Abstract
Viral infections, including β-herpes viruses and community respiratory viruses, are frequent pathogens in lung transplant recipients. These pathogens have become increasingly recognized as having a significant role in long-term outcomes of lung transplantation, which has been limited by the frequent development of infections, and chronic allograft dysfunction. Community respiratory viruses, such as influenza and respiratory syncytial virus have been associated with both acute rejection and chronic allograft dysfunction, particularly if early treatment was not administered. β-herpes viruses, particularly cytomegalovirus (CMV), have long been associated with increased mortality in lung transplant recipients, although the advent of effective antiviral strategies has led to improved morbidity and mortality. Because these pathogens have been associated with altered immune responses against the allograft, a better understanding of immunopathogenesis of viral infections may lead to a broader approach to limit the morbidity from these pathogens.
Keywords: Lung transplantation, respiratory virus, cytomegalovirus
Lung transplantation is the final therapeutic option for select patients with multiple end-stage pulmonary diseases, yet its long-term success is limited by chronic allograft dysfunction and frequent infections, which together account for 25 to 30% of posttransplant mortality after the first year.1 Viral infections, such as β-herpes viruses and community-associated respiratory viruses such as respiratory syncytial virus (RSV) not only contribute to morbidity from acute infection but have also been associated with increased risk of chronic allograft dysfunction, characterized pathologically by obliterative bronchiolitis (OB) and clinically by the bronchiolitis obliterans syndrome (BOS). While cytomegalovirus (CMV), the most common viral pathogen, historically had been associated with worse mortality in lung transplant recipients (LTRs), recent advances in antiviral therapy have markedly improved outcomes associated with CMV infections. On the other hand, community respiratory viruses, for which treatment options are more limited, are increasingly recognized as having an important role in allograft dysfunction. Although a comprehensive review of all viral infections in LTRs is beyond the scope of this review, this discussion will focus on the incidence, pathogenesis, treatment strategies and areas of investigation for viruses that currently confer greatest risk of adverse outcomes, namely the β-herpes virus CMV, and community respiratory viruses (CRVs).
RESPIRATORY VIRAL INFECTIONS
Community respiratory viruses comprise many common RNA and DNA viruses that are usually associated with self-limited upper respiratory tract infections in healthy immunocompetent adults; however, in the immunocompromised host CRV infections can often involve the lower respiratory tract and are associated with significant morbidity and mortality.2 Table 1 summarizes epidemiology and clinical manifestations of common respiratory viruses. Usually transmitted via respiratory droplets, these infections are most common after the first year of transplant, as recipients resume community activities and are thus exposed to persons harboring virus. Sensitive assays for detection of viral pathogens is essential for diagnosis because the clinical syndromes are often nonspecific and can vary in severity in immunocompromised hosts. It is likely that older literature may underestimate the true incidence of CRV in LTRs as newer molecular detection methods are becoming recognized as more sensitive to conventional culture and antibody based methods for detection of many respiratory viruses.3–6 Given that medical treatment options are currently limited and most efficacious when initiated promptly, infectious control measures to limit exposure and spread of virus are of paramount importance.
Table 1.
Epidemiology of Community Respiratory Viral Infections in Lung Transplant Recipients2
| Virus | Incidence | Clinical Features | Diagnosis |
|---|---|---|---|
| Respiratory syncytial virus | 5–10% of LTRs Mortality 10–15% |
URTI, LRTI, BOS (50%), Acute rejection | NP aspirate/BAL |
| Culture+ | |||
| EIA+ | |||
| FA++ | |||
| RT-PCR+++ | |||
| Influenza A/B | 3–4% of LTRs | URTI, LRTI, Bacterial super-infection, BOS (50%), Acute rejection (60–75%), Prolonged shedding | NP aspirate/BAL |
| Culture+ | |||
| EIA++ | |||
| FA++ | |||
| RT-PCR+++ | |||
| Parainfluenza viruses | 2–10% of LTRs | URTI, LRTI, BOS (60%), Acute rejection | NP aspirate/BAL |
| Culture+ | |||
| EIA++ | |||
| FA++ | |||
| RT-PCR+++ | |||
| Metapneumovirus | 4–5% of LTRs | LRTI, BOS (<10%) | NP aspirate/BAL |
| Culture+ | |||
| EIA:NA | |||
| FA++ | |||
| RT-PCR+++ | |||
| Rhinoviruses | 14% of LTRs | URTI, LRTI, Persistent infection with graft dysfunction | NP aspirate/BAL |
| Culture: poor sensitivity | |||
| EIA++ | |||
| FA++ | |||
| RT-PCR+++ |
BAL, bronchoalveolar lavage; BOS, bronchiolitis obliterans syndrome; EIA, enzyme immunoassay; FA, fluorescent antibody; LRTI, lower respiratory tract infection; LTR, lung transplant recipients; NP, nucleoprotein; PCR, polymerase chain reaction; RT, reverse transcription; URTI, upper respiratory tract infection.
Listed are incidence, clinical features, and diagnostic assays for common respiratory viral infections.
Paramyxoviruses
Human RSV, Metapneumovirus, and parainfluenza viruses are enveloped RNA viruses that are members of the Paramyxovirudiae family. Both Metapneumovirus and RSV have been associated with significant graft dysfunction [mean forced expiratory volume in 1 second (FEV1) decline 30%, and 25%, respectively) and mortality from paramyxoviruses may range from 10 to 15% in LTRs.7,8 Respiratory syncytial virus (RSV) is one of the most commonly isolated CRVs, and disease can range from a mild upper respiratory tract infection with rhinorrhea and cough to life-threatening pneumonia associated with acute graft rejection and subsequent obliterative bronchiolitis. Although viral culture, fluorescent antibody, and serologic testing can be used to diagnose acute infection, reverse transcription–polymerase chain reaction (RTPCR) based assays yield greater sensitivity in symptomatic patients.9Metapneumovirus (HMPV) is a recently identified pathogen whose clinical spectrum of disease is similar to that of RSV, although in general, acute HMPV appears to be less severe than RSV and appears to have a lower incidence of postviral obliterative bronchiolitis.10 Parainfluenza virus has been classified into four major serotypes, of which serotype 3 is the most common isolate found in LTRs. The incidence of parainfluenza infections ranges from 2 to 10% of all LTRs, the majority of cases occurring more than 1 year after transplantation, with a seasonal peak in the spring and summer.11 The incidence on concomitant acute rejection as been reported as high as 82% in one series, with a significant portion (30%) going on to develop BOS.11
PATHOGENESIS
The immunopathological mechanisms of respiratory viral infections in LTRs remain incompletely understood. As an example, in normal hosts, RSV primarily infect airway epithelial cells; the subsequent innate and adaptive immune responses may lead to either resolution or chronic airways disease.12 RSV infection of respiratory epithelial cells has been shown to induce innate immune mechanisms, including toll-like receptor (TLR4), which is a potent activator of costimulation pathways for adaptive T-helper 1 (Th1) adaptive immune responses.13,14 This Th1-driven response, characterized by augmented interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-2 (IL-2) production, as well as cytolytic T cell responses, has been associated with clearance of virus and resolution of injury.15,16 In preclinical models of Th1 deficiency, RSV infection has been associated with viral persistence, chronic airway inflammation, characterized by interleukin-10 (IL)-10 and a Th2-driven response.17 Further studies are needed to determine how the altered mucosal immunity in LTRs, which includes suppression of IL-2-mediated responses, influences the pathogenesis of RSV pulmonary infection and subsequent allograft dysfunction and whether similar mechanisms apply to other viral pathogens in this family.
PROPHYLAXIS AND TREATMENT
Vaccine development for RSV and other paramyxoviruses has been hampered by the development of vaccine-associated pulmonary inflammation in early trials, and currently no licensed vaccines are available for clinical use.18,19 Alternatively, a monoclonal RSV-specific antibody, palivizumab, was shown to reduce hospitalization rates among children at high risk for RSV infection in a multicenter, randomized, controlled trial. In immunocompromised patients, palivizumab has an excellent safety profile and may be beneficial in the prevention and/or treatment of RSV infections; however, further clinical trials are needed to determine its efficacy.20 The mainstay of treatment for paramyxoviral infections has been inhaled ribavarin, which has been associated with improved survival in hematopoietic stem cell recipients with RSV infection.21 Weill et al reported stable lung function when LTRs infected with either RSV or parainfluenza were treated with inhaled ribavarin, corticosteroids, and intravenous immunoglobulin (IVIG), with additional palivizumab if RSV was present; however, no control group was available for comparison.22 Due to the inconvenient delivery method and high cost of inhaled ribavarin, oral and intravenous ribavarin for treatment of RSV has also been studied; however, further studies are needed to compare the long-term efficacy of oral versus intravenous versus inhaled therapies.23,24 There are several new agents in early clinical trials for RSV, including RSV604, a novel benzodiazepine, and ALN-RSV01, which is based on small interfering RNA that suppresses viral replication without limiting induction of the memory immune response.25,26 A phase I study of safety of ALN-RSV01 in a multicenter cohort of LTRs was recently completed, and further clinical trials are planned. However, an optimal treatment strategy for paramyxoviruses remains to be determined in LTRs because the majority of studies are limited by small sample sizes, lack of control groups, and variable definitions of efficacy in clinical studies.
Influenza
Seasonal influenza has been reported to cause infection in up to 5% of LTRs, with increased frequency in winter season.2 Although infection in normal hosts is usually a self-limited disease with prominent upper respiratory symptoms along with myalgias and fever, LTRs may have increased lower respiratory tract involvement and prolonged viral shedding.27,28 Diagnosis can be made using rapid antigen-based assays of nasopharyngeal swabs, or by molecular PCR-based methods. The epidemiological pattern of influenza reflects a marked ability of the virus to undergo antigenic drifts and, at times, shifts in their envelope glycoproteins, hemagglutinin (H) and neuraminidase (N), which can result in pandemic outbreaks.29 As an example of an antigenic shift, the recently described novel H1N1 influenza virus has now infected more than 1 million people to date and has been associated with >4000 deaths to date in the United States alone. Although the impact of this strain on immunocompromised hosts has yet to be fully defined, several transplant centers have reported increased severity of infection in transplant recipients.30 Clinical features of H1N1 influenza may be similar to seasonal influenza; however, fever and increased gastrointestinal symptoms such as nausea and diarrhea may be prominent.31 Clinical suspicion of H1N1 is paramount to avoid a delay in treatment because rapid antigen-based nasopharyngeal testing designed for seasonal influenza does not accurately detect novel H1N1.32 Molecular RT-PCR based assays were approved for novel H1N1 in July 2009 but may have longer turnaround time, particularly if testing is not available locally.
PATHOGENESIS
Experimental models of influenza have shown that infection of lung epithelial cells is associated with a toll-like receptor 3–mediated innate immune response, activation of CD8+ cytolytic T lymphocytes, and induction of the type 1 effector cytokines IFN-γ and TNF-α.33 Disease severity appears related to altered innate immunity, excess CD8+ T cell responses, and hypercytokinemia both in seasonal and possibly in novel H1N1 influenza.34 The regulation of this immune response to limit lung injury appears complex because some preclinical studies have demonstrated that attenuation of lung inflammation is dependent on IL-10 production by CD8+ T lymphocytes, whereas others have described development of a protective Th17 response in IL-10 deficient hosts that enhances survival after influenza infection.35,36 In LTRs, the majority of patients with active influenza, but not RSV infection, had concomitant acute allograft rejection.37–39 Furthermore, the inflammatory mediators IFNγ, IL-12p40, and ICAM-1 (inter-cellular adhesion molecule-1) have been shown to be upregulated in both murine respiratory viral models as well as human lung allografts during acute rejection.40–42 Because few studies have examined the antigenic specificity of the immune response during respiratory viral infections in lung allografts, it remains to be determined whether the augmented inflammatory responses are directed against the virus or the allograft, and whether these immune responses are protective due to enhanced viral clearance, or deleterious due to increased lung allograft injury, or both.
PROPHYLAXIS AND TREATMENT
Use of the inactivated trivalent vaccine against seasonal influenza in LTRs has been shown to induce both humoral and cellular immune responses to candidate antigens; however, these responses are attenuated compared with normal controls.43,44 Because periods of augmented immunosuppression (such as with induction agents or cytolytic therapy) may decrease immunogenicity of vaccines, the timing of vaccination should be adjusted accordingly, along with chemoprophylaxis using neuraminidase inhibitors considered depending on local epidemiology and patient exposure.45 Although the effectiveness of novel H1N1 influenza vaccine in immunocompromised hosts is unknown, over 90% of healthy controls achieved significant antibody titers by 3 weeks, and use of the vaccine is strongly encouraged and supported by CDC and International Society for Heart and Lung Transplant (IHSLT) Guidelines.31 Due to the emergence of osteltamivir-resistant seasonal influenza, treatment guidelines for confirmed or possible seasonal influenza include oseltamivir plus amantadine or rimantidine, or zanamivir monotherapy.46 Oseltamivir monotherapy may be acceptable when novel H1N1 is confirmed or predominant in the community, although case reports of resistance have been reported.31 Molecular assays for oseltamivir resistance are under development but not currently available clinically. Although standard treatment duration for both seasonal and novel influenza is 5 days, it is likely that immunocompromised hosts may have prolonged viral shedding. Further studies would be needed to determine optimal length of treatment in LTRs.
IMPACT ON CHRONIC ALLOGRAFT DYSFUNCTION
Respiratory viruses are increasingly being recognized as a significant risk factor for development of BOS.47,48 Obliterative bronchiolitis, the histopathological hallmark of chronic rejection, is a fibroproliferative small airways disease thought to be preceded by inflammation, epithelial injury and mucosal ulcerations, clinically manifested by airflow obstruction, or BOS.49 In addition to the epidemiological studies above, which suggest that respiratory viral infections are a risk factor for BOS, several animal models of lung transplantation have suggested that respiratory viral infections can potentiate airway damage in an allogeneic environment. Winter et al demonstrated that murine Sendai virus, which is closely related to human parainfluenza-1, augmented the airway damage in allogeneic but not syngeneic or non-transplanted rat lung allografts.41 Subsequent studies in a murine orthotopic tracheal transplant model of obliterative airways disease demonstrated increased airway fibrosis in allografts infected with Sendai virus that was attenuated if recipients had the opportunity to develop pretransplant immunity to the virus.50 In other preclinical transplant models, viruses were shown to induce allograft rejection in otherwise tolerant hosts via activation of TLR-dependent pathways.51,52 Further research is needed to determine whether these mechanisms may also contribute to postviral chronic allograft dysfunction in LTRs.
CYTOMEGALOVIRUS AND OTHER β-HERPES VIRUSES
CMV remains a significant cause of morbidity in solid organ transplant recipients because greater than 75% of patients develop primary infection or reactivate CMV after transplantation.53 Moreover, primary CMV infection in naïve recipients from a positive donor (i.e., donor+/recipient−, D+R−) has been shown to be a risk factor for increased 1- and 5-year mortality in LTRs according to registry data from the ISHLT.54 In addition, registry data also indicate that the presence of CMV infection in the graft (i.e., D+R− and D+R+ LTRs) negatively impacts long-term survival in LTRs compared with those without (D−R+ and D−R−), as shown in Fig. 1.54 At the same time, the continued improvement in effective antiviral therapies and prophylaxis strategies has contributed to improved outcomes in LTRs at risk for CMV infection compared with the earlier period of lung transplantation.55
Figure 1.
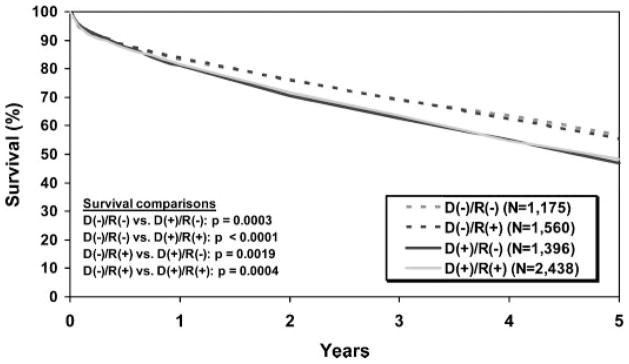
Decreased survival in cytomegalovirus (CMV) donor+ lung transplant recipients. Shown are Kaplan-Meier survival data by donor/recipient CMV status from October 1999 to June 2007. Reprinted with permission from Elsevier, International Society for Heart and Lung Transplantation. Christie et al.111
Epidemiology
The seroprevalence of CMV infection increases with age with rates ranging from 40% in young adults to >90% in those over 80 years of age.56 In immunocompetent hosts primary infection is followed by the establishment of latency, in which viral replication is undetectable by sensitive quantitative assays (i.e., PCR), but virus is not eradicated in the host.57 In LTRs, the risk of CMV infection with pneumonitis is 15 to 55% but varies in different serologic groups, with the great risk for CMV disease occurring in D+R− individuals, as has been reported in all solid organ transplant recipients (SOTRs). The observation of increased susceptibility to active CMV infection in LTRs is perhaps due, in part, to the lung serving as an important reservoir for latent virus.58,59 Early studies prior to the widespread use of CMV antiviral prophylaxis provide clear evidence that LTRs with early significant active infection marked by CMV pneumonitis went on to develop rapid allograft dysfunction and BOS; thus CMV infection has been considered a “probable” risk factor for BOS on the basis of these and subsequent studies that showed a decrease in BOS with the use of prophylactic regimens.60–62 However, more recent studies in the era of routine CMV prophylaxis have not found active CMV infection to impart increased risk for the development of BOS. These apparently conflicting data could be interpreted that serious CMV infection very early after lung transplant can be quite detrimental for allograft function; however, CMV infection at later time points posttransplant may pose less overall risk for BOS development provided prompt treatment is administered. However, many questions remain unanswered regarding the role of CMV infection and BOS, including, What is the optimal antiviral prophylaxis duration posttransplant? Is there a role for continued monitoring after prophylaxis? Does chronic CMV infection in the lung allograft pose increased risk for a subset of individuals? (as is suggested by the increased mortality data in D+ LTRs) (Fig. 1).
PATHOGENESIS
CMV infection is characterized by synthesis of immediate-early, early, and late viral proteins, leading to viral replication and shedding of virus.63 The virus has been shown to infect a variety of cell types, including monocytes, dendritic cells, endothelial cells, and epithelial cells.58,64 The immunopathogenesis of CMV infection is highly complex because it engages both the innate (i.e., natural killer or NK cells) and adaptive (CD8+ and CD4+ T lymphocytes) cellular effector arms of immunity, in addition to humoral immunity from B cells and plasma cells that produce antibody. Although these multiple immune components are likely important for immune control of viral replication during acute primary and chronic infection, CMV has evolved immune evasion mechanisms, to enhance viral transmission in its host.65 In LTRs, intense immunosuppression, as is common during the immediate posttransplant period, might limit the maintenance of sufficient memory T cell immune responses that are important for optimal CMV immune control.66 Several studies have shown an association between CMV infection and allograft dysfunction, with increased levels of chemokines CCL2 and CCL5 in BAL during infection.67 We and others have shown that certain D+R− LTRs are capable of maintaining robust CMV-specific CD8+ and CD4+ T cell and humoral responses that can persist up to years following primary infection. More recently, we have shown that D+R− LTRs are capable of acquiring robust CMV-specific CD8+ T cells during acute primary infection capable of producing the type 1 effector cytokines IFN-γ and TNF-α in response to the CMV phosphoprotein 65 (pp65), and are increased in the lung allograft compared with the blood (Fig. 2). These viral-specific effector memory T cell responses can be detected at the single-cell level in cells obtained from bronchoalveolar lavage fluid (BALF) and using flow cytometric analysis, as demonstrated in airway CD8+ T cells from an LTR following primary CMV infection and during acute influenza infection (Fig. 2). These CMV immune responses have been associated with freedom from CMV disease and preservation of allograft function; however, many questions remain, and the identification of the critical functional and phenotypic correlates of immune protection following transplant need to be further refined. 68–71 For example, a recent report showed that LTRs who develop recurrent episodes of CMV viremia demonstrate significantly decreased long-term survival; therefore, the ability to predict the important correlates of protective immunity could greatly enhance our ability to optimize clinical management strategies for CMV.72
Figure 2.
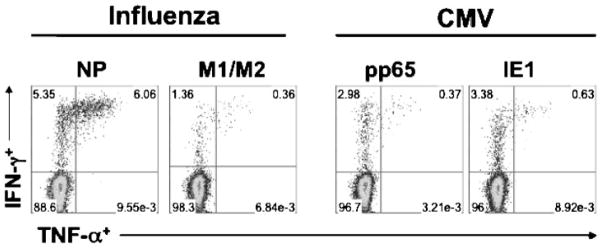
Cytomegalovirus (CMV)-specific and influenza-specific CD8+ effector memory T cells are detectable in the lung allograft using flow cytometric analysis. Bronchoalveolar lavage (BAL) cells were obtained from a lung transplant recipient (LTR) during acute influenza pneumonia and approximately 1 year following primary CMV infection. Cells were restimulated in vitro using pooled peptides to the influenza antigens nucleoprotein (NP) and matrix 1 and matrix 2 (M1/M2) or the CMV antigens phosphoprotein 65 (pp65) and immediate early antigen-1 (IE1) for 6 hours. Cell cultures were then stained for CD3, CD8, and the intracellular cytokines interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). Upper quadrants show the frequencies of cytokine+ cells, following gating on total CD8+ T cells in BAL (left lower quadrant).
DIAGNOSIS
The advent of quantitative molecular techniques for detection of CMV antigens allows for early and more sensitive detection of active viral replication and may correlate with CMV disease, which is defined as symptomatic end-organ involvement attributable to active infection. Although serum-based assays, including quantitative PCR, hybrid capture assays, and the semi-quantitative pp65-antigenemia assay, have all been shown to be effective in LTRs, quantitative methods may be most predictive of disease severity.73–75 Weinberg et al compared the predictive value of quantitative PCR, cultures, and antigenemia for development of CMV disease and found that quantitative PCR-measured DNA viral load increased five- to 10-fold prior to symptomatic disease and decreased with antiviral treatment. 76 However, in clinical use, one must be aware of the potential for interlaboratory variation in quantitative viral load testing because an international reference standard for calibration has not yet been created.77
The most serious manifestation of CMV disease in LTRs is CMV pneumonitis, a relatively common manifestation of either primary or reactivation of chronic pulmonary viral infection in immunocompromised hosts. CMV pneumonitis has been classically defined by the presence of histopathological changes of cytopathic effect or “owl’s eye” viral inclusions in infected cells; however, the low sensitivity of transbronchial biopsies may limit the diagnostic utility of this finding for “definite” pneumonitis. Therefore, the presence of pulmonary symptoms along with microbiological evidence of active CMV infection, in the absence of other causes, fulfills the diagnostic criteria for “probable” pneumonitis.78 Because the lung is a major reservoir for latent CMV, qualitative diagnostic measures, such as a positive viral culture or early antigen assay for CMV, are relatively common in the absence of symptoms but may not be specific for the diagnosis of CMV pneumonitis. 79,80 However, more recent studies examining the role of more sensitive quantitative viral load assays in BALF to either predict pneumonitis or perhaps better quantify subclinical viral infection appear very promising, but further studies are needed before widespread clinical application.81,82
MANAGEMENT STRATEGIES
Despite a paucity of prospective, randomized, controlled trials, cumulative retrospective data suggest that the widespread implementation of CMV prophylaxis and treatment is associated with an overall reduction in incidence of BOS.55,83 Duncan et al first demonstrated a delay in the onset of BOS among CMV seropositive LTRs (D+ or R+) with 90 days of posttransplant ganciclovir compared with acyclovir.84 The oral prodrug valganciclovir is also being widely incorporated into prophylaxis regimens, although direct comparison with ganciclovir has not been performed in LTRs.85 Recent studies have reported a reduction in CMV viremia and CMV disease with prolonged prophylaxis regimens of up to 360 days; however, it is more likely that increasing length of prophylaxis delays onset of CMV infection rather than preventing its onset.86–90 Furthermore, prolonged prophylaxis may carry increased risk of systemic toxicity from medication, may increase risk of UL97 mutations that confer ganciclovir resistance, and may not be necessary in patients that are able to develop protective immune responses against CMV.91
The additive benefit of passive immunoprophylaxis with CMV immunoglobulin has not been well characterized in prospective trials. In several retrospective studies combined prophylaxis with ganciclovir plus CMV immune globulin was compared with a group of historical controls that received ganciclovir alone. In these studies, freedom from disease, obliterative bronchiolitis, and survival were greater in the group that received combination therapy.92,93 Given these uncertainties, commonly used protocols vary from 90 days of prophylaxis with IV ganciclovir in high-risk recipients only (D+R−) to indefinite prophylaxis with CMV immune globulin followed by daily oral valganciclovir in all LTRs. This variability underscores the need for randomized-controlled studies to determine optimal prophylaxis and treatment regimens for CMV because these requirements may vary significantly depending on whether LTRs are at risk for primary infection versus reactivation of chronic infection.
The advent of rapid, sensitive screening assays for CMV infection has led some centers to adopt a preemptive approach that involves frequent screening and prompt treatment of CMV infection as compared with universal prophylaxis. Although studies that have examined this approach differ in their frequency of screening and choice of assay for CMV detection, a solely preemptive approach may be associated with increased CMV pneumonitis and more importantly, chronic allograft dysfunction in D+R− recipients.94,95 Furthermore, despite decreased use of ganciclovir, a preemptive approach has not been shown to reduce the incidence of ganciclovir resistance.96 It remains to be determined whether this approach may be more suitable for R+ recipients, who have lower risk of reactivation. Finally, many centers have adopted a combination of limited prophylaxis, such as 90 days, followed by preemptive therapy. This approach may limit the use of antiviral therapy without adversely impacting long-term outcomes, as suggested in a study by Tamm et al, in which 90 days of prophylaxis with ganciclovir and prompt treatment of CMV pneumonia did not increase the risk of BOS or decrease patient survival in any combination of donor/recipient serological matches.97
Despite the significant progress made in treating CMV-associated complications following lung transplant, challenges still exist in the small recipients who have recurrent episodes of viremia or ganciclovir resistance, which can occur in 5 to 10% of patients. In patients with ganciclovir resistance, foscarnet has been employed with some reduction in viral load, although this approach may impair renal function.98 In the modern era, patients most at risk of developing CMV-associated morbidity and mortality are those who have recurrent viremia and may lack critical immune control mechanisms. Although adoptive T cell immunotherapy has been employed successfully in stem cell recipients who do not respond to standard antiviral treatment, this approach has had limited success in LTRs because it is costly, and autologous CMV-specific effector T cells have been derived from immunocompromised hosts rather than healthy donors.99–101
OTHER β-HERPES VIRUSES
In addition to CMV, several other β-herpes viruses can reactivate in LTRs, with potential for increased complications. Over 90% of adults have been exposed to Epstein-Barr virus; the majority of primary infections in immunocompetent hosts are subclinical and the virus persists in lymphocytes, epithelial cells, and monocytes in a latent phase. The virus is capable of transforming infected B lymphocytes, and is associated with development of posttransplant lymphoproliferative disorder (PTLD) in LTRs and other SOTRs. The risk of PTLD correlates to the degree of immunosuppression, time after transplant, and recipient EBV serostatus, with an overall incidence estimated to be 2 to 9% in LTRs.102 Diagnosis of PTLD continues to require a high degree of suspicion because presenting features may be subtle, with radiologic evidence of a mass, an elevated lactate dehydrogenase (LDH), and/or positive positron emission tomography scanning all supportive of the diagnosis. Monitoring of EBV viral loads may be useful in detecting early disease that is more likely to respond to treatment. In a prospective study by Tsai et al, plasma-based EBNA assays had greatest specificity; however, a complete PCR panel consisting of latent membrane protein 1 (LMP-1), Epstein Barr encoded RNA 1 (EBER-1), and Epstein Barr nuclear antigen 1 (EBNA-1) was most sensitive.103 Whole blood assays may also have excellent sensitivity but may lack sufficient specificity for PTLD.104 Treatment strategies for PTLD include reduction of immunosuppression, surgical resection, standard chemotherapy, and, more recently, use of the humanized anti-CD20 B cell–targeted monoclonal antibody, rituximab.105 Although used frequently in many protocols, antiviral agents such as ganciclovir have not been shown to significantly impact clinical outcome after onset of PTLD but may reduce incidence of PTLD when used in prophylaxis.106 Because of the increased risk of rejection with reduced immunosuppression and risk of toxicity with chemotherapy, the optimal therapy for PTLD in LTRs remains to be determined.
More recently, human herpesvirus 6 (HHV-6) and human herpesvirus 7 (HHV-7) have shown to reactivate following transplantation, and in other solid organs, may be associated with immunomodulatory effects, increased CMV coinfection, and possible graft dysfunction.107,108 A recent study by Humar et al78 documented a 10 to 20% 1-year incidence of HHV-6 and HHV-7 in LTRs treated with valganciclovir prophylaxis; however, the clinical significance of these infections remains controversial. In this retrospective study, investigators noted common coinfection with CMV but did not find any association with acute rejection or BOS at 3 years.55 Other studies have suggested a possible association between HHV-6 and BOS, and HHV-7 and bronchiolitis obliterans organizing pneumonia, but the small cohort size, retrospective data, and variable methods of detection limit the ability to directly compare study results.109,110
CONCLUSIONS
Viral infections continue to contribute to complications after lung transplantation, not only from acute infection, which can cause severe lower respiratory tract infections that are in some instances uncommon in immunocompetent hosts, but also from their potential to induce and/ or augment chronic allograft dysfunction. The cumulative epidemiological data in CMV suggest that the ability to limit viral replication, either with antiviral agents and/or via protective immune responses, may significantly attenuate morbidity and mortality from CMV. Although advances in molecular diagnostics and new treatment options may improve outcomes for select respiratory viruses, further understanding of the mechanisms that regulate the immune response are needed to design broader approaches to limiting the potential immunopathology associated with viral infections in LTRs. These endeavors are critical to limiting chronic allograft dysfunction and improving long-term outcomes following lung transplantation.
References
- 1.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 3.Garbino J, Gerbase MW, Wunderli W, et al. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med. 2004;170:1197–1203. doi: 10.1164/rccm.200406-781OC. [DOI] [PubMed] [Google Scholar]
- 4.Milstone AP, Brumble LM, Barnes J, et al. A single-season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J. 2006;28:131–137. doi: 10.1183/09031936.06.00105505. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Henrickson KJ, Savatski LL. Rapid simultaneous diagnosis of infections with respiratory syncytial viruses A and B, influenza viruses A and B, and human parainfluenza virus types 1, 2, and 3 by multiplex quantitative reverse transcription-polymerase chain reaction-enzyme hybridization assay (Hexaplex) Clin Infect Dis. 1998;26:1397–1402. doi: 10.1086/516357. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg A, Zamora MR, Li S, Torres F, Hodges TN. The value of polymerase chain reaction for the diagnosis of viral respiratory tract infections in lung transplant recipients. J Clin Virol. 2002;25:171–175. doi: 10.1016/s1386-6532(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 7.McCurdy LH, Milstone A, Dummer S. Clinical features and outcomes of paramyxoviral infection in lung transplant recipients treated with ribavirin. J Heart Lung Transplant. 2003;22:745–753. doi: 10.1016/s1053-2498(02)00569-7. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178:876–881. doi: 10.1164/rccm.200711-1657OC. [DOI] [PubMed] [Google Scholar]
- 9.Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–820. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh K, McAdam AJ. Human metapneumovirus—an important new respiratory virus. N Engl J Med. 2004;350:431–433. doi: 10.1056/NEJMp038212. [DOI] [PubMed] [Google Scholar]
- 11.Vilchez RA, Dauber J, McCurry K, Iacono A, Kusne S. Parainfluenza virus infection in adult lung transplant recipients: an emergent clinical syndrome with implications on allograft function. Am J Transplant. 2003;3:116–120. doi: 10.1034/j.1600-6143.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 12.Mahalingam S, Schwarze J, Zaid A, et al. Perspective on the host response to human metapneumovirus infection: what can we learn from respiratory syncytial virus infections? Microbes Infect. 2006;8:285–293. doi: 10.1016/j.micinf.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 14.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 15.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham BS, Rutigliano JA, Johnson TR. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 17.Crowe JE, Jr, Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr Respir Rev. 2003;4:112–119. doi: 10.1016/s1526-0542(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 18.Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 19.Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol. 2007;179:5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- 20.Tsitsikas DA, Oakervee H, Cavenagh JD, Gribben J, Agrawal SG, Mattes FM. Treatment of respiratory syncytial virus infection in haemopoietic stem cell transplant recipients with aerosolized ribavirin and the humanized monoclonal antibody palivizumab: a single centre experience. Br J Haematol. 2009;146:574–576. doi: 10.1111/j.1365-2141.2009.07763.x. [DOI] [PubMed] [Google Scholar]
- 21.Sparrelid E, Ljungman P, Ekelöf-Andström E, et al. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997;19:905–908. doi: 10.1038/sj.bmt.1700752. [DOI] [PubMed] [Google Scholar]
- 22.Liu V, Dhillon GS, Weill D. A multi-drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart-lung transplant recipients. Transpl Infect Dis. 2009 Sep 15; doi: 10.1111/j.1399-3062.2009.00453.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Pelaez A, Lyon GM, Force SD, et al. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant. 2009;28:67–71. doi: 10.1016/j.healun.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glanville AR, Scott AI, Morton JM, et al. Intravenous ribavirin is a safe and cost-effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant. 2005;24:2114–2119. doi: 10.1016/j.healun.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Nokes JD, Cane PA. New strategies for control of respiratory syncytial virus infection. Curr Opin Infect Dis. 2008;21:639–643. doi: 10.1097/QCO.0b013e3283184245. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Tripp RA. RNA interference inhibits respiratory syncytial virus replication and disease pathogenesis without inhibiting priming of the memory immune response. J Virol. 2008;82:12221–12231. doi: 10.1128/JVI.01557-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119:1277–1280. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 28.Vilchez R, McCurry K, Dauber J, et al. Influenza and parainfluenza respiratory viral infection requiring admission in adult lung transplant recipients. Transplantation. 2002;73:1075–1078. doi: 10.1097/00007890-200204150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Hoft DF, Belshe RB. The genetic archaeology of influenza. Engl J Med. 2004;351:2550–2551. doi: 10.1056/NEJMcibr043708. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients - Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:893–896. [PubMed] [Google Scholar]
- 31.Danziger-Isakov LA, Husain S, Mooney ML, Hannan MM ISHLT Infectious Diseases Council. The novel 2009 H1N1 influenza virus pandemic: unique considerations for programs in cardiothoracic transplantation. J Heart Lung Transplant. 2009;28:1341–1347. doi: 10.1016/j.healun.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Faix DJ, Sherman SS, Waterman SH. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361:728–729. doi: 10.1056/NEJMc0904264. [DOI] [PubMed] [Google Scholar]
- 33.Le Goffic R, Pothlichet J, Vitour D, et al. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 34.Mauad T, Hajjar LA, Callegari GD, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181:72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 35.McKinstry KK, Strutt TM, Buck A, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2:287–291. doi: 10.1034/j.1600-6143.2002.20315.x. [DOI] [PubMed] [Google Scholar]
- 38.Kuo JH, Hwang R. Preparation of DNA dry powder for non-viral gene delivery by spray-freeze drying: effect of protective agents (polyethyleneimine and sugars) on the stability of DNA. J Pharm Pharmacol. 2004;56:27–33. doi: 10.1211/0022357022494. [DOI] [PubMed] [Google Scholar]
- 39.Vilchez RA, McCurry K, Dauber J, et al. The epidemiology of parainfluenza virus infection in lung transplant recipients. Clin Infect Dis. 2001;33:2004–2008. doi: 10.1086/324348. [DOI] [PubMed] [Google Scholar]
- 40.Moudgil A, Bagga A, Toyoda M, Nicolaidou E, Jordan SC, Ross D. Expression of gamma-IFN mRNA in bronchoalveolar lavage fluid correlates with early acute allograft rejection in lung transplant recipients. Clin Transplant. 1999;13:201–207. doi: 10.1034/j.1399-0012.1999.130208.x. [DOI] [PubMed] [Google Scholar]
- 41.Winter JB, Gouw AS, Groen M, Wildevuur C, Prop J. Respiratory viral infections aggravate airway damage caused by chronic rejection in rat lung allografts. Transplantation. 1994;57:418–422. doi: 10.1097/00007890-199402150-00018. [DOI] [PubMed] [Google Scholar]
- 42.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med. 2001;193:339–351. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayney MS, Moran J, Wiegert NA, Burlingham WJ. Lung transplant patients’ T cell responses to influenza vaccine viruses between seasons. Vaccine. 2008;26:2596–2600. doi: 10.1016/j.vaccine.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Mazzone PJ, Mossad SB, Mawhorter SD, Mehta AC, Schilz RJ, Maurer JR. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J. 2001;18:971–976. doi: 10.1183/09031936.01.00215201. [DOI] [PubMed] [Google Scholar]
- 45.Khazeni N, Bravata DM, Holty JE, Uyeki TM, Stave CD, Gould MK. Systematic review: safety and efficacy of extended-duration antiviral chemoprophylaxis against pandemic and seasonal influenza. Ann Intern Med. 2009;151:464–473. doi: 10.7326/0003-4819-151-7-200910060-00143. [DOI] [PubMed] [Google Scholar]
- 46.Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant. 2008;27:282–288. doi: 10.1016/j.healun.2007.11.575. [DOI] [PubMed] [Google Scholar]
- 47.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 48.Vilchez RA, Dauber J, Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection—myth or reality? Am J Transplant. 2003;3:245–249. doi: 10.1034/j.1600-6143.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 49.Cooper JD, Billingham M, Egan T, et al. International Society for Heart and Lung Transplantation. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 50.Kuo E, Bharat A, Goers T, et al. Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg. 2006;82:1043–1050. doi: 10.1016/j.athoracsur.2006.03.120. [DOI] [PubMed] [Google Scholar]
- 51.Welsh RM, Markees TG, Woda BA, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossini S, Casazza AP, Engelmann EC, Havaux M, Jennings RC, Soave C. Suppression of both ELIP1 and ELIP2 in Arabidopsis does not affect tolerance to photoinhibition and photooxidative stress. Plant Physiol. 2006;141:1264–1273. doi: 10.1104/pp.106.083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 54.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report—2007. J Heart Lung Transplant. 2007;26:769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Manuel O, Kumar D, Moussa G, et al. Lack of association between beta-herpesvirus infection and bronchiolitis obliterans syndrome in lung transplant recipients in the era of antiviral prophylaxis. Transplantation. 2009;87:719–725. doi: 10.1097/TP.0b013e3181963262. [DOI] [PubMed] [Google Scholar]
- 56.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 57.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonatti H, Tabarelli W, Ruttmann E, et al. Impact of cytomegalovirus match on survival after cardiac and lung transplantation. Am Surg. 2004;70:710–714. [PubMed] [Google Scholar]
- 60.Soghikian MV, Valentine VG, Berry GJ, Patel HR, Robbins RC, Theodore J. Impact of ganciclovir prophylaxis on heart-lung and lung transplant recipients. J Heart Lung Transplant. 1996;15:881–887. [PubMed] [Google Scholar]
- 61.Duncan SR, Paradis IL, Yousem SA, et al. Sequelae of cytomegalovirus pulmonary infections in lung allograft recipients. Am Rev Respir Dis. 1992;146:1419–1425. doi: 10.1164/ajrccm/146.6.1419. [DOI] [PubMed] [Google Scholar]
- 62.Keenan RJ, Lega ME, Dummer JS, et al. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51:433–438. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 63.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 64.Söderberg-Nauclér C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 65.Gold MC, Munks MW, Wagner M, Koszinowski UH, Hill AB, Fling SP. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J Immunol. 2002;169:359–365. doi: 10.4049/jimmunol.169.1.359. [DOI] [PubMed] [Google Scholar]
- 66.Gerna G, Lilleri D, Rognoni V, et al. Preemptive therapy for systemic and pulmonary human cytomegalovirus infection in lung transplant recipients. Am J Transplant. 2009;9:1142–1150. doi: 10.1111/j.1600-6143.2009.02616.x. [DOI] [PubMed] [Google Scholar]
- 67.Weigt SS, Elashoff RM, Keane MP, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant. 2008;8:1512–1522. doi: 10.1111/j.1600-6143.2008.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shlobin OA, West EE, Lechtzin N, et al. Persistent cytomegalovirus-specific memory responses in the lung allograft and blood following primary infection in lung transplant recipients. J Immunol. 2006;176:2625–2634. doi: 10.4049/jimmunol.176.4.2625. [DOI] [PubMed] [Google Scholar]
- 69.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 70.Westall GP, Brooks AG, Kotsimbos T. CD8+ T-cell maturation following lung transplantation: the differential impact of CMV and acute rejection. Transpl Immunol. 2007;18:186–192. doi: 10.1016/j.trim.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Pipeling MR, West EE, Osborne CM, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerschner H, Jaksch P, Karigl G, Popow-Kraupp T, Klepetko W, Puchhammer-Stöckl E. Cytomegalovirus DNA load patterns developing after lung transplantation are significantly correlated with long-term patient survival. Transplantation. 2009;87:1720–1726. doi: 10.1097/TP.0b013e3181a60b4e. [DOI] [PubMed] [Google Scholar]
- 73.Egan JJ, Barber L, Lomax J, et al. Detection of human cytomegalovirus antigenaemia: a rapid diagnostic technique for predicting cytomegalovirus infection/pneumonitis in lung and heart transplant recipients. Thorax. 1995;50:9–13. doi: 10.1136/thx.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guiver M, Fox AJ, Mutton K, Mogulkoc N, Egan J. Evaluation of CMV viral load using TaqMan CMV quantitative PCR and comparison with CMV antigenemia in heart and lung transplant recipients. Transplantation. 2001;71:1609–1615. doi: 10.1097/00007890-200106150-00021. [DOI] [PubMed] [Google Scholar]
- 75.Bhorade SM, Sandesara C, Garrity ER, et al. Quantification of cytomegalovirus (CMV) viral load by the hybrid capture assay allows for early detection of CMV disease in lung transplant recipients. J Heart Lung Transplant. 2001;20:928–934. doi: 10.1016/s1053-2498(01)00283-2. [DOI] [PubMed] [Google Scholar]
- 76.Weinberg A, Hodges TN, Li S, Cai G, Zamora MR. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J Clin Microbiol. 2000;38:768–772. doi: 10.1128/jcm.38.2.768-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pang XL, Fox JD, Fenton JM, Miller GG, Caliendo AM, Preiksaitis JK American Society of Transplantation Infectious Diseases Community of PracticeCanadian Society of Transplantation. Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant. 2009;9:258–268. doi: 10.1111/j.1600-6143.2008.02513.x. [DOI] [PubMed] [Google Scholar]
- 78.Humar A. Reactivation of viruses in solid organ transplant patients receiving cytomegalovirus prophylaxis. Transplantation. 2006;82(2, Suppl):S9–S14. doi: 10.1097/01.tp.0000230432.39447.8b. [DOI] [PubMed] [Google Scholar]
- 79.Solans EP, Garrity ER, Jr, McCabe M, Martinez R, Husain AN. Early diagnosis of cytomegalovirus pneumonitis in lung transplant patients. Arch Pathol Lab Med. 1995;119:33–35. [PubMed] [Google Scholar]
- 80.Erice A, Hertz MI, Snyder LS, Englund J, Edelman CK, Balfour HH., Jr Evaluation of centrifugation cultures of bronchoalveolar lavage fluid for the diagnosis of cytomegalovirus pneumonitis. Diagn Microbiol Infect Dis. 1988;10:205–212. doi: 10.1016/0732-8893(88)90092-2. [DOI] [PubMed] [Google Scholar]
- 81.Chemaly RF, Yen-Lieberman B, Chapman J, et al. Clinical utility of cytomegalovirus viral load in bronchoalveolar lavage in lung transplant recipients. Am J Transplant. 2005;5:544–548. doi: 10.1111/j.1600-6143.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 82.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Human cytomegalovirus load in plasma and bronchoalveolar lavage fluid: a longitudinal study of lung transplant recipients. J Infect Dis. 2004;190:1076–1083. doi: 10.1086/422327. [DOI] [PubMed] [Google Scholar]
- 83.Russo MJ, Sternberg DI, Hong KN, et al. Postlung transplant survival is equivalent regardless of cytomegalovirus match status. Ann Thorac Surg. 2007;84:1129–1134. doi: 10.1016/j.athoracsur.2007.05.037. discussion 1134–1135. [DOI] [PubMed] [Google Scholar]
- 84.Duncan SR, Grgurich WF, Iacono AT, et al. A comparison of ganciclovir and acyclovir to prevent cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 1994;150:146–152. doi: 10.1164/ajrccm.150.1.8025741. [DOI] [PubMed] [Google Scholar]
- 85.Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant. 2004;4:1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 86.Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81:1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 87.Chmiel C, Speich R, Hofer M, et al. Ganciclovir/ valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis. 2008;46:831–839. doi: 10.1086/528689. [DOI] [PubMed] [Google Scholar]
- 88.Zamora MR, Nicolls MR, Hodges TN, et al. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am J Transplant. 2004;4:1635–1642. doi: 10.1111/j.1600-6143.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 89.Speich R, Thurnheer R, Gaspert A, Weder W, Boehler A. Efficacy and cost effectiveness of oral ganciclovir in the prevention of cytomegalovirus disease after lung transplantation. Transplantation. 1999;67:315–320. doi: 10.1097/00007890-199901270-00023. [DOI] [PubMed] [Google Scholar]
- 90.Jaksch P, Zweytick B, Kerschner H, et al. Cytomegalovirus prevention in high-risk lung transplant recipients: comparison of 3- vs 12-month valganciclovir therapy. J Heart Lung Transplant. 2009;28:670–675. doi: 10.1016/j.healun.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 91.Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000;356:645–649. doi: 10.1016/S0140-6736(00)02607-6. [DOI] [PubMed] [Google Scholar]
- 92.Valantine HA, Luikart H, Doyle R, et al. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin plus ganciclovir versus ganciclovir alone. Transplantation. 2001;72:1647–1652. doi: 10.1097/00007890-200111270-00012. [DOI] [PubMed] [Google Scholar]
- 93.Weill D, Lock BJ, Wewers DL, et al. Combination prophylaxis with ganciclovir and cytomegalovirus (CMV) immune globulin after lung transplantation: effective CMV prevention following daclizumab induction. Am J Transplant. 2003;3:492–496. doi: 10.1034/j.1600-6143.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 94.Thomas LD, Milstone AP, Miller GG, Loyd JE, Stephen Dummer J. Long-term outcomes of cytomegalovirus infection and disease after lung or heart-lung transplantation with a delayed ganciclovir regimen. Clin Transplant. 2009;23:476–483. doi: 10.1111/j.1399-0012.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- 95.Monforte V, Román A, Gavaldà J, et al. Preemptive therapy with intravenous ganciclovir for the prevention of cytomegalovirus disease in lung transplant recipients. Transplant Proc. 2005;37:4039–4042. doi: 10.1016/j.transproceed.2005.09.141. [DOI] [PubMed] [Google Scholar]
- 96.Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis. 2002;185:20–27. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- 97.Tamm M, Aboyoun CL, Chhajed PN, Rainer S, Malouf MA, Glanville AR. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2004;170:1120–1123. doi: 10.1164/rccm.200310-1405OC. [DOI] [PubMed] [Google Scholar]
- 98.Reddy AJ, Zaas AK, Hanson KE, Palmer SM. A singlecenter experience with ganciclovir-resistant cytomegalovirus in lung transplant recipients: treatment and outcome. J Heart Lung Transplant. 2007;26:1286–1292. doi: 10.1016/j.healun.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Brestrich G, Zwinger S, Fischer A, et al. Adoptive T-cell therapy of a lung transplanted patient with severe CMV disease and resistance to antiviral therapy. Am J Transplant. 2009;9:1679–1684. doi: 10.1111/j.1600-6143.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 100.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 101.Brestrich G, Zwinger S, Roemhild A, et al. Generation of HCMV-specific T-cell lines from seropositive solid-organ-transplant recipients for adoptive T-cell therapy. J Immunother. 2009;32:932–940. doi: 10.1097/CJI.0b013e3181b88fda. [DOI] [PubMed] [Google Scholar]
- 102.Aris RM, Maia DM, Neuringer IP, et al. Post-transplantation lymphoproliferative disorder in the Epstein-Barr virus-naïve lung transplant recipient. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1712–1717. doi: 10.1164/ajrccm.154.6.8970360. [DOI] [PubMed] [Google Scholar]
- 103.Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8:1016–1024. doi: 10.1111/j.1600-6143.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 104.Stevens SJ, Verschuuren EA, Pronk I, et al. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–1171. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 105.Knoop C, Kentos A, Remmelink M, et al. Post-transplant lymphoproliferative disorders after lung transplantation: first-line treatment with rituximab may induce complete remission. Clin Transplant. 2006;20:179–187. doi: 10.1111/j.1399-0012.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 106.Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5:2894–2900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 107.Deborska-Materkowska D, Lewandowski Z, Sadowska A, et al. Fever, human herpesvirus-6 (HHV-6) seroconversion, and acute rejection episodes as a function of the initial seroprevalence for HHV-6 in renal transplant recipients. Transplant Proc. 2006;38:139–143. doi: 10.1016/j.transproceed.2005.11.093. [DOI] [PubMed] [Google Scholar]
- 108.Kidd IM, Clark DA, Sabin CA, et al. Prospective study of human betaherpesviruses after renal transplantation: association of human herpesvirus 7 and cytomegalovirus co-infection with cytomegalovirus disease and increased rejection. Transplantation. 2000;69:2400–2404. doi: 10.1097/00007890-200006150-00032. [DOI] [PubMed] [Google Scholar]
- 109.Neurohr C, Huppmann P, Leuchte H, et al. Munich Lung Transplant Group. Human herpesvirus 6 in bronchoalveolar lavage fluid after lung transplantation: a risk factor for bronchiolitis obliterans syndrome? Am J Transplant. 2005;5:2982–2991. doi: 10.1111/j.1600-6143.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 110.Ross DJ, Chan RC, Kubak B, Laks H, Nichols WS. Bronchiolitis obliterans with organizing pneumonia: possible association with human herpesvirus-7 infection after lung transplantation. Transplant Proc. 2001;33:2603–2606. doi: 10.1016/s0041-1345(01)02109-1. [DOI] [PubMed] [Google Scholar]
- 111.Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]


