Abstract
The maintenance of CMV-specific T cell memory in lung transplant recipients (LTRs) is critical for host defense and allograft durability, particularly in donor+/recipient− (D+R−) individuals who demonstrate increased mortality. We studied CD4+ and CD8+ CMV-specific memory responses to phosphoprotein 65 (pp65) in a prospective cohort of 18 D+R− LTRs, from bronchoalveolar lavage (BAL)-obtained lung mononuclear cells (LMNC) and PBMC. Unexpectedly, pp65-specific CD4+ and CD8+ IFN-γ memory responses from LMNC were similar, in contrast to persistent CD8+ predominance in PBMC. Unlike the pulmonary CD8+ predominance during acute primary infection, compartmental equalization occurred in the CMV-specific CD8+ memory pool during chronic infection, whereas CMV-specific CD4+ memory was enriched in the bronchoalveolar space. Moreover, CMV-specific CD4+ memory T cells with multifunctional production of IFN-γ, TNF-α, IL-2 and MIP-1β were significantly increased in LMNCs, in contrast to similar intercompartmental CD8+ memory function. Moreover, the absolute number of CMV-specific CD4+IFN-γ+ memory cells in BAL was significantly increased in LTRs exhibiting viral control compared to those with CMV early antigen positivity. Collectively, these data demonstrate both preferential distribution and functional quality of CMV-specific CD4+ memory in the lung allograft during chronic infection, and show an important association with CMV mucosal immunity and viral control.
Keywords: CMV, T cells, lung transplant, memory, viral control
Introduction
Cytomegalovirus, a member of the β-herpesvirus family, remains a significant cause of morbidity and mortality in solid organ transplant recipients (SOTRs) and hematopoietic cell transplant recipients (1–3). Among lung transplant recipients (LTRs), CMV infection is the most common opportunistic pathogen with D+R− LTRs being at highest risk for active viral disease and increased 1- and 5-year mortality (4,5). Moreover, CMV infection is implicated in allograft rejection in SOTRs, including the bronchiolitis obliterans syndrome or chronic allograft rejection in LTRs (6,7). Because of the impact of CMV, the majority of LTRs undergo prophylactic treatment in the immediate posttransplant period, though the duration of therapy widely varies among institutions. A recent study indicates repeated episodes of active CMV replication are associated with decreased survival among LTRs (8). Thus, a key question is whether high-risk D+R− LTRs maintained on life-long immunosuppression are capable of developing and maintaining a functional memory response sufficient for immune control during chronic CMV infection. Lung transplantation provides a unique opportunity to simultaneously evaluate the CMV-specific effector and memory T cell response in both the allograft and systemic circulation, as surveillance bronchoscopy is routinely performed in LTRs allowing collection of bronchoalveolar lavage fluid containing lung mononuclear cells (LMNC) from the allograft for study, unlike other SOTRs.
Previously, we reported the persistence of CMV-specific memory responses in D+R− LTRs following primary infection, including the detection of CD4+ and CD8+ responses from LMNC in a small number of LTRs (9), as well as the acquisition of de novo CMV-specific effector responses during acute primary infection predominated by CD8+ T cells directed toward the major antigen pp65 in the lung allograft (10). While these studies showed the acquisition and persistence of CMV-specific T cells capable of producing IFN-γ, the distribution and quality of memory subsets, i.e. the degree of multifunctionality of antigen-specific cells during chronic CMV infection in this susceptible patient population, are unclear. Recently, several studies have demonstrated that the quality and quantity of the T cell response are a critical factor in determining host protection (11,12), and thus evaluating these characteristics of the CMV-specific memory T cell response in both the lung allograft and peripheral blood may improve our understanding of the capacity for viral immune control in D+R− LTRs in these tissue compartments. Based on our prior studies and work by others, we hypothesized that the magnitude and quality of CMV-specific effector memory cells during chronic infection would be enriched in the bronchoalveolar compartment compared to the systemic circulation, with a predominance of CD8+ memory. To test this, we performed a study in a prospective cohort of 15 D+R− LTRs and assessed CMV-specific acute primary effector T cell responses and memory responses 3–9 months into chronic infection from LMNC and PBMC. Herein, we report our unexpected findings of overall similar CMV-specific CD4+ and CD8+ memory in the bronchoalveolar space, in contrast to CD8+ predominance in the blood. Moreover, we show preferential distribution and functional quality of CMV-specific CD4+ memory responses in the lung allograft during chronic infection, and that these cells contribute to mucosal viral control. Together, these findings reveal dynamic changes in CMV-specific immunity from acute primary into chronic infection, and suggest an important role for CD4+ T cells in viral mucosal immunity.
Materials and Methods
Subjects and tissue samples
LTRs in the Johns Hopkins Lung Transplantation clinic were identified for CMV mismatch status (D+R−) and candidates consented for study participation using an Institutional Review Board-approved consent form. All patients were treated with standard maintenance immunosuppression (see Table 1). All patients undergo routine surveillance bronchoscopy at 1, 3, 6, 9, 12, 18 and 24 months posttransplant, along with any clinically indicated episodes. All patients were treated with routine antiviral prophylaxis therapy for CMV (ganciclovir and/or valganciclovir) for the initial 3 months posttransplant. Upon cessation of antiviral prophylaxis, patients were prospectively monitored weekly for the development of acute primary CMV infection defined as de novo detection of viral replication. The Johns Hopkins Hospital Clinical Virology Laboratory used quantitative PCR of plasma to determine CMV viral loads. Acute primary CMV infection was treated with antiviral therapy until two consecutive weekly quantitative CMV PCR resulted with undetectable viremia. Following that point, patients were considered to have chronic CMV infection. Additionally, none of the surveillance bronchoscopies yielding samples were associated with current or recent acute cellular rejection, nor had any subjects undergone recent immunoaugmentation prior to bronchoscopy.
Table 1.
Characteristics of LTRs following primary CMV infection
| Acute infection samples |
Chronic infection samples |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LTR | Age (years) |
Gender | Primary diagnosisa |
Acute 1° infection samples available (viremia +/−)b |
eAg/CMV stainingc |
Immunosuppressiond | Samples available (# mo. postprimary nfection)b |
CMV igG |
eAg/CMV stainingc |
Viremia |
| 22 | 60 | F | IPF | Y(+) | (+/+) | TAC 2.52, MMF 0.254, Pred 7.51 | Y(6) | (+) | (+/−) | (−) |
| 23 | 59 | M | IPF | Y(+) | (+/+) | N/A | N1 | NA | NA | NA |
| 25 | 34 | F | PHTN | Y(+) | (+/+) | TAC 82, Pred 51 | Y(6) | (+) | (+/−) | (−) |
| 31 | 55 | M | Cystic fibrosis | Y(+) | (−/−) | TAC 32, Pred 51 | Y(4) | (+) | (+/−) | (−) |
| 32 | 60 | F | IPF | Y(+) | (+/+) | N/A | N1 | NA | NA | NA |
| 33 | 51 | F | IPF | Y(+) | (−/−) | TAC 4.52, MMF 0.253, Pred 51 | Y(6) | (−) | (−/−) | (−) |
| 34 | 59 | F | COPD | Y(+) | (+/−) | TAC 2.52, Pred 7.51 | Y(3) | (−) | (−/−) | (−) |
| 35 | 27 | M | Cystic fibrosis | Y(+) | (+/−) | NA | N2 | NA | NA | NA |
| 37 | 56 | F | OB | Y(+) | (+/+) | TAC 2.52, MMF 0.252, Pred 51 | Y(6) | (+) | (−/−) | (−) |
| 40 | 54 | F | COPD | Y(+) | (+/−) | TAC 32, MMF 0.52, Pred 51 | Y(6) | (+) | (−/−) | (−) |
| 41 | 64 | F | Bronchiectasis | N2 | NA | TAC 32, MMF 0.253, Pred 51 | Y(4) | (+) | (−/−) | (−) |
| 42 | 37 | F | Sarcoidosis | Y(+) | (+/+) | TAC 32, MMF 0.252, Pred 7.51 | Y(4) | (+) | (+/−) | (−) |
| 43 | 51 | M | Sarcoidosis | Y(+) | (−/−) | TAC 7.51, MMF 0.53, Pred 17.51 | Y(5) | (+) | (+/−) | (−) |
| 45 | 21 | M | Cystic Fibrosis | Y(+) | (+/−) | TAC 32, MMF 0.52, Pred 12.51 | Y(6) | (+) | (+/−) | (−) |
| 46 | 59 | M | COPD | N3 | NA | TAC 0.52, Pred 101 | Y(7) | (+) | (−/−) | (−) |
| 50 | 64 | M | IPF | N3 | NA | TAC 0.52, MMF0.52, Pred 51 | Y(5) | (−) | (+/−) | (−) |
| 51 | 41 | F | PHTN | N2 | NA | TAC 12, Pred 51 | Y(5) | (+) | (+/−) | (−) |
| 53 | 35 | F | Cystic fibrosis | Y(+) | (−/+) | TAC 1.52, Pred 51 | Y(4) | (+) | (−/−) | (−) |
IPF = idiopathic pulmonary fibrosis; PHTN = pulmonary hypertension; OB = obliterative bronchiolitis
Y = Yes, N = No, 1 = Died, 2 = insufficient sample, 3 = not available
eAg = BAL CMV early antigen status; CMV staining = performed on surgical pathology slides
TAC = tacrolimus (dose in milligrams with superscript times per day); MMF = mycophenolate mofetil (dose in grams); Pred = prednisone (dose in milligrams)
Cell Preparation and antigen stimulation
PBMC preparations were isolated from heparinized blood samples by density gradient centrifugation using Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden). LMNC preparations were obtained via bronchoalveolar lavage and subsequent centrifugation. Pooled overlapping 15 amino acid peptides for pp65 and IE-1 were purchased from JPT (Berlin, Germany). A total of 106 cells per condition were cultured in round bottom tissue culture tubes (Sarstedt, Nümbrecht, Germany) in the presence or absence of pooled peptides or whole CMV lysate or SEB (Toxin Technology, Sarasota, FL, USA) at 1 µg/mL.
Flow cytometry
All stimulations for intracellular cytokine staining (ICS) were performed as previously described (9). Cells were subsequently surface stained with fluorochrome-labeled antibodies anti-CD3-AlexaFluor700, anti-CD4-allophycocyanin-Cy7, anti-CD8-AmCyan and anti-CD45RA-Pacific Blue. CCR7 staining was performed in a two-step method as previously described (13). Live/Dead Fixable Blue Dead Cell Stain was used for gating on viable cells. ICS was performed using anti-IFN-γ-allophycocyanin, anti-TNF-α-PE-cyanine 7, anti-MIP-1B–phycoerythrin and FITC-conjugated anti-IL-2. All antibodies were purchased from BD Biosciences and samples analyzed using a FACSAria cytometer (BD) and FlowJo software (TreeStar).
Statistical analysis
Distributions of all measured variables were not assumed to be Gaussian and comparisons were performed using nonparametric testing. Mean cytokine production and median fluorescence index were compared using ANOVA with post hoc t-test, Mann–Whitney test and Wilcoxon signed-rank analysis. Analysis and presentation of distributions were performed using SPICE version 5.1, downloaded from http://exon.niaid.nih.gov/spice (14). A p-value of less than 0.05 was used to determine statistical significance.
Results
In a prospective cohort of 18 patients, we identified 15 D+R− LTRs during chronic CMV infection previously diagnosed with clinical acute primary CMV infection based on the detection of posttransplant de novo viremia by quantitative CMV PCR, as shown in Table 1. During acute primary infection all study patients had detectable viremia that resolved with antiviral therapy. Evaluation of CMV-specific memory responses during chronic infection were conducted at a mean time of 5.1 months postprimary infection, with all study patients having undetectable plasma CMV viral load by quantitative PCR at the time points assessed. However in the BAL fluid, 8/15 LTRs were positive for CMV eAg with 0/15 staining for CMV on surgical pathology during chronic infection as shown in Table 1. Heterogeneity in posttransplant CMV seroconversion consistent with our previous study (9) was seen, with posttransplant CMV IgG detectable in 12 out of 15 individuals (80%) tested. Ten LTRs were maintained on a three-drug calcineurin inhibitor-based immunosuppressive regimen, while six were on two-drug therapy. Samples during primary and chronic infection could not be obtained in four and three patients respectively. All study patients were diagnosed with primary CMV infection within the first year posttransplant following discontinuation of antiviral prophylaxis, typically at 3 months posttransplant in accordance with established guidelines for antimicrobial prophylaxis in SOTRs (15). Of note, 4/15 patients had >1 episode of relapsing viremia with only two of these patients being maintained on long-term antiviral therapy. Of the four patients with relapsing viremia, two were eAg+ and two were eAg− during chronic time periods tested. Thirteen of the eighteen total patients studied were female with a mean age for all patients of 49 years.
Preferential localization of CMV-specific CD4+ memory in the BAL over the blood during chronic infection differs from CD8+ memory distribution
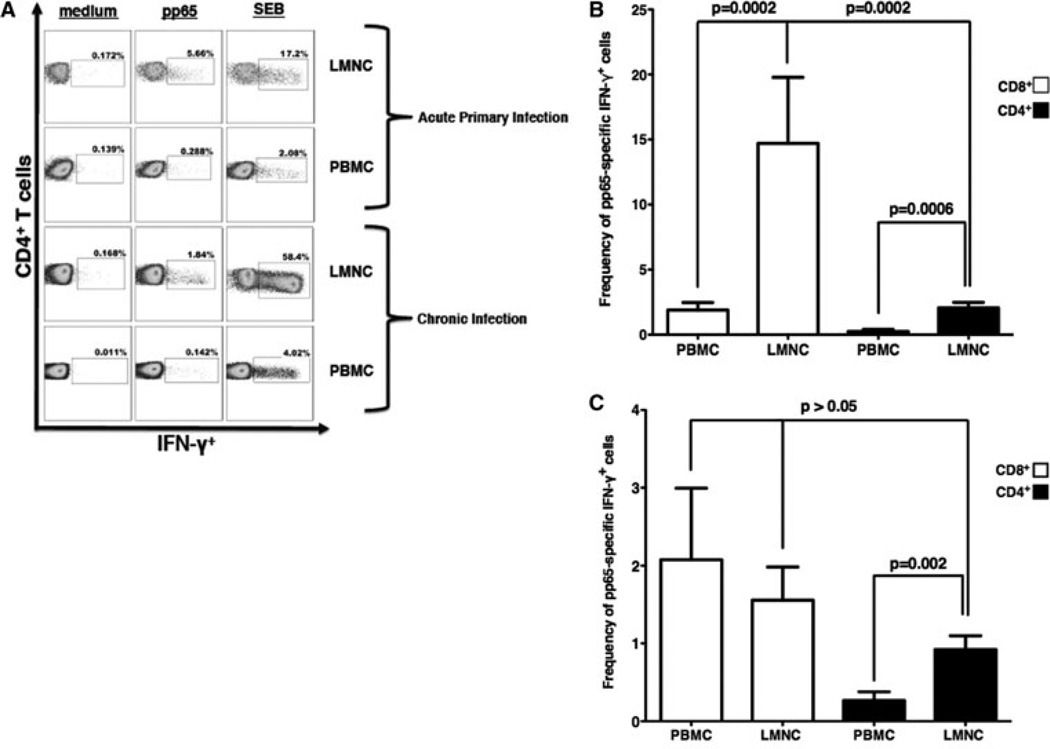
We previously showed a predominance of CMV-specific CD8+ > CD4+ effector T cell responses in the lung allograft over peripheral blood during acute primary infection (10). To determine whether this pattern of distribution persisted during chronic CMV infection in D+R− LTRs, we compared PBMC and LMNC responses during acute primary CMV infection versus chronic infection, and performed in vitro restimulation of PBMC and LMNC obtained from BAL fluid at diagnostic bronchoscopy using pooled peptides of the major CMV antigen pp65, whole CMV lysate or Staphylococcus Enterotoxin B (SEB; positive control) and determined T cell IFN-γ production using ICS. A representative LTR, with CMV-pp65-specific CD4+IFN-γ+ (Figure 1A) effector responses during acute primary infection and memory responses during chronic infection, shows higher responses in LMNC at both time points. Next we evaluated the relative contribution of CD4+ and CD8+IFN-γ+ responses during acute and chronic infection. To do this, we multiplied each subject’s measured pp65-specific IFN-γ+ effector response by the calculated compartmental CD4:CD8 ratio (or the inverse for CD8+ cells) to obtain the adjusted pp65-specific CD4+ and CD8+ effector frequencies. Cumulative pp65-specific IFN-γ+ effector frequency responses were then calculated to determine the adjusted CMV-specific CD4+ and CD8+IFN-γ+ T cell frequencies during both acute primary (Figure 1B; n = 14) and chronic infection (Figure 1C; n = 15). In contrast to acute primary infection where the LMNC CD4: CD8 ratio was comparatively reduced (CD4:CD8 ratio: PBMC–1:1.03, LMNC–1:2.4), during chronic infection the compartmental CD4:CD8 ratios were similar (PBMC–1.3:1 and LMNC–1:1.2). As we previously reported, both CD4+ and CD8+ CMV-pp65-specific effector responses were increased in the lung allograft over blood during primary infection with CD8+IFN-γ+ responses predominating over CD4+IFN-γ+ responses. However, during chronic infection, CMV-specific CD8+IFN-γ+ memory responses contracted sharply in the lung allograft but remained similar in the blood, resulting in compartmental equalization. Similarly, CD4+IFN-γ+ memory responses, contracted in the lung allograft though not significantly in the blood, however, remained preferentially distributed in the bronchoalveolar space over peripheral blood during chronic infection. Additionally, while CMV-specific CD8+IFN-γ+ memory responses remained dominant over the CD4+IFN-γ+ responses within the PBMC compartment (p = 0.002) during chronic infection, LMNC pp65-specific CD4+ and CD8+ memory responses were unexpectedly similar. We also compared pp65-specific CD4+ memory responses toward CMV whole viral lysate and found CMV lysate CD4+IFN-γ+ memory responses had similar frequencies compared to pp65-specific responses (p = NS), with both showing significantly increased memory responses in the lung over the blood (p = 0.016; data not shown). As anticipated, CD8+ CMV lysate-induced responses were detectable but low (≤0.30%), with no significant intercompartmental differences found (data not shown). Thus, while cross-priming of exogenous CMV antigens can occur as previously reported (16), this does not occur at a higher degree in the lung. As an additional control for antigen, we measured CD8+ T cell memory responses in a subset of LTRs (n = 5) to the CMV antigen IE1, using pooled peptides, and found that IE1-specific CD8+IFN-γ+ memory responses were similarly distributed in the LMNC and PBMC compartments (mean frequencies of 1.22 ± 0.15 vs. 1.62 ± 0.62 respectively, p = 1.00; data not shown), consistent with our pp65-specific CD8+ responses. Interestingly, both CD4+ and CD8+ SEB IFN-γ responses were increased in the LMNC over PBMC consistent with a higher number of SEB-reactive memory cells in the lung airways over the blood (p < 0.01; data not shown). Together, these data reveal dynamic changes in the compartmental CMV memory subsets from acute primary into chronic infection, with CMV-specific CD4+ memory responses demonstrating a persistent preferential distribution in the bronchoalveolar space over the blood during chronic infection, unlike CD8+ memory responses.
Figure 1. Dynamic changes in CMV-specific CD8+IFN-γ+ and CD4+IFN-γ+ compartmental responses during acute primary infection into chronic infection with preferential distribution of CMV-specific CD4+IFN-γ+ T cell memory remaining in the lung allograft.
Representative flow cytometric plots of (A) CD4+ LMNC and PBMC from a LTR during acute primary and chronic CMV infection time points. LMNC and PBMC cells from D+R− patients were cultured in the presence or absence of pp65-pooled peptides, whole CMV lysate or SEB followed by ICS as detailed in the Materials and Methods section. Boxed numbers indicate frequencies of populations, with gating on CD3+CD4+ T cells. Cumulative frequencies of CD3+CD8+IFN-γ+ and CD3+CD4+IFN-γ+ pp65-specific responses (adjusted for compartmental CD4:CD8 ratio) in LMNC versus PBMC during acute (B) and chronic infection (C). Bars represent mean ± SEM frequency of IFN-γ+ LMNC or PBMC from 14 (B) and 15 (C) LTRs, respectively. P-values calculated by the Wilcoxon signed-rank test
Greater frequencies of multifunctional CMV-specific CD4+, but not CD8+, T cells are present in the bronchoalveolar space over PBMC during chronic CMV infection
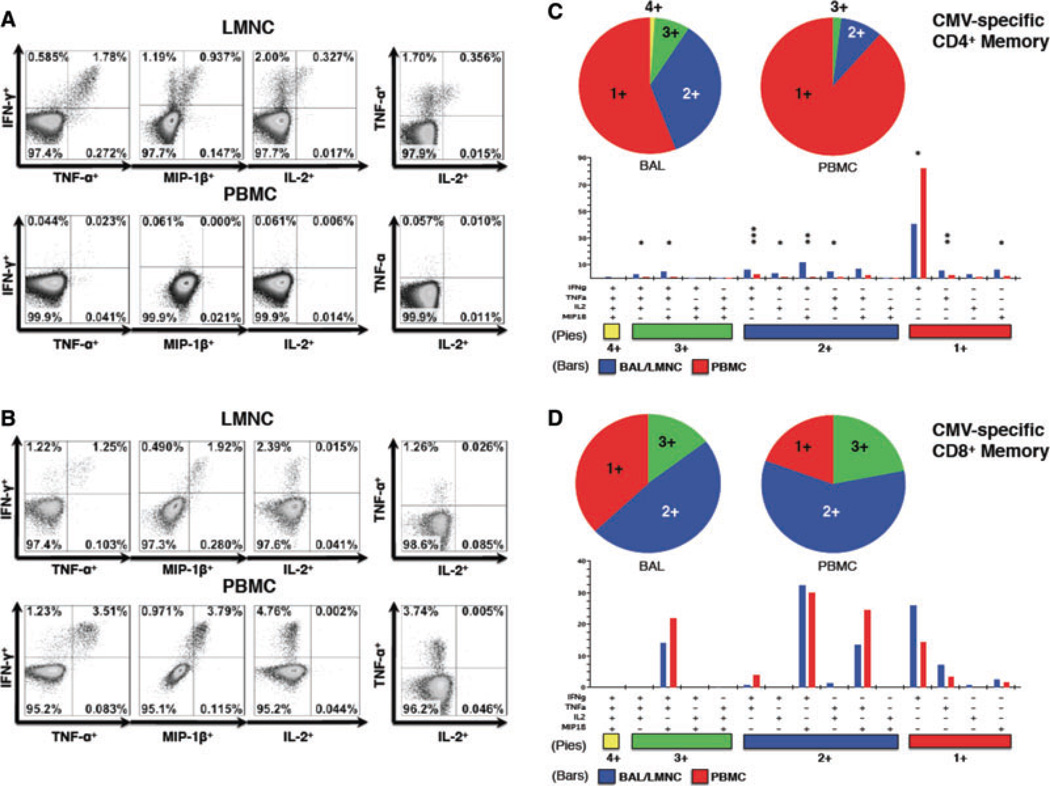
Because durable viral control likely requires the development and maintenance of CMV-specific memory T cells capable of producing other factors beyond IFN-γ, we evaluated the capacity for CD4+ and CD8+ T cells to produce an expanded panel of cytokines and chemokines that included IFN-γ, TNF-α, IL-2 and the CCR5-binding chemokine MIP-1β in 8 D+R− LTRs during chronic infection. As shown in a representative LTR, T cell effector memory responses for the entire panel were detected in CMV-specific CD4+ (Figure 2A) and CD8+ (Figure 2B) T cells during chronic CMV infection, with IL-2 being the cytokine least frequently detected. Because the functional quality of antigen-specific memory responses has been shown to be an important determinant of protection in various human and mouse models of infection (11,17,18), we next analyzed whether compartmental differences existed in the CMV-specific multifunctional cytokine+/chemokine+ populations producing IFN-γ, TNF-α, MIP-1β and IL-2 from CD4+ and CD8+ T cells during chronic CMV infection using Boolean analysis. As shown in Figure 2B/C, both pp65-specific CD4+ and CD8+ T cells were found to include single (1+), double (2+) and triple (3+) cytokine/chemokine producing populations in both tissue compartments, with CMV-specific CD4+ T cells having a modestly wider array (8 vs. 5) compared to CD8+ T cells (Figure 2C/D). Only CMV-specific CD4+ T cells in the lung allograft were found to have a quadruple (4+) cytokine/chemokine producing capacity attributable to the production of IL-2 among multiple CD4+ T cell subsets, whereas detection of CD8+IL-2+ T cells were rare events (<0.05% observed frequency). Overall, when comparing the frequencies of multifunctional T cell memory responses between the LMNC and PBMC compartments, CMV-pp65-specific CD4+ memory T cells showed significant preferential distribution to the lung in six different multifunctional combinations as shown in Figure 2C. In contrast, CMV-specific CD8+ T cells were found to have a similar distribution of T cell effector memory, when comparing 1+, 2+ or 3+ multifunctional cytokine/chemokine production between compartments, as shown in Figure 2D. Together, these data demonstrate increased multifunctional capacities of CMV-specific CD4+, but not CD8+, memory T cells in the bronchoalveolar space compared to the blood during chronic infection.
Figure 2. Diverse multifunctional CMV-specific CD4+T cell effector memory populations are significantly increased in the lung during chronic infection but not among CD8+T cells.
Representative multifunctional flow cytometric plots of (A) CD4+ and (B) CD8+ T cell LMNC and PBMC following restimulation with CMV antigen in two LTRs showing the frequency of IFN-γ by TNF-α, MIP-1 β and IL-2 as well as TNF-α by IL-2 during chronic infection. Individual pie charts show the percentage of pp65-specific CD4+ (C) and CD8+ (D) T cells that produce 1, 2, 3 or 4 functional responses. CMV-specific CD4+ multifunctional responses to pp65-pooled peptides were significantly more frequent in the lung allograft than peripheral blood (p < 0.05) (C), while CD8+ multifunctional responses were equally distributed between the two compartments (D). Using Boolean analysis, the percentage of total for individual multifunctional subset responses for LMNC (blue bars) and PBMC (red bars) are shown in the bar graph for each of the 15 multifunctional subsets. Significant differences when comparing frequencies (mean ± SEM, not shown) of LMNC and PBMC pp65-specific single and multifunctional responses are indicated by a *p < 0.05, **p < 0.01 and ***p < 0.001. Figures represent results from 8 LTRs. All p-values were determined by the Kruskal–Wallis one-way ANOVA or Wilcoxon signed-rank test.
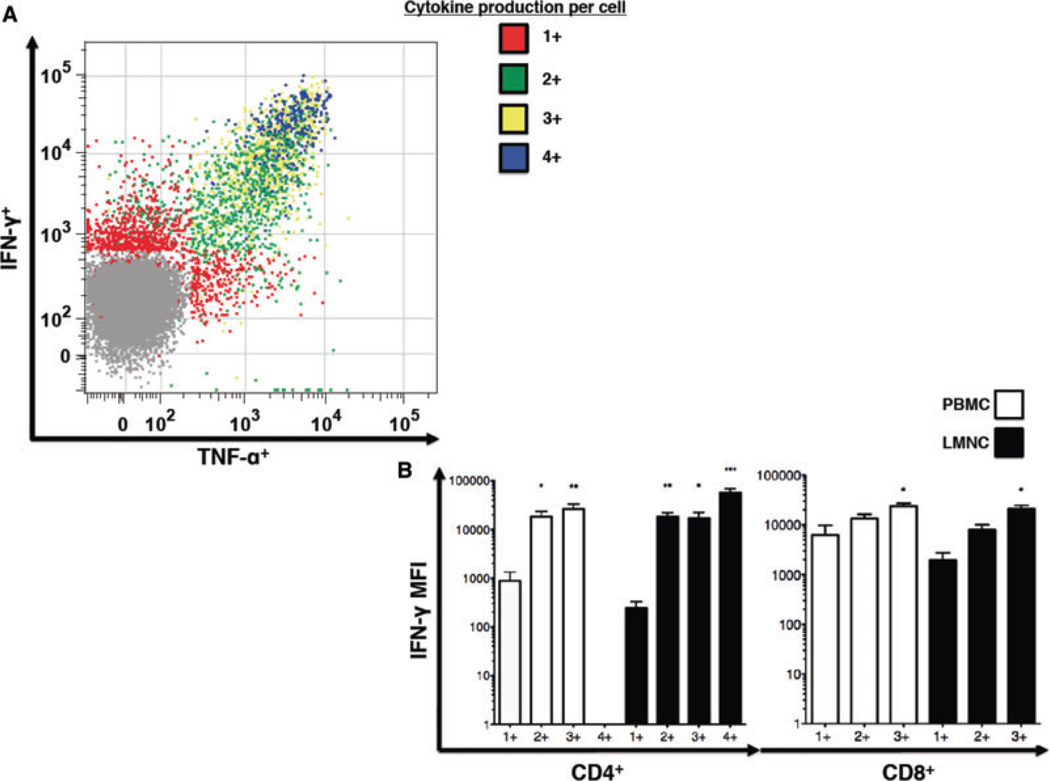
Multifunctional CMV-specific CD4+ effector memory T cells produce more cytokine on a per cell basis compared to single cytokine producers
Recent studies in the mouse and human have shown increased IFN-γ production in multifunctional T cells, indicating higher functional quality (11). We next examined whether multifunctional CMV-specific T cells were capable of increased IFN-γ production at the single-cell level by comparing the median fluorescence intensity (MFI) of intracellular staining for IFN-γ in cells producing this cytokine alone (1+) versus multifunctional cells producing IFN-γ along with other cytokines/chemokines measured (2+, 3+, 4+) in the bronchoalveolar space and peripheral blood. As shown in a representative plot in Figure 3A, multifunctional CMV-specific CD4+ T cells exhibit higher MFI for IFN-γ (and TNF-α) compared to single cytokine producing cells. Cumulative analysis of grouped CMV-specific multifunctional CD4+ and CD8+ T cell subsets demonstrated that CD4+ T cell IFN-γ MFIs were significantly higher in all of the multifunctional T cell groupings found within both the lung and peripheral blood compartments when compared to 1+ grouped T cells. Similar, though less striking differences were observed in CD8+ T cells, with only CD8+ 3+ producers demonstrating significantly higher IFN-γ MFIs compared to 1+ single producers (Figure 3B). Similarly, when evaluating CD4+ and CD8+ TNF-α MFIs, multifunctional CD4+ T cell TNF-α MFIs were found to be significantly higher than single cytokine producing cells (p < 0.05, data not shown). Together, these data show CMV-specific CD4+ and CD8+ multifunctional memory T cells produce more IFN-γ on a per cell basis compared to single cytokine producers and further support higher functional CMV CD4+ memory quality at the lung mucosal site compared to the blood.
Figure 3. Multifunctional CMV-specific CD4+ T cells are more potent effectors on a per-cell basis.
(A) Representative flow cytometric plot of LMNC from LTR # during chronic infection presenting the distribution of T cells producing one (red), two (green), three (yellow) and four (blue) simultaneously measured cytokines/chemokines—IFN-γ, TNF-α, MIP-1β and IL-2. (B) Comparison of mean fluorescence intensity (MFI) of IFN-γ staining in CMV-specific CD4+ and CD8+ single and multifunctional T cell populations (1+, 2+, 3+ and 4+) for each tissue compartment. Bars represent mean ± SEM of MFI for CD4+ and CD8+ LMNC and PBMC IFN-γ production in 8 LTRs *p < 0.05, **p < 0.01 and ***p < 0.001, represents a comparison between single cytokine production and specific multifunctiona grouping. Differences between the groups and p-values were determined by the Kruskal–Wallis one-way ANOVA followed by post hoc Dunns statistic testing.
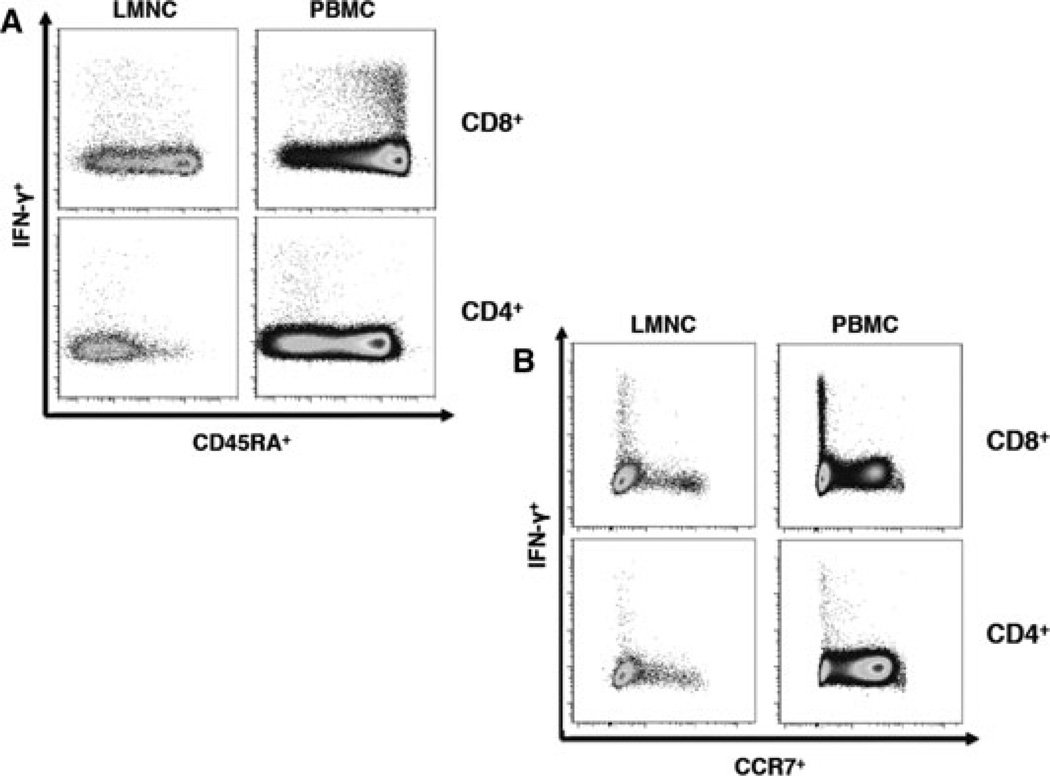
CMV-specific CD4+ effector memory T cells have similar differentiation phenotype in lung and blood in contrast to CD8+ memory
We next investigated the surface phenotype of CMV-specific T cells from the LMNC and PBMC compartments in regard to surface expression of the common leukocyte antigen CD45RA isotype and the chemokine receptor, CCR7. As shown in a representative LTR in Figure 4A, CMV-specific CD4+ T effector memory cells producing IFN-γ in both the lung and blood are CD45RA−. In contrast, the majority of peripheral blood CMV-specific CD8+ T cells are CD45RA+ while lung CD8+IFN-γ+ T cells are predominantly CD45RA− as we previously reported for CD8+ T cells during chronic infection following resolution of viremia (10). Interestingly, we noted approximately 10–60% of total lung CD8+IFN−γ− T cells to be CD45RA+ indicating a variable heterogeneous population within the lung not seen in CD4+ T cells. We also evaluated surface CCR7 expression and found that CMV-specific CD4+ and CD8+ T cells in the lung and blood compartments were CCR7–, consistent with an effector memory phenotype (Figure 4B)(19). Thus, by at least these two major differentiation markers, CMV CD4+ memory T cells have a similar phenotype in the lung airways and systemic circulation, whereas CMV memory CD8+ T cells differ with respect to CD45RA expression between these compartments during chronic infection.
Figure 4. Lung airway and peripheral blood CMV-specific CD4+ effector T cells show similar memory phenotype in contrast CD8+ memory during chronic infection.
Representative flow cytometric plots showing phenotypic analysis from LTR #31. during chronic infection in the absence of active infection showing CMV-specific CD8+ and CD4+ effector T cells in LMNC and PBMC gated (A) CD45RA by IFN-γ and (B) CCR7 by IFN-γ . Plots are representative of six LTRs analyzed during chronic infection following in vitro restimulation with pooled pp65 peptides.
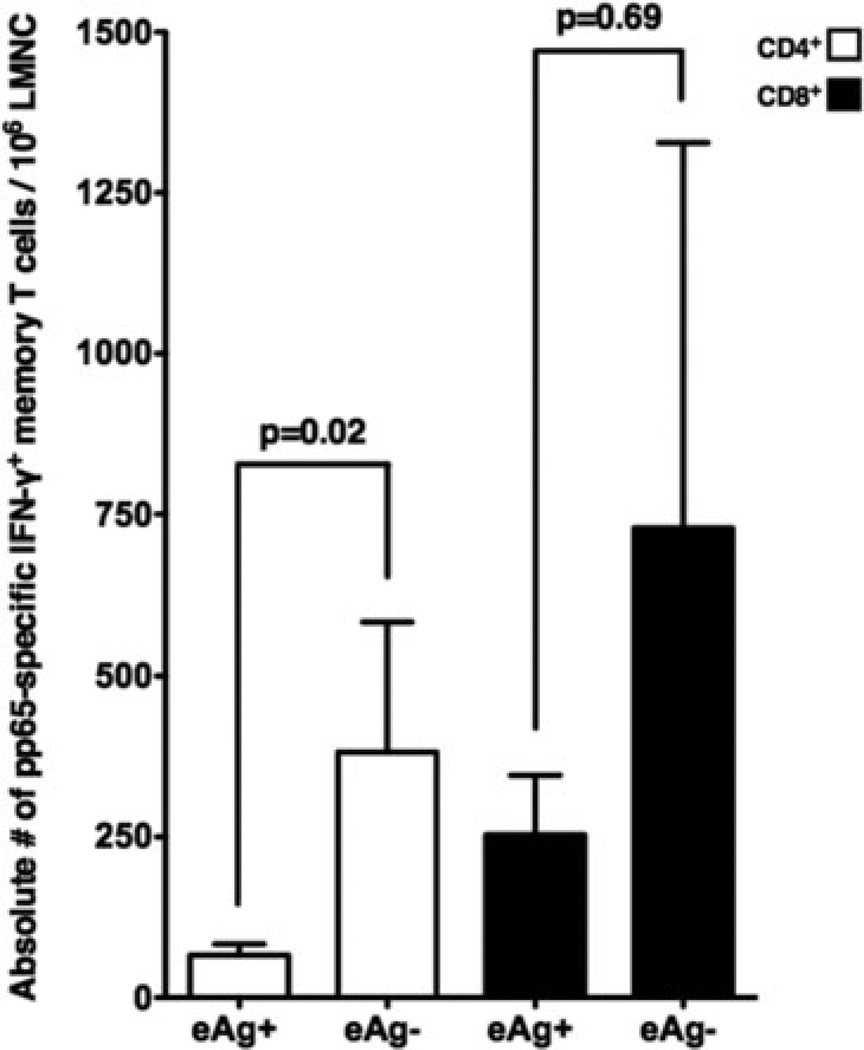
Absolute number of CMV-specific CD4+IFN-γ+ memory cells is increased in LTRs exhibiting mucosal viral control in the lung allograft
Next, we evaluated whether there were differences in CMV-specific memory in LTRs with CMV eAg (+) in BAL versus those with eAg (−). Comparison of the frequencies of pp65-specific IFN-γ+ responses for CD4+ and CD8+ T cells revealed no differences between these two groups (mean frequencies ± SEM, eAg+ vs. eAg-; 0.86 ± 0.14 vs. 1.00 ± 0.35, p = 0.78 and 1.54 ± 0.42 vs. 1.02 ± 0.61, p = 0.34, respectively, data not shown). However, comparison of the absolute number of CD4+ pp65-specific IFN-γ+ memory cells/106 BAL cells revealed significantly increased memory cell numbers in LTRs who were eAg (−) versus those eAg (+), as shown in Figure 5. In contrast, while the absolute numbers of CD8+ pp65-specific IFN-γ+ memory cells/106 BAL cells were also higher in eAg (−) LTRs, this difference was not statistically significant. Together, these data show that CMV-specific CD4+ memory T cells are importantly associated with mucosal viral control in the lung allograft during chronic infection.
Figure 5. The absolute numbers of CMV pp65-specific CD4+IFN-γ+ memory T cells are increased in LTRs exhibiting mucosal viral control in the lung allograft during chronic infection.
Mean absolute CMV-specific effector memory T cells for pp65-specific CD4+ and CD8+IFN-γ+ memory T cells/106 LMNC according to CMV eAg status during chronic infection. Bars represent the mean ± SEM absolute number of effector memory T cells from 15 LTRs. P-values calculated by the Mann–Whitney t-test.
Discussion
Herein we report for the first time that dynamic changes occur in CMV-specific lung memory populations during the transition from acute primary infection to chronic infection, with enriched CD4+ T cell memory in the bronchoalveolar space over the blood in contrast to CD8+ memory. Unexpectedly, we detected similar frequencies of CMV pp65-specific CD4+ and CD8+ memory cells in the bronchoalveolar space, with CD4+ memory significantly contributing to mucosal viral control in the lung allograft during chronic infection. The importance of CD4+ responses for viral control has been previously demonstrated in another mucosal site, the salivary glands and studies showing robust viralspecific pulmonary CD4+ T cell responses during murine CMV infection (20–22). It is plausible that increased distribution of CMV-specific CD4+ memory in the lung is due to this tissue site being a major reservoir for virus during chronic infection (23). These observations extend our previous findings of increased CMV-specific CD4+ memory responses in BAL over blood in four patients, and are consistent with enriched CD4+ memory in the lung and other mucosal sites in the mouse (9,24).
In contrast to CD4+ memory, CMV-specific CD8+ memory cells were found to have a similar distribution between compartments. These findings differ somewhat from previous findings in the mouse showing preferential localization of CD8+ effector memory T cells at nonlymphoid sites including the lung (25). However, our findings are consistent with a recent study showing HIV-specific CD8+ T cells were similarly distributed between the bronchoalveolar space and blood in contrast to HIV-specific CD4+ T cells that were enriched in the BAL during chronic HIV infection (26). In another report, equal distribution of CMV-specific and EBV-specific CD8+ T cells between human lung tissue and blood was found in patients undergoing surgical lung resection (27). Interestingly, this distribution was in contrast to influenza (FLU)-specific and respiratory syncytial virus (RSV)-specific CD8+ T cells that were detected in higher frequencies in the lung compared to blood. Together, these data suggest CD8+ memory to respiratory viruses or a broad array of other antigens (i.e. polyclonal SEB-reactive cells) are continually recruited and populate the airways in an activated state, poised for rechallenge, even in the absence of antigen (28–31). However, in chronic viral infections such as CMV, EBV and HIV, circulating CD8+ memory T cells are equally distributed between the systemic and mucosal compartments for immune surveillance. Thus, heterogeneity exists in human CD8+ T cell memory distribution according to antigen specificity, as previously reported for functional responses (32). It is unclear why CMV-specific CD4+ memory is increased in the bronchoalveolar space. This persistence in the lung mucosa may be due to higher levels of viral antigen, as T cell receptor signaling and MHC class II engagement appear critical for maintenance of CD4+ memory (33–35). Alternatively, other factors may provide an advantage for CD4+ persistence in the lung mucosa such as differential access to key cytokines such as IL-7 and/or IL-15 important for CD4+ memory maintenance (36,37). Additionally, despite low frequencies of CMV-specific CD4+IFN-γ+ T cells in the PBMC of most LTRs studied, we and others have shown these cells are capable of proliferative expansion upon antigen exposure in longer-term cultures (9,38) which may be important to replenish the lung CD4+ memory pool, as airway T cells are reported to possess limited capacity for proliferation (39). Thus, while it remains unclear what factor(s) contribute to preferential localization of CMV-specific CD4+ memory in the lung, an important role for these responses in host defense is suggested.
Several recent studies have demonstrated an important role for multifunctional CD4+ memory responses and host protection against Leishmania major, M. tuberculosis, HIV, EBV and VZV (11,17,40,41). Recently, virus-specific CD4+ blood T cells were shown to exhibit a unique multifunctional profile during chronic CMV infection compared to that observed in other viral infections (42). Our findings are consistent with these reports though we demonstrate increased multifunctional T cell quality in the CMV-specific CD4+ memory pool in the bronchoalveolar space in contrast to CD8+ T cells. We also find an increased breadth of functionality in CMV-specific CD4+ memory cells compared to CD8+ memory largely due to IL-2 production. This increased heterogeneity in CMV-specific CD4+ memory might be due to differential capacities in CD4+ effectors to give rise to long-term memory cells, as demonstrated in previous studies (43,44). Recently, unequal partitioning of critical proteins, such as the transcription factor T-bet during early T lymphocyte division, was shown to influence cell fate (45,46). Additionally, our observation of increased IFN-γ production on a per cell basis, particularly in CD4+ bronchoalveolar T cells, shows functional heterogeneity in the memory pool that may be determined by early priming events.
We also found differences in CD45RA surface expression between CMV-specific CD4+ and CD8+ memory cells. We and others previously reported, upon resolution of viremia, blood CMV-specific memory CD8+ T cells transition to predominantly CD45RA+ unlike blood CMV-specific CD4+ memory in this study (10,13), and consistent with other studies showing blood CMV CD8+ memory is CD45RA+ (10,13,47,48), However, both CMV-specific CD4+ and CD8+ T cells are predominantly CD45RA− in the lung and CCR7− in both compartments consistent with an effector memory phenotype (19).
We should point out several limitations to our study. While our studies predominantly focused on responses to pp65 (49), we recognize the total CMV memory response is considerably larger as additional antigens contribute to a broad CD4+ and CD8+ T cell response to CMV (50). Additionally, though we did not measure cytotoxicity in this current study, it is likely very important in CMV immune control, as recently shown in HIV long-term nonprogressors (51). Lastly, while we found significant differences for CD4+ memory cells in viral control, our study population was small and perhaps larger numbers of subjects, or antigens tested (52), might reveal an important role for CD8+ memory in mucosal viral control. In this regard, we have recently shown that primary CMV-pp65-specific CD8+IFN-γ+ effector responses and inducible CD8+T-bet+ levels predict relapsing viremia in D+R− LTRs during early chronic infection (53). However, our current findings might suggest both CD4+ and CD8+ memory are necessary for optimal control in the lung mucosa. Of course one caveat is that our study subjects were maintained on immunosuppression, which might differentially impact these T cell memory pools. Nonetheless, these data collectively show persistent, multifunctional CMV-specific memory in these high-risk LTRs despite immunosuppressive therapy.
Overall, we show dynamic changes in T cell effectors to memory from acute primary infection into chronic infection with enrichment and high quality CMV-specific CD4+ memory in the bronchoalveolar space, along with an important role for these cells in mucosal antiviral immunity. Our better understanding of compartmental multifunctional CMV-specific T cell memory responses raises the possibility of using both immune and viral monitoring to allow riskstratification of high-risk LTRs that may assist clinicians in individualizing antiviral therapy and improve outcomes in high-risk D+R− patients.
Acknowledgments
This work was supported by National Institutes of Health grants R01-AI079175 (J.F.M.)
We wish to thank the entire Johns Hopkins Lung Transplant clinical team, in particular the nurse coordinators, for their assistance with the patients in this study.
Abbreviations
- BAL
bronchoalveolar lavage
- D+R−
donor+/recipient−
- ICS
intracellular cytokine staining
- LTR
lung transplant recipient
- LMNC
lung mononuclear cell
- SOTR
solid organ transplant recipients.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ljungman P. Beta-herpesvirus challenges in the transplant recipient. J Infect Dis. 2002;186(Suppl 1):S99–S109. doi: 10.1086/342962. [DOI] [PubMed] [Google Scholar]
- 2.Pereyra F, Rubin RH. Prevention and treatment of cytomegalovirus infection in solid organ transplant recipients. Curr Opin Infect Dis. 2004;17:357–361. doi: 10.1097/01.qco.0000136933.67920.dd. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 4.Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant. 2004;4:1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-seventh official adult lung and heart-lung transplant report—2010. J Heart Lung Transplant. 2010;29:1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 7.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010;181:1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerschner H, Jaksch P, Karigl G, Popow-Kraupp T, Klepetko W, Puchhammer-Stockl E. Cytomegalovirus DNA load patterns developing after lung transplantation are significantly correlated with long-term patient survival. Transplantation. 2009;87:1720–1726. doi: 10.1097/TP.0b013e3181a60b4e. [DOI] [PubMed] [Google Scholar]
- 9.Shlobin OA, West EE, Lechtzin N, et al. Persistent cytomegalovirus-specific memory responses in the lung allograft and blood following primary infection in lung transplant recipients. J Immunol. 2006;176:2625–2634. doi: 10.4049/jimmunol.176.4.2625. [DOI] [PubMed] [Google Scholar]
- 10.Pipeling MR, West EE, Osborne CM, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 12.Sund F, Lidehall AK, Claesson K, et al. CMV-specific T-cell immunity, viral load, and clinical outcome in seropositive renal transplant recipients: a pilot study. Clin Transplant. 2010;24:401–409. doi: 10.1111/j.1399-0012.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 13.Jagannathan P, Osborne CM, Royce C, et al. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J Virol. 2009;83:2728–2742. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Ghanekar SA, Suni MA, He XS, Picker LJ, Maino VC. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J Immunol. 2001;166:7268–7275. doi: 10.4049/jimmunol.166.12.7268. [DOI] [PubMed] [Google Scholar]
- 17.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 18.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton SM, Wyrsch P, Munks MW, et al. The dynamics of mouse cytomegalovirus-specific CD4 T cell responses during acute and latent infection. J Immunol. 2008;181:1128–1134. doi: 10.4049/jimmunol.181.2.1128. [DOI] [PubMed] [Google Scholar]
- 22.Arens R, Wang P, Sidney J, et al. Cutting edge: Murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol. 2008;180:6472–6476. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 25.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 26.Brenchley JM, Knox KS, Asher AI, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AD, Woodland DL. Cutting edge: Effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 30.Masopust D, Vezys V, Usherwood EJ, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 31.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg JK, Fast NM, Jordan KA, et al. HIV-specific CD8+ T cell function in children with vertically acquired HIV-1 infection is critically influenced by age and the state of the CD4+ T cell compartment. J Immunol. 2003;170:4403–4410. doi: 10.4049/jimmunol.170.8.4403. [DOI] [PubMed] [Google Scholar]
- 33.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 34.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 35.Lees JR, Farber DL. Generation, persistence and plasticity of CD4 T-cell memories. Immunology. 2010;130:463–470. doi: 10.1111/j.1365-2567.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4 +memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Leeuwen EM, Gamadia LE, Baars PA, Remmerswaal EB, ten Berge IJ, van Lier RA. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol. 2002;169:5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- 39.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195:317–326. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millington KA, Innes JA, Hackforth S, et al. Dynamic relationship between IFN-gamma and IL-2 profile of mycobacterium tuberculosis-specific T cells and antigen load. J Immunol. 2007;178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilton JC, Luskin MR, Johnson AJ, et al. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J Virol. 2007;81:2713–2725. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casazza JP, Brenchley JM, Hill BJ, et al. Autocrine production of beta-chemokines protects CMV-Specific CD4 T cells from HIV infection. PLoS Pathog. 2009;5:e1000646. doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CY, Kirman JR, Rotte MJ, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 44.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 45.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 46.Chang JT, Ciocca ML, Kinjyo I, et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 48.Roos MT, van Lier RA, Hamann D, et al. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein-Barr and human immunodeficiency virus infections in humans. J Infect Dis. 2000;182:451–458. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 49.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185:1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 50.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migueles SA, Weeks KA, Nou E, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83:11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis. 2011;204:1663–1671. doi: 10.1093/infdis/jir624. [DOI] [PMC free article] [PubMed] [Google Scholar]