Abstract
PURPOSE
Men with Gleason score (GS) 8-10 prostate cancer (PCa) are assumed to have a very high risk of micrometastatic disease at presentation. However, local failure is also a major problem. We sought to establish the importance of more aggressive local radiotherapy to ≥80 Gy.
METHODS
There were 226 men treated consecutively with RT ± ADT from 1988 to 2002 for GS 8-10 PCa. Conventional, 3D conformal, or intensity-modulated (IM) RT was used. Radiation dose was divided into three groups: 1: <75 Gy (n=50); 2: 75-79.9 Gy (n=60); or 3: ≥80 Gy (n=116). The endpoints examined included biochemical failure (BF; nadir+2 definition), distant metastasis (DM), cause specific mortality (CSM) and overall mortality (OM).
RESULTS
Median follow-up was 66, 71, and 58 months for groups 1, 2 and 3. On Fine and Gray’s competing risk regression analysis, significant predictors of reduced BF were RT dose ≥80 Gy (p=0.011) and ADT duration ≥24 months (p=0.033). In a similar model of DM, only RT dose ≥ 80 Gy was significant (p=0.007). On Cox regression analysis, significant predictors of reduced OM were RT dose ≥ 80 Gy (p=0.035) and T-category (T3/4 vs. T1, p=0.041). Dose was not a significant determinant of CSM. Results for RT dose were similar in a model with RT dose and ADT duration as continuous variables.
CONCLUSION
The results indicate that RT dose escalation to ≥80 Gy is associated with lower risks of BF, DM, and OM in men with GS 8-10 PCa, independently of ADT.
Keywords: High grade, survival, radiation, dose escalation
Introduction
Men diagnosed with Gleason Score (GS) 8-10 prostate cancer are considered to be at high risk for radiotherapy treatment failure because many are presumed to have occult micrometastatic disease at presentation. Although some gains have been realized with radiotherapy (RT) dose escalation, the results in this population are relatively poorly defined. We hypothesized that local persistence remains a significant concern, even in the setting of RT combined with androgen deprivation therapy (ADT), and that greater gains would be realized by increasing radiation doses to ≥80 Gy.
Several randomized trials have shown a modest benefit with dose escalated RT to doses approaching, but below, 80 Gy (1-4). None of these studies examined RT doses above 80 Gy, and included at most 101 patients with diagnostic biopsy GS 8-10 disease.
The addition of long term ADT to standard dose RT in the treatment of men with high-risk prostate cancer has become the standard of care based on several Phase III randomized studies from the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). In RTOG 92-02, which compared short term ADT to long term ADT + standard dose RT, an overall survival difference was not seen, but a subgroup analysis showed a benefit for long term ADT+RT in men who had GS 8-10 disease (5). However, the 10 yr biochemical failure (BF) rates were quite high with long term ADT + RT, at 56%. An EORTC trial suggested that short term ADT was associated with inferior survival compared to long term ADT + standard dose RT for locally advanced prostate cancer (6). In this trial, the rates of BF for men with GS 8-10 were not specifically reported.
A key clinical question confronted by radiation oncologists on a routine basis is whether to use dose-escalated RT with ADT in men with GS 8-10 disease. What is not known is whether the beneficial effect of long term ADT + RT is due to local or distant effects. If long term ADT acts to reduce local persistence of disease, it is possible that higher doses of radiation may be of little added benefit, but could reduce the length of ADT needed to achieve optimal results. Alternatively, more intense local therapy might further improve the results achieved with long term ADT.
Although there is some evidence from randomized trials that higher RT doses do improve patient outcome in the setting of ADT (4, 5), no Phase III study has examined RT dose escalation to ≥80 Gy with long term ADT. Because the population studied here includes patients treated to these high RT doses, the analysis affords a unique insight into the impact of aggressive local therapy on prostate cancer patients with high grade disease. The purpose was to evaluate the benefit of RT to ≥80 Gy in men with GS 8-10 prostate cancer, with and without ADT for varying periods of time.
Methods
From June 1988 to December 2002, 226 patients were treated consecutively for T1-T4/Tx, N0/NX, M0 GS 8-10 prostate cancer using conventionally fractionated external beam RT at Fox Chase Cancer Center, as per the 2002 AJCC staging guidelines (7). T-category was based on digital rectal exam. If ADT was started prior to the exam, our policy was to score this as Tx. While all study patients had to have a recorded pretreatment initial PSA (iPSA), no exclusions were made with regard to iPSA level. All outside pathology slides were centrally reviewed at Fox Chase Cancer Center.
The conventional and 3D conformal treatment techniques have been previously described (8, 9). Patients received 46-50 Gy to a small pelvis field, followed by a conformal boost to the prostate and seminal vesicles in 2.0 Gy fractions. Typical small pelvis field borders were the middle of the sacroiliac joints superiorly, the bottom on the ischial tuberosities inferiorly, the symphysis pubis anteriorly, the S2/S3 interspace posteriorly, and 1.5 centimeters beyond the pelvic brim laterally. These pelvic fields were shaped only by corner blocks and were delivered with 2-field, 3-field, or 4-field beam arrangements. The planning target volume (PTV) for conformal radiotherapy included the prostate with or without the seminal vesicles, with a margin of 1-1.5 centimeters to the block edge. All conformal treatments utilized 10 to 18 MV photons with a 4-field or 5-field beam arrangement. The radiation dose was prescribed to the 95% isodose line of the beam arrangements. As recommended by the International Commission on Radiation Units and Measurements (ICRU), radiation dose is reported here as the dose delivered to isocenter (10). The ICRU dose was 0.7% to 7.5% (median 5.3%) of the prescribed dose.
The IMRT technique used has also been described previously (11). The primary clinical target volume (CTV1) included the prostate and any extraprostatic extension and the proximal seminal vesicles, defined as the most proximal 9-10 mm of seminal vesicles. Structures included in the CTV2 included the distal seminal vesicles and the CTV3 comprised the periprostatic, periseminal vesicle, external iliac, obturator, and internal iliac lymph nodes. The PTV1, PTV2, and PTV3 margins were 8 mm in all dimensions except posteriorly, in which the margin was 5 mm. PTV1 was planned to receive ≥ 95% of the prescription dose, and PTV2 and PTV3 were planned to receive ≥95% of 56 Gy over the full treatment course. The mean PTV dose for IMRT was used in the MVAs because there is greater heterogeneity (usually greater than 10%) for IMRT (12), overall median and mean doses are similar (13), and mean doses have been used as a descriptor in the past by our group and others (14, 15). In our series, isocenter dose was 8.2% to 21% (median of 16.9%) above the prescribed dose, whereas the mean PTV dose was a median of 5.7% above the prescribed dose.
Image guidance was started in 1998 using the BAT® (Best NOMOS, Pittsburgh, PA) ultrasound system, initially for the cone downs and later for all treatments starting in 2000. Weekly port films were also taken using megavoltage photons for quality assurance purposes. Fiducial markers, e.g. gold seeds in the prostate, were not in use during the study period. IMRT was utilized for all patients for the full duration of treatment starting in 2001. High doses of RT were used prior to the availability of image guidance, with some patients receiving up to 80 Gy at the isocenter using crossfire 3D conformal techniques to spare the normal tissues.
Routine patient follow-up included a DRE and PSA at 3 months post-RT and at 6–12-month intervals thereafter. The Phoenix (PSA nadir + 2.0 ng/mL) definition was used to define BF (16, 17). Late gastrointestinal (GI) and genitourinary (GU) toxicity were scored at each follow up using a modified RTOG/LENT toxicity grading system, which has been published in detail previously (18).
Radiation dose was reported as isocenter dose for conformal and conventional techniques and mean PTV dose for IMRT for dose comparisons. of the mean dose for IMRT was used because of greater inhomogeneity (usually greater than 10%); neither the prescribed dose, nor the isocenter dose, was representative of the delivered dose to the PTV (12). The mean PTV dose has been recommended by the IMRT Collaborative Working Group to approximate the delivered dose (19).
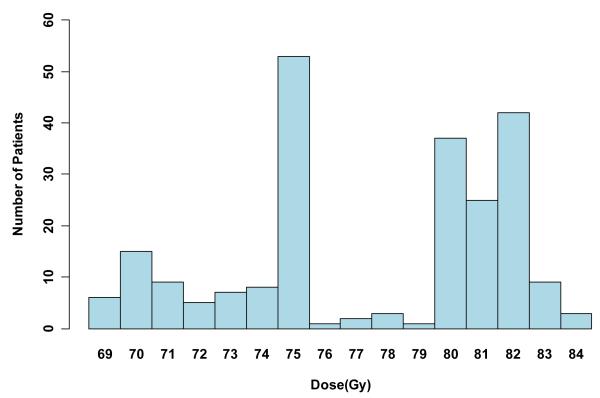
Dose groups of < 75 Gy, 75-79.9 Gy, and ≥80 Gy were determined prior to the analyses. We chose ≥80 Gy as the highest dose group because we were interested in the question of dose escalation above what was used in the randomized trials and because 80 Gy is the prescribed dose currently used for patients at Fox Chase Cancer Center and the University of Miami. We chose a second cutpoint of 75 Gy to establish two lower dose groups for comparison that were even in size, based on the distribution of doses used in this patient cohort (see Figure 1).
Figure 1.
Radiation dose histogram. The doses used and their frequency by year of treatment are represented in this bar graph. For example, the 80 Gy bar represents the number of patients treated with 80-80.9 Gy.
Differences in patient characteristics by dose were determined using Fisher’s exact test for categorical variables and the Kruskal-Wallis test for comparing medians of continuous variables. Primary endpoints were time until BF, distant metastasis (DM), cause specific mortality (CSM) and overall mortality (OM). For OM, cumulative incidence for each dose group was estimated using the Kaplan-Meier method (20); these “one minus survival” curves were compared using the log-rank test. For BF, DM, CSM and toxicity, cumulative incidence was estimated using the competing risk method (21), adjusting for death as a competing risk. This method, used for the non-death endpoints, takes into account that patients who die are no longer at risk for the endpoint. Cumulative incidence curves by dose were compared using Gray’s test (21). Competing risks proportional hazards regression models (22) were used for BF, DM, and CSM, and Cox proportional hazards models (23) were used for OM to assess the independence of RT dose when considered with the other covariates. Two multivariable (MVA) risk models were considered; the first included dose as a categorical variable (dose: <75, 75-79.9, and ≥80 Gy and all other covariates as categorical variables including T-category (T1, T2, T3/4, and TX), RT technique (conventional, 3D-conformal and IMRT), ADT duration: none, <6, 6-<24, and ≥24 months), age (four 10-year age groups), and iPSA (<10, 10-<20, and >20 ng/mL). The second MVA included dose, PSA, age and initial ADT duration as continuous variables. Analyses were done in SAS (version 9.1), Stata (version 10.0) and R (version 2.5.1).
For the overall mortality endpoint, the study had 80% power to detect a hazard ratio of 0.46 or less for ≥80 Gy vs <75 Gy groups, and a hazard ratio of 0.41 for 75-79 vs <75 Gy. These estimates assumed a two-sided test with 1.7% type I error (Bonferroni correction applied), adjusting for correlation of dose with other covariates. For the BF, DM, and CSM endpoints, we are not aware of sample size formulae for Fine and Gray’s model. However, the required sample size is likely similar to that needed for a Cox regression. The detectable subdistribution hazard ratios for BF and DM would likely be more pronounced in absolute magnitude (smaller hazard ratios) since we observed fewer events than we observed for the overall mortality analysis.
Results
Median age was 70.8 years for the 226 assessable patients. Table 1 shows patient characteristics by dose group. Although RT dose generally increased over time, there was considerable overlap. Patients in the <75 Gy dose group were treated between 1988 and 1999, the 75 to 79.9 Gy group between 1992 and 2002, and the ≥80 Gy group between 1997 and 2002 (Table 2). Androgen deprivation therapy was used in a greater percentage and for longer time periods in the ≥80 Gy group. Most patients (215/226 or 95%) were either GS 8 or GS 9. The distribution of GS significantly differed by RT dose group. For example, there was a higher proportion of GS 8 in the ≥80 Gy dose group. Figure 1 further breaks down the various RT doses used over the entire study period and the number of patients at these dose levels.
Table 1.
Patient Characteristics by Dose Group.
| < 75 Gy | 75-79.9 Gy | ≥ 80 Gy | p-value | |
|---|---|---|---|---|
| Number of Patients | 50 | 60 | 116 | |
| T-category | ||||
| T1 | 8 (16) | 7 (12) | 31 (27) | 0.017 |
| T2 | 23 (46) | 33 (55) | 45 (39) | |
| T3/T4 | 18 (36) | 12 (20) | 26 (22) | |
| TX | 1 (2) | 8(13) | 14(12) | |
| Initial PSA (ng/mL) | ||||
| <10 | 15 (30) | 24 (40) | 56 (48) | 0.043 |
| 10-<20 | 16 (32) | 18 (30) | 40 (35) | |
| ≥20 | 19 (38) | 18 (30) | 20 (17) | |
| Gleason Score | ||||
| 8 | 34 (68) | 37 (62) | 99 (85) | 0.003 |
| 9 | 12 (24) | 20 (33) | 13 (11) | |
| 10 | 4 (8) | 3 (5) | 4 (3) | |
| Received ADT | 27 (54) | 39 (65) | 100 (86) | <0.001 |
| ADT Duration, months median |
12.0 | 22.2 | 28.0 | 0.002 |
| RT Technique | ||||
| Conventional | 8 (16) | 0 (0) | 0 (0) | <0.001 |
| 3D Conformal | 42 (84) | 58 (97) | 81 (70) | |
| IMRT | 0 (0) | 2 (3) | 35 (30) | |
| Age, years median (range) |
73 (55-89) | 68 (54-82) | 71 (41-86) | 0.141 |
| Follow-up, months median (range) |
66.2 (11.4-173.5) |
70.9(9.9-126.3) | 58.4 (4.9-105.9) |
0.002 |
Table values are number of patients (percent of dose group) unless otherwise noted. Abbreviations: Gray (Gy), palpation tumor category (T-category), prostate specific antigen (PSA), androgen deprivation therapy (ADT), radiation therapy (RT), intensity modulated RT (IMRT).
Table 2.
Dose by Era
| Yr Treatment |
N | Dose level (Gy) | ||||
|---|---|---|---|---|---|---|
| < 75 | 75-79.9 | ≥ 80 | Median | Range | ||
| n (%) | n (%) | n (%) | ||||
| 1988-1992 | 21 | 15 (71) | 6 (29) | 0 (0) | 72.0 | 69.6-79.0 |
| 1993-1997 | 79 | 33 (42) | 44 (56) | 2 (3) | 75.8 | 69.5-80.0 |
| 1998-2002 | 126 | 2 (2) | 10 (8) | 114 (90) | 82.0 | 73.7-84.2 |
Median doses differ significantly, p<0.001 Kruskal Wallis test
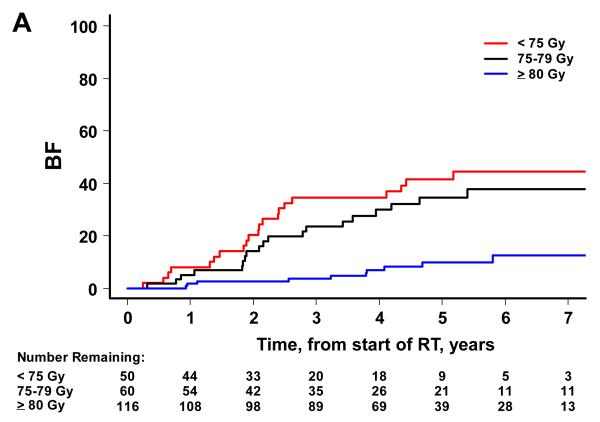
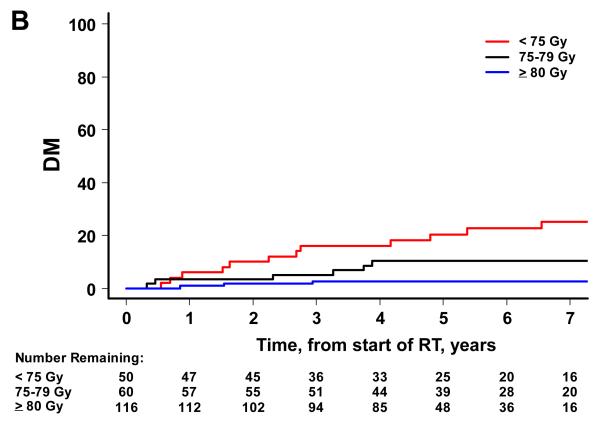
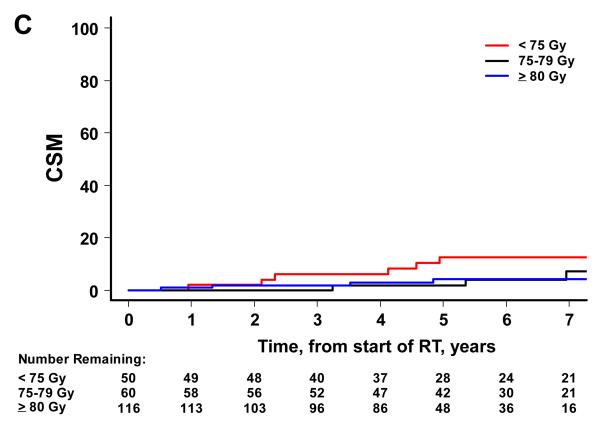
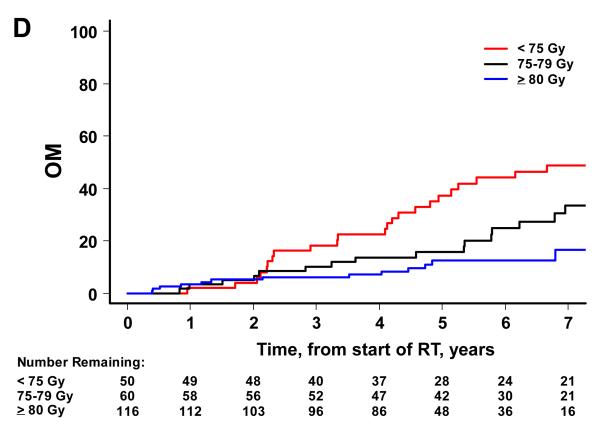
There were 52 biochemical failures, 21 distant metastases, 15 deaths attributable to prostate cancer and 65 deaths from any cause. Higher RT dose was associated with significantly lower risks of BF, DM and OM on univariate analysis, but not CSM (Figure 2). By dose group (1: <75 Gy, 2: 75-79.9 Gy and 3: ≥80 Gy), the 7-year BF estimates were 45% (95% CI=30-58%), 38% (24-51%), and 12% (6-22%) (p<0.001). The 7-year estimates of DM were 25% (14-38%), 10% (4-20%), and 3% (1-7%) for groups 1, 2 and 3 (p<0.001). The 7-year estimates of CSM were 13% (5-24%), 7% (2-18%), and 4% (1-10%). The 7-year estimates of OM were 49% (34-63%), 34% (20-48%) and 17% (8-28%) for groups 1, 2 and 3 (p<0.001).
Figure 2.
Patient outcome by dose group. The cumulative incidence curves by dose group are shown for BF (A), DM (B), and CSM (C) using the competing risks method, and for OM (D) using the Kaplan Meier approach. A significant association is noted with higher RT dose and better outcome for BF (p<0.001), DM (p<0.001), and OM (p<0.001). For CSM, results were not significant (p=0.387).
A landmark analysis was performed to look at time to event from the end of therapy (either end of RT or end of initial ADT), because of the potential bias for different lengths of ADT, for patients who were event free at the end of therapy with follow-up beyond therapy. The median follow-up for the landmark analysis, starting at the end of therapy, was 59.5 months for those who received < 75 Gy, 60.0 months for 75-79.9 Gy, and 33.4 months for 80+ Gy, (p<0.0001, Kruskal-Wallis test). In the landmark analysis, cumulative incidence for BF, DM, and CSM was estimated using the competing risk method, using R version 2.5.1 (24), and the curves compared using Gray’s test. For BF, the p-value was 0.0001; for DM, p=0.0026, and for CSM, p=0.4 (Supplemental Figure 3). However, there were only 15 CSM events. For overall mortality, the log rank p-value from Kaplan Meier analysis was 0.0071. The curves are shown in supplemental Figure 3. The landmark analysis results were similar to when the time to event was calculated from the start of radiotherapy.
Radiation dose retained significance in the MVA models for BF, DM and OM using ADT duration and RT dose as categorical or continuous variables (Tables 3 and 4). In the categorical model, significant predictors of reduced BF were RT dose ≥80 Gy (vs. <75Gy, p=0.011) and ADT duration ≥24 months (vs. none, p=0.033). There was a trend for higher BF with iPSA ≥20 (vs. PSA<10, p=0.084) and T3/T4 category (vs. T1, p=0.079). In the continuous model, higher RT dose (p<0.001) and T3/T4 category (vs. T1 p=0.035) were significant predictors of BF, and there was a trend for iPSA (p=0.053) and ADT duration (p=0.058). The only significant correlate of reduced DM was RT dose ≥80 Gy (p=0.007 in the categorical model and p<0.001 in the continuous model). In the categorical model, RT dose ≥80 Gy (p=0.035) and T3/T4-category (p=0.041) were significant predictors of OM, and there was a trend for age 70-79 (vs. age 40-59, p=0.078). In the continuous model, higher RT dose (p=0.033) and lower age (p=0.002) were significant predictors of reduced OM, and there were trends for greater mortality with higher iPSA (p=0.092) and T3/T4 category (vs. T1, p=0.055). The findings in the landmark analysis were concordant, with RT dose being significant in the categorical and continuous models for BF, DM and OM, but not CSM.
Table 3.
Categorical multivariable analysis
| BF (Competing Risks) | DM (Competing Risks) | OM (Cox) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Groups | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| RT Dose | <75 Gy | Ref | Ref | Ref | ||||||
| 75-79.9 Gy | 0.91 | 0.46-1.80 | 0.795 | 0.37 | 0.13-1.03 | 0.058 | 0.80 | 0.42-1.54 | 0.509 | |
| ≥80 Gy | 0.28 | 0.10-0.74 | 0.011 | 0.09 | 0.01-0.52 | 0.007 | 0.42 | 0.19-0.94 | 0.035 | |
| PSA | <10 | Ref | Ref | Ref | ||||||
| 10-20 | 0.61 | 0.25-1.45 | 0.262 | 0.38 | 0.08-1.84 | 0.228 | 1.40 | 0.71-2.75 | 0.333 | |
| ≥ 20 | 1.78 | 0.93-3.42 | 0.084 | 1.15 | 0.35-3.80 | 0.821 | 1.44 | 0.76-2.71 | 0.266 | |
| T-category | T1 | Ref | Ref | Ref | ||||||
| T2 | 1.07 | 0.38-3.03 | 0.900 | 0.62 | 0.12-3.25 | 0.570 | 1.25 | 0.57-2.75 | 0.584 | |
| T3/T4 | 2.74 | 0.89-8.40 | 0.079 | 3.44 | 0.74-16.05 | 0.116 | 2.40 | 1.04-5.55 | 0.041 | |
| TX | 0.68 | 1.07-4.28 | 0.679 | 0.78 | 0.03-17.91 | 0.879 | 0.68 | 0.14-3.38 | 0.641 | |
| ADT | None | Ref | Ref | Ref | ||||||
| < 6 mo | 0.72 | 0.26-1.95 | 0.511 | 0.37 | 0.06-2.30 | 0.286 | 0.77 | 0.33-1.79 | 0.536 | |
| 6-23 mo | 0.87 | 0.34-2.23 | 0.767 | 3.37 | 0.62-18.38 | 0.160 | 1.19 | 0.56-2.55 | 0.649 | |
| ≥24 mo | 0.38 | 0.15-0.92 | 0.033 | 1.10 | 0.26-4.62 | 0.892 | 0.63 | 0.29-1.39 | 0.254 | |
| Age | 40-59 | Ref | Ref | Ref | ||||||
| 60-69 | 0.59 | 0.19-1.51 | 0.240 | 0.73 | 0.18-3.02 | 0.666 | 1.59 | 0.49-5.12 | 0.440 | |
| 70-79 | 0.46 | 0.16-1.33 | 0.153 | 0.50 | 0.10-2.54 | 0.406 | 2.68 | 0.90-8.00 | 0.078 | |
| 80-89 | 1.19 | 0.27-5.27 | 0.815 | 1.14 | 0.14-9.17 | 0.902 | 2.78 | 0.64-12.04 | 0.171 | |
| Technique | Conventional | 0.61 | 0.17-2.18 | 0.451 | 0.63 | 0.12-3.32 | 0.584 | 1.03 | 0.36-2.92 | 0.954 |
| 3DCRT | Ref | Ref | Ref | |||||||
| IMRT | 0.63 | 0.21-1.92 | 0.420 | 1.10 | 0.30-4.06 | 0.887 | ||||
For technique, the referent group was 3DCRT except for the distant metastasis (DM) endpoint, where 3DCRT and IMRT were combined due to the small number of events. Other abbreviations: Prostate specific antigen (PSA), androgen deprivation therapy (ADT), palpation tumor category (T-category), radiation therapy (RT), intensity modulated RT (IMRT), 3D conformal RT (3DCRT)
Table 4.
Multivariable analysis, with age, PSA, ADT duration and RT dose as continuous variables
| BF (Competing Risks) | DM (Competing Risks) | OM (Cox) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Group | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| RT Dose | Continuous | 0.88 | 0.82-0.94 | <0.001 | 0.83 | 0.75-0.92 | <0.001 | 0.93 | 0.86-0.99 | 0.033 |
| PSA | Continuous | 1.01 | 1.00-1.02 | 0.053 | 1.01 | 0.99-1.03 | 0.420 | 1.01 | 1.00-1.02 | 0.092 |
| T-category | T1 | Ref | Ref | Ref | ||||||
| T2 | 1.28 | 0.51-3.23 | 0.596 | 0.77 | 0.15-3.90 | 0.755 | 1.11 | 0.51-2.42 | 0.794 | |
| T3/T4 | 2.82 | 1.07-7.40 | 0.035 | 3.42 | 0.70-16.68 | 0.129 | 2.20 | 0.98-4.93 | 0.055 | |
| TX | 0.80 | 0.16-4.10 | 0.790 | 1.06 | 0.11-10.70 | 0.961 | 0.62 | 0.13-2.99 | 0.552 | |
| ADT Duration | Continuous | 0.98 | 0.96-1.00 | 0.058 | 1.01 | 0.98-1.03 | 0.605 | 1.00 | 0.98-1.01 | 0.531 |
| Age | Continuous | 0.97 | 0.92-1.01 | 0.136 | 0.99 | 0.93-1.07 | 0.857 | 1.06 | 1.02-1.10 | 0.002 |
| Technique | Conventional | 0.92 | 0.27-3.11 | 0.894 | 0.63 | 0.15-2.70 | 0.535 | 1.07 | 0.42-2.71 | 0.959 |
| 3DCRT | Ref | Ref | Ref | |||||||
| IMRT | 0.83 | 0.29-2.39 | 0.732 | 1.03 | 0.29-3.75 | 0.890 | ||||
Abbreviations: Prostate specific antigen (PSA), androgen deprivation therapy (ADT), palpation tumor stage (T-stage), radiation therapy (RT), intensity modulated radiation therapy (IMRT), 3D conformal radiation therapy (3DCRT), biochemical failure (BF), distant metastasis(DM), overall mortality (OM), referent group (Ref). For technique, the comparison group (i.e. referent group) was 3DCRT for BF and OM; however, for the DM group, 3DCRT and IMRT were grouped together as the comparison group due to the small number of events.
The treatment was well tolerated. There were a total of 21 late GI and 12 late GU grade ≥2 toxicities overall. Actuarial 7 yr late GI grade ≥2 toxicity rates (with 95% confidence intervals) for the <75 Gy, 75-79.9 Gy and ≥80 Gy groups were 12% (5-23%), 12%(5-21%), and 7% (3-13%), respectively (p=0.449). Actuarial 7-year late GU grade ≥2 toxicity rates were 8% (3-18%), 7% (2-15%), and 4% (1-8%), respectively, for the same dose groups (p=0.482).
Discussion
There is no level I evidence that addresses the question of whether high risk prostate cancer patients with GS 8-10 disease benefit from dose escalation, especially when ADT is administered concurrently. Two randomized studies examined RT dose escalation to <80 Gy without ADT. One phase III study at MDACC (1) ,suggested that the benefit of 78 Gy over 70 Gy was most notable in patients with a PSA>10. However, this study did not evaluate the effect by GS. A randomized study by Zietman et al (2) comparing 70.2 Gy vs. 79.2 Gy with RT alone showed a benefit to dose escalation in a mixed group of patients in whom 33 had GS 8-10 disease; there were too few high grade patients to evaluate separately.
Our findings are the first to report that RT doses ≥80 Gy in GS 8-10 patients result in significant gains for BF, DM and OM. On multivariable analysis, RT dose was independent of patient age, iPSA, T-category, ADT duration, and RT technique. RT dose as a categorical or continuous covariate was the most significant determinant of BF and DM, and was also an independent determinant of OM.
Previous studies have evaluated dose escalation in patients with GS 8-10 disease. A study by Fiveash et al examined outcome in 180 patients with GS 8-10 prostate cancer (25). Dose escalation to >70 Gy predicted biochemical control in T1-T2 patients, but not T3-T4 patients. Another study by Roach et al found that among 50 patients with GS 8-10, RT dose >71 Gy was associated with better disease free survival (26). These studies did not evaluate the impact of doses ≥ 80 Gy. Although not specifically focusing on Gleason 8-10 disease, the Memorial Sloan-Kettering dose escalation series reported by Zelefsky et al (27) demonstrated that men with high risk prostate cancer experience a substantial improvement in BF and DM rates when RT doses ≥80 Gy were used.
We did not specifically examine local control as an endpoint because, unless routine prostate biopsies are performed at 2-3 years post-RT, it is not possible to know the true extent of local tumor persistence. As we and others have shown (28-30) local persistence is strongly related to clinical failure ultimately. An indirect measure of local control is the long term effects of RT dose escalation on BF and DM.
Our results indicate a continued pronounced benefit of higher RT doses, most notably at 80 Gy and above. Given that the DM rates for men with prostate cancer are low overall, there has been some debate over whether the risk of potential side effects from RT dose escalation are worth the gains in tumor control (31). The gains we observed here were not at the expense of greater GU or GI morbidity. Moreover, for men with GS 8-10 disease, the progression from local to distant disease is pronounced. As shown in Figure 1, the 7 year DM rates were 25% for <75 Gy, 10% for 75-80 Gy and 3% for ≥80 Gy. Of note, there were no DM failures beyond 3 years for the ≥80 Gy patients and none beyond 4 years in the 75-79 Gy patients, whereas there was no flattening of the DM curve for the <75 Gy patients. There appear to be clinically meaningful incremental gains by increasing radiation dose to ≥80 Gy. The more frequent use of ADT in the high dose group may have contributed to these findings; however, a landmark analysis from the end of all therapy revealed the same outcome patterns. At radiation dose ≥80 Gy, lower incidence of DM was observed, as well as lower OM, but there was no significant difference in CSM. One factor that could have contributed to the OM difference is an imbalance of cormorbid conditions. Another possible explanation is that we did not capture every death due to prostate cancer in this chart review. The full impact of RT dose on these endpoints may become more obvious as the data further mature.
As mentioned above, the gains observed were without increases in toxicity. One might expect greater toxicity at higher doses. However, our data suggest that by reducing exposure of the normal tissues with 3DCRT and IMRT, improved sparing of the critical structures was possible without sacrificing tumor control probability. Of note, about 30% of the patients in the ≥ 80 Gy group were treated with IMRT as opposed to 0% and 3% in the <75 Gy and 75-79.9 Gy groups, respectively. Additional supporting data comes from the experience at Memorial Sloan-Kettering (32). These investigators have reported that 10 yr grade 2 or higher GI toxicity was significantly lower in the patients treated with IMRT compared to those receiving 3DCRT (5% vs. 13%, respectively, p<0.001). The GI toxicity rates they observed are consistent with ours.
The results presented suggest contemporary GS 8-10 patients have a local control problem and are without subclinical distant metastasis at presentation. Knowing that GS 8-10 has a greater propensity for distant spread when not initially controlled provides a convincing rationale for RT dose escalation to ensure complete tumor eradication.
A caveat to our study is that the analysis assumes consistent pathologic grading over the study period, and therefore that patients with high GS have the same level of biological aggressiveness. However, a Gleason score shift has been documented by our group (33). As described in the results section and in Table 2, RT dose increased over time and was highly correlated with year of treatment, even though there was some overlap in the dose groups. When year of treatment was included in the multivariate, neither this covariate or RT dose came out as being significant.
There are obvious imbalances in the dose groups that could have contributed to the finding that RT dose affects the endpoints tested. In particular, there were differences in ADT duration, as well as differences in T-category, PSA, and RT technique. The regression analyses are designed to account for these biases, but are not perfect. While there are differences in follow-up, the lowest dose group did not have the longest follow-up (Table 2). We also looked at the effect of GS 8 vs. higher GS (data not shown), and found that neither was this variable significant in the MVA, nor did it affect the result that dose was a significant predictor of outcome.
The other aim of our study was to determine the impact of dose escalated RT in men with Gleason 8-10, who were often treated with ADT. The effect of ≥80 Gy in patients treated with ADT for GS 8-10 disease is unknown. Two randomized studies evaluated dose escalation to <80 Gy, in which neoadjuvant/adjuvant ADT was allowed. A Dutch randomized study, comparing 78 Gy vs. 68 found a significant benefit in BF at the higher dose despite the use of ADT (3). However, in a post-hoc analysis by risk group, a significant benefit was seen only in those patients with intermediate risk disease. Since ADT was given at the discretion of the treating physician, unaccounted for biases may have had an influence on the results seen. An MRC study in which all patients received short term neodjuvant and concurrent ADT, found a significant reduction in failure for high-risk patients treated to higher RT doses (74 Gy vs 64 Gy), including 96 patients with GS 8-10. However, even the higher dose of 74 Gy used in their study falls within the range of the low dose group in our study.
Several randomized studies have shown an improvement in survival with long term ADT (5, 6, 34). Thus, the standard of care has arguably been to use ADT in all patients with Gleason 8-10 disease. The results presented here indicate that RT dose may be an important adjunct; but, in order to see the maximum effect, RT doses ≥80 Gy appear to be needed. In the MVAs, RT dose was a significant determinant of BF, DM and OM. We conclude that RT doses of ≥80 Gy and ADT should be considered in men with GS 8-10 disease and that clinical trials addressing RT dose escalation and ADT should be high a priority.
Supplementary Material
SFigure 3. Patient outcome by dose group using Landmark analysis. The cumulative incidence curves by dose group are shown for BF (A), DM (B), and CSM (C) using the competing risks method, and for OM (D) using the Kaplan Meier approach. A significant association is noted with higher RT dose and better outcome for BF (p=0.0001), DM (p=0.0026), and OM (p<0.001). For CSM, results were not significant (p=0.4); however, there were only 15 CSM events. For overall mortality, the log rank p-value from Kaplan Meier analysis was 0.0071. Of note: there were 9 patients who had a BF and 10 who had a DM during the initial ADT period that were not included in their respective curves, making the Landmark analysis harder to interpret for these endpoints. Patients lacking PSA follow-up (n=14 for BF analysis) or status follow-up beyond initial ADT (n=2) were not included in the Landmark analysis.
Acknowledgements
This publication was supported in part by Grants CA-006927 and CA101984-01 from the National Cancer Institute, and by Varian Medical Systems, Palo Alto, CA. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Varian Medical Systems. The authors appreciate the advice of Brian L. Egleston, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. Jama. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 3.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 4.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 7.American Joint Commission on Staging of Cancer . Manual for Staging of Cancer. 4 ed JB Lippincott; Philadelphia: 1992. [Google Scholar]
- 8.Soffen EM, Hanks GE, Hunt MA, et al. Conformal static field radiation therapy treatment of early prostate cancer versus non-conformal techniques: a reduction in acute morbidity. International Journal of Radiation Oncology Biology Physics. 1992;24:485–488. doi: 10.1016/0360-3016(92)91063-s. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz EM, Hanlon AL, Pinover WH, et al. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001;92:1281–1287. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Monti AF, Ostinelli A, Frigerio M, et al. An ICRU 50 radiotherapy treatment chart. Radiother Oncol. 1995;35:145–150. doi: 10.1016/0167-8140(95)01541-n. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das IJ, Cheng CW, Chopra KL, et al. Intensity-modulated radiation therapy dose prescription, recording, and delivery: patterns of variability among institutions and treatment planning systems. J Natl Cancer Inst. 2008;100:300–307. doi: 10.1093/jnci/djn020. [DOI] [PubMed] [Google Scholar]
- 13.Senthi S, Gill SS, Haworth A, et al. Benchmarking Dosimetric Quality Assessment of Prostate Intensity-Modulated Radiotherapy. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Kukolowicz PF, Mijnheer BJ. Comparison between dose values specified at the ICRU reference point and the mean dose to the planning target volume. Radiother Oncol. 1997;42:271–277. doi: 10.1016/s0167-8140(97)01905-1. [DOI] [PubMed] [Google Scholar]
- 15.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach M, 3rd, Hanks G, Thames H, Jr., et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Buyyounouski MK, Hanlon AL, Eisenberg DF, et al. Defining biochemical failure after radiotherapy with and without androgen deprivation for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1455–1462. doi: 10.1016/j.ijrobp.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Eade TN, Horwitz EM, Ruth K, et al. A comparison of acute and chronic toxicity for men with low-risk prostate cancer treated with intensity-modulated radiation therapy or (125)I permanent implant. Int J Radiat Oncol Biol Phys. 2008;71:338–345. doi: 10.1016/j.ijrobp.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:447–457. [Google Scholar]
- 21.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annual Statistics. 1988;16:1141–1143. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 23.Cox DR, Oakes D. Analysis of Survival Data. Champman and Hall; London: 1984. Analysis of Survival Data. [Google Scholar]
- 24.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical computing; Vienna, Austria: 2007. [Google Scholar]
- 25.Fiveash JB, Hanks G, Roach M, et al. 3D conformal radiation therapy (3DCRT) for high grade prostate cancer: a multi-institutional review. Int J Radiat Oncol Biol Phys. 2000;47:335–342. doi: 10.1016/s0360-3016(00)00441-7. [DOI] [PubMed] [Google Scholar]
- 26.Roach M, Meehan S, Kroll S. Radiotherapy for high grade clinically localized adenocarcinoma of the prostate. Journal of Urology. 1996;156:1719–1723. [PubMed] [Google Scholar]
- 27.Zelefsky MJ, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71:1028–1033. doi: 10.1016/j.ijrobp.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 28.Pollack A, Zagars GK, Antolak JA, et al. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002;54:677–685. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- 29.Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–1373. doi: 10.1016/j.juro.2007.11.063. discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crook JM, Malone S, Perry G, et al. Twenty-four-month postradiation prostate biopsies are strongly predictive of 7-year disease-free survival: results from a Canadian randomized trial. Cancer. 2009;115:673–679. doi: 10.1002/cncr.24020. [DOI] [PubMed] [Google Scholar]
- 31.Schulz RJ, Kagan AR, et al. Commentary on dose escalation and biochemical failure in prostate cancer: in regard to Eade. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.08.037. [DOI] [PubMed] [Google Scholar]; Int J Radiat Oncol Biol Phys. 2008;70:645. author reply 645-646. [Google Scholar]
- 32.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Chism DB, Hanlon AL, Troncoso P, et al. The Gleason score shift: score four and seven years ago. Int J Radiat Oncol Biol Phys. 2003;56:1241–1247. doi: 10.1016/s0360-3016(03)00268-2. [DOI] [PubMed] [Google Scholar]
- 34.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SFigure 3. Patient outcome by dose group using Landmark analysis. The cumulative incidence curves by dose group are shown for BF (A), DM (B), and CSM (C) using the competing risks method, and for OM (D) using the Kaplan Meier approach. A significant association is noted with higher RT dose and better outcome for BF (p=0.0001), DM (p=0.0026), and OM (p<0.001). For CSM, results were not significant (p=0.4); however, there were only 15 CSM events. For overall mortality, the log rank p-value from Kaplan Meier analysis was 0.0071. Of note: there were 9 patients who had a BF and 10 who had a DM during the initial ADT period that were not included in their respective curves, making the Landmark analysis harder to interpret for these endpoints. Patients lacking PSA follow-up (n=14 for BF analysis) or status follow-up beyond initial ADT (n=2) were not included in the Landmark analysis.