Abstract
Purpose
Recent reports suggest that nephrolithiasis and atherosclerosis share a number of risk factors. There has been no previous examination of the relationship between kidney stones and subclinical atherosclerotic disease Here we assessed the relationship between nephrolithiasis and carotid wall thickness and carotid stenosis assessed by B-mode ultrasound in the general community using data from The Coronary Artery Risk Development in Young Adults (CARDIA) study.
Methods
CARDIA is a U.S. population-based, observational study of 5,115 white and African-American men and women between the ages of 18 and 30 years at recruitment in 1985-1986.
Results
By the year 20 exam, 200 (3.9%) of CARDIA participants had reported ever having kidney stones. Symptomatic kidney stones were associated with greater carotid wall thickness measured at the year 20 exam, particularly of the internal carotid/bulb region. Using a composite dichotomous endpoint of carotid stenosis and/or upper quartile of internal carotid/bulb wall thickness, the association of kidney stones with carotid atherosclerosis was significant (odds ratio=1.6; 95% confidence interval 1.1-2.3; p=0.01) even after adjusting for major atherosclerotic risk factors.
Conclusions
The association between a history of kidney stones and subclinical carotid atherosclerosis in young adults adds further support to the notion that nephrolithiasis and atherosclerosis share common systemic risk factors and/or pathophysiology.
Keywords: urolithiasis, atherosclerosis
Introduction
Kidney stones are a common cause of morbidity in the United States [1]. Their prevalence in young to middle-aged adults is approximately 5-10% [2]. While the focus of nephrolithiasis research has been on biochemical alterations in local urinary constituents leading to stone formation, abnormalities in urine chemistry alone do not explain many aspects of urinary stone disease. Epidemiologic studies have linked nephrolithiasis to vascular risk factors such as hypertension, obesity, and diabetes mellitus [3-5], suggesting that systemic metabolic conditions, in addition to urine composition, strongly influence the pathophysiology of stone formation [6].
Given the association between nephrolithiasis and risk factors for atherosclerotic disease, our hypothesis is that patients with urinary stone disease should be at greater risk for other vascular complications. While clinical cardiovascular events are rare in a younger population, subclinical carotid disease is not uncommon. The relationship between kidney stones and subclinical vascular disease has not been previously assessed in young, community-dwelling adults. We undertook this study to examine the association between urinary stone disease and subclinical carotid atherosclerosis in order to better understand the possible relationship between these two disorders.
Methods
CARDIA study participants and measurements
The Coronary Artery Risk Development in Young Adults (CARDIA) Study was designed as a longitudinal cohort study of the development and evolution of cardiovascular risk factors in young white and African-American adults [7]. In 1985-86, 5,115 participants aged 18–30 years were recruited from four clinical sites located in Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. CARDIA participants were selected to have approximately the same number of people in subgroups of race, gender, education (high school or less and more than high school) and age (18-24 and 25-30). Participants were re-examined at six follow-up examinations with overall retention rates among those surviving of 91% at year 2, 86% at year 5, 81% at year 7, 79% at year 10, 74% at year 15, and 72% at year 20. Data were collected on a variety of medical conditions, lifestyle and dietary factors, and blood measurements believed to be related to cardiovascular disease. Of 3,549 participants who attended the year 20 examination, 3,258 underwent carotid artery ultrasound examination. The study was approved by an Institutional Review Board at all CARDIA sites.
History of kidney stones was assessed by self-report at each exam cycle. Self-report has been shown to be highly accurate [8]. We created a variable based on whether an individual ever reported at any of the exams in which they participated that a doctor or nurse had ever said that the individual had kidney stones; 189 participants reported kidney stones ever. From a review of hospitalization records obtained during CARDIA follow-up, we identified an additional 11 cases, for a total n=200 participants with kidney stones. Of the 153 participants who had at least 1 subsequent visit after stones were reported, 115 did replicate their report at least once; therefore the reliability rate was 115/153 or 75.2%.
For the current analysis, we used cardiovascular risk factor measurements made at the year 0 (baseline) and year 20 follow-up examination [7]. Smoking status was assessed using a standardized questionnaire. Body mass index (BMI; weight (kg)/height (m)2) was calculated from measurements obtained during the physical examination. Standard methods for measuring blood pressure, fasting total cholesterol, HDL cholesterol, triglycerides, glucose, insulin, uric acid, and creatinine were used [9-12]. LDL cholesterol was estimated by the Friedewald equation. The presence of diabetes was defined as fasting blood glucose ≥126 mg/dL or taking insulin and/or oral hypoglycemic agents. Insulin resistance was calculated using the homeostasis model assessment (HOMA = fasting plasma insulin (μU/l) × fasting plasma glucose (mmol/l)/22.5). Serum creatinine was used to estimate GFR and was calculated using the 4-variable MDRD method [13]. Only a small proportion of participants reported diuretic usage (n=32) at the year 0 and year 20 examinations (n=120).
Carotid intimal-medial wall thickness (IMT) was determined by B-mode ultrasound (GE Logiq 700) at the year 20 exam using standard procedures [14]. The maximum IMT of the common carotid, internal carotid and the bulb were defined as the mean of the maximal intima-media thickness of the near and far wall on both the left and right sides. A combined internal carotid artery (ICA)/bulb variable was created consisting of the arithmetic mean of all ICA and bulb measurements. The IMT included the thickness of any atherosclerotic plaque that might be present at the measurement site; plaque is well known to be more likely to be present in the bulb/ICA than in the common carotid, so the common carotid IMT is closer to a pure measure of wall thickness. Presence of any stenosis (mostly 1-24% of the lumen) anywhere in the left or right carotid artery was separately noted. We defined presence of carotid atherosclerosis as any stenosis and/or bulb/ICA in the upper quartile distribution (>1.02 mm).
Statistical analysis
Clinical and socio-demographic characteristics of the study population were evaluated by chi-square analysis for categorical and ANOVA analysis for continuous variables. Associations between ever reporting kidney stones by the year 20 exam and baseline cardiovascular risk factors were evaluated by logistic regression, controlling for other baseline clinical and socio-demographic characteristics. Risk estimates were reported as odds ratios for ever having reported kidney stones by the year 20 examination per indicated unit (SD for continuous variables or category for discrete variables). Multiple linear regression or logistic regression models were undertaken to evaluate association between ever reported kidney stones and the following dependent variables at year 20, adjusted for other baseline or year 20 participant characteristics and risk factors: 1) mean common carotid IMT 2) mean bulb/ICA IMT; 3) presence of carotid stenosis; 4) presence of carotid atherosclerosis defined by any stenosis and/or bulb/ICA in the upper quartile distribution. We also analyzed quartiles of common or bulb/ICA carotid IMT as a categorical variable using polychotomous ordinal logistic regression. All regression analyses were performed using the statistical package Stata/SE11 (Stata Corp., College Station, TX, USA). A p- value of less than 0.05 was considered statistically significant. We additionally considered the relationship of kidney stones that occurred at year 15 or earlier (prior to carotid ultrasound) to carotid atherosclerosis at year 20.
Results
Descriptive characteristics of CARDIA participants
Overall, the mean age at study entry was 25 years, and 55% were women. Current smoking, BMI, blood pressure, and insulin resistance were higher among African-Americans than whites (all p <0.001) (Table 1). By the year 20 exam, 200 (3.9%) participants had reported ever having kidney stones, and the prevalence was 2.6-fold higher among whites (5.7%) than African-Americans (2.3%); p<0.0001.
Table 1. CARDIA participant characteristics at study entry and at most recent follow-up (year 20 examination), by race.
| Exam Year | ||||
|---|---|---|---|---|
|
| ||||
| Year 0 | Year 20 | |||
|
| ||||
| Characteristic | Whites | African-Americans | Whites | African-Americans |
|
| ||||
| Number | 2,478 | 2,637 | 1,898 | 1,651 |
| Mean age, years [range] | 25.4 [17 – 32] | 24.3 [17 – 35] | 45.6 [37 – 52] | 44.5 [37 – 54] |
| Female sex | 1,307 (53) | 1,480 (56) | 1009 (53) | 1005 (61) |
| Education, years ± SD | 14.6 ± 2.4 | 13.0 ± 1.8 | 15.8 ± 2.6 | 14.0 ± 2.2 |
| Clinic | ||||
| Birmingham, AL | 529 (21) | 649 (24) | 379 (20) | 440 (27) |
| Chicago, IL | 557 (22) | 552 (21) | 430 (23) | 363 (22) |
| Minneapolis, MN | 783 (32) | 619 (23) | 603 (32) | 322 (20) |
| Oakland, CA | 609 (25) | 817 (31) | 486 (26) | 526 (32) |
| Smokers | 554 (26) | 689 (33) | 274 (15) | 399 (25) |
| Former | 443 (18) | 233 (9) | 461 (25) | 218 (13) |
| Current | 662 (27) | 884 (34) | 276 (15) | 405 (25) |
| Alcohol consumption (mL/week) | 13.6±21.0 | 10.7 ± 22.7 | 12.4 ± 20.8 | 9.0 + 23.7 |
| Body mass index (kg/m2) | 23.6 ± 4.1 109 ± 11 | 25.3 ± 5.7 | 27.9 ± 6.5 | 31.3 + 7.6 |
| Systolic blood pressure (mm Hg) | 111 ± 11 | 113 ± 13 | 121 ± 16 | |
| Diastolic blood pressure (mm Hg) | 68 ± 9 | 69 ± 10 | 70 ± 11 | 77 ± 12 |
| Total cholesterol (mg/dL) | 176 ± 32 | 177 ± 35 | 187 ± 34 | 184 ±36 |
| LDL cholesterol (mg/dL) | 108 ± 30 | 110 ± 32 | 110 ± 31 | 110 ± 34 |
| HDL cholesterol (mg/dL) | 52 ± 13 | 54 ± 13 | 54 ± 17 | 54 ± 16 |
| Triglycerides (mg/dL) | 79 ± 57 | 67 ± 38 | 119 ± 88 | 98 ± 68 |
| Glucose (mg/dL) | 83 ± 12 | 82 ± 19 | 96 ± 22 | 100 ± 31 |
| Insulin (mg/dL) | 9.3 ± 6.4 | 12.3 ± 9.0 | 15.0 ± 10.1 | 18.4 ± 12.2 |
| Uric acid (mg/dL) | 5.35 ± 1.37 | 5.14 ± 1.37 | 5.67 ± 1.43 | 5.80 ± 1.56 |
| Diabetes | 24 (1.0) | 25 (1.0) | 108 (6) | 167 (10) |
| eGFR (mL/min/1.73 m2) | 110.8 ± 17.9 | 130.6 ± 21.0 | 90.9 ± 19.3 | 104.7 ± 24.7 |
| Kidney stones by self-report or hospitalization | 27 (1.1) | 5 (0.2) | 140 (5.7) | 60 (2.3) |
| Common carotid IMT, mm | ND | 0.66 ± 0.11 | 0.71 ± 0.12 | |
| Bulb/Internal carotid IMT, mm | ND | 0.72 ± 0.18 | 0.74 ± 0.18 | |
| Carotid stenosis | ND | 319/1760* (18) | 263/1484* (18) | |
| Carotid stenosis and/or upper quartile of bulb/internal carotid IMT | ND | 546/1751* (32) | 5033/1458* (35) | |
ND = not determined.
Data are presented as number (%) or mean ± standard deviation, unless otherwise indicated.
The number of participants available for each subclinical atherosclerosis outcome is given in the denominator.
Carotid IMT was greater in African-Americans than whites (p<0.01), but the prevalence of carotid stenosis at year 20 was similar in each population (18%); p=0.82. The prevalence of the composite carotid atherosclerosis outcome of upper quartile of internal carotid/bulb IMT and/or carotid stenosis was 35% among African-American participants and 32% among white participants (p<0.05).
CARDIA participant characteristics and kidney stones
The association between ever reported kidney stones and various baseline demographic, lifestyle and clinical characteristics at the baseline (year 0) CARDIA examination is shown in Table 2. In a minimally adjusted model, age, male sex, white race, clinic site, body mass index, LDL cholesterol, uric acid, and fasting insulin or HOMA index were positively associated with ever reporting kidney stones; baseline HDL cholesterol was negatively predicted ever reporting kidney stones. In a multivariable adjusted regression model, only age and race remained significantly associated with kidney stones. Similar associations with ever reporting kidney stones were obtained when using participant characteristics determined at year 20, with the exception insulin resistance remained significantly associated with kidney stones in the fully-adjusted multivariable model (p=0.01).
Table 2. Associations between ever having kidney stones through year 20 and demographic characteristics and cardiovascular risk factors at baseline (year 0).
| Risk factor | Comparison unit or Standard deviation | Adjusted for age, sex, race | Multivariable adjusted* | ||||
|---|---|---|---|---|---|---|---|
| OR | SE | p | OR | SE | p | ||
| Age | Per additional year | 1.06 | 0.02 | 0.006 | 1.06 | 0.02 | 0.005 |
| Male sex | vs. female sex | 1.48 | 0.22 | 0.007 | 1.39 | 0.27 | 0.10 |
| Self-reported white race | vs. African-American | 2.39 | 0.38 | <0.001 | 2.75 | 0.47 | <0.001 |
| Clinic | 0.08 | 0.12 | |||||
| Chicago | vs. Birmingham | 0.73 | 0.15 | 0.13 | 0.74 | 0.15 | 0.20 |
| Minneapolis | vs. Birmingham | 0.62 | 0.12 | 0.02 | 0.60 | 0.12 | 0.02 |
| Oakland | vs. Birmingham | 0.67 | 0.13 | 0.05 | 0.72 | 0.14 | 0.14 |
| Educational attainment | Per additional year | 1.01 | 0.03 | 0.79 | |||
| Current smokers | vs. never smokers | 1.09 | 0.18 | 0.59 | |||
| Former smokers | vs. never smokers | 0.71 | 0.17 | 0.15 | |||
| Alcohol consumption, mL/day | 21.9 | 0.92 | 0.08 | 0.31 | |||
| BMI, kg/m2 | 5.0 | 1.24 | 0.09 | 0.002 | 1.08 | 0.10 | 0.37 |
| Systolic BP, mm Hg | 10.9 | 1.02 | 0.08 | 0.75 | |||
| Diastolic BP, mm Hg | 9.6 | 1.06 | 0.08 | 0.44 | |||
| LDL cholesterol, mg/dL | 31.2 | 1.16 | 0.08 | 0.03 | 1.11 | 0.08 | 0.18 |
| HDL cholesterol, mg/dL | 13.2 | 0.85 | 0.07 | 0.04 | 0.95 | 0.08 | 0.55 |
| Triglycerides, mg/dL | 48.4 | 1.02 | 0.06 | 0.72 | |||
| Fasting blood glucose, mg/dL | 16.3 | 0.99 | 0.08 | 0.92 | |||
| Fasting insulin, mg/dL | 8.0 | 1.18 | 0.08 | 0.01 | |||
| Uric acid, mg/dL | 1.38 | 1.19 | 0.11 | 0.06 | 1.04 | 0.10 | 0.70 |
| Log(HOMA) | 0.62 | 1.26 | 0.09 | 0.002 | 1.16 | 0.10 | 0.09 |
| eGFR, mL/min/1.73 m2 | 18.6 | 0.96 | 0.12 | 0.77 | |||
OR, odds ratio; SE, standard error; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA, homeostasis model assessment.
Odds ratios were derived from a single multivariable logistic regression model, with all variables listed in the column adjusted for each other. Only variables associated with the outcome at p<0.10 in the minimal model were included in the multivariable model. For the multivariable model, the total number of participants with non-missing values for all baseline covariates is n=4,959, of whom 186 ever reported having kidney stones by year 20.
Kidney stones and subclinical atherosclerosis at the CARDIA year 20 examination When minimally adjusted for age, sex, race, and clinic (Table 3, model A), greater carotid wall thickness, particularly of the internal carotid/bulb region, was associated with a reported history of symptomatic kidney stones. When additional atherosclerotic risk factors (hypertension, blood pressure, cholesterol, insulin resistance, renal function) ascertained at either baseline (model B) or year 20 (model C) were added as covariates to the regression model, there was some attenuation of the apparent relationship between kidney stones and common and internal/bulb IMT, but the association with internal/bulb IMT remained significant. Similar results were obtained when carotid IMT was analyzed according to quartiles using polychotomous logistic regression (Figure 1) There were no significant differences in carotid IMT-symptomatic stone disease association according to race or sex, for either common carotid thickness or bulb/internal carotid thickness (data not shown).
Table 3. Association between history of kidney stones ever by year 20 and continuous carotid wall thickness measures at year 20 examination.
| Atherosclerosis outcome | Model A | Model B | Model C |
|---|---|---|---|
| Bulb/Internal Carotid IMT Regression Coeff ± SE | 0.054 ± 0.018 P=0.003 | 0.048 ± 0.018 P=0.007 | 0.041 ± 0.019 P=0.02 |
| Common Carotid IMT Regression Coeff ± SE | 0.028 ± 0.009 P=0.003 | 0.019 ± 0.009 P=0.04 | 0.014 ± 0.009 P=0.16 |
Abbreviations: IMT, intima-media wall thickness; SE, standard error
Model A: adjusted for age, sex, race, clinic
Model B: adjusted for age, sex, race, clinic, smoking, treated hypertension, systolic blood pressure, BMI, LDL cholesterol, HDL cholesterol, eGFR, uric acid, and HOMA index, covariates assessed at year 0
Model C: adjusted for age, sex, race, clinic, smoking, treated hypertension, systolic blood pressure, BMI, LDL cholesterol, HDL cholesterol, eGFR, uric acid, and HOMA index, covariates assessed at year 20
Figure 1.
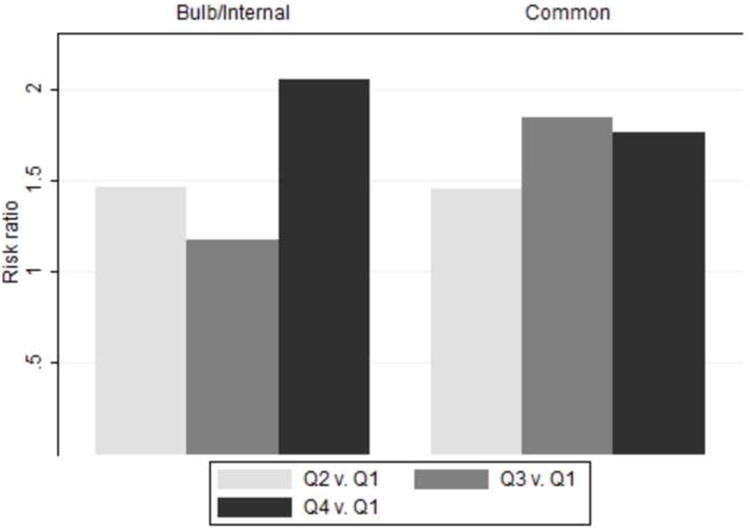
Carotid IMT was analyzed according to quartiles using polychotomous logistic regression.
Kidney stones were associated with a non-significant increased risk of carotid stenosis (Table 4). When a composite binary outcome of “carotid atherosclerosis” was defined as presence or stenosis and/or upper quartile of bulb/internal IMT, a history of kidney stones was associated with a significantly increased 1.6-fold increased risk of carotid atherosclerosis. The association between kidney stones and the composite outcome of carotid atherosclerosis remained significant even after adjusting for all major atherosclerotic risk factors. In a prospective analysis considering the relationship of kidney stones that occurred at year 15 or earlier (n=153) to the occurrence or progression of carotid atherosclerosis at year 20, the association findings were similar to those presented in Tables 3 and 4 (data not shown).
Table 4. Association between history of kidney stones ever by year 20 and dichotomous carotid atherosclerosis measures at year 20 examination.
| Atherosclerosis outcome | Model A | Model B | Model C |
|---|---|---|---|
| Presence of Carotid Stenosis Odds Ratio, 95% CI | 1.37 (0.92 − 2.04) P=0.12 | 1.35 (0.90 − 2.04) P=0.14 | 1.29 (0.84 − 1.98) P=0.24 |
| Presence of Carotid Stenosis and/or Upper Quartile of Bulb/Internal Carotid IMT | 1.67 (1.17 − 2.36) P=0.004 | 1.58 (1.11 − 2.27) P=0.01 | 1.56 (1.06 − 2.28) P=0.02 |
Abbreviations: IMT, intima-media wall thickness; CAC, coronary artery calcium; CI, confidence interval
Model A: adjusted for age, sex, race, and clinic
Model B: adjusted for age, sex, race, clinic, smoking, treated hypertension, systolic blood pressure, BMI, LDL cholesterol, HDL cholesterol, eGFR, uric acid, and HOMA index, covariates assessed at year 0
Model C: adjusted for age, sex, race, clinic, smoking, treated hypertension, systolic blood pressure, BMI, LDL cholesterol, HDL cholesterol, eGFR, uric acid, and HOMA index, covariates assessed at year 20
Discussion
In a community-based study of young to middle-aged white and African-American adults, a history of kidney stones is associated with carotid wall thickness, particularly of the internal carotid/bulb region, and also with a composite measure of carotid stenosis and internal carotid wall thickness. The association between kidney stones and subclinical vascular disease in community-dwelling younger adults from CARDIA adds further support to the notion that nephrolithiasis and atherosclerosis share common systemic risk factors and/or pathophysiology. We also find that whites have a several-fold higher rate of kidney stone formation than African-Americans, consistent with other reports [2,15,16].
The partial attenuation of the association between kidney stones and subclinical atherosclerosis after adjustment for the full set of vascular risk factors suggests that atherosclerosis and nephrolithiasis may operate in part through multiple, shared pathogenic mechanisms. These may include vascular endothelial injury and altered calcium, cholesterol, and/or glucose metabolism. In young adults from CARDIA, age, male sex, dyslipidemia, uric acid, and insulin resistance, either at the baseline or year 20 examination, were associated with a history of kidney stones by year 20. These findings are consistent with recent studies reporting associations between kidney stones and traditional atherosclerotic risk factors, including hypertension, diabetes mellitus, dyslipidemia, and obesity [3-5,17,18]. Taken together, these observations further strengthen the evidence for a link between nephrolithiasis and systemic atherosclerosis and its risk factors.
The apparently stronger association between kidney stones and internal carotid versus common carotid wall thickness noted in CARDA may be noteworthy. Common carotid IMT is a relatively pure measurement of wall thickness, and less indicative of stenosis than internal/bulb IMT. Thus, kidney stones may be specifically or preferentially associated with raised atherosclerotic lesions, as opposed to wall thickening. Confirmation of these findings through additional studies involving a larger number of individuals with subclinical measures of carotid disease is required.
On the basis of several lines of evidence, including kidney stone cholesterol content, histological examination of points of attachment for stones in the renal papilla, and renal blood flow changes based on sleep position which appear to effect the laterality of stone disease, it has been hypothesized that the precipitating factor may be vascular in origin and is localized to the vasa recta of the urinary papilla [6]. However, controversy exists whether the collecting ducts rather than the vasa recta represent the true anatomic point of attachment for forming stones [19]. Therefore, additional study is required to clarify whether the pathogenic similarities between an atherosclerotic plaque and nephrolithiasis suggest a vascular origin of kidney stone formation as an alternative to the shared risk factor model.
Strengths of our study include the large sample size, population-based sampling of young adults without a significant burden of clinical cardiovascular disease, and the availability of several subclinical disease measures as atherosclerotic outcomes. Several possible limitations should also be noted. Kidney stones were ascertained by self-report, and therefore may be subject to measurement error or recall bias. However, self-report of stones has been reported to be >95% accurate in other studies [8]. Moreover, because of the longitudinal nature of the CARDIA study, we were able to confirm a high rate of reproducibility of self-reported kidney stones among participants who had an opportunity for a second confirmatory report.
Another potential limitation of our study is that our ability to detect associations with coronary atherosclerosis may be limited due to the extent of atherosclerosis present in younger to middle-aged adults (for example, only ∼20% of the CARDIA participants had CAC scores >0 detectable at the year 20 examination). It should also be noted that the presence of subclinical atherosclerosis was not assessed at baseline in CARDIA; hence, it is difficult to draw conclusions on the temporal association between kidney stones and atherosclerosis. Nonetheless, we were able to perform a prospective analysis by restricting the number of kidney stones cases to those that occurred or were reported prior to the measurement of carotid and coronary atherosclerosis. Though the prospective analysis was limited by the smaller number of kidney stone cases, the qualitative similarity of the results to the full ‘cross-sectional’ year 20 analysis, are consistent with kidney stones appearing before atherosclerosis, or at least concurrently with atherosclerosis.
While adjustment was performed for major cardiovascular risk factors, residual confounding may account for some of the observed associations between kidney stones and atherosclerosis. Therefore, additional studies are warranted. These should include not only more detailed longitudinal assessment of the occurrence of kidney stones, vascular risk factors, and atherosclerotic disease, but also analysis of population-based samples of older adults with greater atherosclerotic disease burden and large numbers of clinical endpoints such as myocardial infarction and stroke.
Conclusions
we demonstrate that kidney stones are associated with subclinical atherosclerotic disease in young to middle-aged adults. We hypothesize that kidney stones and atherosclerosis tend to co-occur because of shared pathogenic mechanisms (vascular injury and inflammation, calcification) and risk factors (hypertension, altered cholesterol and/or glucose metabolism). Further study of the link between nephrolithiasis and subclinical and clinical CVD and their associated metabolic risk factors could shed further light on the etiology of kidney stone formation and systemic disorders of calcium metabolism and future novel therapeutic interventions for both disorders. Additionally, there could be important public health implications for kidney stone patients with respect to cardiovascular risk assessment.
Acknowledgments
Supported by the CARDIA contract (N01-HC-48047 – N01-HC-48050 and N01-HC-95095), CARDIA Ultrasound Reading Center HHSN268200425204C, and CARDIA Computed Tomography Reading Center N01-HC-05187 and HHSN268200425205C.
Glossary
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- IMT
Study Carotid intimal-medial wall thickness
- ICA
Internal carotid artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project. Urologic diseases in america project: Urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 4.Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32:802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 5.Madore F, Stampfer MJ, Rimm EB, et al. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 6.Stoller ML, Meng MV, Abrahams HM, et al. The primary stone event: A new hypothesis involving a vascular etiology. J Urol. 2004;171:1920–1924. doi: 10.1097/01.ju.0000120291.90839.49. [DOI] [PubMed] [Google Scholar]
- 7.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 8.Taylor EN, Mount DB, Forman JP, et al. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780–789. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 10.Slein M, Cori G, Cori C. A comparative study of hexokinase from yeast and animal tissue. J Biol Chem. 1950;186:763–780. [PubMed] [Google Scholar]
- 11.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 12.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 14.Polak JF, Person SD, Wei GS, et al. Segment-Specific Associations of Carotid Intima-Media Thickness With Cardiovascular Risk Factors. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke. 2010;41:9–15. doi: 10.1161/STROKEAHA.109.566596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarmina I, Spirnak JP, Resnick MI. Urinary lithiasis in the black population: an epidemiological study and review of the literature. J Urol. 1987;138:14–17. doi: 10.1016/s0022-5347(17)42971-5. [DOI] [PubMed] [Google Scholar]
- 16.Soucie JM, Thun MJ, Coates RJ, et al. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893–899. doi: 10.1038/ki.1994.347. [DOI] [PubMed] [Google Scholar]
- 17.Rendina D, Mossetti G, De Filippo G, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern italy: Role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant. 2009;24:900–906. doi: 10.1093/ndt/gfn548. [DOI] [PubMed] [Google Scholar]
- 18.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 19.Evan AP, Coe FL, Lingeman JE, et al. Insights on the pathology of kidney stone formation. Urol Res. 2005;33:383–389. doi: 10.1007/s00240-005-0488-0. [DOI] [PubMed] [Google Scholar]


