Abstract
CD40-ligand/CD154 is predominantly expressed on activated CD4 T cells and plays a central role in regulating CD4 T-cell-dependent responses. To define the relative abilities of CD4 T-cell functional subsets in the induction of CD154—specifically FoxP3− effector, versus FoxP3+ regulatory, CD4 T cells—multiple CD4 T cell preparations were isolated from B6 and B6.FoxP3-GFP mice and stimulated in vitro to examine the kinetics of stimulation-dependent CD154 expression. CD154 was induced in 40–60% of total CD4 T cells in various cell preparations. However, despite similar kinetics of CD154-induced expression, the average percentage of CD154 expression among CD4+ FoxP3+ T regulatory (Treg) cells was only about 4–9%. Such differential, stimulation-dependent CD154 induction by total CD4+ T cells versus CD4+ FoxP3+ Treg cells was consistent, despite multiple stimulation conditions utilizing a variety of cell preparations of different composition. Similar induction of CD154 occurred irrespective of whether the CD4+ FoxP3+ Treg cells were first sorted to 98% purity and stimulated in vitro alone, or stimulated as non-purified cells in the presence of CD4+ FoxP3− T effector cells, suggesting that CD154 induction by CD4+ FoxP3+ Treg cells is regulated by cell-intrinsic mechanisms. Differential CD154 induction may be a key factor in determining the distinguishable functions of FoxP3− T-effector, versus FoxP3+ Treg, CD4+ T cells.
Keywords: CD154, CD4 T regulatory cells, CD4 T effector cells, FoxP3, FoxP3-GFP mouse
1. Introduction
CD154 (CD40 ligand), is a 39 kDa type-II transmembrane glycoprotein of the tumor necrosis factor family most commonly expressed on mature activated CD4 T cells [1,2], rev. in [3]. As the ligand for CD40, CD154 plays a pivotal role as a co-stimulatory molecule for activating productive T-cells and for initiating many B-cell functions in adaptive immunity, including B-cell clonal expansion, germinal center formation, isotype switching, affinity maturation, and generating long-lived antibody-secreting plasma cells [4]. Presumably related to the sensitive nature of the CD154/CD40 interaction and the constitutive expression of CD40 on B cells, CD154 expression on resting CD4 T lymphocytes is limited [5]. Using an anti-CD3-stimulated T helper cell line, CD154 induction was originally shown to be temporally regulated, with CD154 expression peaking between 4 and 8 h and extending up to 48 h [6], a finding also shown in ex vivo studies [5]. This coincides with the well-established timeline [6] of T cell–B cell interactions in an immune response. Owing perhaps in part to the critical functions CD154 provides in T and B cell activation, CD154 is also believed to play a role in autoimmunity. Blocking CD154/CD40 interactions inhibits the development of a number of autoimmune diseases in animal models, rev. in [3].
The CD4+ T regulatory (Treg) cell is critical in preventing autoimmune disorders. CD4+ Treg cells are a class of suppressor cells originally identified as CD25+ CD4+ T cells that can inhibit responses to auto- and allo-antigens, tumor antigens, and determinants of infections, and are important in maintaining immunological self-tolerance [7,8]. Treg cells are either constitutively present, naturally-arising, nTreg cells (of thymic origin) or inducible/adaptive iTreg cells (of peripheral origin), which arise from exposure to exogenous antigen [9]. While the exact mechanisms of suppression continue to be debated, the fundamental effects of Treg cells on CD4+ T effector cells are generally accepted, and include suppression of proliferation and cytokine production [10]. nTreg cells’ vital role in controlling autoimmune disease was clearly revealed by the multi-organ autoimmunity that developed after transfer of CD25+ depleted T cells into athymic nude BALB/c mice [11]. CD4+ Treg cells also have the potential to alter the behavior of CD8 cytotoxic T-lymphocytes [12]. CD4+ nTreg cells, which represent 10–15% of naïve CD4 T lymphocytes, as well as CD4+ iTreg cells, are more specifically identified by the FoxP3 marker, a member of the forkhead/winged felix family of transcriptional regulators [8]. FoxP3 was originally detected as the defective gene in scurfy mice, an X-linked recessive mouse mutant with dysregulated CD4+ T lymphocytes [13]. Thus, the FoxP3 protein is a unique marker of CD4+ Treg cells that programs their development and function [14].
CD154/CD40 signaling appears to be required for generating adaptive CD4+ Treg cells, and athymic nude mice reconstituted with CD154−/− T cells developed autoimmune diseases, while those reconstituted with wild type (w.t.) T cells did not [15]. There is evidence that interactions involving CD154 and CD4+ Treg cells take place in both developing and mature immune systems. CD154 upregulation on CD4+ FoxP3+ Treg cells may play a critical role in clonal expansion of CD4+ Treg cells during thymic development [16]. Furthermore, treatment of adult w.t. mice with a CD154 blocking antibody caused a quick drop in CD4+ CD25+ Treg cell numbers, which was reversible upon antibody withdrawal [17]. In a transplantation model, the blockade of CD154 on a CD4+ CD25+ Treg cell-containing preparation enhanced the immunosuppressive properties of these cells, and played a role in long-term graft acceptance [18]. Conversely, the CD154/CD40 interaction has been argued to play no role in the suppressive capacity of CD4+ Treg cells, based on the observation of full in vitro suppression by CD4+ Treg cells cultured with a CD154 blocking antibody [17]. Taking these reports together, it is unclear as to whether the effects of the CD154/CD40 pair depend on the expression or failure to express CD154 by the CD4+ Treg cells per se, as opposed to secondary effects on CD4+ Treg cells imparted by other CD154+ or CD40+ cells. Thus, the role CD154 may play in CD4+ FoxP3+ Treg cell generation and/or function is not clear. In this paper, we sought to determine the incidence within the cell population, and kinetics, of CD154 expression by naïve versus stimulated CD4+ Treg cells.
2. Materials and methods
2.1. Animals
Male C57BL/6 (B6) mice were purchased from the National Institutes of Health (Bethesda, MD). FoxP3-GFP (on a mixed B6× 129 background) reporter mice were previously described and initially received from Dr. Alexander Rudensky’s lab at the University of Washington School of Medicine, Seattle, WA [19,20]. Derived from these mice, FoxP3-GFP mice fully backcrossed onto the B6 background (B6.FoxP3-GFP mice) were a generous gift from Dr. Mary Jo Turk, Dartmouth Medical School. Animals were housed in the pathogen-free, AAALAC-accredited Animal Resource Center at Dartmouth Medical School and used when 8 to 10 weeks of age. All experimental procedures were approved by the Dartmouth College Institutional Animal Care and Use Committee.
2.2. Splenocyte subpopulation preparations
Splenocytes were isolated from B6 and B6.FoxP3-GFP mice, and then either purified with anti-CD4 and anti-CD19 labeled paramagnetic beads (MACS; Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol, or sorted by the FACSstar plus with TurboSort technique (Becton Dickinson Immunocytometry Systems, San Jose, CA). The following purified cellular subsets were used for cell culture and in vitro stimulation for CD154 induction.
2.2.1. Positively and negatively selected CD4 T cells
Total CD4+ T cells were isolated from either normal B6 splenocytes or B6.FoxP3-GFP splenocytes. Spleen cell suspensions were labeled with anti-CD4 beads and positively selected (MACS). Final selected preparations were ≥98% CD4+ cells, as detected by flow cytometric analysis. CD4+ T cells were negatively selected by labeling unfractionated splenocytes with anti-CD19 beads (to deplete CD19—positive B cells), yielding CD4+ T cell preparations that were enriched to approximately 90% purity.
2.2.2. Purified CD4+ FoxP3-GFP− T effector and CD4+ FoxP3-GFP+ Treg cells
To isolate the CD4+ FoxP3-GFP− T effector and CD4+ FoxP3-GFP+ Treg populations, purified (positively-selected) total splenic CD4+ T cells from B6.FoxP3-GFP donor mice were further sorted into CD4+ FoxP3-GFP− T effector and CD4+ FoxP3-GFP+ Treg cells by a FACSstar instrument coupled with TurboSort. Based on the expression of GFP, the respective sorted populations were ≥98% pure for the CD4+ FoxP3-GFP− T effector, or CD4+ FoxP3-GFP+ Treg, subset.
2.3. Activation of CD4 T cells
The cellular subsets: 1) unfractionated splenocytes, 2) positively and negatively selected CD4+ T cells, and 3) sorted CD4+ FoxP3-GFP− T effector and CD4+ FoxP3-GFP+ Treg cells, all from appropriate donor mice, were each plated in duplicate at 0.5–1×106 cells/well into 96-well flat-bottom plates, with plate-bound anti-CD3 antibody (mAb clone: 145-2C11 10 μg/mL, BD Pharmingen, San Diego), with or without soluble anti-CD28 mAb (37.51 1 μg/mL, BD Pharmingen, San Diego). Culture medium contained 5% FCS, L-glutamine (2.5 mM, Sigma, St. Louis), penicillin G sodium salt (30 μg/mL) and streptomycin sulfate salt (20 μg/mL, Sigma), with or without IL-2 (5 units/mL, Cetus Corp., Emeryville). Cells were harvested and prepared for flow cytometric analyses of CD154 induction at time points specified in the text.
2.4. Flow cytometric analyses of CD154 induction
Spleen cell preparations were incubated with FITC, PE, or APC-conjugated mAbs, and the resulting direct immunofluorescence was assessed by FACSCalibur analysis (BD Bioscience) to detect the expression of murine CD4 (H129.19), CD19 (1D3), CD154 (MR1), and FoxP3 (FJK-16s) (BD Pharmingen or eBioscience, San Diego). FITC, PE, or APC-conjugated Ig isotype control mAbs of irrelevant specificity were used as controls for each experimental mAb. Intracellular staining (ICS) to detect FoxP3 was performed on cell populations from B6 donors as previously reported [21]. Briefly, 0.5–1×106 cells from populations described above were surface labeled with PE-conjugated anti-CD154 and APC-conjugated anti-CD4 followed by permeabilization and intracellular staining with FITC labeled anti-FoxP3 based on the manufacturer’s instructions (FJK-16s eBioscience). Stained cells were analyzed on a FACSCalibur flow cytometry using Cellquest software (BD Bioscience).
3. Results
3.1. Establishing the presence and kinetics of CD154 expression
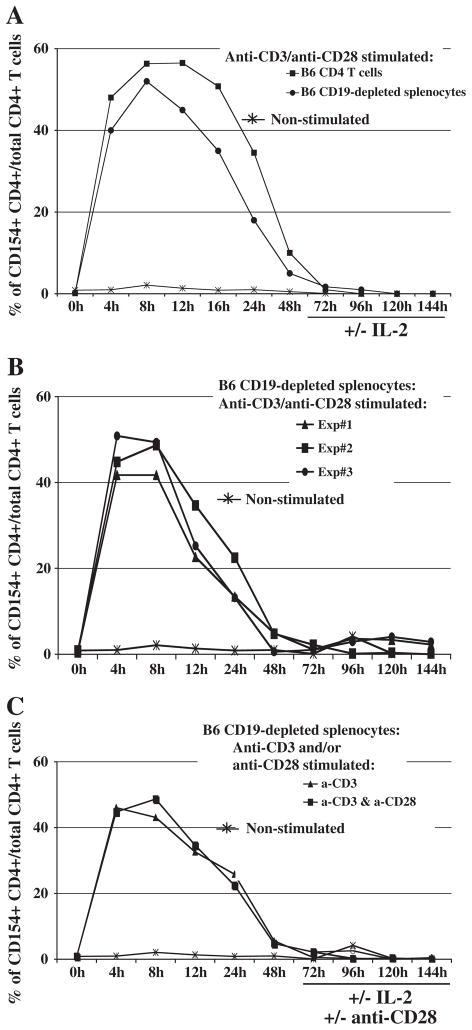
We first confirmed that in vitro culture of CD4+ T cells with anti-CD3 and/or anti-CD28 mAb stimulation resulted in CD154 induction with similar kinetics as previously reported [5]. We used multiple cell preparations to test the possibility of variations in CD154 expression by CD4+ T cells due to the method of CD4 T-cell purification/enrichment and the presence of other splenocyte subsets. The cell preparations we analyzed included positively selected CD4+ T cells and CD19-depleted splenocytes, in which in all cases we found a stimulation-dependent induction of CD154 on CD4+ T cells that occurred between 4 and 48 h (Fig. 1A); we also found comparable kinetics of CD154 expression among unfractionated B6 splenocytes (data not shown). When we extended our studies to 144 h, no additional substantive increases nor second peak of CD154 expression was induced. Over 144 h, we observed reproducible CD154 expression profiles in all the CD4+ T cell preparations in each of our three independent experiments (e.g. Fig. 1B).
Fig. 1.
Reproducible CD154 induction among three different cell preparations in various stimulation conditions. Cells were plated in duplicate with various in vitro stimulations, and harvested for CD4 and CD154 staining after the indicated times of incubation. The non-stimulated group is representative of unfractionated splenocytes. (A) Either positively-selected CD4+ T cells or CD19-depleted splenocytes (“negatively-selected CD4+ T cells”) were cultured with plate-bound anti-CD3 and soluble anti-CD28 mAbs in vitro. When used, soluble IL-2 was added beginning at 72 h incubation. (B) CD19-depleted splenocytes were cultured alone or with plate-bound anti-CD3 and soluble anti-CD28 in vitro stimulation in three independent experiments. (C) Negatively-selected CD4+ T cells were stimulated in various conditions for CD154 induction: including with plate-bound anti-CD3, alone or in combination with soluble anti-CD28, removing the source of primary and/or secondary stimulation at 72 h, or supplementing with soluble IL-2 at 72 h. The data shown are representative of three independent experiments.
To further explore the possibility that a second peak of CD154 expression was induced—as has been reported for human CD4+ T cells [22,23]—we more systematically varied our in vitro culture conditions. In particular, we used stimulation conditions that included removing the source of primary (anti-CD3) and/or co-stimulatory (anti-CD28) mAb activation at 72 h, and/or supplementing with rIL-2 beginning at 72 h, with or without continuing anti-CD3± anti-CD28 stimulation. Regardless of which alternative stimulation conditions we used, a second substantial peak of CD154 expression by CD4+ T cells could not be detected after 72 h of in vitro stimulation (Fig. 1). Because CD154 expression returned to, and remained at, near-baseline levels after 48 h in culture, we chose to focus on hours 0–48 for our subsequent analyses.
In addition, an alloantigenic system: B6/H-2b responders versus BALB/c/H-2d irradiated stimulators, was used to determine whether antigen-induced CD154 expression was fundamentally different from that observed following anti-CD3/± anti-CD28 activation. In initial experiments, we detected alloantigen induction of CD154 expression that was similar in kinetics to anti-CD3/anti-CD28 stimulation, and easily measurable at the expected range of 2.8–3.9% (over the three experiments) of all CD4+ T cells, since the frequency of allo-specific T cells is much smaller than the majority of CD4+ T cells able to be activated polyclonally by anti-TCR complex agonistic mAbs (data now shown). Specifically, stimulation with alloantigen leads to CD154 expression by the CD4+ T cell subset at 8 (3.6% of all CD4+ T cells), 12 (3.9%) and 16 (2.8%) hours post stimulation, with CD154 induction at these three time points reaching significance, compared to other time points and the non-stimulation controls. Further, and most pertinent to our focus on CD154 expression by FoxP3+ Treg cells here (see Section 3.3 below), the percentages of CD154+ FoxP3+ CD4+ T cells, among total FoxP3+ cells, either by alloantigen stimulation or parallel anti-CD3/anti-CD28 stimulation, were very similar (range over the three experiments: 4–9%).
3.2. Effects of in vitro culture on CD4+ Treg cell persistence
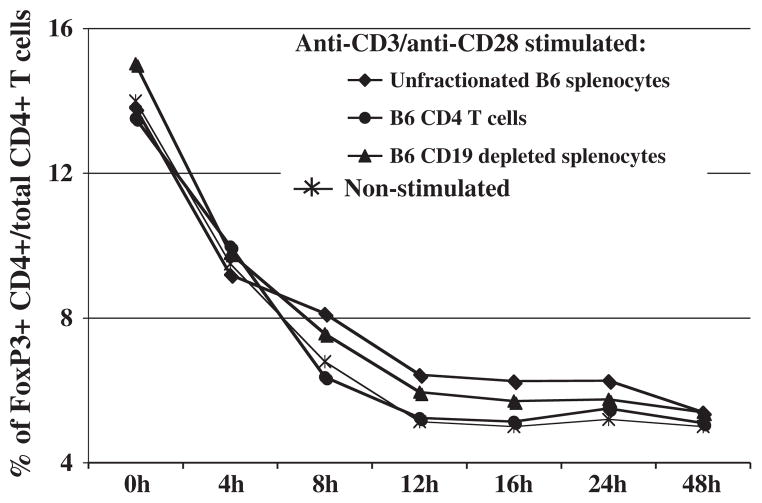
As assessed by FoxP3 expression via ICS analysis, the percentage of CD4+ Treg cells decreased whether examined in unfractionated splenocytes, as positively selected CD4+ T cells, or as negatively selected CD4+ T cells (plus other non-B cells in CD19-depleted splenocyte preparations); this decrease in CD4+ cells was seen even with in vitro stimulation with anti-CD3 and anti-CD28. Initial FoxP3 expression was detected by ~14% of CD4+ T cells and diminished to an average of ~5% after 48 h in culture, irrespective of the presence of other non-CD4 cellular subsets within the culture: i.e. unfractionated splenocytes or as negatively-selected CD4+ T cells/CD19-depleted splenocytes (Fig. 2). Although the percentage of CD4+ FoxP3+ Treg cells in culture thus decreased to a lower persistent level after 12–48 h, the intensity of CD154 expression on the remaining positive subset was undiminished in fluorescence intensity (data not shown). This pattern of declining FoxP3 expression was confirmed in three independent experiments. In addition, the decrease of FoxP3 expression in culture occurred independently of the stimulation conditions (e.g., nonstimulated; anti-CD3, with or without anti-CD28 and IL-2 stimulation, data not shown). This frequency of ~5% CD4+ FoxP3+ Treg cells, with the marker expressed at normally induced levels on a per cell basis, provided a significant and consistent level of sensitivity for further study of CD154 expression.
Fig. 2.
Decline of the percentages of CD4+ FoxP3+ Treg cells upon in vitro incubation. Either unfractionated splenocytes, positively-selected CD4+ T cells, or negatively-selected CD4+ T cells (CD19-depleted splenocytes) were cultured in parallel with in vitro stimulation. Cells were harvested at the indicated times post in vitro stimulation with plate-bound anti-CD3 and soluble anti-CD28 for anti-CD4 and anti-CD154 staining. FoxP3+ cells were identified using intracellular FoxP3 staining (see Materials and methods). Percentages of CD4+ FoxP3+ Treg cells are representative of three independent experiments.
3.3. The expression of CD154 by CD4+ FoxP3+ Treg cells
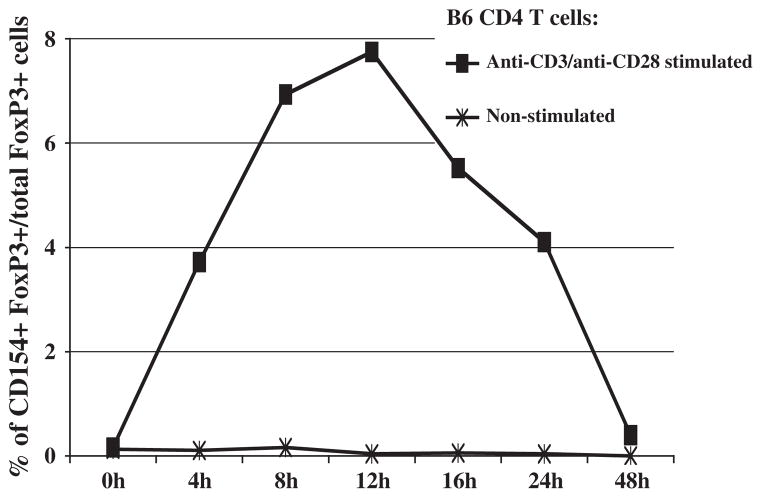
We next assessed the possibility of stimulation-dependent CD154 expression by CD4+ Treg cells. This was accomplished by ICS for FoxP3 expression. Although the kinetics of stimulation-dependent CD154 induction by CD4+ FoxP3+ Treg cells resembled that of the total CD4+ T-cells, a more selective subset of CD4+ FoxP3+ Treg cells expressed CD154 under these conditions (Fig. 3), than the induction of CD154 by 40–60% of cells observed among total CD4+ T cells preparations (Fig. 1A–C). Over several repeat experiments, the average percentage of CD154+ CD4+ FoxP3+ Treg cells among all CD4+ FoxP3+ Treg cells was about 4–9% (Figs. 3 and 4). This was found through several experiments in which we also compared various CD4 T cell-containing splenocyte preparations: i.e. positively selected CD4+ T cells, negatively selected CD4+ T cells by depleting CD19 B cells, and unfractionated splenocytes (data not shown).
Fig. 3.
Kinetics of CD154 expression by CD4+ FoxP3+ Treg cells. Positively-selected CD4+ T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs in vitro. Cells were harvested at various hours post stimulation for anti-CD4 and anti-CD154 staining. FoxP3+ cells were identified using intracellular FoxP3 staining. Percent of total CD4+ FoxP3+ Treg cells that express CD154 is depicted. The same pattern of results was also obtained when unfractionated or CD19-depleted splenocytes were cultured, stimulated, and analyzed for CD4+ FoxP3+ CD154+ T cells (data not shown). The data shown are representative of three independent experiments.
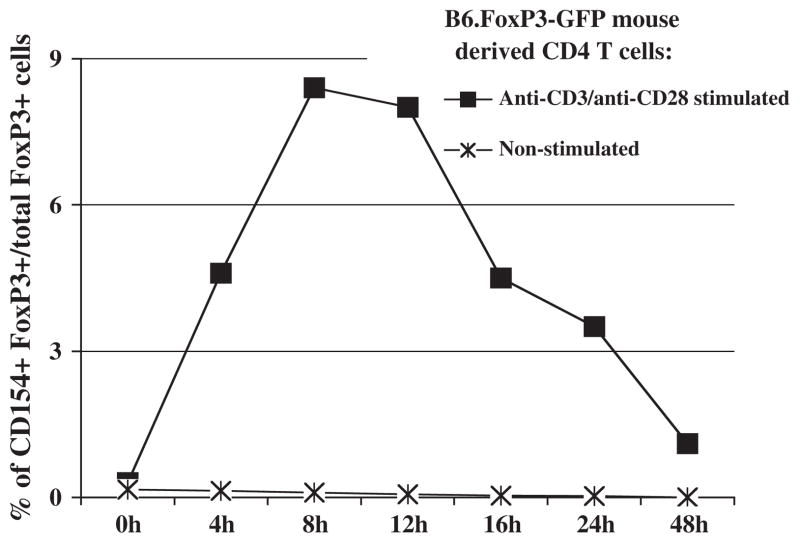
Fig. 4.
CD154 induction upon in vitro stimulation of splenocytes obtained from B6. FoxP3-GFP mice. Positively-selected CD4+ T cells were isolated from B6 FoxP3-GFP donor mice. Cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs in vitro for anti-CD4 and anti-CD154 staining. FoxP3-positive cells from B6.FoxP3-GFP mouse cell cultures were identified based on GFP expression. Percent of total CD4+ FoxP3+ Treg cells that express CD154 is depicted. The data shown are representative of two individual experiments. The same pattern of results was also obtained by analysis of B6.FoxP3-GFP unfractionated splenocytes or positively-selected FoxP3-GFP CD4+ T cells (data not shown).
3.4. Verification of CD154 induction on a subset of CD4+ FoxP3+ Treg cells
In an effort to verify our finding that a subset of CD4+ FoxP3+ Treg cells expressed CD154 after in vitro stimulation, and specifically to use an alternative method to detect FoxP3 that did not depend on the efficiency of the ICS approach, we instead used mice homozygous for a FoxP3-GFP fusion protein knock-in allele (B6.FoxP3-GFP mice). We observed no discernable differences in CD154 induction upon in vitro anti-CD3, with or without anti-CD28, mAb stimulation among all CD4+ T cells between w.t. B6 and B6.FoxP3-GFP mice (data not shown). This validated our experimental approach and verified that the GFP knock-in reporter allele did not disrupt normal CD154 induced expression. The percentages of CD4+ FoxP3-GFP+ Treg cells derived from B6.FoxP3-GFP mice that expressed stimulation-dependent CD154 (Fig. 4) were very similar to the percentages using ICS to identify the CD4+ FoxP3+ Treg cells from w.t. B6 mice (Fig. 3). Again, no second peak of CD154 expression was observed during extended culture to 144 h (data not shown).
3.5. Differences in the extent of CD154 induction by CD4+ FoxP3+ Treg cells versus CD4+ FoxP3− T effector cells
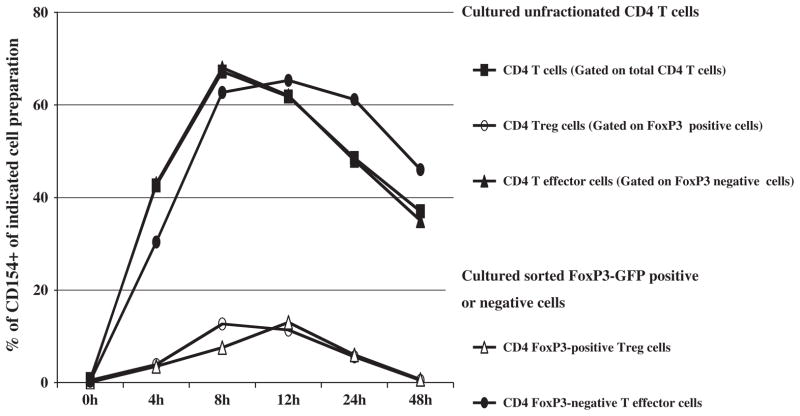
As described above, we found that CD4+ T cells and CD4+ FoxP3+ Treg cells, analyzed from differing cell preparations, exhibited similar kinetics of CD154 induction following anti-CD3 mAb induction, with or without simultaneous anti-CD28 mAb stimulation, although the CD4+ FoxP3+ Treg cells had a substantially reduced percentage of CD154 expression. Here, using B6.FoxP3-GFP mice, we directly compared CD4+ FoxP3− T-effector cells versus FoxP3+ Treg cells. We reproducibly demonstrated that CD154 induction (percent of expressing cells) by CD4+ FoxP3+ Treg cells was on average 5 fold-lower than by CD4+ FoxP3-GFP− T effector cells (Fig. 5). Similar levels of CD154 induction by Treg cells were observed, irrespective of whether the CD4+ FoxP3+ Treg cells were first sorted to 98% purity and stimulated in vitro alone, or cultured with stimulation as non-purified cells with CD4+ FoxP3− T effectors also present (Fig. 5). Similarly, the percentages of cells expressing stimulation-dependent CD154 by FoxP3− T effector cells were essentially at the same high (60%) level regardless of their culture alone or in the context of total CD4+ T cells.
Fig. 5.
Differential CD154 induction by CD4+ FoxP3+ Treg cells versus CD4+ FoxP3− effector T cells is consistent and independent of the presence of other cells during the culture/stimulation phase. Purified (positively-selected) total B6.FoxP3-GFP CD4+ T cells; or further flow cytometrically-sorted CD4+ FoxP3-GFP+ Treg cells, versus CD4+ FoxP3-GFP− effector cells; all from B6.FoxP3-GFP mice; were cultured and stimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs. Three-color flow cytometric analyses were conducted as follows: CD4 and CD154 staining was performed at the indicated various hours post stimulation, with FoxP3+ cells from B6.FoxP3-GFP mouse identified based on GFP expression. The percentages of CD154-expressing cells were determined for either gated (for the unfractionated cell cultures) on or sorted (CD4+ FoxP3+ Treg cells or CD4+ FoxP3-GFP− T effector) cells. The data shown are representative of three independent experiments.
4. Discussion
Because of its rapid and transient expression, CD154 is often difficult to detect following the somewhat asynchronous response to multiple-determinant antigen stimulation in response to exposure to infectious agents or tumor cells—both in vivo and in vitro. However, upon polyclonal, T-cell receptor-signal-dependent activation in vitro by anti-CD3/anti-CD28 mAbs, we were able to consistently detect CD154 induction by 40%–60% of total CD4 T-cells, utilizing a variety of cell preparations. CD154 surface expression peaked at 4–8 h after such stimulation, but gradually diminished after 24 to 48 h (Fig. 1). Using both w.t. B6 and B6.FoxP3-GFP mice, CD154 induction by the FoxP3− T-effector, versus FoxP3+ Treg, CD4+ T-cell subsets was directly compared. In terms of the percentage of expressing cells, induction of CD154 on CD4+ FoxP3+ Treg cells was, on average, about 5-fold lower than on CD4+ FoxP3-GFP− T effector cells, although the intensity of CD154 expression per cell was essentially undiminished, as measured by mean fluorescence intensity. These findings indicate that CD154 is induced differentially between FoxP3− T-effector, and FoxP3+ Treg, CD4+ T-cells. Because this differential induction was observed with various cell preparations containing either purified FoxP3+ Treg cells versus various mixed cell compositions, the collective data suggest that the decreased CD154 induction by CD4+ FoxP3+ Treg cells is regulated by a cell-intrinsic mechanism(s).
Several studies in human systems have suggested a distinct secondary peak of CD154 expression occurring at about 96 h of stimulation [22,23]. However, using murine cells, and upon extended culture, we found that the kinetics of CD154 induction by FoxP3− T-effector, or FoxP3+ Treg, CD4+ T cells from various cell preparations, after in vitro stimulation by anti-CD3 alone or in combination with anti-CD28 supported the existence of only a single broad CD154 induction peak at 4–8 h. Specifically, a second peak of CD154 expression could not be detected at hours 72–144, despite further in vitro stimulation, with or without anti-CD28 and IL-2 (Fig. 1C and data not shown). Thus, regardless of the combination of stimulation conditions, we repeatedly could not detect a significant second peak of CD154 expression in the T-effector or Treg CD4 T-cell subsets.
As for the functional significance of induced CD154 expression by this subset of FoxP3+ CD4+ Treg cells, we have preformed preliminary experiments assessing IFN-γ expression by ICS following anti-CD3/anti-CD28 stimulation. Compared to the robust positive control of FoxP3− CD4+ T effector cell population of IFN-γ, very few FoxP3+ CD4+ Treg cells would produce IFN-γ such that clearly, CD154 expression by activated Treg cells was not sufficient to lead to production of this classic effector cytokines.
In future studies it will also be important to begin to elucidate the functional significance of the differential extent of CD154 expression between naïve Treg cell versus stimulated Treg cell preparations, and their CD154+ versus CD154− FoxP3+ Treg cell subsets, with regard to their suppression capability. Thus, an intriguing question is whether the expression of CD154 by only a relatively small subset of CD4+ Treg cells correlates with varying degrees of suppressive function or utilization of different suppressive mechanisms of the panel that have been attributed to FoxP3+ CD4+ Treg cells—e.g. TGF-β, and/or IL-10 production, versus suppression by cell:cell surface events [24,25]. For example, it may be revealing to initially determine whether CD154+ FoxP3+ Treg cells suppress the proliferation of CD4+ T-effector cells to a greater or lesser extent, compared to CD154− FoxP3+ Treg cells. As discussed in the Introduction here, earlier studies utilizing blocking antibody specific for CD154, or utilizing CD154−/− versus w.t. mice as a source of Treg cells, have provided data to suggest that the outright expression of cellular CD154 by Treg cells at the Treg suppressive effector level, and/or in the cellular development (environment) of Treg cells, affected their suppressive abilities. However, as these early studies utilized cell preparations characterized by their expression of CD25+ (and other markers other than FoxP3, such as GITR and CTLA-4) [25,26], that are now known not to be specific for Treg cells [19,27], it is not clear that the observed effects were mediated by CD154 expression by Treg cells per se [17,18,28]. The answers to these and other questions about a role for Treg expressed CD154 can better be addressed by first purifying the FoxP3-GFP+ Treg cells, then upon 8 h of anti-CD3/CD28 in vitro stimulation, further sorting CD154+ FoxP3+ Treg cells from CD154− FoxP3+ Treg cells, before assessment of their suppressive function and mechanism(s). Owing to the small percentage of FoxP3+ Treg cells that express CD154 after stimulation (Figs. 3–5), and the general decrease in persistence of the numbers of total FoxP3+ Treg cells during in vitro culture over time, whether or not the cell preparations are stimulated (Fig. 2), however, these are very challenging experiments. Thus, the question of whether CD154+ Treg cells are functionally different from CD154− Treg cells is an intriguing possibility that is still under investigation in our lab.
In summary, our results suggest that anti-CD3/anti-CD28, or alloantigen, stimulation leads to CD154 induction by CD4+ FoxP3+ Treg cells, but unlike for CD4+ T-effector cells, in only a small (4–9%) subpopulation of this Treg cell subset; and this more limited expression is regulated by cell-intrinsic mechanism(s). This regulated, differential CD154 induction may be a key factor governing the distinguishable functions of FoxP3− T-effector, versus FoxP3+ Treg, CD4+ T cells. Indeed, among CD4+ T cells, our preliminary results indicated that the vast majority of IFN-γ production was by CD154+ FoxP3− T effectors. Thus, although some FoxP3+ Treg cells become activated and express CD154, IFN-γ production by CD4+ FoxP3+ Treg cells is very low.
Acknowledgments
We wish to thank Melanie Rutkowski, Ling Cao and Kathy Green for critically reading the manuscript, their technical support and helpful discussions. We also thank Alice Givan and Gary Ward for their help with flow cytometry and cell sorting.
This work was supported by the National Institutes of Health Grant CA50157 (to W.R.G.); Wen Li was supported by NIH/NIAID T32 Training Grant AI07363. This work was also supported by a grant from the National Natural Science Foundation of China #81060252 and a Hitchcock Foundation Award (to W.L.). Flow cytometry was performed at Dartmouth Medical School in the Herbert C. Englert Cell Analysis Laboratory, which was established by equipment grants from the Fannie E. Rippel Foundation and the NIH Shared Instrument Program, and is supported in part by the Core Grant (CA 23108) from the National Cancer Institute to the Norris Cotton Cancer Center, and an Immunology COBRE P20 Grant (RR16437) from the NIH National Center of Research Resources.
Abbreviations
- Treg
T regulatory
- w.t
wild type
- B6
C57BL/6
- ICS
intracellular staining
- mAb
monoclonal antibody
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 2.Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–4. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 3.Vogel LA, Noelle RJ. CD40 and its crucial role as a member of the TNFR family. Semin Immunol. 1998;10:435–42. doi: 10.1006/smim.1998.0145. [DOI] [PubMed] [Google Scholar]
- 4.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 5.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–510. [PubMed] [Google Scholar]
- 6.Castle BE, Kishimoto K, Stearns C, Brown ML, Kehry MR. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J Immunol. 1993;151:1777–88. [PubMed] [Google Scholar]
- 7.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 9.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 10.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 12.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M, Yamamoto N, Matsuzuka F, Miyauchi A, Iwatani Y. Decrease of CD154 intensity on peripheral CD4+ T cells in autoimmune thyroid disease. Clin Exp Immunol. 2004;136:555–8. doi: 10.1111/j.1365-2249.2004.02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105:973–8. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guiducci C, Valzasina B, Dislich H, Colombo MP. CD40/CD40L interaction regulates CD4+CD25+ T reg homeostasis through dendritic cell-produced IL-2. Eur J Immunol. 2005;35:557–67. doi: 10.1002/eji.200425810. [DOI] [PubMed] [Google Scholar]
- 18.Jarvinen LZ, Blazar BR, Adeyi OA, Strom TB, Noelle RJ. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation. 2003;76:1375–9. doi: 10.1097/01.TP.0000093462.16309.73. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 21.Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J Immunol. 2008;181:1798–805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDyer JF, Li Z, John S, Yu X, Wu CY, Ragheb JA. IL-2 receptor blockade inhibits late, but not early, IFN-gamma and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-gamma production. J Immunol. 2002;169:2736–46. doi: 10.4049/jimmunol.169.5.2736. [DOI] [PubMed] [Google Scholar]
- 23.Singh K, Laughlin J, Kosinski PA, Covey LR. Nucleolin is a second component of the CD154 mRNA stability complex that regulates mRNA turnover in activated T cells. J Immunol. 2004;173:976–85. doi: 10.4049/jimmunol.173.2.976. [DOI] [PubMed] [Google Scholar]
- 24.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Wing K, Miyara M. Regulatory T cells—a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–23. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 26.Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F. Thymic regulatory T cells. Autoimmun Rev. 2005;4:579–86. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Shevach EM. Regulatory/suppressor T cells in health and disease. Review [13 refs] Arthritis Rheum. 2004;50:2721–4. doi: 10.1002/art.20500. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]