 74% (14) 78% (68-83) 29-95 |
89% (66/74) 19% (18) 15% (3-28) 0.2-73 |
Mean and median refer to the percentage of patients receiving the drug over all agencies which ever used the drug Range refers to the range of use in agencies which ever used the drug.
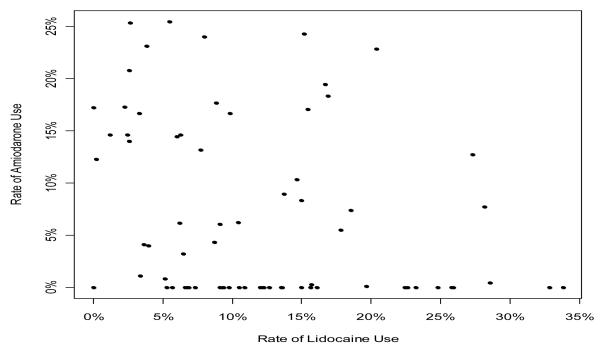
Patients who received lidocaine had a significantly longer call to response time, received fewer shocks, and had longer call to shock time. Overall, 96% of agencies administered lidocaine, 55% administered amiodarone and 54% of agencies administered both lidocaine and amiodarone. Less than half (43%) of agencies only administered one anti-arrhythmic drug. Two agencies did not use either lidocaine or amiodarone during any cardiac arrest (Figure 1).
Figure 1.
Relation between Use of Amiodarone and Lidocaine for all Cardiac Arrests across all Agencies throughout the Epistry ROC
Database
Each point represents one agency with the exception of 2 agencies with zero use of both drugs
Within those agencies using lidocaine, the proportion of patients receiving lidocaine in cases with at least one shock ranged from 1 to 75%. For those who had shock resistant VT/VF, lidocaine administration rates ranged from 1 to 100% between agencies (mean 41 ± 31%).
In agencies using amiodarone, the proportion receiving amiodarone ranged from 0.2 to 63% for patients who received at least one shock, and from 0.4 to 90% for shock resistant VT/VF (mean 26 ± 32%). In patients with shock resistant VT/VF, 22% of those who received amiodarone and 33% of those who received lidocaine had ROSC at the time of ED arrival. Among patients with shock resistant VT/VF, 11% of those who received amiodarone and 15% of those who received lidocaine survived to hospital discharge.
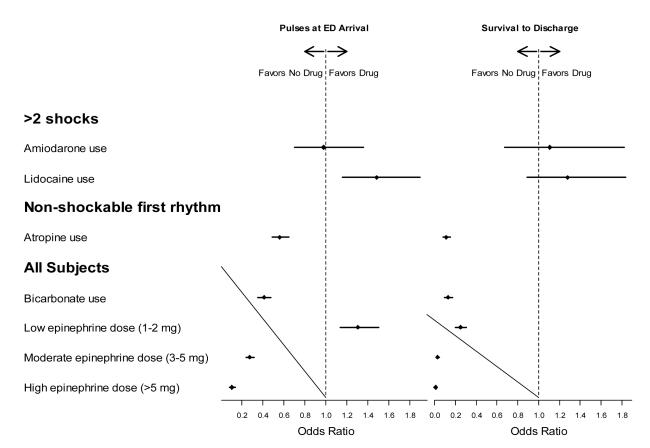
In a multivariable analysis, adjusting for age, gender, EMS witnessed arrest, bystander witnessed arrest, bystander CPR, shockable initial rhythm, time from 911 to EMS arrival and study site, the odds ratio (OR) for survival to hospital discharge after treatment for shock resistant VT/VF was 1.11 (0.67-1.82) for amiodarone and 1.28 (0.89-1.83) for lidocaine (Figure 2).
Figure 2. Forest Plot showing the Odds Ratio of Pulses present at Emergency Department (ED) arrival (left) and survival to Hospital Discharge (right) for the Various Pharmacological Agents administered.
Odds ratios for survival are adjusted for age, sex, EMS witnessed, bystander witnessed, bystander CPR, time from 911 to arrival of first EMS, and VT/VF (except for atropine, since that was all non-VF)
Vasopressors (Tables 1 and 2)
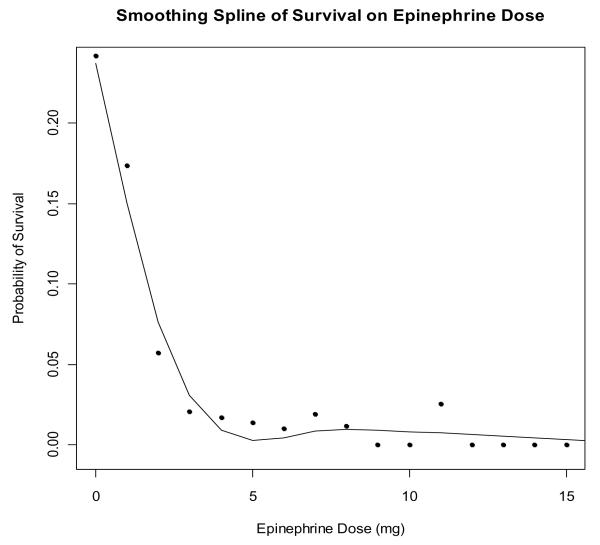
Epinephrine was administered in approximately 80% of ALS treated cardiac arrests (range 57-98% among agencies). All agencies used epinephrine in some cardiac arrests. The mean dose administered was 3.5 mg (± 2.0 mg). Epinephrine dose varied widely across agencies, with a range in the mean epinephrine dose of 1.9-5.5 mg (p < 0.001). There was an inverse association between epinephrine dose and survival to discharge (Figure 3). This relationship persisted after adjusting for age, gender, EMS witnessed arrest, bystander witnessed arrest, bystander CPR, shockable initial rhythm, time from 911 to EMS arrival, the duration of OHCA and study site. Vasopressin was recorded as being used in only 3% of cases; however the use of vasopressin was an optional variable in the data set and the use or non-use was not recorded in all cases.
Figure 3. Relation of Epinephrine dose to Survival.
Each point represents the total dose of epinephrine in mg versus the rate of survival for individuals who received that dose.
Atropine (Tables 1 and 2)
Atropine was administered in 71% (± 14%) of all cardiac arrests. For non VT/VF OHCAs the mean agency rate of use was 74% (± 14%), ranging from 29% to 95%. For patients with a non-shockable rhythm, after adjusting for age, gender, EMS witnessed arrest, bystander witnessed arrest, bystander CPR, shockable initial rhythm, time from 911 to EMS arrival, the duration of OHCA and study site there was an inverse relationship between the administration of atropine and pulses present at hospital arrival (OR 0.56; 0.49-0.65) and survival to hospital discharge (OR 0.11; 0.08-0.15) (Figure 2). An additional multivariable logistic regression analysis that also adjusted for duration of resuscitation gave similar results.
Sodium Bicarbonate
Sodium bicarbonate was administered in 19% of OHCAs. Agency specific rates ranged from 0.3-71% within agencies that ever used the drug. While 7 agencies did not use sodium bicarbonate for any cardiac arrests, 6 agencies, all within one geographical region, used it in approximately half of all cardiac arrests. The administration of sodium bicarbonate for more prolonged cardiac arrests (greater than the median duration) was not significantly higher than for shorter arrests (23% for cardiac arrests greater than 25 minutes versus 18% overall use).
After adjusting for age, gender, EMS witnessed arrest, bystander witnessed arrest, bystander CPR, shockable initial rhythm, time from 911 to EMS arrival, the duration of OHCA and study site, there was an inverse relationship between the administration of sodium bicarbonate and pulses at ED arrival (OR 0.41; 0.36-0.48) and survival to hospital discharge (OR 0.13; 0.10-0.17) (Figure 2). An additional logistic regression that also adjusted for duration of resuscitation gave similar results.
Discussion
This is the largest study describing the contemporary use of pharmacological agents in OHCA. Despite the publication of guidelines designed to help standardise the management of OHCA there is substantial variability in the administration of drugs. Although the reasons for this have never been studied in detail, several factors may account for these findings. The lack of evidence from randomized clinical trials in terms of survival, resulting in class IIb recommendations for the majority of the agents, is likely to be the most significant contributing factor. Other potential factors include a variation in the time taken to incorporate the 2005 guidelines into clinical practice [8] as well as differences in the opinion of medical directors in different agencies. This was demonstrated in this study in terms of significant geographical variability in the use individual pharmacological agents in OHCA despite recommendations made in the guidelines preceding this study period.
The overall lack of evidence in favour of any pharmacological agent administered during CPR may result in different interpretations of the guidelines. A recent trial in which OHCA patients were randomised to receive either ALS with IV drug administration or ALS without access to IV drugs was powered to find an absolute difference of 7% and showed that although those who received IV drugs had a higher survival to hospital admission there was no significant difference in survival to discharge. [5] The early benefits of many of these drugs may be offset by later detrimental effects following resuscitation. Relatively small benefits of drugs, especially among the minority of patients with VT/VF, may require very large sample sizes to be demonstrated. [2] In addition, there are no class I indications for any pharmacological agents during ALS which likely results in a substantial variability in the incorporation of guidelines into agency protocols. The 2005 ALS guidelines recommend that antiarrhythmic agents such as amiodarone can be considered (Class IIb recommendation) if pulseless VT or VF persists after 2 to 3 shocks. If amiodarone is unavailable lidocaine may be considered as an alternative (Class Indeterminate). [3]
The 2010 guidelines do not alter these recommendations [2] and given the important findings in this study it may be plausible that contemporary practice may be unchanged.
These recommendations are based largely on the results of the ALIVE and ARREST trials. [9,10] The ALIVE study was a randomized, controlled, blinded trial comparing IV amiodarone with IV lidocaine in patients with OHCA due to persistent or recurrent VF. Although more than twice as many patients survived to hospital admission in the group who received amiodarone there was no significant difference in survival to hospital discharge. The ARREST trial was a randomized double-blind placebo-controlled trial comparing amiodarone with placebo in OHCA. Although this study also showed an increase in survival to hospital admission with amiodarone (versus placebo), neither trial was sufficiently powered to detect differences in survival to hospital discharge, which differed only slightly between the two groups.
Lidocaine was used more frequently than amiodarone between 2005 and 2007, and a substantial number of agencies did not use amiodarone. Amongst agencies which used either antiarrhythmic drug there was a large variability in use both between agencies and throughout different geographical locations. The adjusted odds ratio for survival to discharge does not indicate a survival advantage for either drug. This type of observational study cannot establish whether any drug is beneficial or harmful even after adjusting for characteristics of OHCA which influence outcome.
Vasopressor agents are used to enhance both coronary and cerebral perfusion pressures during resuscitation by binding predominantly to peripheral α1- and α2 receptors. [11] The recommended agent in the 2005 guidelines is epinephrine. The 2005 and current guidelines recommend that 1 mg epinephrine be administered every 3-5 minutes during cardiac arrest (Class IIb recommendation). [3]
Epinephrine administration is generally high throughout North America with a large variability in the overall dose used. Data from eight randomized clinical studies has shown no improvement in survival to hospital discharge rates or neurologic outcomes with high dose versus standard dose. [12-19] Although ROSC may be achieved, the continued effects of β1-receptor agonism may result in more myocardial dysfunction, tachycardia and hypertension post resuscitation. [19] A recent double-blind randomised placebo-controlled trial of epinephrine in OHCA indicated that there was no benefit in terms of survival to hospital discharge. [20] The 2005 ALS guidelines recommended atropine for use in asystole or slow pulseless electrical activity (ventricular rate < 60 bpm). [3] Although atropine was administered in almost three quarters of non VT/VF cardiac arrests there was substantial variability in use among agencies. Further, while atropine is not recommended for the treatment of VT/VF it was administered in over half of all VT/VF cardiac arrests. This may have been due to a change in the rhythm following defibrillation in which the patient may have developed either asystole or slow PEA. There are no randomised controlled trials which demonstrate an increase in ROSC or survival to hospital discharge associated with the use of atropine in cardiac arrest. The 2010 ALS guidelines have removed atropine from the cardiac arrest algorithm because the routine use of atropine during PEA or asystole is unlikely to have a therapeutic benefit. [2]
The 2005 ALS guidelines state that with the exception of certain special resuscitation situations, such as pre-existing metabolic acidosis, hyperkalemia, or tricyclic antidepressant overdose, bicarbonate is not recommended for the treatment of cardiac arrest (Class III, Level of Evidence B). [3] This recommendation is unchanged in the 2010 guidelines. [2] Sodium bicarbonate was administered in approximately one fifth of all cardiac arrests overall, and in several agencies, in more than half of all cardiac arrests. Out of 19 retrospective studies examining mortality rates and other outcomes in patients administered sodium bicarbonate during cardiac arrest there was no overall benefit demonstrated in any of the studies; 11 showed no difference in outcomes, and eight suggested a deleterious effect. [22]
The final potential explanation which may have contributed to the overall variability in drug administration is a difference in the rate of uptake of the 2005 guidelines between agencies. The mean time from publication of the 2005 guidelines to implementation was over 1 year [8] and therefore it is possible that during the course of this study there was a change in practice. However, temporal trend analysis of the data reveals that the patterns of drug usage did not change significantly from 2005 to 2007.
Limitations
Although this is a large registry describing current practice, this study has several limitations. Dosing data was only available for epinephrine and varying doses of the other drugs may have contributed to the overall drug effects. The multivariable adjustment may not have accounted for all confounding variables with regards to outcomes; in particular we do not have detailed information on specific resuscitation ‘processes’ such as the quantity and quality of chest compressions and interruptions in CPR. Additionally data regarding post resuscitation care, such as therapeutic hypothermia, was not available. Such an observational study can only show an association between variables and outcomes.
Conclusions
Despite the publication of ALS guidelines there is considerable variability in the adherence to recommendations in drug therapy for the treatment of OHCA which may be a result of conflicting results from trials, lack of evidence for many pharmacological agents, differences in the uptake of guidelines, or potential variability in the opinions of medical directors regarding the use of different agents. These variations are large and warrant consideration for the development of large randomised trials examining the effects of these agents on survival to admission and discharge from hospital.
Acknowledgements
We would like to acknowledge the following EMS agencies for their contribution towards the collection of data for this study (listed in alphabetical order of site): Regional Paramedical Services, Alabama; Center Point Fire Dept, Alabama; Birmingham Fire Dept, Alabama; Carrollton Fire Dept, Dallas; Dallas Fire Rescue, Dallas; DeSoto Fire Dept, Dallas; Duncanville Fire Dept, Dallas; Garland Fire Dept, Dallas; Irving Fire Dept, Dallas; Lancaster Fire Dept, Dallas; Mesquite Fire Dept, Iowa; Waterloo Fire Rescue, Iowa; Dallas County EMS, Iowa; West Des Moines EMS, Iowa; Des Moines Fire Dept, Iowa; Siouxland Paramedics, Iowa; Medic EMS, Iowa; Area Ambulance Service, Iowa; Dubuque Fire Dept, Iowa; Sartori Ambulance, Iowa; Henry County EMS, Iowa; Johnson County Ambulance, Iowa; Cedar Rapids Fire Dept, Iowa; Milwaukee Fire Dept, Milwaukee; North Shore Fire Dept, Milwaukee; Wauwatosa Fire Dept, Milwaukee; West Allis Fire Dept, Milwaukee; South Milwaukee Fire Dept, Milwaukee; Franklin Fire Dept, Milwaukee; Oak Creek Fire Dept, Milwaukee; Greenfield Fire Dept, Milwaukee; Niagara EMS, Ottawa; Sudbury EMS, Ottawa; Halton EMS, Ottawa; Essex-Windsor EMS, Ottawa; Lambton County EMS, Ottawa; Waterloo Regional EMS, Ottawa; Frontenac Paramedic Service, Ottawa; Thames EMS, Ottawa; Superior North EMS, Ottawa; Ottawa Paramedic Service, Ottawa; Peterborough EMS, Ottawa; Prescott-Russell EMS, Ottawa; Fayette EMS, Pittsburgh; City of Pittsburgh EMS, Pittsburgh; Ambulance and Chair, Pittsburgh; Mutual Aid Ambulance, Pittsburgh; Hillsboro Fire Dept, Portland; Camas Fire Dept, Portland; AMR Multnomah County, Portland; Clackamas County Fire Dist 1, Portland; Tualatin Valley Fire Rescue, Portland; Portland Fire Rescue, Portland; Gresham Fire Dept, Portland; MetroWest Ambulance, Portland; Vancouver Fire Dept, Portland; AMR Clark County, Portland; El Cajon Fire Dept ALS, San Diego; Escondido Fire Dept ALS, San Diego; Lakeside Fire Protection Dist, San Diego; North County Fire Dept ALS, San Diego; Poway Fire Dept ALS, San Diego; Vista Fire Dept ALS, San Diego; Shoreline Fire KCFD 4, Seattle King County; Redmond Fire Dept, Seattle King County; Bellevue Fire Dept, Seattle King County; Bothell Fire Dept, Seattle King County; Seattle Fire Dept, Seattle King County; South King County Medic 1, Seattle King County; Durham Regional EMS, Toronto; City of Hamilton EMS, Toronto; Region of Peel Ambulance Services, Toronto; Toronto EMS, Toronto; BC Ambulance, Vancouver.
Sources of funding The Resuscitation Outcome Consortium is supported by a series of cooperative agreements with 10 regional clinical centers and one Data Coordinating Center (5U01 HL077863, HL077881, HL077871 HL077872, HL077866, HL077908, HL077867, HL077885, HL077887, HL077873, HL077865) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada and the American Heart Association.
References
- 1.Nichol G, Thomas E, Callaway C, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I, Resuscitation Outcomes Consortium Investigators Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 3.ECC Committee Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;13:IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 4.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 5.Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, Kronick SL, Lavonas EJ, Link MS, Neumar RW, Otto CW, Parr M, Shuster M, Sunde K, Peberdy MA, Tang W, Hoek TL, Böttiger BW, Drajer S, Lim SH, Nolan JP, Advanced Life Support Chapter Collaborators Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S345–421. doi: 10.1161/CIRCULATIONAHA.110.971051. [DOI] [PubMed] [Google Scholar]
- 6.Davis DP, Garberson LA, Andrusiek DL, Hostler D, Daya M, Pirrallo R, Craig A, Stephens S, Larsen J, Drum AF, Fowler R. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11:369–382. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 7.Morrison LJ, Nichol G, Rea TD, Christenson J, Callaway CW, Stephens S, Pirrallo RG, Atkins DL, Davis DP, Idris AH, Newgard C, ROC Investigators Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. 2008;78:161–169. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigham BL, Koprowicz K, Aufderheide TP, Davis DP, Donn S, Powell J, Suffoletto B, Nafziger S, Stouffer J, Idris A, Morrison LJ, ROC Investigators Delayed prehospital implementation of the 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiac care. Prehosp Emerg Care. 2010;14:355–360. doi: 10.3109/10903121003770639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346:884–890. doi: 10.1056/NEJMoa013029. [DOI] [PubMed] [Google Scholar]
- 10.Kudenchuk PJ, Cobb LA, Copass MK, Cummins RO, Doherty AM, Fahrenbruch CE, Hallstrom AP, Murray WA, Olsufka M, Walsh T. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341:871–878. doi: 10.1056/NEJM199909163411203. [DOI] [PubMed] [Google Scholar]
- 11.Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, Niedermeyer E, Rogers MC, Traystman RJ, Weisfeldt ML. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation indogs. Circulation. 1984;69:822–835. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 12.Lindner KH, Ahnefeld FW, Prengel AW. Comparison of standard and high-dose adrenaline in the resuscitation of asystole and electromechanical dissociation. Acta Anaesthesiol Scand. 1991;35:253–256. doi: 10.1111/j.1399-6576.1991.tb03283.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown CG, Martin DR, Pepe PE, Stueven H, Cummins RO, Gonzalez E, Jastremski M. A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. The Multicenter High-Dose Epinephrine Study Group. N Engl J Med. 1992;327:1051–1055. doi: 10.1056/NEJM199210083271503. [DOI] [PubMed] [Google Scholar]
- 14.Stiell IG, Hebert PC, Weitzman BN, Wells GA, Raman S, Stark RM, Higginson LA, Ahuja J, Dickinson GE. High-dose epinephrine in adult cardiac arrest. N Engl J Med. 1992;327:1045–1050. doi: 10.1056/NEJM199210083271502. [DOI] [PubMed] [Google Scholar]
- 15.Callaham M, Madsen CD, Barton CW, Saunders CE, Pointer J. A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. JAMA. 1992;268:2667–2672. [PubMed] [Google Scholar]
- 16.Lipman J, Wilson W, Kobilski S, Scribante J, Lee C, Kraus P, Cooper J, Barr J, Moyes D. High-dose adrenaline in adult in-hospital asystolic cardiopulmonary resuscitation: a double-blind randomised trial. Anaesth Intensive Care. 1993;21:192–196. doi: 10.1177/0310057X9302100210. [DOI] [PubMed] [Google Scholar]
- 17.Choux C, Gueugniaud PY, Barbieux A, Pham E, Lae C, Dubien PY, Petit P. Standard doses versus repeated high doses of epinephrine in cardiac arrest outside the hospital. Resuscitation. 1995;29:3–9. doi: 10.1016/0300-9572(94)00810-3. [DOI] [PubMed] [Google Scholar]
- 18.Sherman BW, Munger MA, Foulke GE, Rutherford WF, Panacek EA. High-dose versus standard-dose epinephrine treatment of cardiac arrest after failure of standard therapy. Pharmacotherapy. 1997;17:242–247. [PubMed] [Google Scholar]
- 19.Gueugniaud PY, Mols P, Goldstein P, Pham E, Dubien PY, Deweerdt C, Vergnion M, Petit P, Carli P. A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. European Epinephrine Study Group. N Engl J Med. 1998;339:1595–1601. doi: 10.1056/NEJM199811263392204. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–43. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Stueven HA, Tonsfeldt DJ, Thompson BM, Whitcomb J, Kastenson E, Aprahamian C. Atropine in asystole: human studies. Ann Emerg Med. 1984;13:815–817. doi: 10.1016/s0196-0644(84)80447-3. [DOI] [PubMed] [Google Scholar]
- 22.Levy MM. An evidence-based evaluation of the use of sodium bicarbonate during CPR. Crit Care Clin. 1998;14:457–483. doi: 10.1016/s0749-0704(05)70011-7. [DOI] [PubMed] [Google Scholar]