SUMMARY
Thymically derived Foxp3+ regulatory T (Treg) cells have a propensity to recognize self-peptide:MHC complexes, but their ability to respond to epitope-defined foreign antigens during infectious challenge has not been demonstrated. Here we show that pulmonary infection with Mycobacterium tuberculosis (Mtb), but not Listeria monocytogenes (Lm), induced robust lymph node expansion of a highly activated population of pathogen-specific Treg cells from the pre-existing pool of thymically derived Treg cells. These antigen-specific Treg cells peaked in numbers 3 weeks after infection but subsequently underwent selective elimination driven, in part, by interleukin-12-induced intrinsic expression of the Th1-cell-promoting transcription factor T-bet. Thus, the initial Mtb-induced inflammatory response promotes pathogen-specific Treg cell proliferation, but these cells are actively culled later, probably to prevent suppression during later stages of infection. These findings have important implications for the prevention and treatment of tuberculosis and other chronic diseases in which antigen-specific Treg cells restrict immunity.
INTRODUCTION
Regulatory T (Treg) cells, a subset of CD4+ T cells characterized by their stable expression of the transcription factor Foxp3, prevent autoimmune disease (Sakaguchi et al., 2008) but can also restrict immunity to infectious microbes (Belkaid and Tarbell, 2009). During infections, Treg cells appear to play a dichotomous role: on the one hand, they benefit the host by curbing excessive inflammation that could be deleterious to host tissues (Belkaid and Tarbell, 2009). On the other hand, by limiting potentially protective immune responses, they can facilitate pathogen replication and persistence, as shown for several chronic infections, including tuberculosis (Belkaid and Tarbell, 2009; Kursar et al., 2007; Scott-Browne et al., 2007). Strategic manipulations of Treg cells that promote pathogen clearance while avoiding detrimental consequences to the host could provide new avenues to prevent or treat persistent infections. One approach would be to exploit their microbial antigen specificity, because T-cell-receptor (TCR)-mediated signals are required for their suppressive function (Sakaguchi et al., 2008), but the specific antigens recognized by Treg cells during infection are largely unknown, and in most cases it is not even clear whether Treg cells recognize microbe-derived antigens or primarily respond to self-antigens.
A fundamental question in immunology, one that also raises practical considerations that impact protective immunity and vaccination, is whether thymically derived Treg cells can respond to microbe-derived antigens during infection. During homeostatic conditions, commensal biota-specific Treg cells accumulate in the gut-associated lymphoid system. Some studies suggest that these cells are peripherally induced Treg cells (Atarashi et al., 2011; Lathrop et al., 2011; Round and Mazmanian, 2010), although a recent study suggests that they are thymically derived Treg cells (Cebula et al., 2013). During chronic lymphocytic choriomeningitis virus (LCMV) infection, Treg cells have been shown to recognize a self-antigen rather than a virus-specific antigen (Punkosdy et al., 2011). This finding may reflect the fact that thymically derived Treg cells are selected by high-affinity interactions with self-antigens within the thymus (Bautista et al., 2009; DiPaolo and Shevach, 2009) and therefore have a propensity for recognizing self-antigens in the periphery (Hsieh et al., 2004, 2006; Killebrew et al., 2011; Korn et al., 2007). Nonetheless, thymically derived Treg cells specific for foreign epitopes have been detected in the naive population (Ertelt et al., 2009; Moon et al., 2011; Zhao et al., 2011), but their expansion during infection has not been shown.
Multiple studies with different infectious models have failed to definitively identify microbe-specific thymically derived Treg cells (Ertelt et al., 2009; Antunes et al., 2008). For Salmonella (Johanns et al., 2010) and neurotropic mouse hepatitis virus (Zhao et al., 2011) infections, low frequencies of microbe-specific Foxp3+CD4+ T cells have been reported; however, whether these populations represented thymically derived or peripherally induced Treg cells was not clear. During Leishmania infection, thymically derived Treg cells were shown to proliferate specifically to Leishmania-infected dendritic cells, suggesting that they recognized a microbe-derived antigen (Suffia et al., 2006), and similar results were recently reported showing specific proliferation of Treg cells from influenza-infected mice after stimulation with influenza-infected dendritic cells (Betts et al., 2012). In both cases, however, the specificity of these expanded Treg cells were not identified, leaving the possibility that infection of dendritic cells induced the expression of novel self-proteins that were the actual target of Treg cell recognition. Finally, after Mycobacterium tuberculosis (Mtb) infection, we showed that pathogen-specific Treg cells from TCR transgenic mice, but not Treg cells with irrelevant specificities, proliferate robustly in infected mice (Shafiani et al., 2010). However, Mtb specificity was not directly demonstrated among the endogenous Treg cell population. Thus, the question of whether endogenous Treg cells from the thymically derived Treg cell pool recognize microbe-derived antigens during responses to infectious challenge remains unanswered.
In this study, we found that early after Mtb infection, a substantial fraction of the CD4+ T cells in the pulmonary lymph node (pLN) recognizing an immunodominant Mtb epitope expressed high amounts of Foxp3 and markers of Treg cell activation. These cells arose from the thymically derived Treg cell population in a context-dependent manner; pulmonary infection with recombinant Listeria monocytogenes (Lm) expressing the same Mtb-derived epitope resulted in pLN expansion of antigen-specific effector T cells but not Treg cells. The Mtb-specific Treg cells peaked in numbers 3 weeks after infection and declined thereafter, a process driven in part by interleukin-12 (IL-12)-induced T-bet expression. Our results suggest a model in which Mtb-induced inflammation promotes proliferation of pathogen-specific Treg cells when adaptive immunity is initiated, but the subsequent host response selectively culls these highly suppressive Treg cells so that they cannot restrict immunity during later stages of infection.
RESULTS
Mtb-Specific Treg Cells Expand in the pLNs of Infected Mice
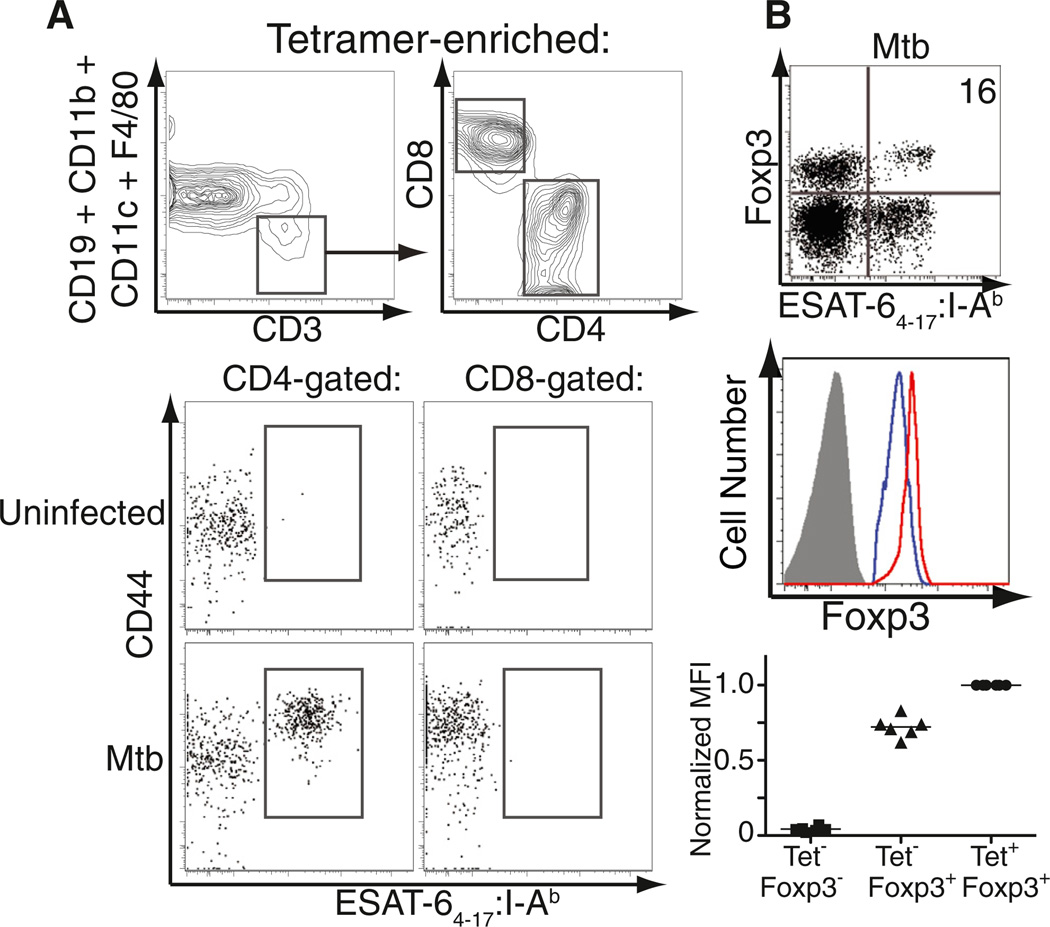
First we sought to determine whether Mtb-specific Foxp3+ cells were present within endogenous CD4+ T cell populations in mice infected with a low dose of aerosolized Mtb. Because we previously showed that transfer of small numbers of transgenic Mtb-specific Treg cells impaired immunity by restricting priming of effector T cells in the pLN (Shafiani et al., 2010), we focused on the pLN during early infection. To overcome the fact that Mtb-specific CD4+ T cells comprise only a small population in the pLN (Figure S1A available online), we employed a tetramer-based approach to enrich rare populations of antigen-specific T cells (Moon et al., 2007, 2009). Pulmonary LN cells from multiple Mtb-infected mice on day 21 after infection were pooled, enriched for ESAT-64–17:I-Ab tetramer-binding cells with magnetic beads and columns, stained for cell surface markers, and permeabilized for subsequent intracellular Foxp3 staining. Although some cells bound the magnetized column nonspecifically, a large population of CD44hi tetramer-binding CD4+ T cells was found after first gating on CD3+ cells and excluding non-T cell lineage cells from the analysis (Figure 1A). This population was not observed in uninfected mice or within CD8-gated T cells from infected mice (Figure 1A). A defined population of tetramer-binding CD4+ T cells in the pLN on day 21 after infection was consistently observed to express Foxp3 (usually 5%–20% of the tetramer-binding CD4+ T cells), and these Mtb-specific Treg cells expressed higher amounts of Foxp3 than did non-tetramer-binding Treg cells in the same lymph node (Figure 1B). These findings were not unique to this antigen because Foxp3+ CD4+ T cells with specificity for the Mtb epitope Ag85B240–254IAb were also observed, albeit at a slightly lower frequency (Figure S1B). Thus, Foxp3-expressing Treg and effector T cells recognizing the same immunodominant Mtb epitopes both undergo robust expansion in the pLN after aerosolized infection.
Figure 1. Detection of ESAT-64–17-Specific Treg Cells in the pLNs of Mtb-Infected Mice.
(A) Gating strategy used to identify CD3+CD4+ESAT-64–17-specific cells in the pLNs of B6 mice, 21 days after aerosol infection. PLNs from three Mtb-infected mice or all lymph nodes from an uninfected control mouse were pooled and enriched for ESAT-64–17-specific cells.
(B) Binding of tetramer and expression of Foxp3 by CD4+ cells within pLN cells (day 21 after infection) after enrichment of tetramer-binding cells. The percentage of tetramer-binding cells expressing Foxp3 is shown. Histograms denote the amount of Foxp3 expression in tetramer-binding Treg cells (red), non-tetramer-binding Treg cells (blue), and Foxp3−CD4+ T cells (solid gray) in the pLN. Graph depicts mean fluorescent intensity (MFI) of Foxp3 within each of these three populations. Cumulative data from six independent experiments are shown. MFI values are normalized to those obtained for tetramer-binding Treg cells. Each solid square, triangle, or circle represents an experiment and the bars represent the mean of the six experiments performed. See also Figure S1.
Mtb-Specific Treg Cells Are Activated but Do Not Produce IFN-γ
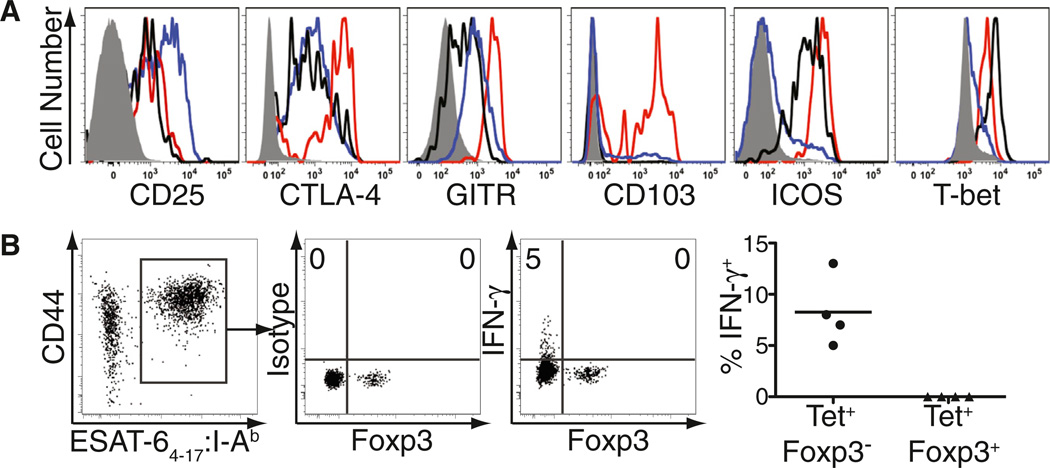
We next assessed tetramer-binding CD4+Foxp3+ T cells in the pLN for cell surface and intracellular molecules characteristic of Treg cells and their activation. Consistent with their identity as bona fide Treg cells, tetramer-binding Foxp3+ cells expressed high amounts of cell surface CD25, CTLA-4, GITR, CD103, and ICOS (Figure 2A, red histograms), compared to Foxp3-negative CD4+ T cells in the pLN that either did or did not bind the tetramer (Figure 2A, black and gray histograms, respectively). However, these antigen-specific Treg cells expressed less CD25 but higher amounts of CTLA-4, GITR, CD103, and ICOS than other Treg cells in the pLN that did not bind the tetramer (Figure 2A, blue histograms). Mtb-specific tetramer-binding Treg cells also expressed T-bet at only slightly lower levels than those observed in tetramer-binding effector T cells (Figure 2A). Thus, Treg cells recognizing Mtb antigens exhibit a more activated phenotype than other Treg cells in the same lymph node.
Figure 2. ESAT-64–17-Specific Treg Cells Express an Activated Phenotype.
(A) Expression of the indicated activation molecules by CD4+Foxp3+ cells that either bind (red) or do not bind (blue) the tetramer or by CD4+Foxp3− cells that either bind (black) or do not bind (solid gray) the tetramer in the pLN of Mtb-infected mice, 21 days after infection. Data are representative of three independent experiments.
(B) ESAT-64–17-specific cells among CD4+ tetramer-enriched pLNs were gated for subsequent analysis of IFN-γ production (left). Middle panels depict direct ex vivo detection of intracellular IFN-γ by Foxp3-expressing or Foxp3− cells 21 days after infection. Staining with an isotype antibody of irrelevant specificity (clone MOPC-21) was used as negative control. Numbers represent the percentage of tetramer-binding Foxp3− or Foxp3+ cells producing IFN-γ in this representative experiment. Cumulative data from four independent experiments are also shown; each circle or triangle represents the percentage of IFN-γ within the indicated population. Each solid circle or triangle represents an experiment and the bars represent the mean of the four experiments performed. See also Figure S2.
Because T-bet expression is strongly associated with IFN-γ production (Glimcher, 2007), we next asked whether T-bet-expressing Mtb-specific Treg cells produced IFN-γ in vivo. Tetramer-binding CD4+ T cells were analyzed directly ex vivo (without in vitro restimulation) for intracellular IFN-γ after harvesting pLN of Mtb-infected mice in the presence of a Golgi-mediated transport inhibitor (brefeldin A). For comparison we assessed effector T cells and consistently observed a population of tetramer-binding Foxp3-negative CD4+ T cells producing IFN-γ with no observed staining by using an isotype-matched control antibody (Figure 2B). At day 21 after infection, 5% of these effector CD4+ T cells were producing IFN-γ, and at day 25 this frequency increased to 14% (Figure S2). In contrast, we did not observe IFN-γ production at any time in tetramer-binding Foxp3+ cells (Figures 2B and S2). These results are consistent with our previous findings that Treg cells from Mtb-infected mice do not produce IFN-γ even after polyclonal stimulation in vitro (Scott-Browne et al., 2007) and support the idea that in contrast to the plasticity of Treg cells recently described in parasitic and viral infections (Oldenhove et al., 2009; Zhao et al., 2011), Treg cells do not produce IFN-γ during tuberculosis despite expressing high levels of T-bet (Koch et al., 2012).
Mtb-Specific Foxp3+CD4+ T Cells Arise from the Thymically Derived Treg Cell Lineage
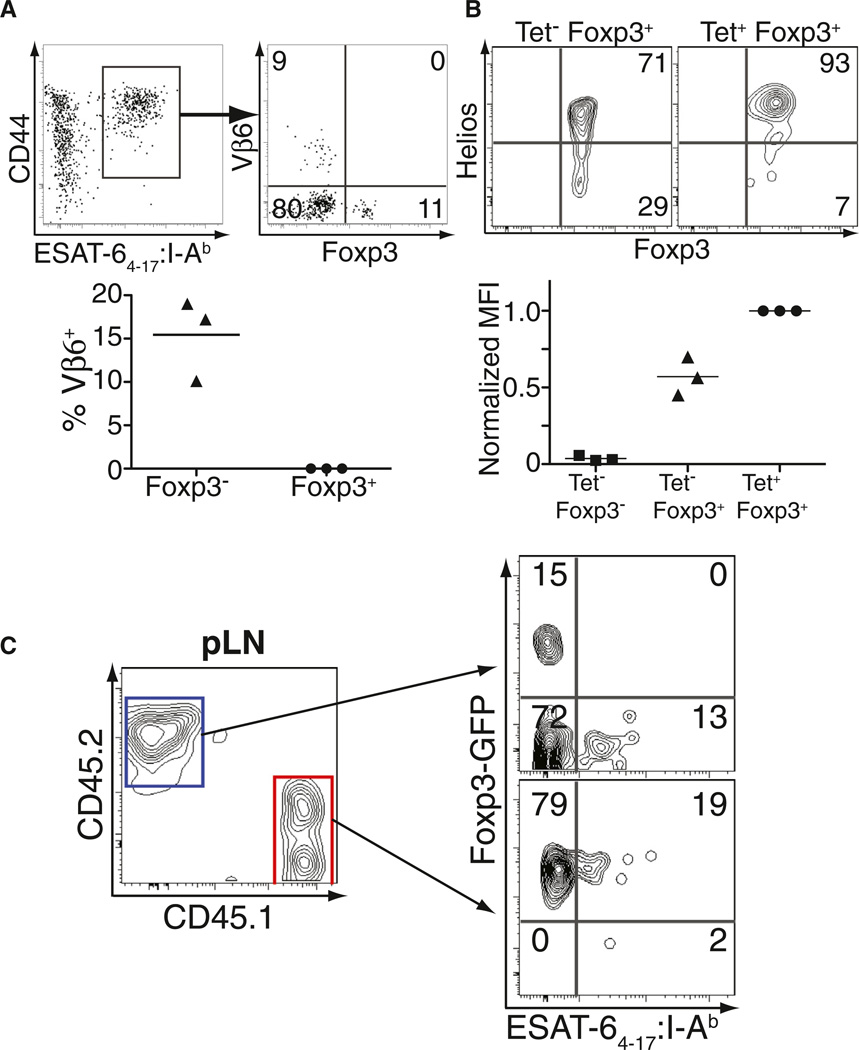
We next investigated the origin of expanded Mtb-specific Treg cells to determine whether they arise from the thymically derived or the peripherally induced Treg cell populations. Initially, the TCR usage of tetramer-binding Foxp3− and Foxp3+CD4+ T cells specific for the same Mtb epitope were characterized because a previous report showed that a subset of the ESAT-6-specific effector CD4+ T cells in Mtb-infected C57BL/6 mice express TCRs containing Vβ6 (Winslow et al., 2003). Consistent with these results, we observed an expanded population of Vβ6+CD4+ T cells within the Foxp3−, but not within the Foxp3+, tetramer-binding population (Figure 3A). These findings indicate that tetramer-binding Foxp3-negative and Foxp3+CD4+ T cells do not have identical TCR repertoires, and suggest they do not originate from the same precursor population.
Figure 3. ESAT-64–17-Specific Treg Cells Arise from Pre-existing Foxp3+ Precursors.
(A) Gated on ESAT-64–17-specific CD4+ T cells (left), the expression of Foxp3 and Vβ6 is shown in the tetramer-enriched pLN of mice 21 days after infection. Numbers represent the percentage of cells within each quadrant. Cumulative data from three independent experiments are also shown; each symbol represents the percentage of Vβ6+ T cells within the indicated population. Each solid triangle or circle represents an experiment and the bars represent the mean of the three experiments performed.
(B) Expression of Foxp3 and Helios is shown gated on non-tetramer-binding Foxp3+CD4+ T cells or the tetramer-binding Foxp3+ T cells from the enriched pLN on day 21 after infection. Numbers represent the percentage of cells in the quadrant. Cumulative data from three independent experiments show relative Helios expression by non-tetramer-binding Foxp3− cells, non-tetramer-binding Foxp3+ cells, and tetramer-binding Foxp3+ cells within the CD4-gated population of the same pLN. Because of variability in the MFI of Helios expression between individual experiments, values are normalized to those obtained for tetramer-binding Foxp3+ cells. Each solid square, triangle, or circle represents an experiment and the bars represent the mean of the three experiments performed.
(C) CD45.2 and CD45.1 expression on CD4-gated T cells from tetramer-enriched pLN of T cell reconstituted Tcrb−/− Tcrd−/− mice on day 21 after infection. Foxp3-GFP expression and ESAT-64–17:I-Ab tetramer binding is shown for cells transferred as either Foxp3− precursors (CD45.2, blue gate) or Foxp3+ precursors (CD45.1, red gate). Numbers represent the percentage of cells within each quadrant. The experiment was performed twice and representative data are shown. See also Figure S3.
We next assessed intracellular expression of Helios, a transcription factor reported to be highly expressed by thymically derived Treg cells but not peripherally induced Treg cells (Thornton et al., 2010). Helios was highly expressed in the majority of tetramer-binding Foxp3+CD4+ T cells (Figure 3B), whereas other pLN Treg cells showed a distribution of Helioshi and Helioslo cells consistent with published data (Thornton et al., 2010). These results support the idea that Mtb-specific Treg cells expand from the thymically derived Treg cell population.
Finally, we cotransferred congenically marked CD4+Foxp3− (CD45.2) and Foxp3+ (CD45.1) T cells from Foxp3-GFP reporter mice (Fontenot et al., 2005) into T cell-deficient (Tcra−/−Tcrd−/−) recipients and infected them with Mtb on the following day. ESAT-64–17:I-Ab-specific T cells were enriched from the pLN on day 21 after infection and Foxp3 expression was assessed in each congenically marked population (Figure 3C). The population initially transferred as Foxp3− cells gave rise only to tetramer-binding Foxp3−CD4+ T cells. In contrast, the Foxp3+ transferred cells gave rise to a population of tetramer-binding CD4+ T cells that continued to express Foxp3, directly indicating that Mtb-specific Treg cells expand from the pool of Foxp3+ CD4+ T cells present before infection (Figure 3C). Moreover, ESAT-64–17:I-Ab-specific Treg cells were observed in the pLN of Mtb-infected Tcra+/− mice (Figure S3), excluding the possibility that Treg cells recognizing this epitope were selected in the thymus by an alternative TCR with a different TCRα chain. Taken together, the results suggest that Mtb-specific Treg cells expand from the thymically derived Treg cell lineage.
Proliferation of Antigen-Specific Treg Cells Is Context Dependent
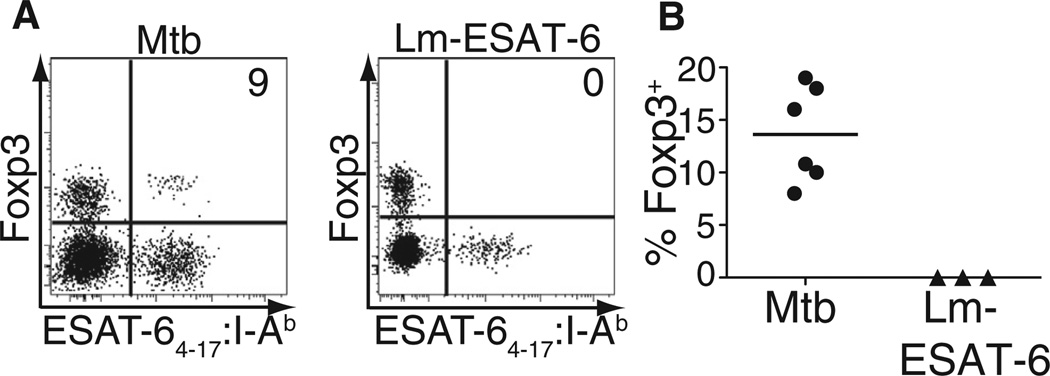
To investigate whether expansion of ESAT-64–17:I-Ab-specific Treg cells could reflect either unique properties of the ESAT-6 epitope or alternatively the lung tissue environment, we generated a recombinant strain of Lm expressing the ESAT-6 epitope (Lm-ESAT-6) and infected mice intranasally to establish a sublethal pulmonary infection. Although ESAT-64–17:I-Ab-specific effector T cells were readily detected in the pLN of Lm-ESAT-6-infected mice beginning on day 5 after infection, tetramer-binding Foxp3+ cells were not observed at any time during the infection, which is cleared between days 8 and 10 (Figure 4 and data not shown). In addition, only small numbers of Treg cells specific for an endogenous Lm antigen were observed (Figure S4). Thus, the finding that Mtb induces a sizeable population of microbe-specific thymically derived Treg cells cannot be explained by inherent properties of the ESAT-6 antigen itself, or primary infection within the lung, but instead suggests that Mtb selectively induces an inflammatory milieu that is conducive to the expansion of antigen-specific thymically derived Treg cells.
Figure 4. Expansion of ESAT-64–17-Specific Treg Cells Is Context Dependent.
(A) Foxp3 expression and ESAT-64–17:I-Ab tetramer binding within CD4-gated tetramer-enriched pLN of Mtb-infected or Lm-ESAT-6-infected mice at day 21 or day 9 after infection, respectively. Pooled pLNs from each group were enriched for tetramer-binding cells, and the experiment was performed six times for Mtb and three times for Lm-ESAT-6. Numbers represent the percentage of tetramer-binding T cells that express Foxp3.
(B) Cumulative data from six experiments with Mtb-infected and three experiments with Lm-ESAT-6-infected mice are shown. Each circle or triangle represents a time point. See also Figure S4.
Mtb-Specific Treg Cells Undergo Subsequent Contraction
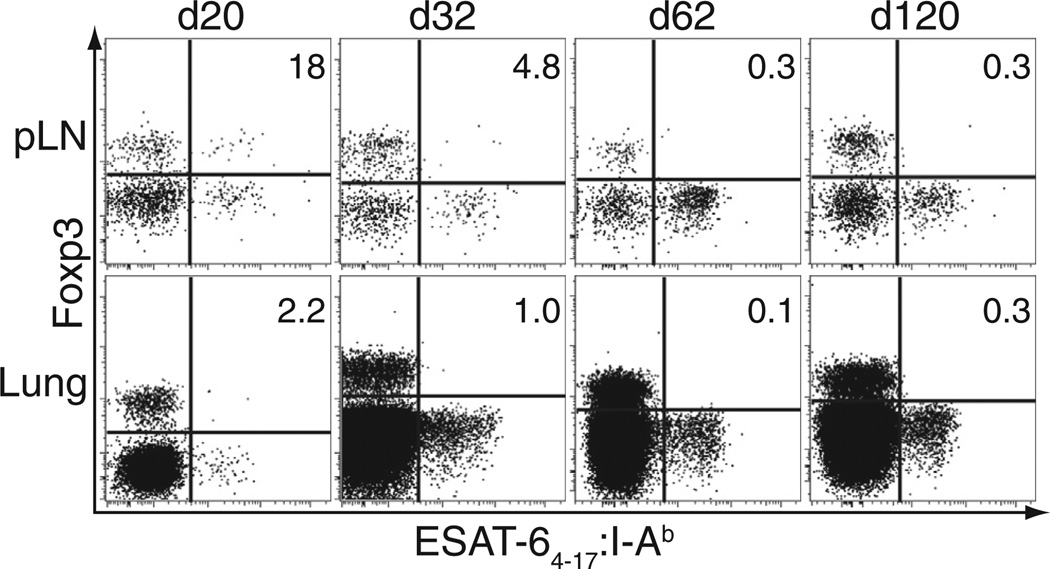
We next monitored the location and abundance of antigen-specific Treg cells throughout the course of Mtb infection, focusing on the pLN and lung (the primary site of infection). In the pLN, the percentage of tetramer-binding CD4+ T cells that expressed Foxp3 was highest early after infection, up to 20% (150–300 cells per lymph node) at day 20 (Figure 5). By day 32 after infection, this percentage fell to less than 5% (5–35 cells), and tetramer-binding Treg cells were only inconsistently detected at these later time points. In the lung, however, there was a relative paucity of tetramer-binding Treg cells at any time point. In some experiments, up to 5% of tetramer-binding CD4+ T cells expressed Foxp3 at day 20, but very few, if any, of these cells could be detected later (Figure 5). Thus, Mtb-specific Treg cells undergo initial expansion in the pLN during the period when rapid priming and expansion of Mtb-specific effector T cells is critical to establish early control of infection (Cooper, 2009; Shafiani et al., 2010; Urdahl et al., 2011). However, these cells are short lived and do not accumulate in the lung.
Figure 5. Mtb-Specific Treg Cells Exhibit Early but Short-Lived Accumulation in the pLN.
Mtb-infected mice were sacrificed at the indicated time points after infection, and cells from their lungs and tetramer-enriched pLNs were analyzed for tetramer binding and Foxp3 expression. Numbers in the quadrants represent the percentage of tetramer-binding cells that express Foxp3. Experiments were performed ten times at day 20 or 21, six times at day 32–35, and twice at each later time point.
IL-12-Induced Intrinsic T-bet Expression Drives Mtb-Specific Treg Cell Contraction
We next asked whether contraction of Mtb-specific Treg cells could be due to the lack of available IL-2, a cytokine that drives Treg cell proliferation and survival (Cheng et al., 2011). We administered IL-2 plus IL-2 antibody complexes to Mtb-infected mice during the peak of the antigen-specific Treg cell response. Although we observed expansion of Treg cells with other specificities in the pLN, the Mtb-specific Treg cells still underwent contraction (Figure S5). These results are consistent with our observation that Mtb-specific Treg cells express lower amounts of CD25 than do other Treg cells in the pLN (Figure 2A) and suggest that contraction is not the result of IL-2 deprivation.
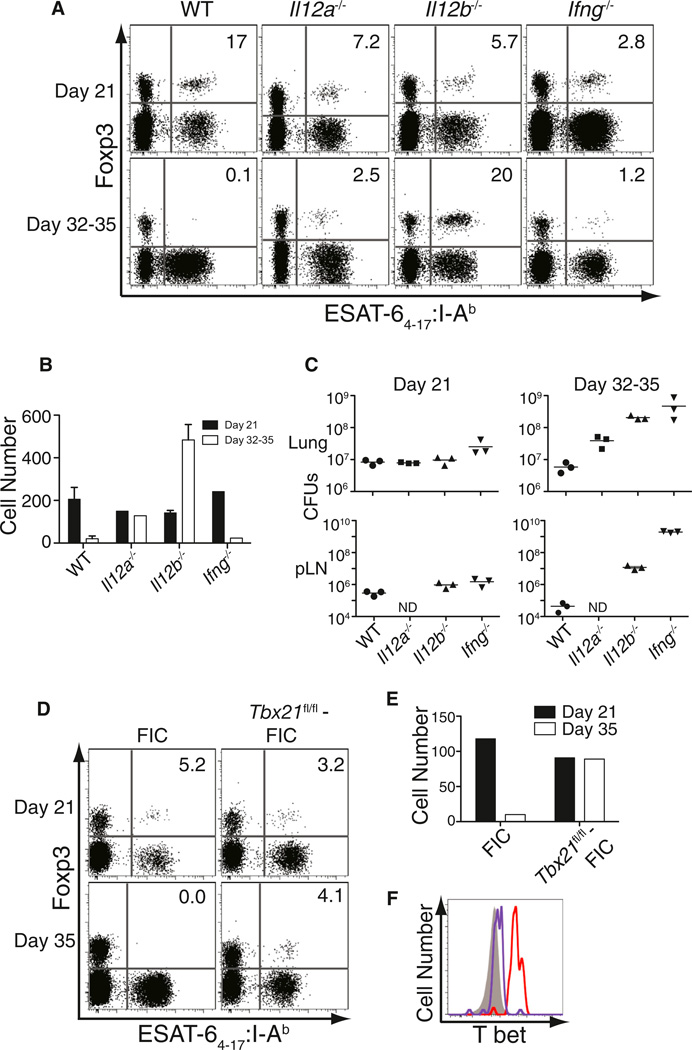
To investigate the possibility that other inflammatory cytokines drive the disappearance of Mtb-specific Treg cells, we infected mice deficient in either IFN-α/β receptor (Ifnar−/−) or the cytokines IL-6 (Il6−/−), IL-12p35 (Il12a−/−), and IL-12p40 (Il12b−/−), which are known to participate in the inflammatory response to Mtb infection (Cooper et al., 2011). The contraction tempo of Mtb-specific Treg cells in mice lacking either the IFN-α/β receptor or IL-6 was comparable to WT controls (Figure S6A). By contrast, Mtb-specific Treg cells did not appear to undergo contraction in IL-12p35-deficient mice; they were observed in similar numbers at days 21 and 35 after infection (Figures 6A and 6B). In IL-12p40-deficient mice, not only did Mtb-specific Treg cells persist, but they continued to expand from day 21 through day 35 in the pLN and were also observed in the lungs (Figure S6). The lack of Treg cell contraction in IL-12-deficient mice could not be explained by increased bacterial and antigenic load because IFN-γ-deficient mice had similar or even higher bacterial burdens in their lungs and pLN (Figure 6C) but exhibited contraction of Treg cells similar to WT mice. These results suggest that IL-12 (p40:p35 heterodimer) is essential for Mtb-specific Treg cell contraction and that the p40 molecule further impedes the continued expansion of these cells in an IL-12-independent manner.
Figure 6. Mtb-Specific Treg Cell Contraction Is Driven by IL-12-Induced T-bet Expression.
(A) FACS plots represent column-enriched pLNs from Mtb-infected control wild-type (WT) B6 mice and mice deficient in IL-12p35 (Il12a−/−), IL-12p40 (Il12b−/−), and IFN-γ (Ifng−/−) analyzed for tetramer binding cells and Foxp3 expression. Mice were harvested at day 21 and 35 after infection with the exception of IFN-γ-deficient mice that were analyzed at day 32 because they had lost 20% of their body weight and appeared near death. Numbers in the quadrants represent the percentage of Foxp3+ cells within the tetramer-binding population. Experiments were performed four times for the WT and Il12b−/− mice, twice for Ifng−/− mice, and once for the Il12a−/− mice. Representative plots are shown.
(B) Bar graphs represent absolute number of tetramer-binding Treg cells at the two time points in all the above groups. Mean ± SEM is shown for the WT and Il12b−/− groups.
(C) Mtb CFUs in the lungs and pulmonary lymph nodes of the four groups of mice at the two time points are depicted (ND, not determined). Each solid circle, square, or triangle represents a mouse and the bar represents the mean of the group.
(D) FACS plots represent column-enriched pLNs from Mtb-infected control Foxp3-IRES-Cre mice (FIC), and mice with conditional deficiency of T-bet expression in Foxp3-expressing Treg cells (Tbx21fl/fl:FIC), analyzed for tetramer-binding cells and Foxp3 expression. Mice were harvested at day 21 and 35 after infection. Numbers in the quadrants represent the percentage of Foxp3+ cells within the tetramer-binding population. Experiments were performed once for each time point.
(E) Bar graphs represent absolute number of tetramer-binding Treg cells at the two time points in both groups.
(F) Histograms denote the levels of T-bet expression in tetramer-binding Treg cells in either control FIC mice (red) or Tbx21fl/fl-FIC (purple) and CD44−Foxp3−CD4+ T cells (solid gray) in the pLN at day 21 after infection. See also Figure S6.
T-bet expression was 2-fold higher in Mtb-specific Treg cells in WT mice compared to mice lacking IL-12 (MFI values of 2,430 and 1,330, respectively; data not shown), an observation consistent with the finding that IL-12 is required for high T-bet expression in Treg cells (Koch et al., 2012). Because T-bet can exhibit proapoptotic properties in some settings (Joshi et al., 2007; Oldenhove et al., 2009), the dynamics of Mtb-specific Treg cells were examined in mice lacking T-bet specifically in Foxp3+ Treg cells (Tbx21fl/fl:Foxp3-IRES-Cre mice) (Intlekofer et al., 2008; Wing et al., 2008). We found that Mtb-specific Treg cells with a targeted deficiency in T-bet persisted until day 35 after infection, directly paralleling the response in IL-12p35-deficient mice (Figures 6D–6F). Taken together, these findings demonstrate that IL-12-induced T-bet expression drives the contraction of pathogen-specific Treg cells during persistent Mtb infection.
DISCUSSION
Here we show that antigen-specific thymically derived Treg cells, defined to the epitope level, can expand to a foreign antigen during infection. This finding is supported by three lines of evidence. (1) Conventional and Treg cells recognizing the same Mtb epitope (i.e., ESAT-64–17:I-Ab) utilized discordant TCR repertoires, suggesting that they arose from separate lineages. (2) ESAT-64–17:I-Ab-specific Treg cells expressed high amounts of Helios, a transcription factor associated with thymically derived Treg cells (Thornton et al., 2010). (3) Reconstitution studies demonstrated that ESAT-64–17:I-Ab-specific Treg cells expanded from the pre-existing pool of Foxp3+ T cells present in uninfected mice.
Mtb-specific Treg cells exhibited a highly activated phenotype with increased Foxp3, Helios, CTLA-4, GITR, CD103, and ICOS expression compared with other Treg cells in the pLN. These results suggest that antigen recognition is critical and that Treg cells are not activated nonspecifically by the inflammatory milieu of Mtb infection. Expression of each of these molecules has been associated with enhanced Treg cell immunosuppression (Chauhan et al., 2009; Miyara and Sakaguchi, 2007; Vignali et al., 2008; Wing et al., 2008; Zabransky et al., 2012), and this activation state may explain why small numbers of adoptively transferred Mtb-specific Treg cells, but not Treg cells with irrelevant specificities, were capable of restricting protective immunity against Mtb (Shafiani et al., 2010). T-bet expression by Mtb-specific Treg cells is likely to be important in their functional specialization and has been shown to augment their proliferative and suppressive properties (Koch et al., 2009). In contrast to reports in Toxoplasma gondii (T. gondii) and neurotropic mouse hepatitis infections (Oldenhove et al., 2009; Zhao et al., 2011), Treg cells activated during Mtb infection do not acquire the ability to produce IFN-γ. Thus, inflammation associated with Mtb infection seems to confer a measured degree of Treg cell differentiation compared with other infections and results in an activated but suppressive pathogen-specific Treg cell population.
Studies showing that the TCR repertoire of the thymically derived Treg cell population is at least as diverse as that of conventional CD4+ T cells (Hsieh et al., 2006; Pacholczyk et al., 2007) may help to explain their capacity to cross react to foreign antigens despite their positive selection by high-affinity interactions with self-antigens within the thymus (Bautista et al., 2009; DiPaolo and Shevach, 2009). Our finding that pre-existing thymically derived Treg cells specific for a pathogen-derived epitope undergo proliferation in the context of pulmonary infection with Mtb, but not Lm, suggests that the nature of the inflammatory environment may dictate whether or not antigen-specific Treg cells become activated. Despite the fact that IL-6 has been shown to inhibit Treg cell responses in other experimental systems (Korn et al., 2007; Pasare and Medzhitov, 2003) and despite our demonstration that IL-12p40 can drive contraction of Treg cells in the context of tuberculosis, expansion of Lm-specific Treg cells was not rescued in mice deficient in either IL-6 or IL-12p40 (data not shown). Thus, the inflammatory milieu elicited by Lm probably contains multiple redundant factors that contribute to the inhibition of Lm-specific Treg cell proliferation. In the case of Mtb infection, it is tempting to speculate that Mtb itself manipulates the inflammatory response to promote the pLN proliferation of these suppressive T cells during the period of effector T cell priming.
In addition to governing whether antigen-specific Treg cells expand in numbers, the quality and magnitude of the inflammatory response probably also dictates whether these highly immunosuppressive cells are allowed to persist. Long-term survival of antigen-specific Treg cells is permitted during the alternative inflammatory milieu of pregnancy when their activity is advantageous in promoting fetal tolerance in subsequent pregnancies (Rowe et al., 2012). By contrast, in the infectious setting of tuberculosis, potentially deleterious antigen-specific Treg cells are actively eliminated. IL-12 and intrinsic T-bet expression within Mtb-specific Treg cells are each required for their elimination, suggesting that the culling of immune-suppressive Treg cells is driven by the Th1 cell inflammatory response. These results are consistent with recent studies illustrating that intermediate T-bet expression in Treg cells can be induced by IFN-γ and STAT-1 signaling, but that high expression, such as we observed in Mtb-specific Treg cells, requires IL-12 receptor signaling (Koch et al., 2012). It seems likely that the disappearance of Mtb-specific Treg cells represents a true contraction, as indicated by the fact that high T-bet expression is proapoptotic in some settings (Joshi et al., 2007; Oldenhove et al., 2009). However, future studies are needed to exclude the possibility that pathogen-specific Treg cells simply turn off Foxp3 expression and join the Mtb-specific effector T cell pool (Zhou et al., 2009).
In addition to the unambiguous roles of IL-12 and T-bet in driving antigen-specific Treg cell contraction, the robust expansion of Treg cells at later time points in IL-12p40-deficient mice suggests that the p40 molecule further restricts the ongoing proliferation of antigen-specific Treg cells. This restriction of pathogen-specific Treg cells occurs despite the fact that other Treg cells in the pLN continue to increase in numbers (Scott-Browne et al., 2007). The p40 molecule can partner with p19 to form IL-23 heterodimers, and IL-23 has been shown to be necessary to sustain Th17 cell responses during tuberculosis (Khader et al., 2005). In addition, p40 homodimers play an important role in activating dendritic cells during Mtb infection and promote migration of Mtb-infected dendritic cells from the lung to the LDLN (Khader et al., 2006). Further studies are needed to elucidate the mechanisms by which p40 restricts the expansion of antigen-specific Treg cells during Mtb infection.
In contrast to the selective culling of pathogen-specific Treg cells observed during Mtb infection, T. gondii infection, which elicits an even stronger Th1 cell inflammatory response than does tuberculosis, drives the collapse of the entire Treg cell population (Oldenhove et al., 2009). This collapse is driven, at least in part, by IL-2 deprivation during the extreme Th1 cell environment of T. gondii infection and leads to lethal inflammatory dysregulation. During tuberculosis, however, the global Treg cell population undergoes continued expansion at the primary sites of infection in the lungs and pLNs (Kursar et al., 2007; Scott-Browne et al., 2007) despite the selective culling of pathogen-specific Treg cells. Furthermore, Treg cell contraction during Mtb infection cannot be explained by the lack of IL-2 availability but is driven by intrinsic expression of T-bet within Mtb-specific Treg cells. Nearly all Treg cells exhibit high T-bet expression during infection with T. gondii, whereas high expression of T-bet is restricted almost exclusively to pathogen-specific Treg cells during Mtb infection. Taken together, our results suggest that a tempered Th1 cell inflammatory response can mediate the targeted culling of pathogen-specific Treg cells without compromising the global Treg cell population and unleashing lethal immunopathology.
Although the Mtb-specific Treg cell response that we observed was short lived, their expansion in the pLN coincides with the initiation of the effector T cell response at this site (Cooper, 2009). This is also precisely where and when adoptively transferred Mtb-specific transgenic Treg cells (fewer than 100 transferred cells in the pLN) were shown to impair protection by slowing the kinetics of effector T cell priming and arrival in the lung (Shafiani et al., 2010). Here we report that 150–300 endogenous Treg cells in the pLN are specific for a single Mtb-derived epitope and that Treg cells recognizing other Mtb antigens also exist. Thus, the relatively high number of endogenous Mtb-specific Treg cells that accumulate in the pLN is likely to have a profound effect on the rate at which effector T cells are primed and reach the lung. This is important because the rapidity of the effector T cell response to pulmonary Mtb infection is strongly correlated with long-term protection (Cooper, 2009; Urdahl et al., 2011). When Mtb effector T cells are late to arrive in the lung, a prolonged period of unrestrained bacterial replication ensues and probably contributes to Mtb persistence by allowing the bacteria to establish a lung niche.
Antigen-specific Treg cells have the capacity to persist as memory cells when stimulated in the alternative environment of pregnancy (Rowe et al., 2012). This raises the question of whether memory Treg cell responses may be induced to myco-bacteria in less inflammatory scenarios when IL-12p40 levels are lower or less sustained. Mycobacterium bovis BCG, the tuberculosis vaccine currently used, stimulates much less inflammation compared with Mtb but shares many common antigens (Andersen and Doherty, 2005). Likewise, nontuberculous mycobacteria that also share many antigens with Mtb are relatively ubiquitous in the environment (Falkinham, 2009) and human exposure, usually occurring in the gastrointestinal tract, causes minimal inflammation. Future studies are needed to determine whether BCG immunization or exposure to nontuberculous mycobacteria can induce the expansion of antigen-specific Treg cells, and if so, whether they are long lived and can mount a recall response that restricts immunity to Mtb infection.
Our findings have important implications for tuberculosis and other chronic diseases in which antigen-specific Treg cells restrict immunity. If the immune pathways that promote antigen-specific Treg cell expansion during early tuberculosis can be identified, pharmacologic manipulation of these pathways in recently exposed individuals may provide new avenues to hasten the protective T cell response, achieve earlier control of Mtb replication in the lung, and ultimately improve infection outcomes. Conversely, insights into the IL-12p40-dependent pathways that mediate the selective culling of antigen-specific Treg cells during tuberculosis could inform therapeutic strategies for other chronic diseases, such as cancer. Targeted ablation of tumor-specific Treg cells could enhance tumor clearance with less risk of triggering autoimmunity compared to current approaches that suppress or eliminate Treg cells nonspecifically (Byrne et al., 2011).
EXPERIMENTAL PROCEDURES
Mice
C57BL/6, B6.SJL-Ptprca Pepcb/BoyJ (CD45.1), B6.129P2-Tcrbtm1Mom Tcrdtm1Mom/J (Tcrb−/− Tcrd−/−), B6.129S2-Tcratm1Mom/J (Tcra−/−), and mice deficient in IL-6 (B6.129S2-Il6tm1Kopf/J), IL-12p40 (B6.129S1-Il12btm1Jm/J), IL-12p35 (B6.129S1-Il12atm1Jm/J), and IFN-γ (B6.129S7-Ifngtm1Ts/J) were purchased from Jackson Laboratories. Foxp3-GFP mice (bred 12 times to C57BL/6 mice) were provided by A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY), and some of these mice were bred to homozygosity with B6.SJL-Ptprca Pepcb/BoyJ mice (CD45.1). Mice deficient in the IFN-α/β receptor (Müller et al., 1994) were provided by A. Aderem (Seattle Biomedical Research Institute). Mice homozygous for the TCRα gene deletion were crossed with C57BL/6 mice and the F1 generation (TCRα+/−) was used for Mtb infection. Mice with loxp sites flanking both alleles encoding T-bet (Tbx21fl/fl) on C57BL/6 background (Intlekofer et al., 2008) were crossed with Foxp3-IRES-Cre (FIC) transgenic mice on C57BL/6 background (Wing et al., 2008) to generate Tbx21fl/fl:FIC mice with a specific knockout of Tbx21 in Foxp3-expressing cells. All mice were housed and bred under specific-pathogen-free conditions at the University of Washington and Seattle Biomedical Research Institute, and all experiments were performed in compliance with the respective Institutional Animal Care and Use Committees.
Bacteria and Aerosol Infections
A stock of Mtb strain H37Rv was sonicated before use and mice were infected as described earlier (Shafiani et al., 2010). For Mtb CFU determination, lungs were homogenized in PBS containing 0.05% Tween 80, and serial dilutions were plated out on 7H10 agar plates. Colonies were counted after 21 days of incubation at 37°C. In some experiments, mice were anesthetized with ketamine and xylazine and then intranasally administered recombinant Listeria monocytogenes engineered to stably express and secrete Mtb ESAT-61–20 (Lm-ESAT-6; 5 × 106 CFUs in 30 µl total volume). Lm-ESAT-6 was constructed by PCR amplifying the promoter and coding regions for ESAT-61–20 from the pAM401-based expression construct (Orr et al., 2007), subcloning into the temperature-sensitive plasmid pKSV7, and selection for clones with homologous recombination after electroporation into Lm-OVA as described (Rudd et al., 2011). The number of viable Lm-ESAT-6 in the lungs was performed on a couple of mice 30 min after infection after plating serial dilutions of the lung homogenate onto BHI agar and overnight incubation at 37°C. In some experiments, an attenuated strain of Lm lacking the expression of the virulence determinant ActA and expressing the ESAT-6 antigen heterologously was used (1 × 108 CFUs in 50 µl total volume).
Lung and pLN Cell Isolation
Single-cell preparations of lungs and pLNs were prepared as described earlier (Urdahl et al., 2003). In experiments where IFN-γ was being detected directly ex vivo, brefeldin A (Sigma, 10 µg/ml) was added to all media during tissue processing.
Tetramer and Surface Staining
PE or APC-labeled MHC class II tetramers (I-Ab) containing the stimulatory residues 4 to 17 (QQWNFAGIEAAASA) of the early secreted antigenic target 6 kDa (ESAT-6) and amino acids 240 to 254 (FQDAYNAAGGHNAVF) of Antigen 85B of Mtb were generous gifts from M. Jenkins (Moon et al., 2007) and the NIH tetramer facility, respectively. APC-labeled MHC class II tetramers (I-Ab) containing the stimulatory residues 190 to 201 (NEKYAQAYPNVS) of the Listeriolysin (LLO) antigen of Listeria monocytogenes was kindly provided by M. Pepper. Single-cell preparations from lungs and pLNs were incubated for 1 hr at room temperature with tetramers (5–10 nM) in 1:1 Fc block (2.4G2) and sorter buffer (PBS containing 0.1% NaN3 and 2.5% fetal bovine serum), followed by washing in sorter buffer. Lung cells were then stained with anti-CD3 (eBioscience), anti-CD4 (Invitrogen), anti-CD44 (eBioscience), anti-CD8 (eBioscience), and a non-T cell cocktail containing anti-F4/80, anti-CD19, anti-CD11c, and anti-CD11b (eBioscience). ESAT-64–17 or Ag85B240–254-specific T cells were identified as CD3+, non-T cell cocktail-negative, CD4+, CD8−, tetramer+, CD44hi events. Because of the lower frequency of antigen-specific T cells in the lymph nodes, after the tetramer staining step, the pLNs were enriched for antigen-specific cells as described earlier (Moon et al., 2009) and stained for surface markers, as described for lungs. Additionally, in some experiments, antibodies were used to detect CD25 (BD Biosciences), GITR (BD Biosciences), CD103 (BD Biosciences), ICOS (BD Biosciences), Vβ6 TCR (BD Biosciences), CD45.1 (eBioscience), and CD45.2 (eBioscience) markers. Samples were fixed and analyzed with a FACSCanto or LSR-II (BD Biosciences) and FlowJo (Treestar) software.
Intracellular Staining
In most experiments, tetramer and surface staining was followed by staining for intracellular markers according to manufacturer’s recommendations (eBioscience). Cells were fixed and permeabilized with eBiosciences Fix/Perm buffer for 1 hr, followed by staining for Foxp3 by Foxp3 antibodies (eBioscience) in Permeabilization/Wash buffer (eBiosciences) for 30 min. Stained cells were acquired and analyzed as described above. In some experiments, IFN-γ (BD Biosciences), Helios (Biolegend), CTLA-4 (BD Biosciences), and T-bet (Biolegend) antibodies were added in addition to Foxp3 to allow the detection of these markers.
Adoptive Transfer
For adoptive transfer experiments, CD4+ T cells from Foxp3-GFP reporter mice on congenic CD45.1 and CD45.2 backgrounds were negatively enriched to >95% purity from freshly isolated spleen and LN cells, using magnetic microbeads and subsequent column purification according to the manufacturer’s protocol (Miltenyi Biotec). GFP+ cells (Treg cells) and GFP− cells (conventional CD4+ T cells) were then sorted from the CD45.1- and CD45.2-expressing populations, respectively, on a FACSAria (BD Biosciences). Sorted cells were then analyzed for purity, mixed, and adoptively cotransferred into recipient TCRβ−/−TCRδ−/− mice (1.5 × 106 GFP+ and 10 × 106 Foxp3-GFP− cells per mouse).
IL-2-Anti-IL-2 Antibody Complex Treatment
IL-2 cytokine and anti-IL-2 antibody were purchased from eBioscience and Bio X Cell, respectively. Starting on day 20 after Mtb infection, mice received daily intraperitoneal doses of a mixture of IL-2 (1.5 µg) and IL-2 antibody (15 µg) per mouse (200 µl volume) for 5 consecutive days before being harvested on day 25 after infection. Prior to injection, the IL-2 cytokine and IL-2 antibody were mixed and incubated at 37°C for 30 min. Control mice were administered equal volume of PBS.
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Stohr for technical assistance; M. Jenkins, M. Pepper, and H. Chu for technical advice regarding tetramer staining; M. Jenkins, R. Larson, J. Lund, and L. Ramakrishnan for comments on the manuscript. This work was funded by grants to K.B.U. (National Institutes of Health R01AI076327 and from the Paul G. Allen Family Foundation), to S.S.W. (NIH R01AI087830 and R01AI100934 and the Burroughs Wellcome Fund Infectious Disease Program), and to D.J.C. (NIH R01AI085130). I.S. was supported by Award Number D43TW000924 from the Fogarty International Center.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2013.06.003.
REFERENCES
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- Antunes I, Tolaini M, Kissenpfennig A, Iwashiro M, Kuribayashi K, Malissen B, Hasenkrug K, Kassiotis G. Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology. Immunity. 2008;29:782–794. doi: 10.1016/j.immuni.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu. Rev. Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- Betts RJ, Prabhu N, Ho AW, Lew FC, Hutchinson PE, Rotzschke O, Macary PA, Kemeny DM. Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response. J. Virol. 2012;86:2817–2825. doi: 10.1128/JVI.05685-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71:6915–6920. doi: 10.1158/0008-5472.CAN-11-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol. Rev. 2011;241:63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo RJ, Shevach EM. CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur. J. Immunol. 2009;39:234–240. doi: 10.1002/eji.200838772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt JM, Rowe JH, Johanns TM, Lai JC, McLachlan JB, Way SS. Selective priming and expansion of antigen-specific Foxp3-CD4+ T cells during Listeria monocytogenes infection. J. Immunol. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO., 3rd Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 2009;107:356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Glimcher LH. Trawling for treasure: tales of T-bet. Nat. Immunol. 2007;8:448–450. doi: 10.1038/ni0507-448. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killebrew JR, Perdue N, Kwan A, Thornton AM, Shevach EM, Campbell DJ. A self-reactive TCR drives the development of Foxp3+ regulatory T cells that prevent autoimmune disease. J. Immunol. 2011;187:861–869. doi: 10.4049/jimmunol.1004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar M, Koch M, Mittrücker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 2007;178:2661–2665. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Hataye J, Pagán AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat. Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Dash P, Oguin TH, 3rd, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MT, Orgun NN, Wilson CB, Way SS. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J. Immunol. 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Punkosdy GA, Blain M, Glass DD, Lozano MM, O’Mara L, Dudley JP, Ahmed R, Shevach EM. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc. Natl. Acad. Sci. USA. 2011;108:3677–3682. doi: 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Žugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc. Natl. Acad. Sci. USA. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdahl KB, Liggitt D, Bevan MJ. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/−Db−/− mice, but provide minimal protection. J. Immunol. 2003;170:1987–1994. doi: 10.4049/jimmunol.170.4.1987. [DOI] [PubMed] [Google Scholar]
- Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–293. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J. Immunol. 2003;170:2046–2052. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, Tam AJ, Getnet D, Drake CG. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS ONE. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhao J, Fett C, Trandem K, Fleming E, Perlman S. IFN-γ- and IL-10-expressing virus epitope-specific Foxp3(+) T reg cells in the central nervous system during encephalomyelitis. J. Exp. Med. 2011;208:1571–1577. doi: 10.1084/jem.20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.