Abstract
Cellular nucleotide pools are often contaminated by base analog nucleotides which interfere with a plethora of biological reactions, from DNA and RNA synthesis to cellular signaling. An evolutionarily conserved inosine triphosphate pyrophosphatase (ITPA) removes the non-canonical purine (d)NTPs inosine triphosphate and xanthosine triphosphate by hydrolyzing them into their monophosphate form and pyrophosphate. Mutations in the ITPA orthologs in model organisms lead to genetic instability and, in mice, to severe developmental anomalies. In humans there is genetic polymorphism in ITPA. One allele leads to a proline to threonine substitution at amino acid 32 and causes varying degrees of ITPA deficiency in tissues and plays a role in patients’ response to drugs. Structural analysis of this mutant protein reveals that the protein is destabilized by the formation of a cavity in its hydrophobic core. The Pro32Thr allele is thought to cause the observed dominant negative effect because the resulting active enzyme monomer targets both homo- and heterodimers to degradation.
Keywords: nucleotide pool, ITPA gene polymorphism, pharmacogenetics, base analogs, mercaptopurines, protein stability, dominant negative
1. Introduction
Maintenance of cellular nucleotide pools is critical for preserving the integrity of the genome and for allowing normal cellular processes to occur. Inosine triphosphate pyrophosphatase (ITPA) is one of several enzymes whose job is to cleanse the nucleotide pool. ITPA acts on the non-canonical purines inosine and xanthosine triphosphate (ITP/XTP) and their deoxy forms (dITP/dXTP) [1]. ITPA has homologs in all domains of life. Further proof of its importance was demonstrated by the negative effect of mutations in the ITPA orthologs in model organisms [2, 3]. A particularly dramatic example was seen in Itpa knockout mice [4]. Loss of ITPA activity in humans has been implicated in disease and in the degree of adverse reactions of patients to several drugs [5, 6]. A polymorphism responsible for the most dramatic reduction in ITPA activity leads to a proline to threonine substitution at amino acid number 32 [7, 8]. In vitro studies of human cells containing this variant have confirmed that ITPA is necessary for protecting the genome from damage [9]. The crystal structure of Pro32Thr ITPA helps explain its dominant negative effect on enzymatic activity in cells [10].
2. ITPA history, function, and structure
2.1. A brief history of ITPA
In 1964 Liakopoulou and Alivisatos discovered the presence of an ITPase in human erythrocytes [11]. This ITPase activity was separable from ATPase activity, dependent on the presence of Mg2+ ions and competitively inhibited by adenine derivatives. In 1970, Vanderheiden demonstrated by using partially purified preparations of the ITPase from human erythrocytes that the enzyme released PPi and therefore was an ITP pyrophosphatase [12]. Then, in 1979, this ITP pyrophosphatase was purified 2,300-fold from human erythrocytes and was then characterized in terms of kinetics, optimal reaction conditions, and substrate specificity. ITPA was found to be highly specific for ITP, with only minimal activity against ATP, GTP, CTP, and UTP [13]. In the same year, Holmes and coworkers studied the tissue distribution and subcellular localization of ITPA. They found that there was large variation between individuals in the level of ITPA activity. In general erythrocytes had low levels of activity and other cell types, such as bone marrow fibroblasts, had substantially greater activity. They also localized ITPA activity to the soluble cytoplasmic fraction of cells. Lastly they confirmed Vanderheiden’s results that ITPA was highly specific for ITP and dITP, and also demonstrated that XTP was a substrate [14]. These would be the last major developments in human ITPA characterization until the turn of the century.
In 2001, the human ITPA gene was cloned and functionally expressed in E. coli [1]. The human ITPA gene was localized to chromosome 20p. Purified recombinant ITPA specifically cleaved ITP, dITP and XTP. With the sequence of the gene in hand, the level of ITPA expression in various tissues was measured with northern blots. Expression varied greatly among the 24 tissues tested. The highest levels were found in the heart, liver, pancreas, thyroid, testis/ovary, and adrenal glands. The purification of recombinant ITPA made the enzyme easily available and set the stage for future developments, including the identification of additional substrates, such as the triphosphates of mutagenic base analogs 6-hydroxylaminopurine (HAP) and 2-amino-6-hydroxylaminopurine (AHA) [15].
The last major advancement was the crystallization of ITPA and the subsequent visualization of its structure in detail. Open, nucleotide-free ITPA was solved independently by Porta et al. in 2006 [16] and Stenmark et al. in 2007 [17]. Stenmark and coworkers also solved a crystal structure of ITPA with ITP bound. Together, these structures show the conformational changes upon ligand binding, help explain the specificity of ITPA for the non-canonical purines ITP and XTP and explain why ITPA does not seem to discriminate between NTPs and dNTPs. These aspects will be described next.
2.2. ITPA function and structure
ITPA catalyzes the following reactions:
The optimal parameters for maximal activity have varied somewhat among different studies, but in general ITPA has an alkaline pH optimum (8.5 or greater) and a requirement for Mg2+ ions, with around 50 mM providing the greatest activity [1, 13, 14]. The kinetic parameters, viz. the Michaelis constant (Km), the maximum rate (Vmax), and turnover number (kcat), have also varied to a degree among reports (Table 1).
Table 1.
Summary of studies on ITPA catalytic properties
| Reference | Liakopoulou [11] |
Vanderheiden, 1970 [12] |
Vanderheiden, 1979 [13] |
Holmes [14] |
Lin [1] | Stepchenkova [18] |
Herting [15, 19] |
|---|---|---|---|---|---|---|---|
| Protein source |
Hemolysate | Partially purified from hemolysate |
Purified from hemolysate |
Hemolysate | Purified from E. coli |
Purified from E. coli |
Purified from E. coli |
| Km | 1 × 10−4 M | 6 × 10−4 M | 1.3 × 10−4 M | 7 × 10−5 M | 5.1 × 10−4 M | 4.0 × 10−4 M | 3.25 ×10−5 M |
| Vmax | N/A | N/A | 1.2 × 10−9 M/min | N/A | 1520 µmol/(min·mg) | N/A | N/A |
| kcat | N/A | N/A | N/A | N/A | 580 s−1 | 91 s−1 | 79.6 s−1 |
| pH optimum | 7.5 | 8.5 (9.5 with DTT) | 8.6 in Tris-HCl 9.6 in glycine | N/A | 10.0 | N/A | N/A |
| Mg2+ optimum | 50 mM | 50 mM | 100 mM | 60 mM | 30 – 100 mM | N/A | N/A |
| Substrate inhibition | No | Yes | Slight | No | Yes | Yes | Yes |
| Reducing agent activation | N/A | Yes | No | Yes | Yes | N/A | N/A |
The substrate was ITP in all cases except for Herting, where dITP was used.
Differences in the kinetic parameters listed in Table 1 are likely due to the different sources, purity levels and the assay used. Early studies were done using ITPA purified from erythrocytes at various levels of purity. Later studies used highly purified recombinant human ITPA [1, 15, 18, 19]. It is possible that ITPA is post-translationally modified and that the lack of these modifications in a recombinant system may alter the kinetics [1]. The assay method likely has an effect on the values obtained. For example, Vanderheiden [13] used radiolabeled ITP as the substrate and directly measured IMP with high-voltage paper electrophoresis and liquid scintillation counting [20]. The studies of Holmes [14], Lin [1, 15] and Stepchenkova [18], used a coupled assay where the PPi produced by ITPA is further broken down by inorganic pyrophosphatase to Pi, which is then measured colorimetrically. This assay assumes that the inorganic pyrophosphatase reaction is not rate limiting. Further, ITP is known to inhibit human inorganic pyrophosphatase [21] and may therefore account for the substrate inhibition observed in some studies (see Table 1). Given the inhibitory action of IDP on ITPA (see below), the presence of IDP in the ITP used as substrate could also account for substrate inhibition, as suggested by some authors [1, 14]. Lastly, the various buffer systems and different reaction conditions used likely account for some variability, especially given the different pH optimum observed by Vanderheiden for glycine versus Tris-HCl buffers.
Various inhibitors of the ITPA reaction have been identified. When ITPase activity was originally discovered, adenine, adenosine, and the mono-, di-, and triphosphate forms were found to inhibit the activity in a competitive fashion [11]. However, later work by Holmes and colleagues found that adenosine and its nucleotides had very little effect on the activity of ITPA [14]. They showed, though, that IDP is a strong competitive inhibitor of the ITPA reaction, with a dissociation constant of 1.2 × 10−5 M. Several divalent cations were found to also inhibit ITPA [13]. In the presence of 50 mM Mg2+, as little as 1 mM Cd2+ and Cu2+ inhibited the enzyme by 62% and 40%, respectively. Ca2+ also functions as an inhibitor, but a concentration of 10 mM was required to give inhibition. Cd2+ is a soft Lewis acid and therefore interacts well with soft bases, such as sulfur atoms [22]. A study of Cd2+ inhibition of the DNA mismatch repair enzyme complex Msh2-Msh6 found that about 100 Cd2+ ions were bound per protein complex, likely binding to Cys and Met side chains, amino and carbonyl groups, and so on, leading to non-specific inhibition [23]. This is likely the case with ITPA. Cu2+ is a borderline acid and Ca2+ is a hard acid [24]. This could explain the similar, but slightly reduced inhibition from Cu2+ compared to Cd2+, and Ca2+ is thus likely to inhibit by a different mechanism, such as competing for the Mg2+ binding site.
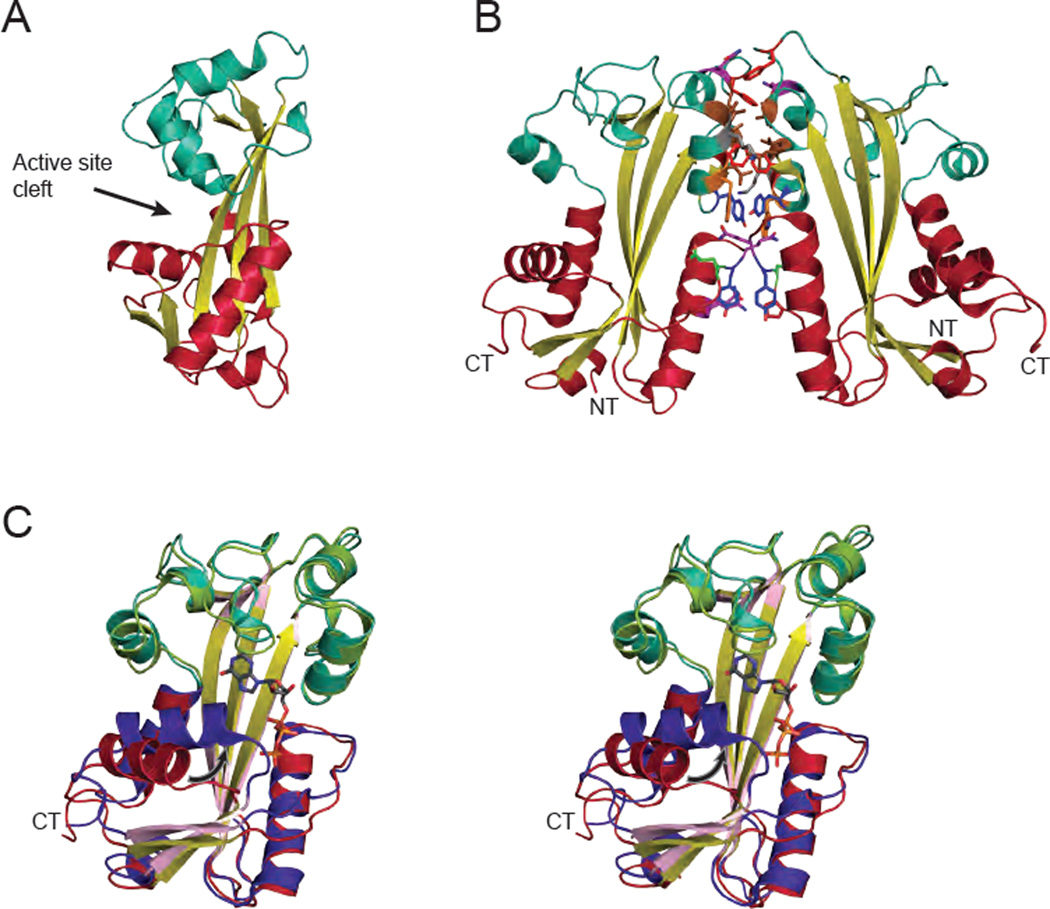
The ITPA monomer is 21.5 kDa and is composed of 194 amino acids. A homodimeric quaternary structure was first observed by gel filtration, where the calculated mass was twice that expected for a monomer [1]. The dimeric nature of ITPA was observed in all crystal structures [16, 17]. The protein consists of a long central β-sheet forming the floor of the active site, with two mainly α-helical lobes flanking the active site (upper and lower lobes, Fig. 1A). The monomers in the dimer are related by a 2-fold symmetry axis. The dimer interface involves mainly the upper lobe, with a buried surface area of 1080 Å2 and involves 9 hydrogen bonds, 9 bridging waters, an aromatic ring-stacking interaction, and numerous hydrophobic contacts (Fig. 1B) [17]. This extensive interface holds the dimer together, even in dilute solutions, and the enzyme functions as a dimer [18].
Fig. 1.
The structure of human ITPA. (A) The structure of ITPA (PDB code 2CAR [17]) without substrate. The protein is composed of a central β-sheet (yellow) with the active site flanked by two predominantly α-helical lobes: an upper lobe in light blue and a lower lobe in red. (B) In the ITPA dimer the monomers are related by a 2-fold symmetry axis. The majority of the dimerization interactions occur in the upper lobe (light blue). Residues in the interface are colored as follows: glutamic acid = green, glutamine = purple, lysine = gray, leucine = brown, phenylalanine = red, tryptophan = orange, tyrosine = dark blue. This interface buries 1080 Å2 of surface area and involves numerous hydrophobic contains and hydrogen bonds. (C) Stereopair of the structure of ITPA with ITP bound (PDB code 2J4E [17]), shown in purple, pink, and green, reveals several structural changes upon substrate binding. The most prominent is in the lower lobe with the closing of the central α-helix towards ITP, indicated by the arrow. The α-carbons of the two structures align with an r.m.s.d. of 1.87 Å. Ribbon figures were generated with PyMOL [134].
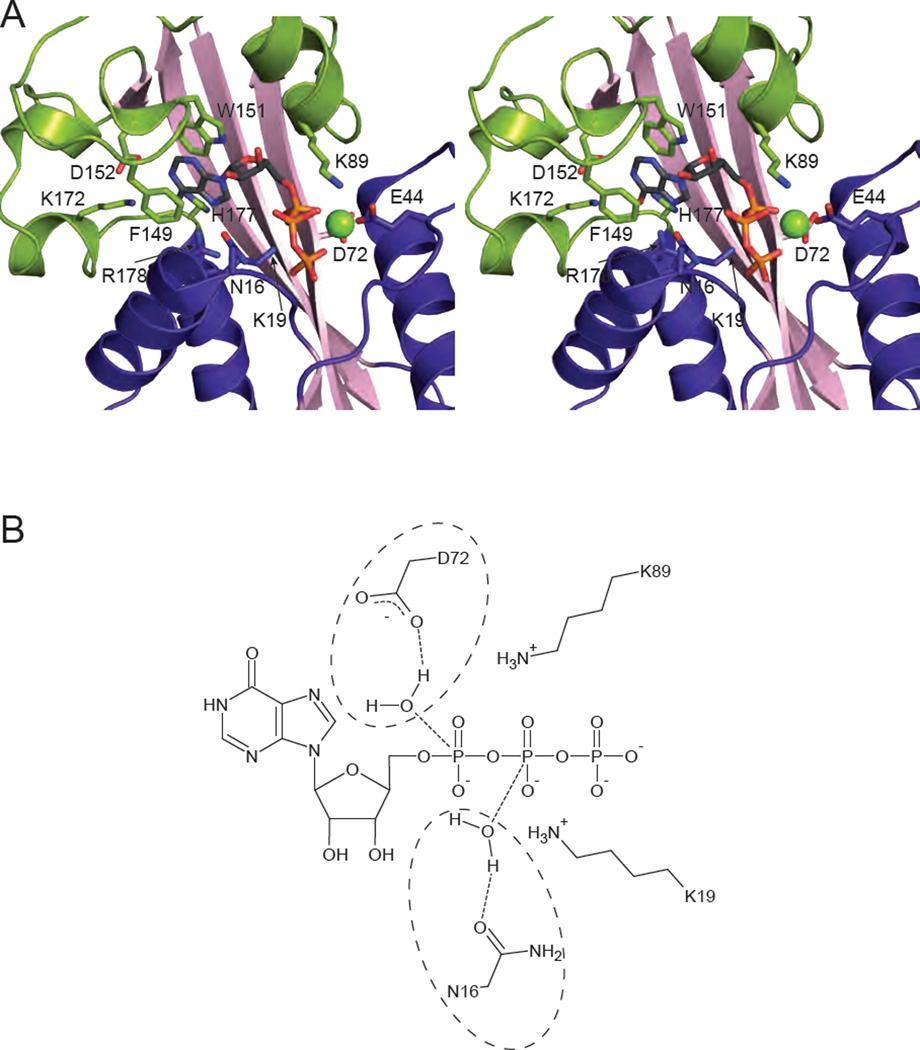
The structure with ITP bound reveals that the substrate binds in the cleft between the two lobes (Fig. 1A). Upon substrate binding, the enzyme shifts to a closed position in which the lower lobe moves 25° towards the upper dimerization lobe. Most notable is the movement of the α-helix formed by residues 16 to 27 toward the substrate (Fig. 1C, arrow). The nucleotide base is clamped by ring-stacking interactions involving Phe149 and Trp151 (Fig. 2A; Table 2). The Mg2+ cation is coordinated by the β and γ phosphates of ITP and the side chain of Glu44. Substrate specificity is explained as follows. Lys172, His177, and Arg178 are within hydrogen bonding distance of the 6-keto oxygen of ITP and XTP. ATP contains an amino group at this position which would not allow for hydrogen bonding. Although the protein was not crystallized with XTP, a potential hydrogen bond can be identified between the 2-keto oxygen and the backbone amide of Asp152. GTP is excluded from the active site because an amino group in this position would not be accommodated. Neither of the ribose hydroxyl groups makes strong contacts with the protein and instead point out toward the solvent (Fig. 2A). This explains the ability of ITPA to act on both NTPs and dNTPs [17].
Fig. 2.
Substrate binding and catalysis. (A) Stereopair of ITPA with ITP bound (PDB code 2J4E [17]) is shown with relevant residues involved in substrate binding and discrimination shown in stick form. A Mg2+ cation is shown as a green sphere. (B) Potential reaction mechanisms for ITPA are shown schematically. Due to the low resolution of the structure, a single catalytic mechanism cannot be determined. Asp 72 is in position to coordinate a water molecule for attack on the α phosphate (circled on the top), while Asn-16 is positioned to coordinate a water for attack on the β phosphate (circled on the bottom). Either would result in the scission of the α-β phosphoanhydride bond. The positively charged ε-amino groups of Lys 19 and Lys 89 are positioned to stabilize the negatively charged intermediate.
Table 2.
Assignment of function of active site residues in human ITPA
| Role | Residues |
|---|---|
| Inosine ring stacking | Phe149, Trp151 |
| Inosine specificity | Asp152, Lys172, His177, Arg178 |
| Ribose | Asn16 |
| α-phosphate | Lys19, Glu44/Mg2+, Asp72 |
| β-phosphate | Asn16, Lys19, Glu44/Mg2+, Lys89 |
| γ-phosphate | Thr14, Gly 15, Glu44/Mg2+, Lys56 |
Strictly conserved residues are shown in bold
Lastly, the structure of ITPA with bound ITP allows for a potential reaction mechanism to be proposed (Fig. 2B). Unfortunately the resolution of 2.8 Å prevents exact details, such as the role of water, from being described [17]. The nucleotide is stably bound in the active site in a conformation amenable for catalysis. Two possibilities exist for the location of the hydrolytic water. Asp72 could coordinate a water molecule for nucleophilic attack on the α-phosphate, whereas Asn16 is positioned to coordinate a water molecule for attack on the β-phosphate. In either case, the result is the scission of the α-β phosphoanhydride bond. The residues Lys89 and Lys19 are in a position such that they could stabilize the negatively charged intermediate of the reaction. Clearly a higher resolution structure is needed to resolve the specifics of the reaction.
Structures of ITPA analogs from other organisms have been solved, including Thermatoga maritima (PDB code 1VP2), Pyrococcus horikoshii (PDB codes 1V7R, 2DVP, 2DVO, 2DVN, and 2ZTI [25]), Methanococcus jannaschii (PDB codes 1B78 and 2MJP [26]), and E. coli (PDB codes 1K7K, 2PYU, and 2Q16 [27]). These structures show a remarkable degree of conservation of tertiary structure. In all cases the structure forms a 2-fold symmetric homodimer with a large buried surface area of about 1000 Å2. These structural similarities indicate that ITPA and its homologs are performing an important and evolutionarily conserved function.
3. Origins of non-canonical nucleotides
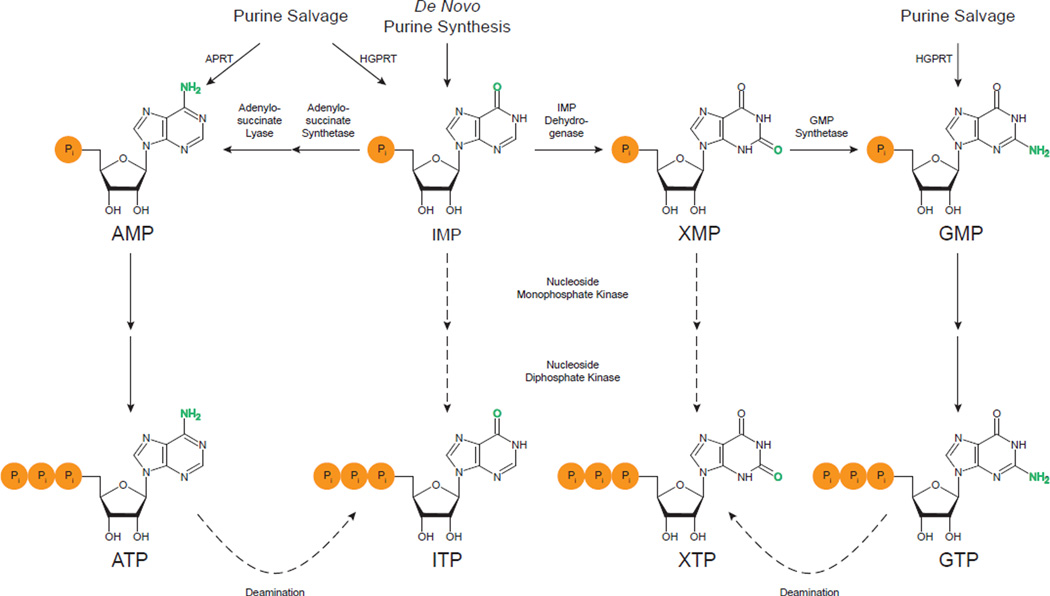
Given that a ubiquitous enzyme exists to remove the non-canonical nucleotides ITP and XTP, the question that arises is how they are formed in the first place. IMP is the first nucleotide produced in the de novo synthesis pathway by a series of eleven reactions (Fig. 3). IMP can be used for either the synthesis of AMP or GMP. The formation of AMP from IMP requires changing the 6-keto group to an amino group. For this to occur, aspartate is connected by its amino group to the C6 of IMP by adenylosuccinate synthetase in a GTP-requiring reaction. Then adenylosuccinate lyase cleaves off fumarate, leaving AMP. To form GMP the C2 of IMP must first be oxidized to a keto group by IMP dehydrogenase with the concomitant reduction of NAD+ to NADH forming XMP. In the second step, the keto group in converted to an amino group by GMP synthetase using glutamine and ATP [28].
Fig. 3.
Purine metabolism and the formation of ITP and XTP. IMP is the first purine nucleotide formed in the de novo synthesis pathway. IMP is converted to either AMP or GMP, the latter through XMP as an intermediate. IMP and GMP can also be made from the free bases hypoxanthine and guanine by hypoxanthine-guanine phosphoribosyltransferase (HGPRT) in the salvage pathway. Similarly, AMP can be synthesized from adenine by adenine phosphoribosyltransferase (APRT). AMP and GMP are sequentially phosphorylated to ATP and GTP by nucleoside monophosphate kinases and nucleoside diphosphate kinase. These enzymes may also work on IMP and XMP, providing one means of generating ITP and XTP. Highlighted in green are the functional groups that differ between ATP and ITP and between GTP and XTP. ATP and GTP contain amino groups, while ITP and XTP contain keto groups. The amino groups can undergo deamination to keto groups. Hence, this deamination of ATP and GTP is another mechanism that can produce ITP and XTP.
IMP, AMP, and GMP are also formed from the purine bases hypoxanthine, adenine, and guanine, respectively, by the purine salvage pathway (Fig. 3). These bases are formed during normal nucleic acid turnover, and the salvage pathway allows them to be reused, thus circumventing the need for the energetically costly de novo pathway. No matter which pathway is used, once AMP and GMP are formed, the two additional phosphate groups are added sequentially by base-specific nucleoside monophosphate kinases, forming ADP and GDP, and nucleoside diphosphate kinase, producing the triphosphate forms. Lastly, the nucleoside diphosphates (NDPs) can be converted to the corresponding deoxynucleoside diphosphates (dNDPs) by ribonucleotide reductase. The dNDPs can then be phosphorylated by NDP kinase to dNTPs for use in DNA synthesis [28]. Although these reactions can potentially directly convert IMP and XMP to ITP and XTP, their impact on the overall level of noncanonical triphosphates in living cells is not known.
Early studies showed that erythrocytes were capable of synthesizing ITP from inosine [29, 30]. Two possibilities for the initial formation of IMP were suggested: (1) the direct phosphorylation of inosine by an “inosine kinase” and (2) via free hypoxanthine generated by the phosphorolytic cleavage of inosine by purine nucleoside phosphorylase. They also suggested that the following two phosphorylation steps are performed by nucleoside monophosphate kinase and NDP kinase with ATP serving as the phosphate donor. Next researchers found that ITP could be synthesized from hypoxanthine [31–33] favoring the second theory for the generation of IMP. All of these studies focused on erythrocytes.
In 1979, Vanderheiden sought to examine the enzymatic mechanism for ITP formation in a variety of cells [34]. Using conditions that inhibit the activity of ITPA, levels of IMP, IDP, and ITP were measured over time and compared to the levels of GMP, GDP, and GTP in cells incubated with guanosine. The rates of formation and disappearance of IMP, IDP, and ITP differed from those of GMP, GDP, and GTP, indicating differences in the enzymatic pathways used or in the affinity of the enzyme for the particular nucleotide. Unfortunately, the data could be interpreted two ways: (1) that ITP is formed by the sequential phosphorylation of IMP or (2) that ITP is formed by the direct pyrophosphorylation of IMP. Further experiments are therefore needed to unequivocally establish the enzymatic pathway from inosine or hypoxanthine to ITP.
There has been some evidence along these lines. Agarwal et al. found that IMP can serve as a substrate for human erythrocyte guanylate kinase to form IDP [35]. However, the reaction is very slow, with a Vmax approximately 0.2% that of the reaction for GMP. The Km was also two orders of magnitude higher for IMP than for GMP. More recently, human adenylate kinase-6 was shown to phosphorylate IMP, but, as with guanylate kinase, to a much lower extent than its preferred substrate (d)AMP [36]. A study with HeLa cell nuclear extracts showed that dIDP is rapidly converted to dITP in the presence of ATP, presumably by NDP kinase [37]. The high ATP levels in cells may be driving the following reactions towards the formation of ITP:
Finally, there is another mechanism for the formation of (d)ITP and (d)XTP. (d)ATP contains a 6-amino group while (d)ITP has a keto group in the same position. Similarly, (d)GTP contains a 2-amino group whereas (d)XTP has a 2-keto group. Deamination of the amino groups can thus convert (d)ATP to (d)ITP and (d)GTP to (d)XTP. Nitrous acid is known to react with adenine and guanosine, resulting in the formation of hypoxanthine and xanthine, respectively [38]. Other mechanisms of deamination include hydrolytic deamination and nitrosative deamination by reactive nitrogen species produced during inflammation, such as nitric oxide [39]. The deamination of adenosine residues in DNA to inosine has been observed [40]. One should note that deamination of ADP and ATP could have led to the formation of IDP and ITP seen in the studies mentioned in the preceding paragraphs. Namely, IMP formed from hypoxanthine could be converted to AMP as shown in Fig. 3, which could then be phosphorylated to ADP and ATP, which in turn could be deaminated to IDP and ITP. However, erythrocytes lack adenylosuccinate synthetase and thus cannot form AMP from IMP [33]. Nonetheless, this pathway to ITP is a possibility for the other cell types examined.
It is clear that (d)ITP and (d)XTP can be produced in cells, by either enzymatic phosphorylation reactions or by deamination of (d)ATP and (d)GTP. The next logical question is “What problems can be caused by these (d)NTPs that cells need an enzyme to remove them?”
4. Problems arising from non-canonical nucleotides
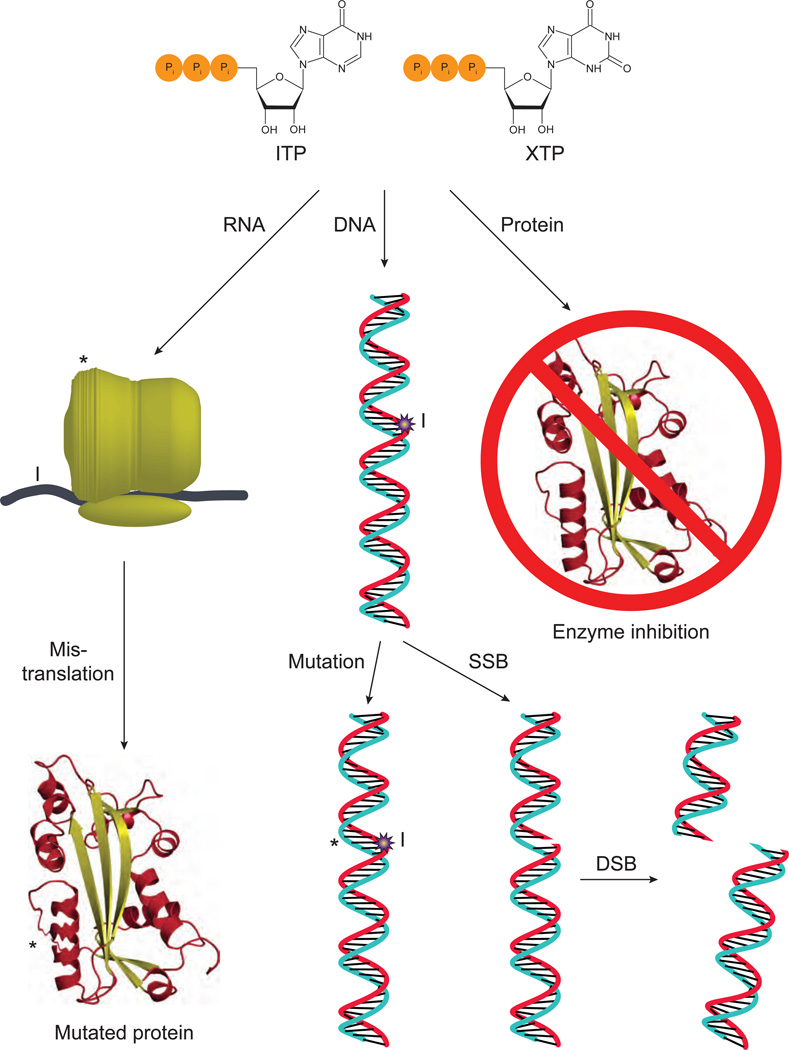
In the genetic code, only four NTPs are the precursors of RNA and likewise only four dNTPs are the precursors of DNA. Thus, the presence of (d)NTPs other than these canonical ones could produce adverse effects (Fig. 4). Several assumptions are implicit here. First is that non-canonical nucleotides can base pair with the canonical ones. It is known, for example, that inosine can form Watson-Crick base pairs with cytosine, although only two hydrogen bonds are formed and their interaction is weaker than standard canonical base pairs [41]. Another assumption is that RNA and DNA polymerases are able to utilize (d)ITP and insert it into the nascent strand. Human RNA polymerase II has been shown to incorporate inosine opposite cytosine with a similar Km and Vmax as for inserting the canonical guanine. However, the incorporation of inosine inhibited elongation of the chain, allowing for the proofreading action of RNA polymerase II [42]. It has also been found that HeLa nuclei and partially purified DNA polymerase α are able to incorporate dITP, although at a substantially reduced rate compared to dGTP. Also of note, in the reaction dITP could substitute for dGTP, but not dATP [37, 43, 44]. Major yeast replicative DNA polymerases δ and ε incorporate dITP opposite template C only 2–4 fold less efficiently than the correct dGTP, and only polymerase ε can incorporate dITP opposite template T (a putative mutagenic intermediate) but with a hundred fold less efficiency than the correct dATP (C. Grabow and Y. Pavlov, unpublished data). It is difficult to estimate the rate of incorporation of dITP in vivo, because it is present at low concentration in the pool of precursors and has to compete with normal nucleotides. Nevertheless, in sensitized genetic backgrounds one inosine per 10,000 bases is detected in yeast DNA[45]. DNA polymerases can also incorporate dXTP opposite C albeit at very low efficiency [46]. This is consistent with xanthosine also forming a Watson-Crick base pair with cytosine. The incorporation of (d)ITP into RNA and DNA was found in ITPA knockout organisms and will be described in detail later.
Fig. 4.
Problems caused by non-canonical NTPs. If ITP or XTP accumulate in cells, they can be inserted into ribosomal RNA, potentially leading to structural alterations in the ribosome (indicated by *), or mRNA (indicated by I). Either of these could lead to mistranslation and altered proteins. Alternatively, they can inhibit enzymes that normally use ATP or GTP. If dITP or dXTP accumulate, they could be inserted into DNA (indicated by I). If not repaired, this could lead to mutations (indicated by *). Alternatively, excision of the abnormal base creates a SSB, which can lead to DSBs if repair is not completed before replication.
The presence of noncanonical bases in RNA or DNA has deleterious effects. Their presence in RNA could affect the structure of large RNA complexes, such as the ribosome, or could lead to mistranslation of proteins by altering the codons of mRNA or changing the properties of tRNA [47]. In terms of effects on DNA, the most obvious would be the induction of mutations. Incorporation of dITP is non-mutagenic per se, because dITP is predominantly incorporated opposite cytosine and behaves like G in subsequent replication cycles [48]. However, when cellular systems attempt repair of incorporated dITP, DNA breaks are produced as intermediate products that can lead to induction of recombination and chromosomal rearrangements in sensitized genetic backgrounds (see below) [49, 50]. Because dXMP in DNA is difficult to bypass, it can cause DNA polymerase stalling and induction of further repair or damage tolerance mechanisms, leading to mutagenesis.
Deamination of adenine in DNA results in hypoxanthine, which codes as G and thus changes genetic information. When a human c-HA-ras gene containing xanthine was transfected into NIH3T3 cells, the presence of xanthine was mutagenic, with a mutation frequency of 30–50%, and led to mainly G→A transition mutations, indicating that thymine was incorporated opposite xanthine [51, 52]. Similarly, when hypoxanthine appears in the c-HA-ras gene the mutation frequency was 38–50%, with mainly A→G transition mutations, again consistent with hypoxanthine base pairing with cytosine [52, 53]. Another study in E. coli using an oligonucleotide with randomly located inosine residues found that A/T→G/C mutations were formed [54].
Another possibility is that the presence of hypoxanthine and xanthine bases in DNA could lead to DNA breaks and chromosomal instability. To see how this could happen, consider the following scenario: hypoxanthine or xanthine is recognized as a non-standard component of DNA by a DNA repair pathway and is excised, creating a single-strand break (SSB). If a replication fork encounters this SSB before it is repaired, the fork collapses. When the replication fork from the other direction encounters this and collapses, the result is a double-strand break (DSB) in one of the daughter chromosomes. This DSB can lead to deletions, translocations and other forms of chromosomal instability [55–57]. The enzyme endonuclease V in E. coli recognizes hypoxanthine and xanthine bases in DNA and nicks the backbone at the second phosphodiester bond on the 3’ end, creating a SSB [49, 58, 59]. A similar enzyme exists in rodents and humans, but its enzymatic activity is a subject of debate. Two reports states that it has genuine EndoV-like specificity towards inosine [60–62], while another group described its ability to bind branched DNA [63]. E. coli also have a hypoxanthine-DNA glycosylase which releases hypoxanthine from DNA, creating an abasic site [64]. A similar enzyme was also found in calf thymus [40]. Based on phylogenetic analysis, all three domains of life possess a member of the uracil-DNA glycosylase superfamily that has hypoxanthine-DNA glycosylase activity [65]. Lastly, hypoxanthine was found to be released from inosine-containing DNA incubated with HeLa cell nuclear extracts, implying the existence of a hypoxanthine-DNA glycosylase in human cells [37]. The 3-methyladenine-DNA glycosylase ANPG in human cells has been shown to efficiently remove hypoxanthine bases from DNA [66]. The resulting abasic site can then be recognized and an AP (apurinic/apyrimidinic) endonuclease or AP lyase can initiate repair, opening the DNA backbone and creating a SSB [67]. This topic will be addressed further in relation to ITPA deficiency. ITP has also been identified as a component of the clastogenic (chromosome breaking) factor found in the plasma of patients with scleroderma [68, 69].
Lastly, besides the effects from incorporation into DNA or RNA, ITP and XTP could potentially have negative effects on the numerous proteins that utilize ATP and GTP (Fig. 4, right). Several examples of this have been found. For example, the enzyme l-glutamic acid decarboxylase, involved in the synthesis of the neurotransmitter γ-aminobutyric acid, from rat brain is inhibited by ITP [70]. Rabbit psoas muscle fibers did not contract well and had disordered striations in the presence of ITP. This is likely due, at least in part, to the slow rate of ITP hydrolysis by actomyosin compared to ATP [71]. ITP was also found to inhibit Drosophila DNA topoisomerase II [72], which could explain, in part, its clastogenic activity. ITP is known to bind to the F1 subunit of ATP synthase [73], but no studies have shown a detrimental effect. Lastly, ITP and XTP are both able to activate G-proteins involved in signal transduction. Again, no direct negative effects have been reported, but given the different binding affinities and rates of hydrolysis of GTP, XTP, and ITP, it is possible that these NTPs could function as differential signal amplifiers [74].
The non-canonical nucleotides (d)ITP and (d)XTP can form in cells. Once formed, they can lead to a variety of problems (Fig. 4). Thus, the need for an enzyme to remove them is apparent. Homologs of ITPA have been identified in all three domains of life. This includes the archaea Methanococcus jannaschii [26] and the bacteria E. coli [49], as well as the eukaryotes Saccharomyces cerevisiae [3, 75], mice [76], rats [77], rabbits [78], and humans [1]. These facts lead to the conclusion that the function of ITPA must be important. This will be seen by looking at the effect of removing ITPA from model organisms.
5. Effects of ITPA deficiency in model organisms
5.1. E. coli
The ITPA homolog in E. coli is known as RdgB (Rec-dependent growth B). It was originally identified as being a gene necessary for recombination-defective recA-mutant E. coli to be viable [2]. RdgB has (d)NTP pyrophosphohydrolase activity and, like human ITPA, is highly specific for ITP, dITP, and XTP, with minimal activity on canonical (d)NTPs [15, 27]. Alignment of the protein sequences of RdgB and ITPA shows 21 strictly conserved residues, many of which are involved in substrate binding and discrimination or catalysis (see section 2.2). Lastly, the crystal structure of RdgB showed a structure highly homologous to human ITPA (r.m.s.d.=1.87 Å). RdgB is therefore a bona fide homolog of ITPA.
What happens when rdgB is mutated and there is a loss of enzyme activity? Single rdgB mutants are viable and grow normally, with normal DNA synthesis. However, the mutant strains had higher levels of intrachromosomal rearrangements than wild type, as well as a greater induction of the SOS response [2]. The SOS response is a complex pathway in bacteria that is induced by DNA damage and helps the bacteria survive and repair the DNA. The RecA protein is a key regulator of the SOS response, as well as being a major player in the repair of DSBs by homologous recombination [79]. Hence, E. coli lacking RdgB function seem to have higher levels of DNA damage, which requires the SOS response. The greater level of intrachromosomal rearrangements would suggest that the damage is repaired by some recombination event mediated by RecA [2].
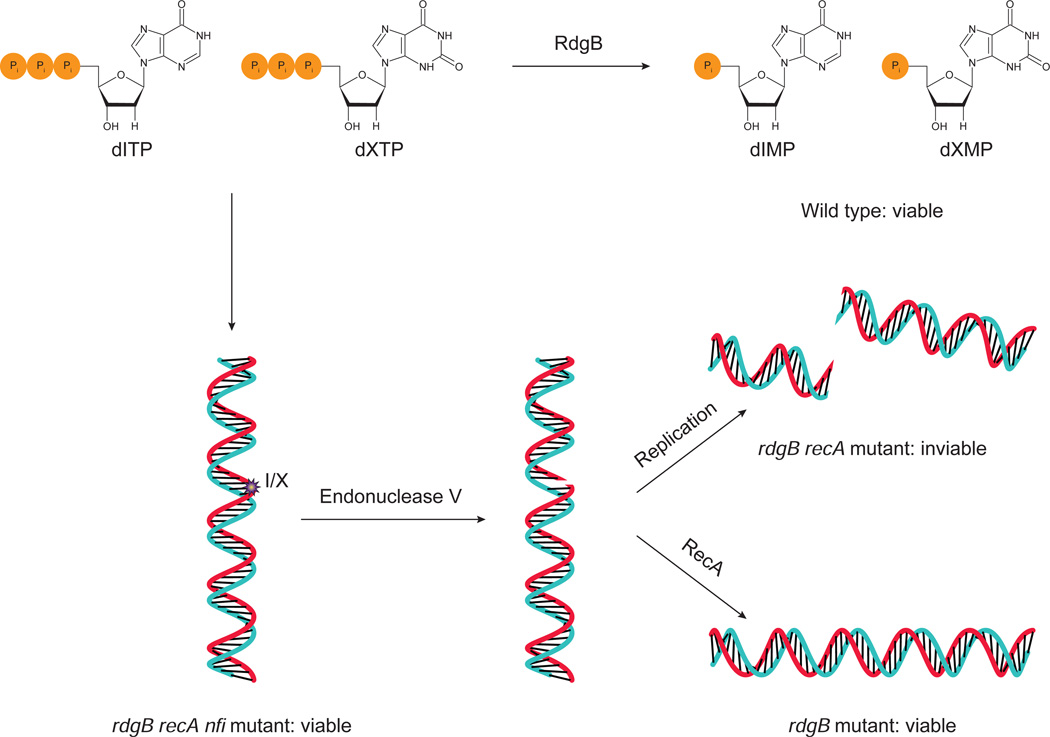
Mutating rdgB alone induced DNA damage requiring RecA for repair, so it makes sense that the rdgB recA double mutant E. coli are non-viable. What is of interest is the fact that additional mutations in the nfi gene, which encodes the endonuclease V protein, suppress the lethality of the rdgB recA double mutant [49, 59] leading to the model presented in Fig. 5. In the absence of RdgB, dITP and dXTP accumulate because they are not converted to dIMP and dXMP. The elevated levels of dITP and dXTP lead to their incorporation into DNA, either during replication or repair. If endonuclease V is present, it can create a SSB in the process of removing the inosine or xanthosine residue. The SSB can become a DSB if replication occurs before it is repaired. If RecA is functional, this damage can be repaired, explaining the viability of rdgB single mutants. On the other hand, if RecA is absent, the bacteria die due to fragmentation of the chromosome and hence rdgB recA double mutants are inviable [50]. When nfi is additionally mutated, the lethality is suppressed because the SSBs are not created and the chromosome cannot become fragmented. Also, E. coli lacking RdgB and one of the enzymes that converts IMP to AMP or XMP (i.e. adenylosuccinate synthetase or IMP dehydrogenase, see Fig. 3) showed large increases in the amount of hypoxanthine bases in the DNA and RNA [45]. These data lend strong support to the ideas presented in previous sections, namely that dITP and dXTP are formed in vivo, that they can be inserted into DNA, and that once in DNA they can have harmful effects.
Fig. 5.
E. coli rdgB mutant, synthetic lethality, and rescue. Wild type E. coli do not accumulate dITP or dXTP because RdgB catalyzes their conversion to dIMP and dXMP. In rdgB mutant E. coli, dITP and dXTP accumulate, leading to their incorporation into DNA (indicated by I/X). Endonuclease V recognizes these lesions and creates SSBs. If the RecA protein is present, the DNA can be repaired, and so rdgB mutants are viable. If recA is also mutated, then DSBs can accumulate, leading to the inviability of rdgB recA double mutants. However, mutating the nfi gene, which codes for endonuclease V, prevents SSBs from forming and thus rescues the synthetic lethality of rdgB recA double mutants. Based on [59].
5.2. Yeast
The ITPA homolog in Saccharomyces cerevisiae was named HAM1 [3, 75]. When HAM1 was mutated the yeast became supersensitive to the mutagenic effects of the purine analog 6-N-hydroxylaminopurine (HAP) [3]. Alignment of the HAM1 protein sequence with the sequence later identified as human ITPA shows several regions of high sequence similarity [80]. HAM1 was shown to catalyze the pyrophosphohydrolysis of dITP and dHAPTP, with minimal effect on canonical nucleotides [15]. Although HAM1 mutant yeast are hypersensitive to the mutagenic effects of HAP, the mutant yeast displayed no increase in spontaneous mutation or recombination [3, 75]. This is contrary to what was seen in E. coli, indicating a difference between the two organisms, either in their degree of dITP/dXTP accumulation or in how they deal with the incorporation of non-canonical bases in DNA. It is noteworthy that yeast do not possess an ortholog for Endo V, the enzyme responsible for nicking DNA with non-canonical purines in bacteria. It was proposed that HAP is taken up by yeast, converted to HAP monophosphate by the salvage pathway, and then phosphorylated, reduced, and phosphorylated again to eventually form dHAPTP. With HAM1 present, this dHAPTP can be detoxified by conversion back to a monophosphate. In the absence of HAM1, dHAPTP can be incorporated into the DNA where it leads to mutations [75].
E. coli and S. cerevisiae are both unicellular organisms and both are viable when their ITPA-homolog gene is mutated. Differences in the phenotype of the mutants indicate differences between prokaryotes and eukaryotes. What would be the phenotype in a multicellular organism?
5.3. Mice
In 2009, mice with both alleles of the Itpa gene knocked out (Itpa−/−) were developed [4]. Sakumi et al (2010) reviewed these results so only a brief summary is provided here [47]. Contrary to the results in E. coli and yeast, greater than half of the Itpa−/− mice died before birth, and those that were born were smaller and died within two weeks. Itpa−/− mice displayed ataxia, abnormal breathing, immature hair follicles, testicular hypoplasia, and other abnormalities. The hearts of knockout mice had irregular, asynchronous contractions and they died of cardiac failure. Their hearts were hypoplastic, with thin ventricular walls and disorganized cardiac sarcomeres. Normally the heart and brain express high levels of ITPA (see section 2.1) [1, 76] and these organs showed the most severe phenotype. Rabbit psoas muscle fibers had disordered striations and impaired contractions in the presence of ITP (section 4) [71]. This is also consistent with the impaired cardiac function of Itpa−/− mice. These observations in Itpa−/− mice show conclusively that ITPA is serving an important and necessary role in higher organisms.
As further evidence of the fact that ITP can form spontaneously in vivo, Behmanish et al. examined the contents of the erythrocyte nucleotide pool [4]. The Itpa−/− mice had a definite increase in ITP, amounting to 10% of the ATP. Additionally, total heart RNA was found to have elevated inosine (1% of the adenosine level), providing concrete evidence that ITP can be incorporated into RNA. If the presence of inosine were due solely to deamination of adenosine residues already in RNA, then one would not expect to see an increase in the knockout mice.
After the Itpa−/− mice were created, primary mouse embryonic fibroblasts (MEFs) were isolated and characterized [81]. Itpa−/− MEFs grew significantly more slowly and tended to accumulated in G2/M phase. Examination of the MEFs for DNA content and chromosomal abnormalities revealed that the Itpa−/− MEFs had an increase in ploidy, SSBs, and abnormal chromosomes, particularly ones containing chromatid gaps or breaks.
The MEFs became spontaneously immortalized after 30–40 passages and then they appeared like wild type in terms of proliferation, cell cycle, SSBs, and chromosomal abnormalities. NUDT16 protein expression was markedly higher in immortalized knockout MEFs compared to primary knockout MEFs. In a previous study, the same group had identified human NUDT16 as an enzyme that binds to ITP. Biochemical characterization of NUDT16 found that it specifically hydrolyzes (d)IDP to (d)IMP, with (d)ITP serving as a substrate to a lesser extent [82]. Knockdown of NUDT16 expression by siRNA reverted the phenotype of immortalized knockout MEFs to that observed in the primary knockout MEFs. The authors proposed that ITPA and NUDT16 have overlapping roles in protecting cells from (d)ITP. When ITPA is not present, NUDT16 could help reduce the levels of (d)ITP by preventing the buildup of (d)IDP, so that it is not further phosphorylated to (d)ITP [81].
6. ITPA deficiency in humans
6.1. Human ITPA polymorphism and the Pro32Thr variant
Since the role of ITPA in humans cannot be assessed as directly as with model organisms, one must rely on “natural experiments” where genetic changes lead to ITPA deficiency. The first hint that ITPA deficiency exists in humans came in 1965 when Vanderheiden observed elevated levels of ITP in the erythrocytes of two siblings [83]. He then examined over 6000 blood samples and found seven that had a high ITP concentration [84]. Other studies confirmed that low ITPA activity seemed to run in families [31, 32, 85]. A major breakthrough came in 2002 when the underlying genetic cause for ITPA deficiency was determined (Table 3). Several variants in the sequence of the ITPA gene were found [7]. Two were silent and one was in the 3’-untranslated region and did not segregate with ITPA activity. A mutation in intron 2 (IVS2+21A→C) was found that led to 60% residual ITPA activity in heterozygotes. The greatest reduction in activity was due to a mutation in exon 2 (94C→A), where heterozygotes had 22.5% residual activity and homozygotes had zero ITPA activity. The activity in compound heterozygotes (94C→A/IVS2+21A→C) was 10%. Another study published later that same year also identified the 94C→A mutation as a cause of ITPA deficiency [8]. The frequency of this allele is highest in Asian populations (11–15%) and lowest in Central/South American populations (1–2%), with Caucasian and African populations falling in the middle (6–7%) [86]. The Pro32 residue is conserved in mice and Drosophila [1]. Several other alleles have been identified that lead to ITPA deficiency (see Table 3). No mutations leading to deficiency have been identified in the ITPA promoter [87].
Table 3.
ITPA polymorphisms associated with decreased activity
| Genotype | Residual Activity | Reference |
|---|---|---|
| 94C→A | Heterozygote: 22.5% Homozygote: <1% |
[7, 8] |
| IVS2+21A→C | Heterozygote: 60% | [7] |
| IVS2+68T→G | Heterozygote: 30% | [131] |
| IVS2+68T→C | Heterozygote: 60% | [132] |
| 359_366dupTCAGCACC* | Heterozygote: 30% | [133] |
| 97T→C† | Heterozygote: 22.8% | [119] |
Duplication of the eight listed residues (359 to 366)
Leads to a Cys to Arg substitution at amino acid 33
Table is based on [118]
Some evidence for how these alleles cause ITPA deficiency has been found. With the intronic mutations the most obvious hypothesis is that they affect mRNA splicing. Indeed the IVS2+21A→C mutation leads to reduction in splicing efficiency, with the ratio for the C genotype being 80% that of the A genotype [88]. This modest decrease is consistent with the relatively modest decrease in activity observed. A later study quantified the amount of misspliced transcripts for both the IVS2+21A→C and the 94C→A alleles [89]. The former led to missplicing of exon 2, while the latter led to missplicing of exons 2 and 3. The variant missing exon 2 was present at 1–5% in wild type individuals, but was increased to 16% in an individual homozygous for IVS2+21A→C. The variant missing exons 2 and 3 was present at 17% for wild type patients and at 39% and 61% for heterozygotes and homozygotes for 94C→A, respectively. The authors propose that this mutation removes an exonic splicing silencing element, which leads to increased missplicing of exons 2 and 3. Clearly, removing an exon from the mRNA would result in a segment of the protein not being transcribed, producing a nonfunctional enzyme [89].
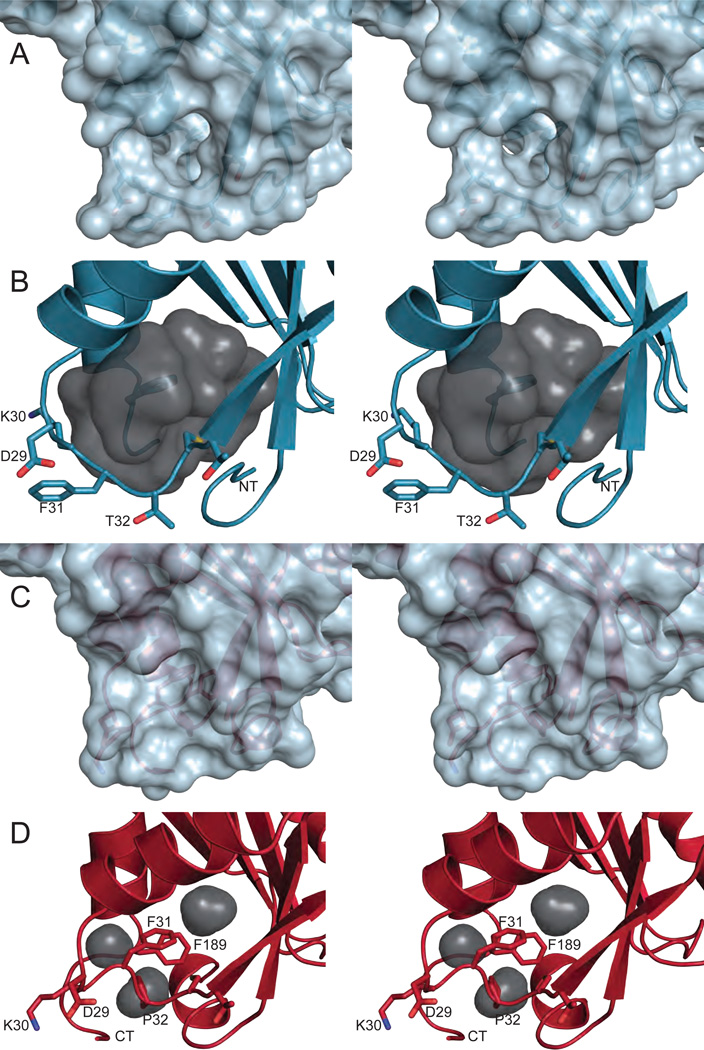
Missplicing accounts for some of the reduced activity seen with the 94C→A allele, but it does not explain why homozygotes have no detectable activity. The 94C→A polymorphism leads to a Pro32Thr substitution in the protein. This implies that the Pro32Thr change in the protein brings about the additional reduction in activity. Surprisingly, Pro32Thr ITPA purified from bacteria possessed robust ITPase activity, suggesting that enzymatic deficiency in erythrocytes does not stem from the loss of catalysis [18, 19]. Given the approximately 25% activity in heterozygotes and the fact that the Pro32Thr change would alter local secondary structure, it was postulated that the mechanism of catalytic deficiency was caused by an impairment of dimer formation between wild type and variant proteins [7]. This has been ruled out after it was shown that Pro32Thr ITPA forms a stable dimer, like wild type, but has a lower thermal stability than the wild type enzyme [18]. Structural analysis revealed the cause of this destabilization [10]. The amino acid substitution causes unfavorable steric clashes and simultaneously relaxes the torsional constraints of the peptide backbone, with the latter likely being the most relevant. These lead to large structural changes in the vicinity of the mutation, with the major feature being the movement of Phe31 from the protein’s hydrophobic core out into the solvent (Fig. 6). The region around Thr32 and the protein termini become disordered and there is a reduction in secondary structure. The solvent exposure of Phe31 destabilizes the protein by leaving a cavity in its core and also by causing unfavorable interactions between the hydrophobic side chain of Phe31 and water, likely increasing the degree of proteasomal degradation. It was also proposed that this exposure of Phe31 could lead to Pro32Thr being directly targeted to the proteasome or sequestered by chaperones. This is consistent with the observation that human fibroblasts expressing Pro32Thr ITPA have approximately 10-fold lower protein levels than wild type fibroblasts [18].
Fig. 6.
Disruption of the hydrophobic core in Pro32Thr ITPA. (A) The surface of Pro32Thr ITPA shows a hole in the protein structure due to Phe 31 and Thr 32 moving from the hydrophobic core out into the solvent. (B) An inverse view generated by HOLLOW [135] clearly exhibits the large cavity (gray) left in the protein’s core. The volume of the cavity is 887 Å3 as determined by VOIDOO [136]. (C) By comparison, the surface of wild type ITPA does not reveal any holes. (D) Only small cavities (gray) exist in the core of wild type ITPA (volume = 159 Å3). Wall-eyed stereo pairs were generated with PyMOL [134].
6.2. Potential implications of ITPA deficiency in human disease
ITPA deficiency was initially identified by the presence of elevated ITP concentrations in erythrocytes, a seemingly innocuous condition [83, 84]. On the other hand, overexpression of ITPA has been identified in several cancer cell lines (including colon, lung, liver, pancreatic, brain, and others) [90] and MLL-amplified acute myeloid leukemia [91]. Likewise, ITPA expression was higher in stage III melanoma samples having a poor prognosis compared to samples having a good prognosis, although this barely achieved statistical significance (P=0.049) [92]. It should be pointed out that this overexpression does not necessarily mean that ITPA is a causal factor in cancers. In HeLa cells, inhibition of ITPA by shRNA leads to sensitivity to HAP-caused DNA breaks and apoptosis [93]. However, evidence for a role for ITPA deficiency in human disease is mostly indirect and will be briefly summarized here.
The first evidence came in 1975, when Fraser and coworkers found that 16 out of 100 (16%) erythrocyte samples from a mentally retarded population accumulated high ITP levels, compared to only 4 out of 80 (5%) in a normal population [31]. The following year, a study found that the incidence of erythrocyte ITPA deficiency was 2.3 times higher in a psychiatric population versus a normal population. The study included 3477 psychiatric patients and 6774 normal patients. Within the psychiatric population, the occurrence of ITPA deficiency was greatest among schizophrenics (particularly paranoid schizophrenics) and a mentally retarded population. The authors of the study pointed out that Lesch-Nyhan syndrome, which is characterized by mental retardation and self-mutilation, is caused by hypoxanthine-guanine phosphoribosyltransferase (HGPRT) deficiency. Since HGPRT is involved in IMP synthesis (section 3), it is not unreasonable that deficiency of another enzyme involved in inosine metabolism, namely ITPA, could lead to psychological disorders [94].
A follow up study examined ITPA deficiency in three groups: 100 nonpsychiatric patients, 38 chronic paranoid schizophrenic, and 44 chronic undifferentiated schizophrenic. The mean activity of ITPA was lower among the schizophrenic population compared the normal population. Within the schizophrenic group, the paranoid schizophrenics had lower ITPA activity. The comparisons achieving statistical significance were between normal and all schizophrenics, normal and paranoid schizophrenics, and paranoid and undifferentiated schizophrenics [95]. As mentioned in section 4, ITP was shown to inhibit rat brain l-glutamic acid decarboxylase. Thus, ITPA deficiency could lead to ITP build up in the brain, which could inhibit synthesis of the neurotransmitter γ-aminobutyric acid, possibly producing neurological symptoms [70].
In contrast, a study by a different group trying to verify these results did not find a greater incidence of low ITPA activity among schizophrenic or mentally retarded populations. The study included 30 schizophrenic patients, 35 mentally retarded patients, and a random population of 150 [14]. Unfortunately, no study on this topic has been done since 1980, leaving the question of whether or not the results are valid up in the air.
It was recently observed that three out of six patients with pulmonary Langerhans’ cell histiocytosis had ITPA deficiency, which was confirmed by genotyping. This is in contrast to a reference population which only had 11% ITPA deficiency. Interestingly, the same three patients with ITPA deficiency also had an unfavorable clinical outcome [96]. Although an intriguing result, further work is needed with larger sample sizes to see if this association is genuine. If it is, additional work will be required to elucidate the mechanism of how ITPA polymorphisms cause the disease.
Lastly, some indirect evidence comes from cell line studies. A human fibroblast cell line homozygous for the 94C→A polymorphism (producing Pro32Thr ITPA) was examined and found to have a higher level of spontaneous DNA breaks and treatment with HAP induced more breaks than in wild type fibroblasts [9]. So it is possible that the combination of an ITPA polymorphism with a polymorphism in a DNA repair enzyme could lead to disease.
The fact that ITPA deficiency does not seem to produce a severe phenotype like that seen in Itpa−/− mice deserves consideration. One possibility is that humans express higher levels of NUDT16 (see section 5.3), which could help reduce (d)ITP levels [81]. Another likely explanation relies on the fact that the measurement of ITPA activity for the various polymorphisms was done in erythrocytes. In other cell types, partial loss of ITPA activity could be compensated by higher levels of expression. This is supported by the observation that although low erythrocyte ITPA activity is correlated with lower activity in other cells, the absolute activity in these cells is substantially higher than in erythrocytes [85]. Hence, even though 94C→A homozygotes may have zero erythrocyte ITPA activity, the activity in other cells is likely higher and could protect from the phenotype observed in knockout mice.
There is some evidence for a direct role of ITPA deficiency in human disease in that ITPA polymorphism is related to mutagenesis in the mitochondrial genome and blood cancers. A study of patients with hematological malignancies found that the 94C→A polymorphism was associated with a significantly higher number of mutation in the mitochondrial DNA (149 mutation in patients carrying the 94C allele vs. 20 mutation in 94A homozygotes). Additionally, compared to a normal population the frequency of the 94C→A polymorphism was higher in patients with myelodysplastic syndrome, although this did not reach statistical signifance [97]. Overall, there is still much to be learned about the effects of different variants of ITPA in human disease. There is growing evidence that ITPA deficiency is an important pharmacogenetic phenotype, leading to either an increase or a decrease in adverse reactions, depending on the drug used.
6.3. ITPA pharmacogenetics
ITPA deficiency has been found to play a role in negative responses to several drugs. Marinaki and coworkers were the first to discover an association between the 94C→A ITPA variant and increased occurrence of adverse reactions to azathioprine used in the treatment of inflammatory bowel disease [5, 98]. Specifically, ITPA 94C→A was associated with flu-like symptoms, rash, and pancreatitis. The IVS2+21A→C polymorphism did not predict toxicity. Azathioprine and other mercaptopurine drugs are also used in the treatment of leukemias and autoimmune disorders and are well known to produce negative side-effects such as rash, myelotoxicity, pancreatitis, and hepatotoxicity in some patients. Another enzyme, thiopurine S-methyltransferase (TPMT) also functions in mercaptopurine metabolism and its deficiency due to polymorphism leads to hematopoietic toxicity [99, 100]. Many studies have been published analyzing the roles of ITPA and TPMT in mercaptopurine intolerance. Although the contribution of TPMT is well accepted, the results for ITPA have been contradictory.
Gearry and colleagues examined a larger population of patients with inflammatory bowel disease and failed to find a significant association between ITPA 94C→A and any adverse reactions [101]. Additional studies found that ITPA 94C→A was associated with early drop-out due to intolerance [102] or leukopenia [103], while others failed to find a significant association between ITPA 94C→A and adverse reactions [104, 105]. A meta-analysis of these studies also did not find a significant association [106]. Based on these data, it would seem that the data against an association outweigh the data for an association.
Many other studies have come out which lend more support to the association of ITPA 94C→A with adverse reactions to mercaptopurines. One found a significant association with flulike symptoms in an analysis of 207 inflammatory bowel disease patients [107]. Another evaluated children with acute lymphoblastic leukemia and showed a higher probability of severe febrile neutropenia in patients with ITPA 94C→A whose mercaptopurine dosage had been adjusted based on TPMT genotype [108]. Similarly, a study of Malaysian acute lymphoblastic leukemia patients demonstrated that those with the IVS2+21A→C allele had a greater likelihood of developing fever and liver toxicity [109]. This study is interesting in that it examines an Asian population, which has a higher frequency of the ITPA 94C→A allele (section 6.1). Thus, the chance of identifying an association may be higher in an Asian population. A final study on acute lymphoblastic leukemia found that a high concentration of 6-methylmercaptopurine nucleotides is associated with hepatotoxicity, and the concentration was highest in patients with wild type TPMT alleles and heterozygous for ITPA 94C→A [110]. It may be that ITPA only has a role in mercaptopurine toxicity in acute lymphoblastic leukemia patients. However, a recent study in inflammatory bowel disease patients found that low ITPA activity is significantly associated with adverse reactions, with decreased activities accompanied by leukopenia and very low activities accompanied by increased liver enzymes [111]. It is important to note that this study looked at ITPA activity rather than a specific genotype.
The discrepancies between the various studies merit an explanation. Stocco et al. suggest that inconsistencies in previous studies may be due to a failure to account for TPMT genotype. Their study adjusted mercaptopurine doses based on the TPMT genotype of the patients while other studies did not [108]. It could be that the effects of the TPMT genotype mask the effects due to ITPA when the dose is not adjusted. Another possibility is that the low frequency of variant ITPA alleles in some populations makes it difficult to achieve a statistically significant result. Lastly, Shipkova and coworkers propose that the disagreement between studies is likely due to the fact that most studies only looked for the two most common ITPA polymorphisms (94C→A and IVS2+21A→C) [111]. Other polymorphisms are known that cause ITPA deficiency (Table 3) and there may be others that are not yet known. Variation in activity levels within a given genotype may also play a role. Hence, ITPA activity is likely a better metric than ITPA genotype for assessing the probability of mercaptopurine toxicity.
Two other interesting studies have been published regarding ITPA and azathioprine. A case report of a liver transplant recipient on azathioprine who presented fifteen years later with nodular regenerative hyperplasia found that both the patient and the donor liver were heterozygous for ITPA 94C→A [112]. This indicates that genotyping of ITPA in both transplant recipients and donor organs may help reduce long-term complications. Other studies demonstrated that the ITPA 94C→A polymorphism in Japanese populations was associated with an improved response to low-dose azathioprine in the treatment of systemic lupus erythematosus [113, 114]. They postulated that accumulation of the metabolite 6-thioITP could augment the immunosuppression of azathioprine leading to a synergistic effect rather than toxicity at low doses.
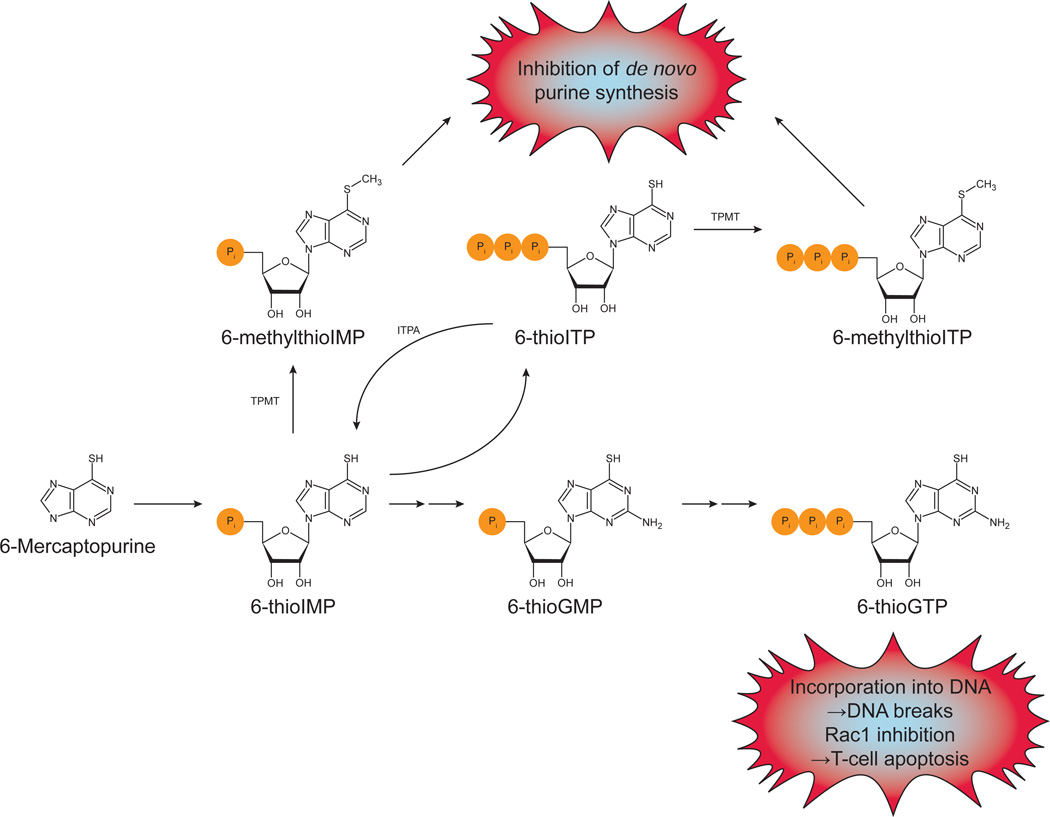
A simplified pathway for the metabolism of mercaptopurines is shown in Fig. 7. The active metabolites are the 6-thioguanine nucleotides. 6-thioGTP can be incorporated into DNA and cause strand breaks, producing immunosuppression by killing immune cells. This metabolite also inhibits the small GTPase Rac1, leading to apoptosis in T-cells. The metabolites 6-methylthioIMP, -IDP, and -ITP inhibit de novo purine synthesis [115–117]. ITPA is known to be able to catalyze the pyrophosphohydrolysis of 6-thioITP, although with a greater Km and reduced Vmax compared to ITP, but 6-methylthioITP is a poor substrate [118, 119]. It is thought that ITPA deficiency leads to accumulation of 6-thioITP and 6-methylthioITP. This agrees with the increase in 6-methylmercaptopurine nucleotides in ITPA 94C→A heterozygotes [110]. In wild type individuals, 6-thioITP can be converted back to 6-thioIMP, preventing its accumulation and subsequent methylation by TPMT. With ITPA deficiency, this conversion is severely reduced.
Fig. 7.
Mercaptopurine metabolism and mechanisms of cytotoxicity. After being taken up into cells, 6-mercaptopurine is converted to 6-thioIMP. (Azathioprine is a prodrug of 6-mercaptopurine.) 6-thioIMP can be converted to 6-thioGMP, which can be phosphorylated to 6-thioGTP. 6-thioGTP is known to cause DNA strand breaks by being incorporated into DNA and to induce T-cell apoptosis by Rac1 inhibition. 6-thioIMP can also be phosphorylated to 6-thioITP. ITPA can catalyze the conversion of this back to 6-thioIMP. TPMT can methylate both 6-thioIMP and 6-thioITP. These methylated forms inhibit de novo purine synthesis. In ITPA deficient patients, 6-thioITP and 6-methylthioITP can accumulate and lead to adverse reactions. Based on [108, 116, 117].
A role for ITPA in methotrexate treatment for rheumatoid arthritis has been examined in several studies. The first found an association between the wild type ITPA gene (the 94C→A polymorphism was examined) and a good clinical response to methotrexate. Methotrexate is believed to help rheumatoid arthritis by increasing the release of the anti-inflammatory agent adenosine. ITPA may influence the concentrations of AMP and adenosine by affecting IMP levels, since IMP is a precursor for AMP (see section 3 and Fig. 3), providing a possible explanation for why ITPA deficiency is not associated with a good response [120]. As with azathioprine, some reports have confirmed this result [121, 122] and others have not [123]. Possible reasons could include different study designs and insufficient sample sizes. Another study found no association between the IVS2+21A→C polymorphism and methotrexate efficacy [124]. Given the moderate phenotype associated with this allele the result is not surprising. One of the studies also noted an association between ITPA 94C→A and gastrointestinal toxicity [122]. The evidence seems to be in favor of ITPA playing a role in methotrexate efficacy, but further work needs to be done.
Lastly, the most recent discovery was the finding by Felley et al. that the ITPA deficiency alleles 94C→A and IVS2+21A→C protect against hemolytic anemia induced by ribavirin used in treating hepatitis C [6]. Hemolytic anemia is a treatment-limiting side effect, requiring ribavirin doses to be lowered to less efficacious levels. This result has been corroborated by several studies [125–129]. Ribavirin is a nucleoside analog, so it is possible that ITP could compete with ribavirin triphosphate in some process or processes that lead to erythrocyte lysis [6]. However, it has been shown that ribavirin reduces erythrocyte ATP levels and that ITP can substitute for GTP (which is also reduced by ribavirin) in the adenylosuccinate synthetase reaction that is part of the de novo ATP synthesis pathway (see section 3 and Fig. 3). Thus, in ITPA deficient patients, ITP accumulates in the erythrocytes and helps restore ATP levels, preventing lysis due to loss of ATP and the many processes that require it [130]. A contribution from the other mechanism mentioned cannot currently be ruled out, though.
ITPA is an important enzyme in the metabolism of several drugs. The conditions treated by these drugs are fairly common and assessing ITP levels and ITPA genotyping may be helpful for decisions on what drugs and what doses to give.
7. Concluding remarks
It is likely that non-canonical (d)ITP and (d)XTP are constantly produced in cells, causing genetic instability, producing aberrant proteins and altering signaling pathways that rely on ATP. ITPA removes these problematic (d)NTPs. Its importance is underscored by conservation in all species and the severe biological consequences of ITPA loss in model organisms. The human 94C→A (Pro32Thr) polymorphism leads to attenuation of ITPA activity in tissues (up to zero activity in erythrocytes) and has been identified as an important pharmacogenetic polymorphism, altering the efficacy and/or adverse effects of several chemotherapies. In fact, assessing a patient’s ITPA activity or ITPA genotype may become a critical first step in designing certain treatment plans. Further accumulation of data on the distribution of the 94C→A allele, and other polymorphs, in the human population will answer whether it is linked to any pathologies. The discovery that the Pro32Thr ITPA variant has a disrupted hydrophobic core, which likely leads to degradation in cells, provides a handle to correct the defect in patients. It may be possible to improve the stability of Pro32Thr ITPA with a small molecule that could be co-administered with primary drugs to reduce the degree of adverse effects in patients with this variant of ITPA. The creation of a Pro32Thr ITPA mouse would provide a valuable model system for examining the role of this mutant protein in disease and to evaluate possible treatments.
Research Highlights.
-
►
ITPA cleanses nucleotide pools of naturally-occurring, dangerous base analogs
-
►
Mutations in model organisms lead to genomic instability, heart defect and death
-
►
Reduced ITPA activity/polymorphism are linked to adverse reactions to certain drugs
-
►
Pro32Thr ITPA variant has incorrectly folded structure and limited action in cells
-
►
Dominant negative effect of P32T variant in cells caused by mixed dimer degradation
Acknowledgements
This work was supported by the following research grants: UNMC Eppley Cancer Center seed grants (YIP and GEOB), NCI Eppley Cancer Center Support Grant P30CA036727 (GEOB), Department of Education GAANN grant P200A070554 (PDS), National Center for Research Resources grant 5P20RR016469 and the National Institute for General Medical Science grant 8P20GM103427 (GEOB and CAB); and NCI grant CA129925, by the Russian federal program “Innovation scientific personnel” State contracts 14.740.11.0916 and the Federal Grant-in-Aid Program «Human Capital for Science and Education in Innovative Russia» (Government Contract No. 8654) (YIP).
Abbreviations
- ITPA
inosine triphosphate pyrophosphatase
- ITP
inosine triphosphate
- XTP
xanthosine triphosphate
- HAM1
Saccharomyces cerevisiae ITPA homolog
- RdgB
E. coli ITPA homolog (Rec-dependent growth B)
- HAP
base analog 6-hydroxylaminopurine
- HGPRT
hypoxanthine-guanine phosphoribosyltransferase
- TPMT
thiopurine S-methyltransferase
- SSB
single-strand break
- DSB
double-strand break
- MEF
mouse embryonic fibroblasts
- NUDT16
nudix (nucleoside diphosphate linked moiety X)-type motif 16
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the contributors to this article has any conflicting interests.
References
- 1.Lin S, McLennan AG, Ying K, Wang Z, Gu S, Jin H, Wu C, Liu W, Yuan Y, Tang R, Xie Y, Mao Y. Cloning, expression, and characterization of a human inosine triphosphate pyrophosphatase encoded by the ITPA gene. J. Biol. Chem. 2001;276:18695–18701. doi: 10.1074/jbc.M011084200. [DOI] [PubMed] [Google Scholar]
- 2.Clyman J, Cunningham RP. Escherichia coli K-12 mutants in which viability is dependent on recA function. J. Bacteriol. 1987;169:4203–4210. doi: 10.1128/jb.169.9.4203-4210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlov YI. Saccharomyces cerevisiae mutants highly sensitive to the mutagenic action of 6-N-hydroxylaminopurine. Sov. Genet. 1986;22:1099–1107. [Google Scholar]
- 4.Behmanesh M, Sakumi K, Abolhassani N, Toyokuni S, Oka S, Ohnishi YN, Tsuchimoto D, Nakabeppu Y. ITPase-deficient mice show growth retardation and die before weaning. Cell Death Differ. 2009;16:1315–1322. doi: 10.1038/cdd.2009.53. [DOI] [PubMed] [Google Scholar]
- 5.Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, Shobowale-Bakre e-M, Escuredo E, Fairbanks LD, Sanderson JD. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase) Pharmacogenetics. 2004;14:181–187. doi: 10.1097/00008571-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M, Warner A, Muir AJ, Brass C, Albrecht J, Sulkowski M, McHutchison JG, Goldstein DB. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405–408. doi: 10.1038/nature08825. [DOI] [PubMed] [Google Scholar]
- 7.Sumi S, Marinaki AM, Arenas M, Fairbanks L, Shobowale-Bakre M, Rees DC, Thein SL, Ansari A, Sanderson J, De Abreu RA, Simmonds HA, Duley JA. Genetic basis of inosine triphosphate pyrophosphohydrolase deficiency. Hum. genet. 2002;111:360–367. doi: 10.1007/s00439-002-0798-z. [DOI] [PubMed] [Google Scholar]
- 8.Cao H, Hegele RA. DNA polymorphisms in ITPA including basis of inosine triphosphatase deficiency. J. Hum. Genet. 2002;47:620–622. doi: 10.1007/s100380200095. [DOI] [PubMed] [Google Scholar]
- 9.Waisertreiger IS-R, Menezes MR, Randazzo J, Pavlov YI. Elevated Levels of DNA Strand Breaks Induced by a Base Analog in the Human Cell Line with the P32T ITPA Variant. J. Nucleic Acids. 2010;2010 doi: 10.4061/2010/872180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simone PD, Struble LR, Kellezi A, Brown CA, Grabow CE, Khutsishvili I, Marky LA, Pavlov YI, Borgstahl GE. The human ITPA polymorphic variant P32T is destabilized by the unpacking of the hydrophobic core. J Struct Biol. 2013;23:00078-6. doi: 10.1016/j.jsb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liakopoulou A, Alivisatos SG. Distribution of Nucleoside Triphosphatases in Human Erythrocytes. Biochim. Biophys. Acta. 1964;89:158–161. doi: 10.1016/0926-6569(64)90112-9. [DOI] [PubMed] [Google Scholar]
- 12.Vanderheiden BS. Human erythrocyte "ITPase": an ITP pyrophosphohydrolase. Biochim. Biophys. Acta. 1970;215:555–558. doi: 10.1016/0304-4165(70)90109-1. [DOI] [PubMed] [Google Scholar]
- 13.Vanderheiden BS. Purification and properties of human erythrocyte inosine triphosphate pyrophosphohydrolase. J. Cell. Physiol. 1979;98:41–47. doi: 10.1002/jcp.1040980106. [DOI] [PubMed] [Google Scholar]
- 14.Holmes SL, Turner BM, Hirschhorn K. Human inosine triphosphatase: catalytic properties and population studies. Clin. Chim. Acta. 1979;97:143–153. doi: 10.1016/0009-8981(79)90410-8. [DOI] [PubMed] [Google Scholar]
- 15.Burgis NE, Cunningham RP. Substrate specificity of RdgB protein, a deoxyribonucleoside triphosphate pyrophosphohydrolase. J. Biol. Chem. 2007;282:3531–3538. doi: 10.1074/jbc.M608708200. [DOI] [PubMed] [Google Scholar]
- 16.Porta J, Kolar C, Kozmin SG, Pavlov YI, Borgstahl GE. Structure of the orthorhombic form of human inosine triphosphate pyrophosphatase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006;62:1076–1081. doi: 10.1107/S1744309106041790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenmark P, Kursula P, Flodin S, Graslund S, Landry R, Nordlund P, Schuler H. Crystal structure of human inosine triphosphatase. Substrate binding and implication of the inosine triphosphatase deficiency mutation P32T. J. Biol. Chem. 2007;282:3182–3187. doi: 10.1074/jbc.M609838200. [DOI] [PubMed] [Google Scholar]
- 18.Stepchenkova EI, Tarakhovskaya ER, Spitler K, Frahm C, Menezes MR, Simone PD, Kolar C, Marky LA, Borgstahl GE, Pavlov YI. Functional study of the P32T ITPA variant associated with drug sensitivity in humans. J. Mol. Biol. 2009;392:602–613. doi: 10.1016/j.jmb.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herting G, Barber K, Zappala MR, Cunningham RP, Burgis NE. Quantitative in vitro and in vivo characterization of the human P32T mutant ITPase. Biochim. Biophys. Acta. 2010;1802:269–274. doi: 10.1016/j.bbadis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderheiden BS. Micro assay of ITP pyrophosphohydrolase by liquid scintillation. Anal. Biochem. 1972;49:459–466. doi: 10.1016/0003-2697(72)90449-6. [DOI] [PubMed] [Google Scholar]
- 21.Herz F. Effect of heat and nucleotides on human erythrocyte inorganic pyrophosphatase. Proc. Soc. Exp. Biol. Med. 1967;125:68–70. doi: 10.3181/00379727-125-32015. [DOI] [PubMed] [Google Scholar]
- 22.Andersen O. Chelation of cadmium. Environ. Health Perspect. 1984;54:249–266. doi: 10.1289/ehp.8454249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieland M, Levin MK, Hingorani KS, Biro FN, Hingorani MM. Mechanism of cadmium-mediated inhibition of Msh2–Msh6 function in DNA mismatch repair. Biochemistry. 2009;48:9492–9502. doi: 10.1021/bi9001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miessler GL, Tarr DA. Inorganic Chemistry. Third ed. Upper Saddle River, New Jersey: Pearson Prentice Hall; 2004. [Google Scholar]
- 25.Lokanath NK, Pampa KJ, Takio K, Kunishima N. Structures of dimeric nonstandard nucleotide triphosphate pyrophosphatase from Pyrococcus horikoshii OT3: functional significance of interprotomer conformational changes. J. Mol. Biol. 2008;375:1013–1025. doi: 10.1016/j.jmb.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Hwang KY, Chung JH, Kim SH, Han YS, Cho Y. Structure-based identification of a novel NTPase from Methanococcus jannaschii. Nat. Struct. Biol. 1999;6:691–696. doi: 10.1038/10745. [DOI] [PubMed] [Google Scholar]
- 27.Savchenko A, Proudfoot M, Skarina T, Singer A, Litvinova O, Sanishvili R, Brown G, Chirgadze N, Yakunin AF. Molecular basis of the antimutagenic activity of the house-cleaning inosine triphosphate pyrophosphatase RdgB from Escherichia coli. J. Mol. Biol. 2007;374:1091–1103. doi: 10.1016/j.jmb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry. Upgrade ed. New York, New York: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 29.Vanderheiden BS. Phosphate esters of human erythrocytes: synthesis of ITP-14C from inosine-8-14C. Nature. 1967;216:1036–1037. doi: 10.1038/2161036a0. [DOI] [PubMed] [Google Scholar]
- 30.Zachara B, Lewandowski J. Isolation and identification of inosine triphosphate from human erythrocytes. Biochim. Biophys. Acta. 1974;353:253–259. doi: 10.1016/0005-2787(74)90190-7. [DOI] [PubMed] [Google Scholar]
- 31.Fraser JH, Meyers H, Henderson JF, Brox LW, McCoy EE. Individual variation in inosine triphosphate accumulation in human erythrocytes. Clin. Biochem. 1975;8:353–364. doi: 10.1016/s0009-9120(75)93685-1. [DOI] [PubMed] [Google Scholar]
- 32.Soder C, Henderson JF, Zombor G, McCoy EE, Verhoef V, Morris AJ. Relationships between nucleoside triphosphate pyrophosphohydrolase activity and inosine triphosphate accumulation in human erythrocytes. Can. J. Biochem. 1976;54:843–847. doi: 10.1139/o76-121. [DOI] [PubMed] [Google Scholar]
- 33.Henderson JF, Zombor G, Fraser JH, McCoy EE, Verhoef V, Morris AJ. Factors affecting inosinate synthesis and inosine triphosphate accumulation in human erythrocytes. Can. J. Biochem. 1977;55:359–364. doi: 10.1139/o77-049. [DOI] [PubMed] [Google Scholar]
- 34.Vanderheiden BS. Inosine di- and triphosphate synthesis in erythrocytes and cell extracts. J. Cell. Physiol. 1979;99:287–301. doi: 10.1002/jcp.1040990303. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal RP, Scholar EM, Agarwal KC, Parks RE., Jr Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of monophosphate nucleotides of purine analogs. Biochem. Pharmacol. 1971;20:1341–1354. doi: 10.1016/0006-2952(71)90261-9. [DOI] [PubMed] [Google Scholar]
- 36.Ren H, Wang L, Bennett M, Liang Y, Zheng X, Lu F, Li L, Nan J, Luo M, Eriksson S, Zhang C, Su XD. The crystal structure of human adenylate kinase 6: An adenylate kinase localized to the cell nucleus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:303–308. doi: 10.1073/pnas.0407459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myrnes B, Guddal PH, Krokan H. Metabolism of dITP in HeLa cell extracts, incorporation into DNA by isolated nuclei and release of hypoxanthine from DNA by a hypoxanthine-DNA glycosylase activity. Nucleic Acids Res. 1982;10:3693–3701. doi: 10.1093/nar/10.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro R, Pohl SH. The reaction of ribonucleosides with nitrous acid. Side products and kinetics. Biochemistry. 1968;7:448–455. doi: 10.1021/bi00841a057. [DOI] [PubMed] [Google Scholar]
- 39.Dedon PC, Barth M, Chen B, De Mott M, Dendroulakis V, Dong M, Kalinga S, Elmquist E, Margolin Y, Pang B, Zhou X. In: Diverse Mechanisms of Endogenous Nucleobase Deamination in DNA and RNAin Advances in Molecular Toxicology. James CF, editor. Elsevier; 2006. pp. 25–63. [Google Scholar]
- 40.Karran P, Lindahl T. Hypoxanthine in deoxyribonucleic acid: generation by heatinduced hydrolysis of adenine residues and release in free form by a deoxyribonucleic acid glycosylase from calf thymus. Biochemistry. 1980;19:6005–6011. doi: 10.1021/bi00567a010. [DOI] [PubMed] [Google Scholar]
- 41.Martin FH, Castro MM, Aboul-ela F, Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985;13:8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas MJ, Platas AA, Hawley DK. Transcriptional fidelity and proofreading by RNA polymerase II. Cell. 1998;93:627–637. doi: 10.1016/s0092-8674(00)81191-5. [DOI] [PubMed] [Google Scholar]
- 43.Dierick H, Stul M, De Kelver W, Marynen P, Cassiman JJ. Incorporation of dITP or 7-deaza dGTP during PCR improves sequencing of the product. Nucleic Acids Res. 1993;21:4427–4428. doi: 10.1093/nar/21.18.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bessman MJ, Lehman IR, Adler J, Zimmerman SB, Simms ES, Kornberg A. Enzymatic Synthesis of Deoxyribonucleic Acid. Iii. The Incorporation of Pyrimidine and Purine Analogues into Deoxyribonucleic Acid. Proc Natl Acad Sci U S A. 1958;44:633–640. doi: 10.1073/pnas.44.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pang B, McFaline JL, Burgis NE, Dong M, Taghizadeh K, Sullivan MR, Elmquist CE, Cunningham RP, Dedon PC. Defects in purine nucleotide metabolism lead to substantial incorporation of xanthine and hypoxanthine into DNA and RNA. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2319–2324. doi: 10.1073/pnas.1118455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T, Yoshida M, Yamada M, Ide H, Kobayashi M, Kanaori K, Tajima K, Makino K. Misincorporation of 2'-deoxyoxanosine 5'-triphosphate by DNA polymerases and its implication for mutagenesis. Biochemistry. 1998;37:11592–11598. doi: 10.1021/bi980971f. [DOI] [PubMed] [Google Scholar]
- 47.Sakumi K, Abolhassani N, Behmanesh M, Iyama T, Tsuchimoto D, Nakabeppu Y. ITPA protein, an enzyme that eliminates deaminated purine nucleoside triphosphates in cells. Mutat. Res. 2010;703:43–50. doi: 10.1016/j.mrgentox.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Budke B, Kuzminov A. Hypoxanthine incorporation is nonmutagenic in Escherichia coli. J Bacteriol. 2006;188:6553–6560. doi: 10.1128/JB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgis NE, Brucker JJ, Cunningham RP. Repair system for noncanonical purines in Escherichia coli. J. Bacteriol. 2003;185:3101–3110. doi: 10.1128/JB.185.10.3101-3110.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budke B, Kuzminov A. Production of clastogenic DNA precursors by the nucleotide metabolism in Escherichia coli. Mol Microbiol. 2010;75:230–245. doi: 10.1111/j.1365-2958.2009.06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamiya H, Shimizu M, Suzuki M, Inoue H, Ohtsuka E. Mutation Induced by Deoxyxanthosine in Codon 12 of a Synthetic c-Ha-ras Gene. Nucleosides Nucleotides. 1992;11:247–260. [PubMed] [Google Scholar]
- 52.Kamiya H. Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary. Nucleic Acids Res. 2003;31:517–531. doi: 10.1093/nar/gkg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamiya H, Miura H, Kato H, Nishimura S, Ohtsuka E. Induction of mutation of a synthetic c-Ha-ras gene containing hypoxanthine. Cancer Res. 1992;52:1836–1839. [PubMed] [Google Scholar]
- 54.Nordmann PL, Makris JC, Reznikoff WS. Inosine induced mutations. Mol. Gen. Genet. 1988;214:62–67. doi: 10.1007/BF00340180. [DOI] [PubMed] [Google Scholar]
- 55.Caldecott KW. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 56.Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8241–8246. doi: 10.1073/pnas.131009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 58.He B, Qing H, Kow YW. Deoxyxanthosine in DNA is repaired by Escherichia coli endonuclease V. Mutat. Res. 2000;459:109–114. doi: 10.1016/s0921-8777(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 59.Bradshaw JS, Kuzminov A. RdgB acts to avoid chromosome fragmentation in Escherichia coli. Mol. Microbiol. 2003;48:1711–1725. doi: 10.1046/j.1365-2958.2003.03540.x. [DOI] [PubMed] [Google Scholar]
- 60.Moe A, Ringvoll J, Nordstrand LM, Eide L, Bjoras M, Seeberg E, Rognes T, Klungland A. Incision at hypoxanthine residues in DNA by a mammalian homologue of the Escherichia coli antimutator enzyme endonuclease V. Nucleic Acids Res. 2003;31:3893–3900. doi: 10.1093/nar/gkg472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mi R, Alford-Zappala M, Kow YW, Cunningham RP, Cao W. Human endonuclease V as a repair enzyme for DNA deamination. Mutat Res. 2012;735:12–18. doi: 10.1016/j.mrfmmm.2012.05.003. Epub 2012 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao W. Endonuclease V: an unusual enzyme for repair of DNA deamination. Cell Mol Life Sci. 2012;20:20. doi: 10.1007/s00018-012-1222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosnes I, Rowe AD, Vik ES, Forstrom RJ, Alseth I, Bjoras M, Dalhus B. Structural basis of DNA loop recognition by endonuclease V. Structure. 2013;21:257–265. doi: 10.1016/j.str.2012.12.007. Epub 2013 Jan 11. [DOI] [PubMed] [Google Scholar]
- 64.Karran P, Lindahl T. Enzymatic excision of free hypoxanthine from polydeoxynucleotides and DNA containing deoxyinosine monophosphate residues. J. Biol. Chem. 1978;253:5877–5879. [PubMed] [Google Scholar]
- 65.Lee HW, Dominy BN, Cao W. New family of deamination repair enzymes in uracil-DNA glycosylase superfamily. J. Biol. Chem. 2011;286:31282–31287. doi: 10.1074/jbc.M111.249524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saparbaev M, Mani JC, Laval J. Interactions of the human, rat Saccharomyces cerevisiae and Escherichia coli 3-methyladenine-DNA glycosylases with DNA containing dIMP residues. Nucleic Acids Res. 2000;28:1332–1339. doi: 10.1093/nar/28.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daley JM, Zakaria C, Ramotar D. The endonuclease IV family of apurinic/apyrimidinic endonucleases. Mutat. Res. 2010;705:217–227. doi: 10.1016/j.mrrev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Auclair C, Gouyette A, Levy A, Emerit I. Clastogenic inosine nucleotide as components of the chromosome breakage factor in scleroderma patients. Arch. Biochem. Biophys. 1990;278:238–244. doi: 10.1016/0003-9861(90)90253-u. [DOI] [PubMed] [Google Scholar]
- 69.Emerit I, Filipe P, Meunier P, Auclair C, Freitas J, Deroussent A, Gouyette A, Fernandes A. Clastogenic activity in the plasma of scleroderma patients: a biomarker of oxidative stress. Dermatology. 1997;194:140–146. doi: 10.1159/000246083. [DOI] [PubMed] [Google Scholar]
- 70.Vanderheiden BS. Possible implication of an inosinetriphosphate metabolic error and glutamic acid decarboxylase in paranoid schizophrenia. Biochem. Med. 1979;21:22–32. doi: 10.1016/0006-2944(79)90051-6. [DOI] [PubMed] [Google Scholar]
- 71.Burton K, White H, Sleep J. Kinetics of muscle contraction and actomyosin NTP hydrolysis from rabbit using a series of metal-nucleotide substrates. J. Physiol. 2005;563:689–711. doi: 10.1113/jphysiol.2004.078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osheroff N, Shelton ER, Brutlag DL. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J. Biol. Chem. 1983;258:9536–9543. [PubMed] [Google Scholar]
- 73.Weber J, Senior AE. Bi-site catalysis in F1-ATPase: does it exist? J. Biol. Chem. 2001;276:35422–35428. doi: 10.1074/jbc.M104946200. [DOI] [PubMed] [Google Scholar]
- 74.Klinker JF, Seifert R. Functionally nonequivalent interactions of guanosine 5'-triphosphate, inosine 5'-triphosphate, and xanthosine 5'-triphosphate with the retinal G-protein, transducin, and with Gi-proteins in HL-60 leukemia cell membranes. Biochem. Pharmacol. 1997;54:551–562. doi: 10.1016/s0006-2952(97)00205-0. [DOI] [PubMed] [Google Scholar]
- 75.Noskov VN, Staak K, Shcherbakova PV, Kozmin SG, Negishi K, Ono BC, Hayatsu H, Pavlov YI. HAM1, the gene controlling 6-N-hydroxylaminopurine sensitivity and mutagenesis in the yeast Saccharomyces cerevisiae. Yeast. 1996;12:17–29. doi: 10.1002/(SICI)1097-0061(199601)12:1%3C17::AID-YEA875%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 76.Behmanesh M, Sakumi K, Tsuchimoto D, Torisu K, Ohnishi-Honda Y, Rancourt DE, Nakabeppu Y. Characterization of the structure and expression of mouse Itpa gene and its related sequences in the mouse genome. DNA Res. 2005;12:39–51. doi: 10.1093/dnares/12.1.39. [DOI] [PubMed] [Google Scholar]
- 77.Vanderheiden BS. ITP pyrophosphohydrolase and IDP phosphohydrolase in rat tissue. J. Cell. Physiol. 1975;86:167–175. doi: 10.1002/jcp.1040860118. [DOI] [PubMed] [Google Scholar]
- 78.Chern CJ, MacDonald AB, Morris AJ. Purification and properties of a nucleoside triphosphate pyrophosphohydrolase from red cells of the rabbit. J. Biol. Chem. 1969;244:5489–5495. [PubMed] [Google Scholar]
- 79.Michel B. After 30 years of study, the bacterial SOS response still surprises us. PLoS Biology. 2005;3:e255. doi: 10.1371/journal.pbio.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kozmin SG, Schaaper RM, Shcherbakova PV, Kulikov VN, Noskov VN, Guetsova ML, Alenin VV, Rogozin IB, Makarova KS, Pavlov YI. Multiple antimutagenesis mechanisms affect mutagenic activity and specificity of the base analog 6-N-hydroxylaminopurine in bacteria and yeast. Mutat. Res. 1998;402:41–50. doi: 10.1016/s0027-5107(97)00280-7. [DOI] [PubMed] [Google Scholar]
- 81.Abolhassani N, Iyama T, Tsuchimoto D, Sakumi K, Ohno M, Behmanesh M, Nakabeppu Y. NUDT16 and ITPA play a dual protective role in maintaining chromosome stability and cell growth by eliminating dIDP/IDP and dITP/ITP from nucleotide pools in mammals. Nucleic Acids Res. 2010;38:2891–2903. doi: 10.1093/nar/gkp1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsuchimoto D, Iyama T, Nonaka M, Abolhassani N, Ohta E, Sakumi K, Nakabeppu Y. A comprehensive screening system for damaged nucleotide-binding proteins. Mutat. Res. 2010;703:37–42. doi: 10.1016/j.mrgentox.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 83.Vanderheiden BS. Inosine triphosphate in human erythrocytes: A genetic trait, Proceedings of the 10th Congress of the International Society of Blood Transfusion. Stockholm. 1965;1964:540. [Google Scholar]
- 84.Vanderheiden BS. Genetic studies of human erythrocyte inosine triphosphatase. Biochem. Genet. 1969;3:289–297. doi: 10.1007/BF00521144. [DOI] [PubMed] [Google Scholar]
- 85.Verhoef VL, Fuller SA, Morris AJ. Individual variation of nucleoside triphosphate pyrophosphohydrolase activity in human erythrocytes, granulocytes, lymphocytes, and platelets. Biochem. Genet. 1980;18:235–245. doi: 10.1007/BF00484239. [DOI] [PubMed] [Google Scholar]
- 86.Marsh S, King CR, Ahluwalia R, McLeod HL. Distribution of ITPA P32T alleles in multiple world populations. J. Hum. Genet. 2004;49:579–581. doi: 10.1007/s10038-004-0183-y. [DOI] [PubMed] [Google Scholar]
- 87.von Ahsen N, Oellerich M, Armstrong VW. Characterization of the inosine triphosphatase (ITPA) gene: haplotype structure, haplotype-phenotype correlation and promoter function. Ther. Drug Monit. 2008;30:16–22. doi: 10.1097/FTD.0b013e318161a21a. [DOI] [PubMed] [Google Scholar]
- 88.Heller T, Oellerich M, Armstrong VW, von Ahsen N. Rapid detection of ITPA 94C>A and IVS2 + 21A>C gene mutations by real-time fluorescence PCR and in vitro demonstration of effect of ITPA IVS2 + 21A>C polymorphism on splicing efficiency. Clin. Chem. 2004;50:2182–2184. doi: 10.1373/clinchem.2004.039685. [DOI] [PubMed] [Google Scholar]
- 89.Arenas M, Duley J, Sumi S, Sanderson J, Marinaki A. The ITPA c.94C>A and g.IVS2+21A>C sequence variants contribute to missplicing of the ITPA gene. Biochim. Biophys. Acta. 2007;1772:96–102. doi: 10.1016/j.bbadis.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Shichijo S, Azuma K, Komatsu N, Kawamoto N, Takedatsu H, Shomura H, Sawamizu H, Maeda Y, Ito M, Itoh K. Identification of two novel tumor-associated antigens recognized by HLA-B46-restricted cytotoxic T lymphocytes. Int. J. Mol. Med. 2003;12:895–902. [PubMed] [Google Scholar]
- 91.Poppe B, Vandesompele J, Schoch C, Lindvall C, Mrozek K, Bloomfield CD, Beverloo HB, Michaux L, Dastugue N, Herens C, Yigit N, De Paepe A, Hagemeijer A, Speleman F. Expression analyses identify MLL as a prominent target of 11q23 amplification and support an etiologic role for MLL gain of function in myeloid malignancies. Blood. 2004;103:229–235. doi: 10.1182/blood-2003-06-2163. [DOI] [PubMed] [Google Scholar]
- 92.John T, Black MA, Toro TT, Leader D, Gedye CA, Davis ID, Guilford PJ, Cebon JS. Predicting clinical outcome through molecular profiling in stage III melanoma. Clin. Cancer Res. 2008;14:5173–5180. doi: 10.1158/1078-0432.CCR-07-4170. [DOI] [PubMed] [Google Scholar]
- 93.Menezes MR, Waisertreiger IS, Lopez-Bertoni H, Luo X, Pavlov YI. Pivotal role of inosine triphosphate pyrophosphatase in maintaining genome stability and the prevention of apoptosis in human cells. PLoS ONE. 2012;7:e32313. doi: 10.1371/journal.pone.0032313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vanderheiden BS, Zarate-Moyano C. Erythrocyte ITP pyrophosphohydrolase deficiency in a psychiatric population. Biol. Psychiatry. 1976;11:755–765. [PubMed] [Google Scholar]
- 95.Vanderheiden BS, Bora G. Erythrocyte ITP pyrophosphohydrolase in chronic paranoid and undifferentiated schizophrenics: a biological difference. Biochem. Med. 1980;23:76–86. doi: 10.1016/0006-2944(80)90057-5. [DOI] [PubMed] [Google Scholar]
- 96.Bakker JA, Bierau J, Drent M. A role for ITPA variants in the clinical course of pulmonary Langerhans' cell histiocytosis? Eur. Respir. J. 2010;36:684–686. doi: 10.1183/09031936.00026610. [DOI] [PubMed] [Google Scholar]
- 97.Zamzami MA, Duley JA, Price GR, Venter DJ, Yarham JW, Taylor RW, Catley LP, Florin TH, Marinaki AM, Bowling F. Inosine Triphosphate Pyrophosphohydrolase (ITPA) polymorphic sequence variants in adult hematological malignancy patients and possible association with mitochondrial DNA defects. J Hematol Oncol. 2013;6:24. doi: 10.1186/1756-8722-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marinaki AM, Duley JA, Arenas M, Ansari A, Sumi S, Lewis CM, Shobowale-Bakre M, Fairbanks LD, Sanderson J. Mutation in the ITPA gene predicts intolerance to azathioprine. Nucleosides Nucleotides Nucleic Acids. 2004;23:1393–1397. doi: 10.1081/NCN-200027639. [DOI] [PubMed] [Google Scholar]
- 99.Krynetski EY, Evans WE. Pharmacogenetics of cancer therapy: getting personal. Am. J. Hum. Genet. 1998;63:11–16. doi: 10.1086/301941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui CH, Evans WE. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J. Natl. Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 101.Gearry RB, Roberts RL, Barclay ML, Kennedy MA. Lack of association between the ITPA 94C>A polymorphism and adverse effects from azathioprine. Pharmacogenetics. 2004;14:779–781. doi: 10.1097/00008571-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 102.von Ahsen N, Armstrong VW, Behrens C, von Tirpitz C, Stallmach A, Herfarth H, Stein J, Bias P, Adler G, Shipkova M, Oellerich M, Kruis W, Reinshagen M, Schutz E. Association of inosine triphosphatase 94C>A and thiopurine S-methyltransferase deficiency with adverse events and study drop-outs under azathioprine therapy in a prospective Crohn disease study. Clin. Chem. 2005;51:2282–2288. doi: 10.1373/clinchem.2005.057158. [DOI] [PubMed] [Google Scholar]
- 103.Zelinkova Z, Derijks LJ, Stokkers PC, Vogels EW, van Kampen AH, Curvers WL, Cohn D, van Deventer SJ, Hommes DW. Inosine triphosphate pyrophosphatase and thiopurine S-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin. Gastroenterol. Hepatol. 2006;4:44–49. doi: 10.1016/j.cgh.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 104.van Dieren JM, van Vuuren AJ, Kusters JG, Nieuwenhuis EE, Kuipers EJ, van der Woude CJ. ITPA genotyping is not predictive for the development of side effects in AZA treated inflammatory bowel disease patients. Gut. 2005;54:1664. [PMC free article] [PubMed] [Google Scholar]
- 105.Hindorf U, Lindqvist M, Peterson C, Soderkvist P, Strom M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423–1431. doi: 10.1136/gut.2005.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Dieren JM, Hansen BE, Kuipers EJ, Nieuwenhuis EE, Van der Woude CJ. Meta-analysis: Inosine triphosphate pyrophosphatase polymorphisms and thiopurine toxicity in the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2007;26:643–652. doi: 10.1111/j.1365-2036.2007.03412.x. [DOI] [PubMed] [Google Scholar]
- 107.Ansari A, Arenas M, Greenfield SM, Morris D, Lindsay J, Gilshenan K, Smith M, Lewis C, Marinaki A, Duley J, Sanderson J. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2008;28:973–983. doi: 10.1111/j.1365-2036.2008.03788.x. [DOI] [PubMed] [Google Scholar]
- 108.Stocco G, Cheok MH, Crews KR, Dervieux T, French D, Pei D, Yang W, Cheng C, Pui CH, Relling MV, Evans WE. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 2009;85:164–172. doi: 10.1038/clpt.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wan Rosalina WR, Teh LK, Mohamad N, Nasir A, Yusoff R, Baba AA, Salleh MZ. Polymorphism of ITPA 94C>A and risk of adverse effects among patients with acute lymphoblastic leukaemia treated with 6-mercaptopurine. J. Clin. Pharm. Ther. 2011;37:237–241. doi: 10.1111/j.1365-2710.2011.01272.x. [DOI] [PubMed] [Google Scholar]
- 110.Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K, Jacqz-Aigrain E. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br. J. Clin. Pharmacol. 2011;71:575–584. doi: 10.1111/j.1365-2125.2010.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shipkova M, Franz J, Abe M, Klett C, Wieland E, Andus T. Association between adverse effects under azathioprine therapy and inosine triphosphate pyrophosphatase activity in patients with chronic inflammatory bowel disease. Ther. Drug Monit. 2011;33:321–328. doi: 10.1097/FTD.0b013e31821a7c34. [DOI] [PubMed] [Google Scholar]
- 112.Buster EH, van Vuuren HJ, Zondervan PE, Metselaar HJ, Tilanus HW, de Man RA. Thiopurine-methyltransferase and inosine triphosphate pyrophosphatase polymorphism in a liver transplant recipient developing nodular regenerative hyperplasia on low-dose azathioprine. Eur. J. Gastroenterol. Hepatol. 2008;20:68–72. doi: 10.1097/MEG.0b013e32825a6a8a. [DOI] [PubMed] [Google Scholar]
- 113.Okada Y, Nakamura K, Hiromura K, Nojima Y, Horiuchi R, Yamamoto K. Pro32Thr polymorphism of inosine triphosphate pyrophosphatase gene predicts efficacy of low-dose azathioprine for patients with systemic lupus erythematosus. Clin. Pharmacol. Ther. 2009;85:527–530. doi: 10.1038/clpt.2008.261. [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto K, Okada Y, Nakamura K, Hiromura K, Nojima Y, Nakamura T. Inosine triphosphate pyrophosphatase 94C>A polymorphism: clinical implications for patients with systemic lupus erythematosus treated with azathioprine. Expert Opin Drug Saf. 2010;9:447–457. doi: 10.1517/14740330903544474. [DOI] [PubMed] [Google Scholar]
- 115.Derijks LJ, Wong DR. Pharmacogenetics of thiopurines in inflammatory bowel disease. Curr. Pharm. Des. 2010;16:145–154. doi: 10.2174/138161210790112773. [DOI] [PubMed] [Google Scholar]
- 116.Dewit O, Starkel P, Roblin X. Thiopurine metabolism monitoring: implications in inflammatory bowel diseases. Eur. J. Clin. Invest. 2010;40:1037–1047. doi: 10.1111/j.1365-2362.2010.02346.x. [DOI] [PubMed] [Google Scholar]
- 117.Stocco G, Crews KR, Evans WE. Genetic polymorphism of inosine-triphosphate-pyrophosphatase influences mercaptopurine metabolism and toxicity during treatment of acute lymphoblastic leukemia individualized for thiopurine-S-methyl-transferase status. Expert Opin. Drug Saf. 2010;9:23–37. doi: 10.1517/14740330903426151. [DOI] [PubMed] [Google Scholar]
- 118.Bierau J, Lindhout M, Bakker JA. Pharmacogenetic significance of inosine triphosphatase. Pharmacogenomics. 2007;8:1221–1228. doi: 10.2217/14622416.8.9.1221. [DOI] [PubMed] [Google Scholar]
- 119.Bakker JA, Lindhout M, Habets DD, van den Wijngaard A, Paulussen AD, Bierau J. The effect of ITPA polymorphisms on the enzyme kinetic properties of human erythrocyte inosine triphosphatase toward its substrates ITP and 6-Thio-ITP. Nucleosides Nucleotides Nucleic Acids. 2011;30:839–849. doi: 10.1080/15257770.2011.606789. [DOI] [PubMed] [Google Scholar]
- 120.Wessels JA, Kooloos WM, De Jonge R, De Vries-Bouwstra JK, Allaart CF, Linssen A, Collee G, De Sonnaville P, Lindemans J, Huizinga TW, Guchelaar HJ. Relationship between genetic variants in the adenosine pathway and outcome of methotrexate treatment in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2006;54:2830–2839. doi: 10.1002/art.22032. [DOI] [PubMed] [Google Scholar]
- 121.Wessels JA, van der Kooij SM, le Cessie S, Kievit W, Barerra P, Allaart CF, Huizinga TW, Guchelaar HJ. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum. 2007;56:1765–1775. doi: 10.1002/art.22640. [DOI] [PubMed] [Google Scholar]
- 122.Dervieux T, Wessels JA, van der Straaten T, Penrod N, Moore JH, Guchelaar HJ, Kremer JM. Gene-gene interactions in folate and adenosine biosynthesis pathways affect methotrexate efficacy and tolerability in rheumatoid arthritis. Pharmacogenet. Genomics. 2009;19:935–944. doi: 10.1097/FPC.0b013e32833315d1. [DOI] [PubMed] [Google Scholar]
- 123.Lee YC, Cui J, Costenbader KH, Shadick NA, Weinblatt ME, Karlson EW. Investigation of candidate polymorphisms and disease activity in rheumatoid arthritis patients on methotrexate. Rheumatology. 2009;48:613–617. doi: 10.1093/rheumatology/ken513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kooloos WM, Wessels JA, van der Straaten T, Allaart CF, Huizinga TW, Guchelaar HJ. Functional polymorphisms and methotrexate treatment outcome in recent-onset rheumatoid arthritis. Pharmacogenomics. 2010;11:163–175. doi: 10.2217/pgs.09.139. [DOI] [PubMed] [Google Scholar]
- 125.Ochi H, Maekawa T, Abe H, Hayashida Y, Nakano R, Kubo M, Tsunoda T, Hayes CN, Kumada H, Nakamura Y, Chayama K. ITPA polymorphism affects ribavirin-induced anemia and outcomes of therapy--a genome-wide study of Japanese HCV virus patients. Gastroenterology. 2010;139:1190–1197. doi: 10.1053/j.gastro.2010.06.071. [DOI] [PubMed] [Google Scholar]
- 126.Thompson AJ, Fellay J, Patel K, Tillmann HL, Naggie S, Ge D, Urban TJ, Shianna KV, Muir AJ, Fried MW, Afdhal NH, Goldstein DB, McHutchison JG. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology. 2010;139:1181–1189. doi: 10.1053/j.gastro.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakamoto N, Tanaka Y, Nakagawa M, Yatsuhashi H, Nishiguchi S, Enomoto N, Azuma S, Nishimura-Sakurai Y, Kakinuma S, Nishida N, Tokunaga K, Honda M, Ito K, Mizokami M, Watanabe M. ITPA gene variant protects against anemia induced by pegylated interferon-alpha and ribavirin therapy for Japanese patients with chronic hepatitis C. Hepatol. Res. 2010;40:1063–1071. doi: 10.1111/j.1872-034X.2010.00741.x. [DOI] [PubMed] [Google Scholar]
- 128.Suzuki F, Suzuki Y, Akuta N, Sezaki H, Hirakawa M, Kawamura Y, Hosaka T, Kobayashi M, Saito S, Arase Y, Ikeda K, Chayama K, Kamatani N, Nakamura Y, Miyakawa Y, Kumada H. Influence of ITPA polymorphisms on decreases of hemoglobin during treatment with pegylated interferon, ribavirin, and telaprevir. Hepatology. 2011;53:415–421. doi: 10.1002/hep.24058. [DOI] [PubMed] [Google Scholar]
- 129.Chayama K, Hayes CN, Abe H, Miki D, Ochi H, Karino Y, Toyota J, Nakamura Y, Kamatani N, Sezaki H, Kobayashi M, Akuta N, Suzuki F, Kumada H. IL28B but not ITPA polymorphism is predictive of response to pegylated interferon, ribavirin, and telaprevir triple therapy in patients with genotype 1 hepatitis C. J. Infect. Dis. 2011;204:84–93. doi: 10.1093/infdis/jir210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hitomi Y, Cirulli ET, Fellay J, McHutchison JG, Thompson AJ, Gumbs CE, Shianna KV, Urban TJ, Goldstein DB. Inosine Triphosphate Protects Against Ribavirin-Induced Adenosine Triphosphate Loss by Restoring Adenylosuccinate Synthase Function. Gastroenterology. 2011;140:1314–1321. doi: 10.1053/j.gastro.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 131.Maeda T, Sumi S, Ueta A, Ohkubo Y, Ito T, Marinaki AM, Kurono Y, Hasegawa S, Togari H. Genetic basis of inosine triphosphate pyrophosphohydrolase deficiency in the Japanese population. Mol. Genet. Metab. 2005;85:271–279. doi: 10.1016/j.ymgme.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 132.Shipkova M, Lorenz K, Oellerich M, Wieland E, von Ahsen N. Measurement of erythrocyte inosine triphosphate pyrophosphohydrolase (ITPA) activity by HPLC and correlation of ITPA genotype-phenotype in a Caucasian population. Clin. Chem. 2006;52:240–247. doi: 10.1373/clinchem.2005.059501. [DOI] [PubMed] [Google Scholar]
- 133.Atanasova S, Shipkova M, Svinarov D, Mladenova A, Genova M, Wieland E, Oellerich M, von Ahsen N. Analysis of ITPA phenotype-genotype correlation in the Bulgarian population revealed a novel gene variant in exon 6. Ther. Drug Monit. 2007;29:6–10. doi: 10.1097/FTD.0b013e3180308554. [DOI] [PubMed] [Google Scholar]
- 134.Schrödinger LLC. The PyMOL Molecular Graphics System, Version 1.4.1. 2011 [Google Scholar]
- 135.Ho B, Gruswitz F. HOLLOW: Generating Accurate Representations of Channel and Interior Surfaces in Molecular Structures. BMC Struct. Biol. 2008;8:49. doi: 10.1186/1472-6807-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kleywegt GJ, Jones TA. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr. D Biol. Crystallogr. 1994;50:178–185. doi: 10.1107/S0907444993011333. [DOI] [PubMed] [Google Scholar]