Abstract
Objective
Sirolimus (SRL) is an immunosuppressant drug used to prevent rejection in organ transplantation and neointimal hyperplasia when delivered from drug eluting stents (DES). Major side effects of SRL include edema and local collection of intimal lipid deposits at the DES site suggesting that SRL impairs endothelial barrier function (EBF). The aim of this study was to address the role of SRL on impaired EBF and the potential mechanisms involved.
Approach and Results
Cultured human aortic endothelial cells (HAEC) and intact human and mouse endothelium was examined to determine the effect of SRL, which binds FKBP12.6 to inhibit the mammalian target of rapamycin (mTOR), on EBF. EBF, measured by transendothelial electrical resistance (TEER), was impaired in HAEC when treated with SRL or siRNA for FKBP12.6 and reversed when pretreated with ryanodine, a stabilizer of RyR2 intracellular calcium release channels. Intracellular calcium increased in HAEC treated with SRL and normalized with ryanodine pretreatment. SRL treated HAEC demonstrated increases in PKCα phosphorylation, a calcium sensitive serine/threonine kinase important in VE cadherin barrier function through its interaction with p120-catenin (p120). Immunostaining of HAEC, human coronary and mouse aortic endothelium showed disruption of p120-VE cadherin interaction treated with SRL. SRL impairment of HAEC EBF was reduced with PKCα siRNA. Mice treated with SRL demonstrated increased vascular permeability by Evans blue albumin extravasation (EBAE) in the lungs, heart and aorta.
Conclusions
SRL-FKBP12.6 impairs EBF by activation of PKCα and downstream disruption of the p120-VE cadherin in vascular endothelium. These data suggest this mechanism may be an important contributor of SRL side effects related to impaired EBF.
Keywords: Sirolimus, Endothelium, Barrier Function, PKCα
Introduction
Endothelial barrier function (EBF) is required for vascular homeostasis while its dysfunction can lead to pathologic conditions such as atherosclerosis and edema1, 2. Sirolimus (SRL) is a mammalian target of rapamycin (mTOR) inhibitor used to prevent organ transplantation rejection and restenosis after percutaneous coronary intervention when delivered from drug eluting stents (DES). The predominant side effect of systemic sirolimus use is edema while local elution with DES can result in collections of foamy macrophages within the neointima (termed “neoatherosclerosis”) contributing to late thrombotic events3-5. These side effects limit the therapeutic use of sirolimus and suggest that the drug, when given both systemically and locally, impairs endothelial barrier function (EBF). While the major therapeutic mechanism of SRL is through mTOR inhibition, it is not clear whether mTOR inhibition itself leads to increased vascular permeability6, 7. Therefore understanding the underlying mechanisms by which SRL impairs EBF may clarify whether these effects are directly related to mTOR inhibition or may represent off-target effects of SRL.
SRL inhibits mTOR through specific binding of the FKBP12, a ubiquitous, cytosolic 12-KD FK506 binding protein and key stabilizing component of ryanodine (RyR2) intracellular calcium release channels in various cell types 8, 9. SRL has subnanomolar affinity to FKBP12 with 50% inhibitory concentration (IC50) for the mTOR signaling pathway at this subnanomolar dose range10, 11. In addition systemic use of SRL can lead to alteration of vascular intracellular calcium levels via displacement of the FKBP12.6, a vasculature specific isoform12, resulting in decreased endothelial dependent relaxation responses via protein kinase C activation through a calcium dependent mechanism8, 13. The alpha isoform of protein kinase C (PKCα) is a calcium sensitive threonine/serine kinase whose activation plays an important role in increasing vascular permeability, both through calcium-dependent and independent mechanisms14-17. Studies suggest that PKCα activation leads to p120-catenin (p120) dissociation from VE cadherin resulting in loss of VE cadherin homotypic interaction at the adherens junction, VE cadherin degradation, and impaired EBF18, 19.
In this study, we hypothesized that SRL-FKBP12.6 interaction would impair EBF by increasing intracellular calcium via RyR2 destabilization and activation of PKCα leading to disruption of p120-VE cadherin interaction. To test this we used human aortic endothelial cells (HAECs) to measure PKCα phosphorylation and the association of p120 and VE cadherin with and without SRL treatment. Additionally we measured EBF in HAEC with transendothelial electrical resistance (TEER) in addition to intracellular calcium levels under both the influence of SRL and pharmacologic RyR2 stabilization with ryanodine. Furthermore siRNA for FKBP12.6, PKCα and selective mTOR inhibition with a ATP-competitive inhibitor, torin2,20 were used to determine their respective mechanistic roles. Finally vascular permeability was measured in a C57BL/6 mouse model after SRL treatment in addition to immunostaining for p120-VE cadherin in intact mouse aortic and human coronary endothelium after SRL treatment.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Sirolimus activates PKCα and alters the interaction of p120-catenin with PKCα and VE Cadherin
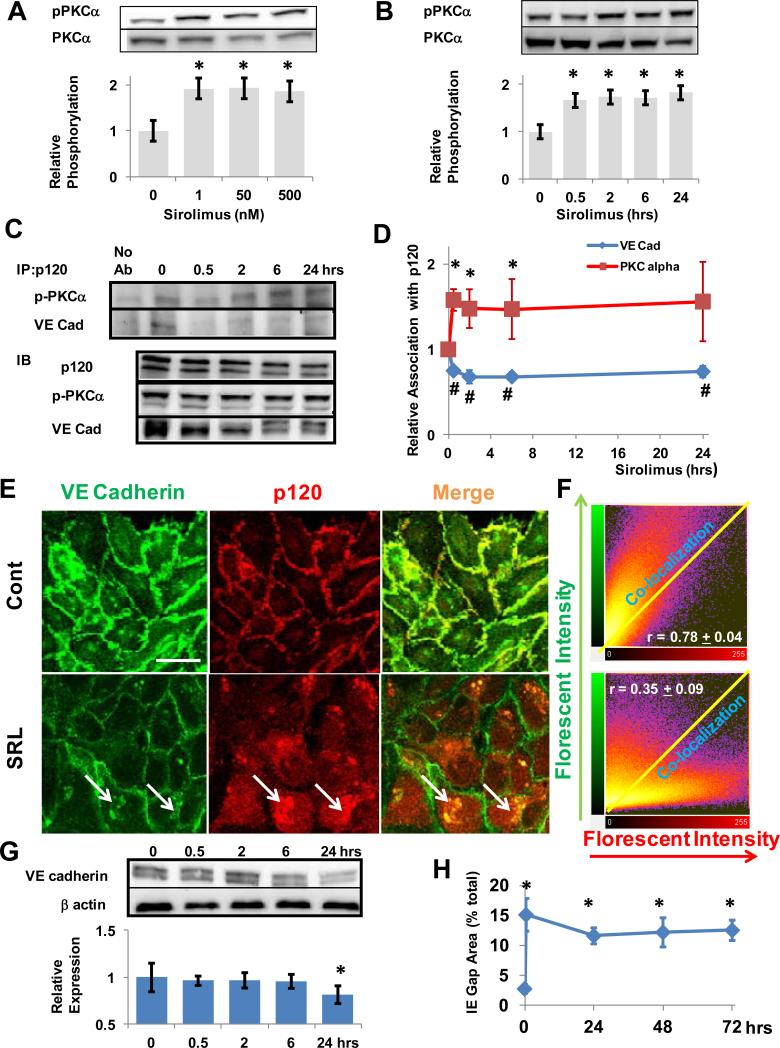
VE cadherin interacts with p120 to maintain EBF by repressing signals for VE cadherin endocytosis and degradation2, 18. Activation of PKCα, a serine/threonine kinase, is involved destabilizing p120-VE cadherin interactions18. When HAECs were treated with sirolimus (SRL), activation of PKCα occurred at all dose tested (i.e. 1 nmol/L to 500 nmol/L) (figure 1A). PKCα activation was also seen as quickly as 30 minutes after SRL treatment and remained activated at 24 hours (figure 1B). The interaction of p120 with phosphorylated PKCα (pPKCα) and VE cadherin in SRL-treated HAECs was examined using immunoprecipitation for p120. The interaction of p120 with pPKCα significantly increased after 30 minutes while its interaction with VE cadherin significantly decreased during this period (figure 1C-D). This suggests that SRL activates PKCα in HAECs which is associated with a sustained increase in pPKCα-p120 but a decrease in p120-VE cadherin interaction.
Figure 1.
SRL Activates PKCα, Disrupts the Interaction of p120 with VE Cadherin and the Endothelial Barrier. (A) Human aortic endothelial cells (HAECs) were immunoblotted for phosphorylated PKCα (Ser 657) after treatment with SRL at the indicated range of doses for 24 hours (1 - 500 nmol/L). Densitometry was performed (mean ± SD, n = 3, * p < 0.05). (B) HAECs were treated with SRL (500 nmol/L) from 30 minutes to 24 hours and immunoblotted for pPKCα at the times shown. Densitometry was performed (mean ± SD, n = 3, * p < 0.05). (C) p120 was immunoprecipitated from HAEC lysates at the indicated times after SRL treatment (500 nmol/L) and precipitates were immunoblotted for pPKCα and VE-cadherin. Total cell lysates were also immunoblotted (IB) for the respective antibodies and representative examples shown. (D) Densitometry was performed for the association of p120 with pPKCα and VE-cadherin (mean ± SD, n = 3, * and # p < 0.05 compared to 0 hrs). (E) Immunofluorescent imaging of HAECs with VE Cadherin (green) and p120 (red) was performed after no SRL treatment (cont) and 24 hours treatment (SRL) shown in 20x. White arrows denoted intraendothelial deposits with increased p120 and VE cadherin content. White bar indicates 20 mm. (F) Representative 2-D florescent intensity plots for immunoflourescent images each treatment group (shown in E) with pearson correlation coefficients (r) for the co-localization of p120 and VE cadherin pixels shown in inset (mean ± SD, p < 0.01 for cont v. SRL, n > 4 fields). (G) VE cadherin protein expression (relative to endothelial beta actin expression)levels were decreased atafter 24 hours of SRL treatment as measured by immunoblotting. Densitometry was performed for the relative expression of VE cadherin to beta actin (mean ± SD, n = (experiments repeated 4, * p < 0.05). times) (H) Interendothelial (IE) gap areas were assessed after SRL treatment at the indicated times by immunoflorescent imaging of membrane VE cadherin (mean ± SD, n > 4 fields, * p < 0.05 compared to t = 0).
Sirolimus Treatment Mobilizes p120 from the Membrane to the Cytosol and Increases Interendothelial Gap Area
To confirm these observations, we next examined the interaction of p120 and VE cadherin by immunostaining in HAECs treated with SRL. SRL treatment at 24 hours increased p120 (red) mobilization from the HAEC membrane to the cytosol when compared to no treatment (figure 1E). In addition we observed evidence of intracellular deposits with increased p120-VE cadherin staining suggestive of membrane disruption (white arrow, figure 1E). There was a significant decrease in colocalization of p120 and VE cadherin as measured by pearson's correlation coefficient (figure 1F). At 24 hours, there was decrease in overall protein expression of VE cadherin (figure 1G). Additionally the interendothelial gap areas measured by VE cadherin immunostaining remained increased up to 72 hours (figure 1H), suggesting disruption of endothelial barrier. Similar to SRL treatment, removal FKBP12.6 via siRNA showed p120-VE cadherin disruption compared with non-targeting siRNA (Scr) with immunostaining and an increase in pPKCα–p120 interaction but a decrease in p120-VE cadherin interaction using immunoprecipitation with p120 (supplemental figure I A-C).
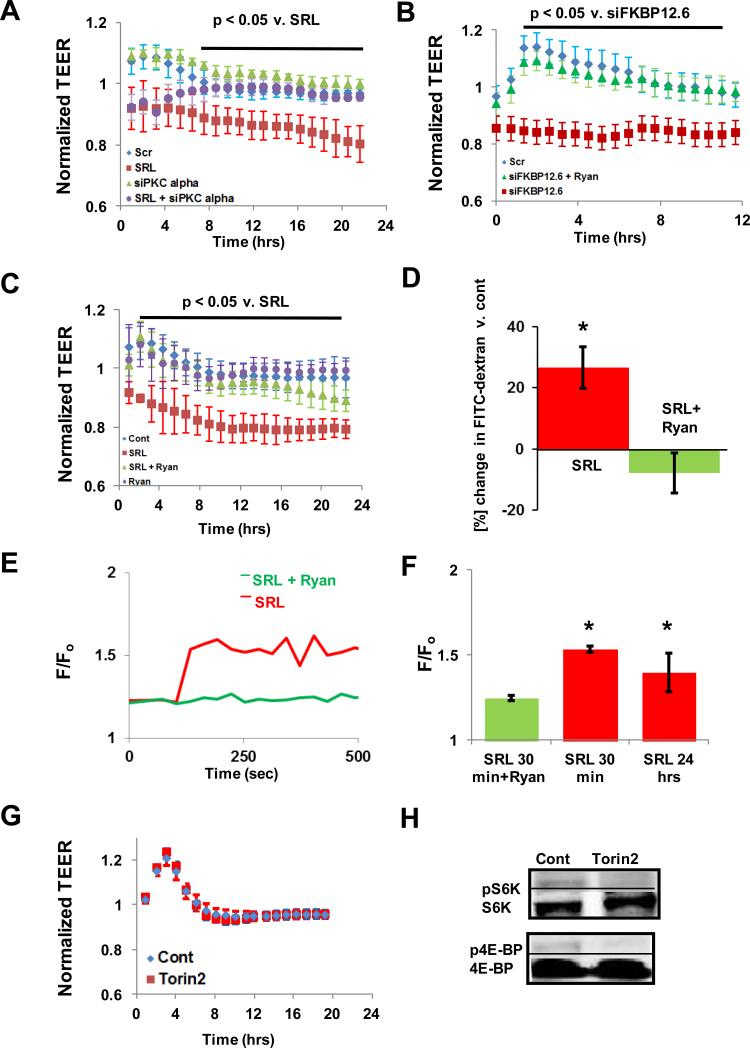
Sirolimus Treatment or FKBP12.6 Knockdown but Not Selective mTOR inhibition Impairs EBF in a PKCα Dependent Manner
Sirolimus treatment or removal of FKBP12.6, a FK506 binding protein which normally binds RyR2 in the vasculature12, with siRNA impaired HAEC EBF with significant reduction in normalized TEER, respectively, over the measured period (figure 2A-C, supplemental table I). This impairment was reversed when HAEC were pre-treated with ryanodine (ryan), a stabilizer of RyR2 calcium release channels (figure 2C). There was an initial rise in TEER with the control and ryanodine-treated groups suggesting an initial barrier stabilizing effect which was seen not in the treated groups (figure 2B-C). SRL impairment of HAEC EBF was also attenuated when PKCα was removed via siRNA (figure 2A-B) suggesting PKCα is required for this effect. Transwell permeability of HAEC monolayers was also increased with SRL treatment and improved when pre-treated with ryanodine (figure 2D, supplemental table 2). In HAECs, we observed a significant increase in intracellular calcium content after SRL treatment up to 24 hours (figure 2F-G). Pre-treatment with ryanodine ameliorated the initial increase in intracellular calcium levels up to 30 minutes (figure 2F-G). Torin2, a selective ATP-competitive inhibitor of the mammalian target of rapamycin (mTOR) which does not bind FKBP12/12.621, did not impair EBF while inhibiting the downstream targets of mTOR signaling pathway and endothelial proliferation similar to SRL22 (figures 2G-H, supplemental figure 2A-B). Collectively, these results suggest that displacement of FKBP12.6 from RyR2 calcium release channels by SRL, rather than mTOR inhibition, induces an endothelial intracellular calcium leak and increases PKCα phosphorylation leading to impaired EBF.
Figure 2.
SRL-FKBP12.6 Impairs Endothelial Barrier Function (EBF) by Modulating Intracellular Calcium Concentration via RyR2 Channels. (A) Treatment with SRL impairs EBF as measured by reduced normalized transendothelial electrical resistance (TEER) while PKCα siRNA reduces SRL-induced decrease in TEER (mean ± SEM, n = 3 wells, normalization was to each individual baseline value, t = 0). (B) Treatment with FKBP12.6 siRNA decreases TEER which is reversed by a RyR2 stabilization with ryanodine (50 μmol/L for 1 hour) pre-treatment (mean ± SEM, n = 3 wells). (C) Pre-treatment with ryanodine reduces the SRL-induced decrease in TEER (mean ± SEM, n = 3 wells). (D) Ryanodine pre-treatment prevents SRL-induced increase in HAEC transwell permeability (mean ± SD, n = 4 wells, * p < 0.05 compared to change in FITC-Dextran in control wells). (E-F) SRL induces an intracellular calcium leak which is ameliorated by ryanodine pre-treatment in HAECs (mean emission ratio [F/Fo = Fluo3/Fura Red] ± SD, n > 10 cells, * < 0.05 v. SRL + Ryan). (G) Treatment of HAEC with Torin2, a selective ATP-competitive inhibitor of mTOR, does not impair EBF compared with control (mean ± SEM, n = 3 wells). (H) Representative western blots of activated S6K (pS6K) and 4E-BP (p4E-BP) representing major downstream signaling products of the mTOR pathway after Torin2 treatment for 24 hours (n = 4).
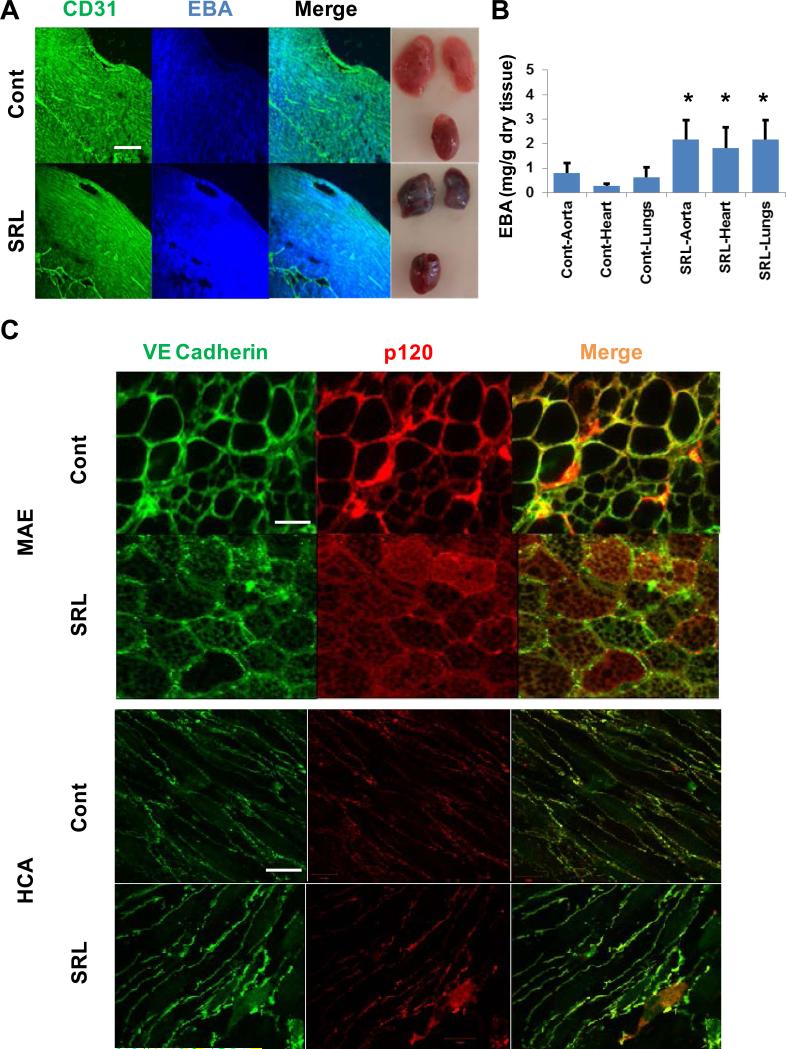
Sirolimus Treatment Increased Vascular Permeability by Disrupting the p120-VE Cadherin Interaction
We examined the effect of SRL treatment EBF in adult male C57BL/6 mice (1 mg/kg/day intraperitoneal for 3 days). Dosing was based on previous experimental data to achieve steady state clinical drug levels without significant immune suppression7, 23, 24 SRL treatment qualitatively increased Evans blue albumin (EBA) extravasation in the myocardium both in the microvascular and macrovascular beds as seen using CD31 staining (figure 3A) in addition to significantly increasing EBA extravasation in homogenates of the myocardial, aortic and pulmonary tissue (figure 3B). In addition, in mouse aortas there was an increase in both p120 (red) and VE cadherin (green) mobilization from the membrane into the cytosol when compared with vehicle-treated mice resulting in decreased co-localization of these proteins (Figure 3C and supplemental figure III A) as compared to aortas of control (i.e. vehicle) treated mice. Additionally we examined human coronary arteries which were collected 4 hours post-mortem and treated ex vivo with SRL (500 nmol/L) or vehicle for 24 hours. SRL treatment decreased p120 (red) and VE Cadherin (green) co-localization during this time interval (figure 3E and supplemental figure III B).
Figure 3.
SRL Induces Increased Vascular Permeability and Disrupts the Interaction of VE-cadherin and p120 in vivo and ex vivo. (A) Intraperitoneal injection of SRL (1 mg/kg/day for 3 days) and vehicle-treated mouse myocardium showing increased Evans blue albumin (EBA) extravasation both in the micro- and microvasculature stained with CD31 (green) at 4x magnification. Gross example of the heart and lungs in each group are also shown. White bar indicates 100 μm. (B) Vascular permeability of different endothelial beds (aortic, heart, lungs) was increased in SRL-treated mice when compared to vehicle as measured by EBA content in the respective tissue homogenate (mean ± SD, n = 6 mice,* p < 0.05 compared to vehicle (cont)). (C) SRL treatment disrupts the interaction of p120 (red) and VE cadherin (green) in C57BL/6 mice aortic endothelium (MAE) when compared with vehicle treated mice (top) shown in 20x. White bar indicates 10 μm. (D) Human coronary endothelium treated ex vivo with SRL (500 nmol/L) for 24 hours or vehicle showing increased p120 (red) decreased co-localization with VE cadherin (green) in SRL-treated arteries compared with vehicle (cont). Magnification at 40x with white bar indicating 10 μm.
Discussion
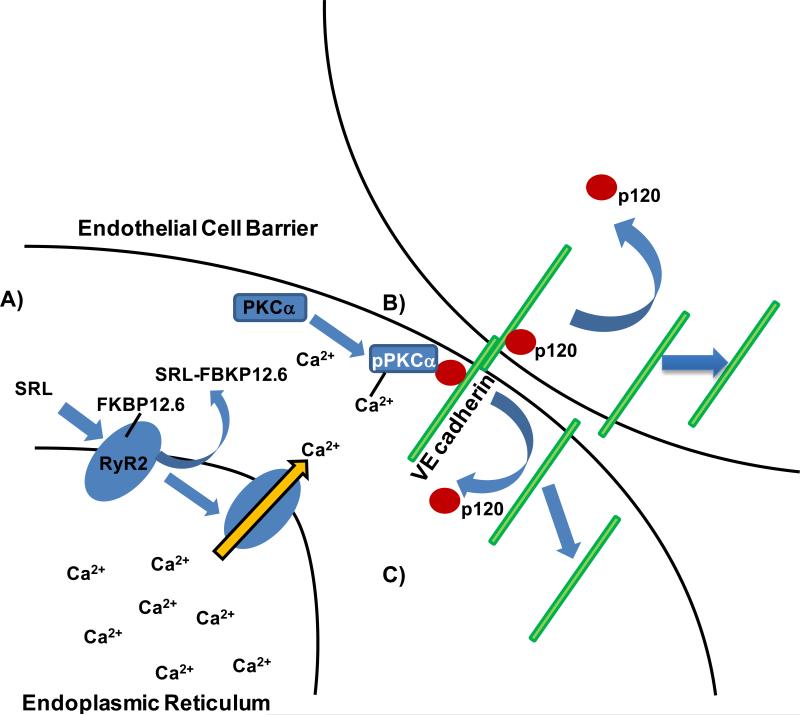
This study is the first to propose a novel mechanism by which SRL-FKBP12.6 impairs EBF independent of the mTOR signaling pathway (figure 4A-C). We show evidence that pharmacologically displacing FKBP12.6 with SRL in vascular endothelial cells leads to calcium-dependent activation of PKCα, disruption p120-VE cadherin interaction and impaired EBF. We suggest that this mechanism involves destabilization of the RyR2 intracellular calcium release channels by FKBP12.6 displacement leading to increased intracellular calcium and found impaired EBF to be improved through RyR2 stabilization with ryanodine. These finding were recapitulated through removal of FKBP12.6 via siRNA again showing disruption of the p120-VE cadherin interaction and impaired EBF. Furthermore removal of PKCα via siRNA abrogates the effect of SRL on EBF. Additionally we observed that a selective ATP-competitive mTOR inhibitor, that does not require FKBP12/12.6 binding20, 21, did not affect EBF suggesting that SRL induced impairment of EBF is unrelated to mTOR inhibition. Finally we show increased vascular permeability with SRL treated mice in different vascular beds and disruption of p120-VE cadherin in intact aortic and coronary endothelium after SRL treatment.
Figure 4.
Proposed Mechanism of Sirolimus to Impair Endothelial Barrier Function. (A) Sirolimus (SRL) displaces FKBP12.6 from RyR2 calcium release channel (blue oval) in vascular endothelial cells results in increased intracellular release of free Ca2+ from the endoplasmic reticulum. (B) PKCα is activated and destabilized the p120-VE cadherin interaction. (C) p120 and eventually VE cadherin move from the membrane to the intracellular space leading to impaired endothelial barrier function.
Previous studies examining EBF and SRL have centered on the mTOR signaling pathway in with equivocal results6, 7, 25, 26. Downstream effectors of the mTOR signaling pathway such as Akt/PKB, a serine/threonine kinase and downstream effector of mTOR complex 2 (mTORC2), have been proposed to regulate endothelial permeability however results differ6, 7, 25-27. VE cadherin content however are consistently decreased by SRL treatment suggesting the SRL may affect VE cadherin content and vascular endothelial homeostasis regardless of the model used6, 7, 25. Studies suggest that p120-catenin interaction with VE cadherin may act as a set point for endothelial homeostasis and cellular VE cadherin content28. PKC, a family of serine/threonine kinases, and its alpha isoform are key regulators of endothelial function both in the macro- and microvasculature29 and has been shown to modulate EBF through disruption of the p120-VE cadherin interaction in addition to affecting endothelial cytoskeleton dynamics by activating myosin light chain-2 (MLC-2) through myosin light chain kinase18, 30, 31. Our study suggests SRL's effects on EBF are mediated through alteration of p120-VE cadherin interaction and likely disruption of vascular homeostasis in multiple vascular beds (figure 3A-B)8, 18, 29. While we do not see any significant activation of MLC-2 (supplemental figure IV) with SRL treatment of HAECs, there probably are likely SRL-mediated alterations in endothelial cytoskeleton dynamics given the impaired intracellular calcium concentration leading to persistent interendothelial gaps (figure 1H)31, 32. In vivo, p120-VE cadherin disruption and increased vascular permeability was observed in different vascular beds in C57BL/6 mice treated with SRL (1 mg/kg/day for 3 days) compared to vehicle treated control. This dose was chosen to achieve steady state clinical drug levels and suppression of mTOR signaling products without significant immune suppression7, 23, 24. In the mouse aortic endothelium, there is decreased p120-VE cadherin co-localization within the membrane in the treated animals. Finally we observed a similar disruption in p120-VE cadherin interaction within a human coronary endothelium treated ex vivo with SRL (500 nmol/L for 24 hours) suggesting that this effect not only occurs in intact human endothelium but also has an acute time course (< 24 hours). This is consistent with our proposed mechanism as opposed to one which involves mTOR/Akt inhibition which requires longer treatment duration33.
Clinical Implications
SRL is an immunosuppressant with diverse systemic and local effects. Its main therapeutic mechanism is through the allosteric inhibition of mTOR by SRL-FKBP12 complex similar to the inhibition of calcineurin by FK506-FKBP12 complex34. Interestingly, while SRL and its analogs (i.e. everolimus) have gained increasing use over calcineurin-inhibitors (CNI) such as FK506 (i.e. tacrolimus) in the prevention of solid organ transplant rejection, edema represents the most common adverse reaction in both classes of medications3, 35. In a retrospective registry of heart transplant recipients, edema was the most frequent cause for discontinuation of mTOR inhibitors3. Additionally when comparing SRL, to newer analogs such as everolimus, a 40-O-hydroxyethyl-derivative of SRL, there has been shown increased tolerability to everolimus with respect to edema36. This is likely in because of the overall decreased affinity of everolimus to FKBP12/12.6 compared to SRL 11. These clinical findings suggest that edema is related to systemic inhibition of endothelial FKBP12.6. Additionally up to 1/3 of coronary stents that elute mTOR inhibitors have evidence of intimal lipid deposits within the stent, again suggesting poor endothelial barrier function and contributing to late thrombotic events 4, 5. This also implies that SRL analogs, such as everolimus, which have reduced affinity to FKBP12/12.6 may also likely have reduced local adverse effects compared SRL however this has not yet been studied11. A role for specific mTOR inhibitors, such as ATP-competitive mTOR inhibitors, as therapeutic options for local elution in DES however should be considered20.
Conclusions
SRL-FKBP12.6 impairs EBF by PKCα activation and disruption of the p120-VE cadherin in the vascular endothelium. This mechanism may be an important contributor of SRL side effects related to impaired EBF.
Supplementary Material
Significance.
Sirolimus is an mTOR inhibitor used to prevent rejection in organ transplantation and neointimal hyperplasia when delivered from drug eluting stents (DES). However major side effects of sirolimus such as edema and local collection of intimal lipid deposits at the DES site suggesting that sirolimus impairs endothelial barrier function (EBF) which limits its therapeutic use. Our study suggests a novel mechanism by which sirolimus impairs EBF through disruption of key interactions between p120-VE cadherin which maintain endothelial barrier function. This proposed mechanism is independent from mTOR inhibition which may aid in the development of selective, better tolerated mTOR inhibitors for clinical use.
Acknowledgements
Emory University Integrated Cellular Imaging (ICI) Core for their assistance with live cell imaging and intracellular calcium quantification.
Funding Sources
This study was supported by the Carlyle Fraser Heart Center, CVPath Inc., American Heart Association and US NIH grant RO1 HL096970-01A.
Non-Standard Abbreviations
- DES
drug eluting stents
- EBA
Evans blue albumin
- EBF
endothelial barrier function
- IE
Interendothelial
- HAEC
human aortic endothelial cells
- MAE
mouse aortic endothelium
- mTOR
mammalian target of rapamycin
- mTORC2
mammalian target of rapamycin complex 2
- p120
p120 catenin
- RyR2
ryanodine receptor 2
- SRL
sirolimus
- TEER
transendothelial electrical resistance
Footnotes
Disclosures
AVF has sponsored research agreements with Medtronic CardioVascular and Boston Scientific. He is also an advisory board member to Medtronic CardioVascular. AH is supported with an AHA Postdoctoral Fellowship grant (Greater Southeast Affiliate). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Nieuw Amerongen GP, Vermeer MA, Negre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VW. Simvastatin improves disturbed endothelial barrier function. Circulation. 2000;102:2803–2809. doi: 10.1161/01.cir.102.23.2803. [DOI] [PubMed] [Google Scholar]
- 2.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiological reviews. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Vilchez F, Vazquez de Prada JA, Almenar L, et al. Withdrawal of proliferation signal inhibitors due to adverse events in the maintenance phase of heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:288–295. doi: 10.1016/j.healun.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. Journal of the American College of Cardiology. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: A final common pathway of late stent failure. Journal of the American College of Cardiology. 2012;59:2051–2057. doi: 10.1016/j.jacc.2011.10.909. [DOI] [PubMed] [Google Scholar]
- 6.Oroszlan M, Bieri M, Ligeti N, Farkas A, Koestner SC, Meier B, Mohacsi PJ. Proliferation signal inhibitor-induced decrease of vascular endothelial cadherin expression and increase of endothelial permeability in vitro are prevented by an anti-oxidant. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27:1311–1318. doi: 10.1016/j.healun.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained akt signaling and inhibited by rapamycin. Cancer cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long C, Cook LG, Wu GY, Mitchell BM. Removal of fkbp12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1580–1586. doi: 10.1161/ATVBAHA.107.144808. [DOI] [PubMed] [Google Scholar]
- 9.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by fk506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 10.Bierer BE, Mattila PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, Schreiber SL. Two distinct signal transmission pathways in t lymphocytes are inhibited by complexes formed between an immunophilin and either fk506 or rapamycin. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler W, Sedrani R, Cottens S, Haberlin B, Schulz M, Schuurman HJ, Zenke G, Zerwes HG, Schreier MH. Sdz rad, a new rapamycin derivative: Pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S. Selective binding of fkbp12.6 by the cardiac ryanodine receptor. The Journal of biological chemistry. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- 13.Long C, Cook LG, Hamilton SL, Wu GY, Mitchell BM. Fk506 binding protein 12/12.6 depletion increases endothelial nitric oxide synthase threonine 495 phosphorylation and blood pressure. Hypertension. 2007;49:569–576. doi: 10.1161/01.HYP.0000257914.80918.72. [DOI] [PubMed] [Google Scholar]
- 14.Hempel A, Lindschau C, Maasch C, Mahn M, Bychkov R, Noll T, Luft FC, Haller H. Calcium antagonists ameliorate ischemia-induced endothelial cell permeability by inhibiting protein kinase c. Circulation. 1999;99:2523–2529. doi: 10.1161/01.cir.99.19.2523. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and pkcalpha activate increased endothelial permeability by disassembly of ve-cadherin junctions. The Journal of physiology. 2001;533:433–445. doi: 10.1111/j.1469-7793.2001.0433a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinase c activation. The Journal of clinical investigation. 1990;85:1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase c phosphorylation. The EMBO journal. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. Pkcalpha activation of p120-catenin serine 879 phospho-switch disassembles ve-cadherin junctions and disrupts vascular integrity. Circulation research. 2012;111:739–749. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. Ve-cadherin-p120 interaction is required for maintenance of endothelial barrier function. American journal of physiology. Lung cellular and molecular physiology. 2004;286:L1143–1153. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Xu C, Kirubakaran S, et al. Characterization of torin2, an atp-competitive inhibitor of mtor, atm and atr. Cancer research. 2013 doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, Sabatini DM, Gray NS. Discovery of 9-(6-aminopyridin-3-yl)-(3(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1h)-one (torin2) as a potent, selective, and orally available mammalian target of rapamycin (mtor) inhibitor for treatment of cancer. Journal of medicinal chemistry. 2011;54:1473–1480. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habib A, Karmali V, Polavarapu R, Akahori H, Nakano M, Yazdani S, Otsuka F, Pachura K, Davis T, Narula J, Kolodgie FD, Virmani R, Finn AV. Metformin impairs vascular endothelial recovery after stent placement in the setting of locally eluted mammalian target of rapamycin inhibitors via s6 kinase-dependent inhibition of cell proliferation. Journal of the American College of Cardiology. 2013;61:971–980. doi: 10.1016/j.jacc.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker H, Sidorowicz A, Sehgal SN, Vezina C. Rapamycin (ay-22,989), a new antifungal antibiotic. Iii. In vitro and in vivo evaluation. The Journal of antibiotics. 1978;31:539–545. doi: 10.7164/antibiotics.31.539. [DOI] [PubMed] [Google Scholar]
- 24.Chen BJ, Morris RE, Chao NJ. Graft-versus-host disease prevention by rapamycin: Cellular mechanisms. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2000;6:529–536. doi: 10.1016/s1083-8791(00)70062-0. [DOI] [PubMed] [Google Scholar]
- 25.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase akt. The Journal of clinical investigation. 2006;116:334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phung TL, Eyiah-Mensah G, O'Donnell RK, Bieniek R, Shechter S, Walsh K, Kuperwasser C, Benjamin LE. Endothelial akt signaling is rate-limiting for rapamycin inhibition of mouse mammary tumor progression. Cancer research. 2007;67:5070–5075. doi: 10.1158/0008-5472.CAN-06-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiojima I, Walsh K. Role of akt signaling in vascular homeostasis and angiogenesis. Circulation research. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 28.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. The Journal of cell biology. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geraldes P, King GL. Activation of protein kinase c isoforms and its impact on diabetic complications. Circulation research. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase signaling in endothelial barrier dysfunction. Medicinal research reviews. 2012 doi: 10.1002/med.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annual review of physiology. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 32.Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin ii in cultured bovine corneal endothelial cells. Experimental eye research. 2004;79:477–486. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 34.Vilella-Bach M, Nuzzi P, Fang Y, Chen J. The fkbp12-rapamycin-binding domain is required for fkbp12-rapamycin-associated protein kinase activity and g1 progression. The Journal of biological chemistry. 1999;274:4266–4272. doi: 10.1074/jbc.274.7.4266. [DOI] [PubMed] [Google Scholar]
- 35.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: Focus on improving renal function and nephrotoxicity. Clinical transplantation. 2008;22:1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 36.Moro JA, Almenar L, Martinez-Dolz L, Sanchez-Lazaro I, Aguero J, Salvador A. Tolerance profile of the proliferation signal inhibitors everolimus and sirolimus in heart transplantation. Transplantation proceedings. 2008;40:3034–3036. doi: 10.1016/j.transproceed.2008.09.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.