Abstract
Rationale
Social environment influences alcohol consumption in humans, however, animal models have only begun to address biological underpinnings of these effects.
Objectives
We investigated whether social influences on alcohol drinking in the prairie vole are specific to the sex of the social partner.
Methods
In Experiment 1, control, sham, and gonadectomized voles were placed either in mesh-divided housing with a same-sex sibling or isolation with access to ethanol. In Experiment 2 animals were given an elevated plus maze test (EPM) and then females were paired with a castrated male followed by isolation or mesh-divided housing with access to ethanol. In Experiment 3, subjects categorized as low or high drinkers based on initial ethanol intake were placed in mesh-divided housing with an opposite-sex partner of the same or opposite drinking group and ethanol access. Subjects were then moved back to isolation for a final ethanol access period.
Results
Same-sex pairs showed social facilitation of drinking similar to previous reports. Gonadectomy did not affect alcohol drinking. Opposite-sex paired animals in Experiment 2 did not differ in alcohol drinking based on social housing. EPM measures suggested a relationship between anxiety-like behaviors and drinking that depended on social environment. Experiment 3 identified moderate changes in alcohol preference based on social housing, but these effects were influenced by the animal’s own drinking behavior and were independent of their partner’s drinking.
Conclusions
Social influences on alcohol self-administration in prairie voles differ based on the sex of a social partner, consistent with human drinking behavior.
Keywords: Prairie voles, social behavior, alcohol self-administration, social influence
Introduction
There is substantial evidence for a mutual relationship between the social environment and alcohol drinking in both the human (Bushman and Cooper 1990; Homish and Leonard 2008; McCrady et al. 2006; Steele and Southwick 1985) and animal literature (Anacker and Ryabinin 2010; Miczek et al. 1993; Pepino et al. 2002; Pfaus and Pinel 1989). Although it is well established that adult relationships play a substantial role in human drinking (Homish and Leonard 2008; Leonard and Eiden 2007; McCrady et al. 2006), a common limitation of human studies is the inability to determine the causal roles and biological underpinnings of this bidirectional relationship. Animal models are therefore a useful tool, although to date there has been a lack of research on the relationship between adult attachment and alcohol drinking (Anacker and Ryabinin 2010).
The monogamous prairie vole forms selective emotional attachments toward an adult pair-mate and is an excellent animal model for understanding social influences on ethanol drinking. There is striking overlap in the neural circuitry of pair bonding and affiliation in the prairie vole and that of reward and addiction (Young et al. 2011a). Striatal dopamine systems have been shown to mediate interactions between pair bonding behaviors and amphetamine (Liu et al. 2010, 2011; Young et al. 2011b). Recent work has demonstrated a bidirectional interaction between social environment and alcohol self-administration in this species (Anacker et al. 2011a,b,c). Prairie voles that are housed in same-sex pairs show a robust correlation in alcohol consumption and higher levels of both alcohol consumption and preference compared to animals housed in isolation (Anacker et al. 2011b). Furthermore, under certain social conditions, a prairie vole will alter its own consumption to match the intake of another, even into a subsequent isolation period, demonstrating that the drinking behavior of one animal can exert a direct and persistent effect on a social partner (Anacker et al. 2011c).
It is important to note that these previous studies on social drinking in the prairie vole have used same-sex pairs. In prairie voles the sex of a social partner can have different effects on behavioral and neurophysiological measures (aggression: Bowler et al. 2002; isolation behavior: Hostetler and Bales 2012; corticosterone: DeVries et al. 1995, 1997; CART peptide: Hostetler et al. 2011). However, other studies have found responses to the social environment are independent of the sex of a conspecific (tyrosine hydroxylase: Cavanaugh and Lonstein 2010; immediate early genes: Northcutt and Lonstein 2009). These studies suggest that behavioral consequences of the social environment are often, but not necessarily, sensitive to the sex of the social partner. Therefore, we aimed to explore whether social influences on prairie vole drinking behavior differ based on the sex of the social partner. To address this issue in the current study we repeat our previous studies on social drinking behavior, the first experiment using same-sex pairs, and the final two experiments using male-female pairs of prairie voles. In humans, individual differences in anxiety can influence drinking behavior (Kushner et al. 2000), and personality factors may even modulate the relationship between drinking and the social environment (Homish and Leonard 2008; Russell et al. 1997). Therefore we also explored whether individual differences in anxiety-like behavior were related to drinking behaviors in prairie voles, and whether this relationship differed based on social conditions.
Reproductive confounds such as pregnancy and the presence of pups present a significant problem for long-term studies on male-female pairs. The use of gonadectomized animals presents a possible solution, and pairs of gonadectomized voles still form a successful bond (DeVries and Carter 1999). However, it is unknown how gonadal hormones influence ethanol drinking in prairie voles. Therefore, our first experiment also explored the effects of gonadectomy on ethanol self-administration in each isolated and socially housed animals.
Materials and Methods
Subjects
The subjects used in this study were from a breeding colony housed at the Portland Veterans Affairs Medical Center Veterinary Medical Unit. Animals were weaned at 21 days and housed in same sex sibling groups in cages (27 cm × 27 cm × 13 cm) under controlled temperature, humidity, and 14L:10D light conditions. Food (LabDiet Hi-Fiber Rabbit chow, cracked corn, and oats) and water were available ad libitum throughout the experiments. All subjects had access to cotton nestlets throughout the experiments. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Portland Veterans Affairs Medical Center. Subjects were tested as adults (60–100 days of age at start of testing). Different subjects were used for each experiment.
Housing conditions
For all experiments, animals were placed either in isolation or in mesh-divided social housing. Social housing consisted of a cage (27 cm × 27 cm × 13 cm) with a mesh divider in the middle separating each animal in a pair as previously described (Anacker et al. 2011b). Keeping the animals separated allowed individual monitoring of fluid consumption, and the mesh allowed animals to maintain contact. For isolation housing, subjects were placed alone in ‘shoebox’ mouse cages (27 cm × 16.5 cm × 13 cm).
Gonadectomy surgeries
All gonadectomy and sham surgeries were performed under isoflurane anesthesia. Castrated males received a single incision in the scrotum and both testes were removed. Sham castration was performed using the same procedure, with the exception of the removal of the testes. Ovariectomies were performed via a single midline dorsal incision and bilateral perineum incisions, with the uterine horns, ovaries, and associated fat cauterized and removed. Sham ovariectomies included all procedures described above, with the exception of the removal of any tissue. Animals were housed in mesh divider cages during the recovery period to prevent the cage mate from disturbing the surgical area, but allow social contact between the two. All subjects were fully recovered (at least 7–10 days post-operation) before experimental testing.
Two-bottle choice test
Throughout each experiment, animals had continuous access to two 25mL glass cylinders fitted with a metal sipper tube and rubber stopper. One bottle contained tap water. The second bottle contained a solution of ethanol, saccharin, or quinine, depending on experimental conditions (described below). Water access in Experiment 1 (days 12–14) was performed with a single bottle. Fluid volume for each bottle was monitored every 24 hours, and the bottles were refilled and rotated (to avoid side preference bias) at this time.
Elevated plus maze
The elevated plus maze (EPM) test allows assessment of anxiety-like behavior and locomotor activity. The EPM used in the current study consisted of two opaque black high-walled arms and two white open arms (51 cm long × 8 cm wide; Med Associates, Inc., St. Albans, Vermont). The entire maze was elevated 60 cm off the ground. The animal was placed in the center of the apparatus and behavior scored continuously for location (center, open arm, closed arm) and autogrooming by Behavior Tracker 1.5 software (www.behaviortracker.com). Subjects that fell off the apparatus were removed from analysis (n=6).
Study procedures
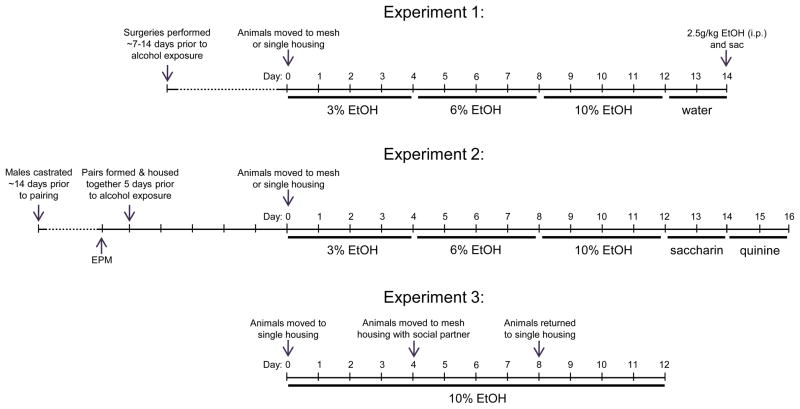
Experiment 1: effects of gonadectomy on ethanol self-administration
Subjects were adult male and female prairie voles. Surgeries were performed as above. Control animals received no surgery or anesthesia, but were placed in mesh divider cages for a minimum of seven days (comparable to the surgical recovery period) prior to the beginning of the experiment. The sample size was 6 animals per sex, housing, and surgical status, with the exception of isolated and ovariectomized females (n=5) and socially housed control females (n=8).
At the beginning of the experiment, all animals were moved either to a new mesh divider cage with a familiar same-sex sibling or to isolation housing. All subjects were then given a series of 2-bottle choice tests with increasing concentrations of ethanol (3%, 6%, and 10%, for four days each), followed by two days of ad libitum water access. To determine whether surgery alters ethanol metabolism, on the final day of the study all subjects received a 2.5 g/kg i.p. injection of ethanol (20% volume/volume in physiological saline). Following injection, animals were returned to their home cage, and euthanized by CO2 at either 30 minutes or 120 minutes post-injection (Figure 1). Serum from trunk blood samples was analyzed for blood ethanol concentration (BEC) using an Analox Analyzer (Analox Instruments, Luneburg, MO, USA).
Figure 1.
Timeline of experimental procedures.
Experiment 2: effects of social housing on ethanol intake in male-female pairs
This experiment tested effects of social housing on alcohol drinking in male-female pairs. To control for reproductive confounds such as pregnancy and the presence of pups, and based on the lack of effect of gonadectomy on drinking behavior in Experiment 1, males were castrated approximately two weeks prior to social pairing. All subjects received a five-minute EPM prior to social pairing and ethanol exposure. The day immediately following EPM testing, each subject was introduced to their novel opposite sex partner by placing them in standard housing for five days undisturbed. This time was based on previous experiments in same-sex pairs and is longer than the 6–24 hours normally sufficient for pair bond formation (Anacker et al. 2011b; DeVries and Carter 1999). Weights were collected the day of pairing, and subsequently collected every three days until the end of the experiment. Following the initial five day pairing period, half of the pairs were placed in mesh-divided cages and half were placed in isolation housing. All subjects were then given a series of 2-bottle choice tests with increasing concentrations of ethanol (3%, 6%, and 10%, for four days each), followed by (0.05%) saccharin and (0.0025%) quinine (two days each; Figure 1). Sample size for each group was 15 animals per sex per housing condition.
Experiment 3: effects of partner’s drinking on ethanol intake in male-female pairs
Given the results in Experiment 2 showing that social facilitation and coordination in male-female pairs differs from patterns seen in same-sex pairs in Experiment 1 and in previous experiments (Anacker et al. 2011b), we explored the effects of influential drinking behavior on ethanol intake in male-female pairs, using a different paradigm previously tested in same-sex pairs (Anacker et al. 2011c). All subjects in this experiment were gonadally intact. Subjects were weighed and placed in single housing for four days with access to tap water and 10% ethanol in a 2-bottle choice test. Animals were subsequently weighed every four days. At the end of the initial four-day period, each animal was categorized as a low, medium, or high drinker based on their ethanol intake. Low drinkers had an average consumption of less than 5 g/kg/day, and high drinkers had an average consumption of over 9 g/kg/day. Medium drinkers were removed from the remainder of the study.
Subjects were then placed in mesh-divided housing with a novel opposite-sex partner of either the same or opposite drinking group. Hence, there were four groups of male-female pairs: high-high, high-low, low-high, and low-low. Subjects within a pair were assigned randomly (except that no pairs were siblings) and placed for four days in mesh-divided housing with continuous access to 10% ethanol in the 2-bottle choice test. After four days of social housing, subjects were moved back to single housing for an additional four days of 2-bottle choice access. Hence, there were three four-day housing conditions: Isolation 1, Pairing, and Isolation 2 (Figure 1). Sample sizes were 9 high/high pairs, 8 high/low pairs, 8 low/high pairs, and 6 low/low pairs.
Statistical analyses
A single average for each dose consumed (g/kg/day) and preference score was calculated for each fluid condition. Some data from individual days were removed from analysis for two reasons. First, bottles would occasionally leak, leading to inaccurate readings and these data were removed. Second, if animals ‘escaped’ from one side of their mesh divider to the other, volumes for both subjects for that day were not used for analysis. Given that each condition involved multiple observation days, removal of single observation days did not affect our ability to calculate an overall average consumption and preference score for each condition. However, loss of repeated observations led to removal of data from two subjects for each quinine and saccharin preference scores in Experiment 2. Data were analyzed in SAS 9.2 (SAS Institute, Cary, NC).
Experiment 1
Alcohol consumption and preference were analyzed via repeated measures ANOVA with sex, housing, and surgery as between subjects factors and ethanol concentration as the repeated measure. Post-hoc comparisons were investigated using Least Square Means. Water consumption (g/kg) was analyzed via three-way ANOVA with sex, housing, and surgery as between subjects factors. Litter effects were included as a random effect in each analysis, but were dropped from the models due to lack of significant effects.
In order to determine whether members within a pair exhibited coordinated drinking, as previously described (Anacker et al. 2011b), correlations between each pair member’s average consumption were performed. Isolated animals were compared to the familiar same-sex sibling they were housed with prior to isolation. In order to limit the number of analyses, we focused on g/kg intake at 10%, as this was the main finding previously reported (Anacker et al. 2011b). Given that animals in these dyads are given the same treatment, we aimed to reduce variability and type II error due to random assignment of each animal within a pair. Within each pair, the animal with the lower average intake at 10% was assigned as the X variable, and the other animal was assigned the Y variable. Correlation coefficients and p-values were obtained using a Fisher z-score based bias adjustment. Correlation coefficients from isolation- and mesh-housed subjects were directly compared by computing a z-score (Fisher 1921).
BECs were analyzed via three-way ANOVA with uptake time, housing, and surgery as between subjects factors. Sex was not examined as this factor does not influence BECs (Anacker et al. 2011b). Litter effects were included as a random effect in each analysis, as they were found to be significant.
Experiment 2
Alcohol consumption and preference were each analyzed via repeated measures ANOVA with sex and housing as between subjects factors and ethanol concentration as the repeated measure. Saccharin and quinine preference scores were each analyzed via two-way ANOVA with sex and housing as between subjects factors. Litter effects were included as a random effect in each analysis, but were dropped from the models due to non-significant effects. Post-hoc comparisons were investigated using Least Square Means.
Correlations between each pair member’s average intake (g/kg/day) were analyzed for each concentration of ethanol, with the male as the X variable and the female as the Y.
To examine whether individual differences in anxiety and locomotor activity are associated with drinking behavior, we performed correlation analyses between EPM measures and each consumption and preference scores. EPM measures included the proportion of time spent in the open arm (= open arm time divided by the total time spent in either arm), frequency of arm entries, and time spent autogrooming. We ran separate correlation analyses for each sex and housing condition, leading to four groups: single-housed males, mesh-housed males, single-housed females, and mesh-housed females.
Experiment 3
Alcohol consumption and alcohol preference were analyzed via repeated measures ANOVA with sex, subject’s drinking status (high or low), and partner’s drinking status (high or low) as between subjects factors and pairing period (Isolation 1, Pairing, Isolation 2) as the repeated measure. Litter effects were included as a random effect in each analysis, but were dropped from the models due to lack of significant effects. Post-hoc comparisons were investigated using paired t-tests.
Results
Experiment 1
Animals in social housing had both higher ethanol intake and preference than those in isolated housing, in a concentration dependent manner. Males had higher ethanol intake and preference than females, and this was also dependent on ethanol concentration. Surgical history had no effect on any alcohol drinking measures (Figure 2).
Figure 2.
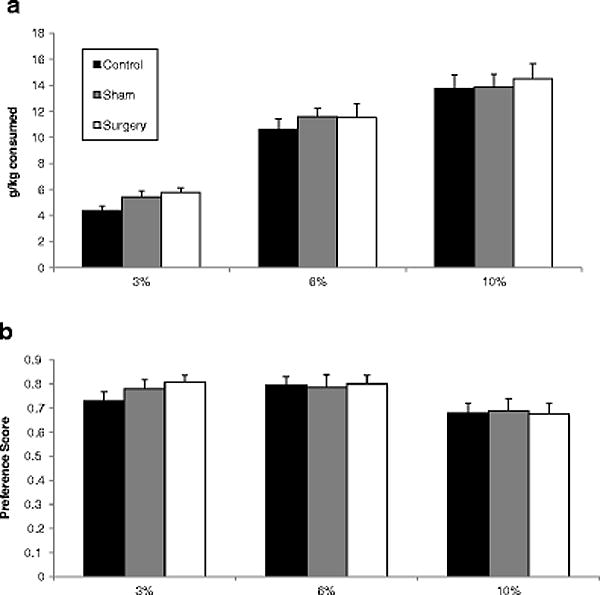
Alcohol intake (a) and preference scores (b) across three alcohol concentrations among same-sex pairs in Experiment 1. Groups are collapsed by sex and housing status. Sample sizes range from 23–27 per group. No differences were observed based on surgical status on any drinking measure.
For ethanol consumption, there was a main effect of social housing (F1,68=4.13, p=0.04), indicating that animals in social housing had significantly higher g/kg intake than animals in isolation. There were no main effects for either sex (F1,68=1.52, p=0.22) or surgery (F2,68=0.58, p=0.56). We also found a main effect of concentration (F2,136=206.26, p<0.0001), as well as an interaction between concentration and each sex (F2,136=9.25, p=0.0002) and social housing (F2,136=6.47, p=0.002), but not surgery. Post-hoc comparisons within each concentration indicated that sex and social housing differences were only present at 10% ethanol, with males consuming more than females (p<0.001), and mesh-housed subjects having higher intake than isolation-housed animals (p<0.001; all other comparisons p≥0.17; Figure 3a).
Figure 3.
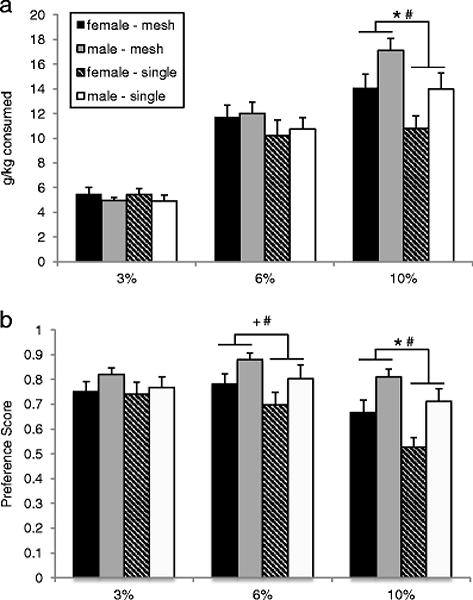
Alcohol intake (a) and preference scores (b) across three alcohol concentrations among same-sex pairs in Experiment 1. Groups are collapsed by surgery status. Sample sizes range from 17–20 per group. *significant effect of housing (p≤0.01),+trend for effect of housing (p=0.07), #significant effect of sex (p≤0.02).
For ethanol preference, there was a main effect of both sex (F1,68=6.95, p=0.01) and housing (F1,68=3.88, p=0.05). Overall, males had higher preference than females, and socially housed animals had a higher ethanol preference than those in isolation housing. There was no main effect of surgery (F2,68=0.11, p=0.89). There was also a main effect of concentration (F2,136=23.76, p<0.0001), and interaction of concentration with each sex (F2,136=5.62, p=0.004), and social housing (F2,136=3.09, p=0.05), but not surgery. Post-hoc comparisons within each concentration indicated that sex and social housing differences were present at 6% and 10% ethanol, with males exhibiting a higher preference for alcohol over water than females (6%: p=0.02; 10%: p=0.0009), and mesh-housed subjects having higher preference than isolation-housed animals (6%: trend only: p=0.07; 10%: p=0.01; Figure 3b). There were no sex or housing effects of alcohol preference for 3% ethanol (p≥0.26). We found no effects of any factor on water consumption.
There was a significant positive correlation between pairs’ 10% alcohol consumption in each mesh- (r=0.78, df=15 p<0.0001) and isolation-housing (r=0.54, df=15, p=0.02). The correlation coefficient of the mesh-housed population was significantly higher than that of the isolated subjects (z=2.918, p=0.0035).
There were main effects for both uptake time (F1,40=284.6, p<0.0001) and housing (F1,40=4.10, p<0.05) on BEC. BECs obtained 30 minutes after ethanol treatment were significantly higher than BECs collected after 120 minutes, as expected. Socially housed animals (30 min: 354 ± 20 mg/dl; 120 min: 134 ± 16 mg/dl) had lower BECs than isolated animals (30 min: 386 ± 14 mg/dl; 120 min: 156 ± 15 mg/dl). There were no main effects of surgery on BEC.
Experiment 2
Alcohol drinking was not affected by social housing or by sex in male-female pairs. There were no significant main effects of sex or housing on alcohol consumed at any concentration of alcohol (Figure 4a). There was a main effect of alcohol concentration (F2,106=105.0, p<0.0001), with g/kg consumed increasing with increased concentration. Alcohol consumption significantly increased between each concentration (p<0.0001 for all comparisons). There was also a significant interaction between alcohol concentration and housing (F2,106=4.34, p=0.015). However, post-hoc comparisons reflected no difference in consumption within each concentration between single- and mesh-housed subjects (p≥0.18 for all comparisons). Alcohol consumption was not significantly correlated between partners, regardless of housing conditions (trend in 3% for mesh-housed subjects: r=0.46, p=0.08; all other comparisons p≥0.12).
Figure 4.
Social housing does not affect alcohol self-administration in male-female pairs of prairie voles. Neither alcohol consumed (g/kg; a) or preference scores (b) differed in isolated versus socially housed voles, regardless of sex. Animals housed with their partner had significantly lower quinine preference scores than animals housed in isolation, regardless of sex (*p<0.05). Sample sizes are 14–15 per group.
For alcohol preference, there were no significant main effects of sex or housing (Figure 4b). There was a significant main effect of concentration on ethanol preference scores (F2,106=10.87, p<0.0001), with preference decreasing across increasing concentrations (p<0.0001 for all comparisons). There was a significant interaction between concentration and housing (F2,106=3.59, p=0.03). Similar to consumption, post-hoc comparisons indicated that there were no significant differences between single- and mesh-housed subjects within any concentration (p≥0.13 for all comparisons).
Saccharin preference was not affected by either sex or housing. In contrast, we did find a significant main effect of housing on quinine preference (F1,54=4.57, p=0.03; Figure 1b), with animals in mesh-divided cages having lower quinine preference scores than isolated animals. There were no main effects of sex on quinine preference.
Performance on the EPM was correlated with alcohol drinking most consistently in single-housed males (Tables 1 and 2). Specifically, there were significant correlations between number of arm entries and each alcohol intake (−0.67≤r≤−0.60 for all three concentrations) and preference (r=0.58 at 3%). Alcohol intake at 3% concentration was also positively correlated with autogrooming (r=0.65) in these males. There were no significant correlations between any alcohol and behavioral measures for single-housed females. Among mesh-housed females, the number of arm entries was negatively correlated with alcohol preference at 3% and 6% (−0.67 and −0.66, respectively).
Table 1.
Correlations between alcohol consumption (g/kg) of increasing concentrations (columns) and EPM behaviors (rows). Each housing condition per sex was analyzed separately. EPM measures include open arm proportion (defined as time spent in open arms divided by total time spent in either the closed or open arm), number of entries into either arm, and time spent autogrooming. For each comparison, the first number presented is the correlation co-efficient (r), and the second number presented in parentheses is the p-value. Significant correlations (p ≤ 0.05) are bolded. Sample size is n=13–15 per group.
| Males
|
Females
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-housed | Mesh-housed | Single-housed | Mesh-housed | |||||||||
| 3% | 6% | 10% | 3% | 6% | 10% | 3% | 6% | 10% | 3% | 6% | 10% | |
| Open Arm Proportion | −0.52 (0.08) | −0.31 (0.32) | −0.37 (0.24) | −0.70 (0.01) | −0.33 (0.27) | 0.11 (0.73) | 0.29 (0.28) | −0.13 (0.65) | −0.16 (0.56) | −0.12 (0.67) | 0.13 (0.67) | −0.16 (0.59) |
|
| ||||||||||||
| Arm Entries | −0.67 (0.02) | −0.61 (0.04) | −0.60 (0.04) | −0.16 (0.06) | 0.35 (0.24) | 0.59 (0.04) | 0.19 (0.49) | −0.09 (0.74) | −0.18 (0.50) | −0.43 (0.13) | −0.44 (0.11) | −0.50 (0.07) |
|
| ||||||||||||
| Autogrooming | 0.65 (0.02) | 0.54 (0.07) | 0.46 (0.13) | −0.25 (0.40) | −0.13 (0.68) | 0.55 (0.05) | 0.28 (0.31) | 0.25 (0.37) | 0.15 (0.58) | −0.08 (0.78) | −0.12 (0.68) | −0.02 (0.95) |
Table 2.
Correlations between alcohol preference scores of increasing concentrations (columns) and EPM behaviors (rows). Each housing condition per sex was analyzed separately. EPM measures include open arm proportion (defined as time spent in open arms divided by total time spent in either the closed or open arm), number of entries into either arm, and time spent autogrooming. For each comparison, the first number presented is the correlation co-efficient (r), and the second number presented in parentheses is the p-value. Significant correlations (p ≤ 0.05) are bolded. Sample size is n=13–15 per group.
| Males
|
Females
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-housed | Mesh-housed | Single-housed | Mesh-housed | |||||||||
| 3% | 6% | 10% | 3% | 6% | 10% | 3% | 6% | 10% | 3% | 6% | 10% | |
| Open Arm Proportion | −0.36 (0.25) | −0.21 (0.51) | −0.20 (0.53) | −0.60 (0.03) | 0.02 (0.94) | 0.07 (0.82) | 0.41 (0.12) | −0.16 (0.58) | −0.25 (0.37) | −0.23 (0.43) | −0.13 (0.65) | −0.30 (0.30) |
|
| ||||||||||||
| Arm Entries | −0.58 (0.05) | −0.51 (0.09) | −0.44 (0.15) | 0.03 (0.93) | 0.39 (0.19) | 0.32 (0.29) | 0.13 (0.65) | −0.21 (0.47) | −0.19 (0.49) | −0.67 (0.01) | −0.66 (0.01) | −0.45 (0.10) |
|
| ||||||||||||
| Autogrooming | 0.26 (0.41) | 0.37 (0.24) | 0.26 (0.42) | −0.67 (0.01) | −0.51 (0.08) | −0.20 (0.52) | −0.23 (0.41) | −0.13 (0.64) | −0.03 (0.89) | 0.08 (0.78) | 0.07 (0.82) | 0.08 (0.80) |
Experiment 3
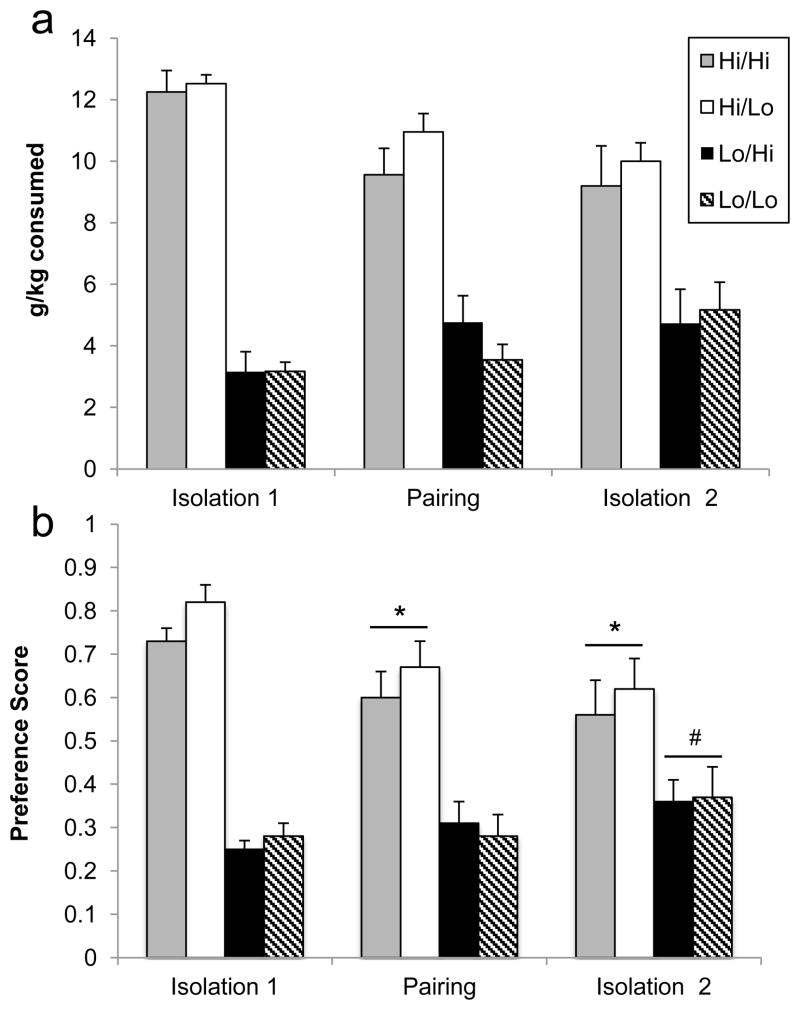
Alcohol intake was not affected by social housing, and there were no influences of the social partner’s drinking category on any measures. For alcohol intake, there was a significant effect of the subject’s own drinking status (F1,57=41.22, p<0.0001; Figure 5a), although this was expected as animals were placed in categories based on their initial alcohol drinking behavior. There were no main effects of either sex or partner’s drinking category. There was no significant interaction between the subject’s own drinking status and the drinking status of their partner.
Figure 5.
Alcohol intake (a) and preference scores (b) across three housing conditions in Experiment 3. There were no direct effects of the social partner’s drinking status on the subject’s drinking. Groups are collapsed across sex. Gray bars: high drinkers paired with high drinkers; white bars: high drinkers paired with low drinkers; black bars: low drinkers paired with high drinkers; striped bars: low drinkers paired with low drinkers. Sample sizes range from 12–18 per group. *significant difference from Isolation 1 (Hi subjects only, p<0.001), #significant difference from both Isolation 1 and Pairing (Lo subjects only, p≤0.03).
For all housing periods, there were no significant interactions between the subject and partner’s drinking category on alcohol intake. There was no main effect of housing period on ethanol intake, nor any interaction effects between housing and the other factors investigated.
For alcohol preference, there was a significant effect of the subject’s own drinking status, as expected (F1,57=57.71, p<0.0001; Figure 5b). There were no main effects of either sex or partner’s drinking category. There was a significant interaction between the subject’s drinking category and the housing period on alcohol preference (F2,114=12.31, p<0.0001). Post-hoc comparisons revealed that high drinkers showed a decrease in preference following Pairing (p<0.0001), but no change from Pairing to Isolation 2 (p=0.36). Low drinkers did not change alcohol preference between Isolation 1 and Pairing (p=0.29), but there was a significant increase from Pairing to Isolation 2 (p=0.03). These directional changes are also indicated by an overall difference in preference between Isolation 1 and Isolation 2 (highs: p<0.001; lows: p=0.02). There was no significant interaction between the subject’s own drinking status and the drinking status of their partner.
Discussion
The present research investigated social influences on alcohol drinking in the prairie vole. We found social facilitation and coordination in same-sex pairs that were housed together, consistent with previous research (Figure 3; Anacker et al. 2011b). In contrast, social facilitation and coordination are not present in opposite-sex pairs (Figure 4). Using a different social housing paradigm in Experiment 3, we found moderate changes in alcohol drinking relative to social housing with an opposite-sex partner, but these were independent of their partner’s drinking (Figure 5). These findings are in contrast to very specific influences of a same-sex partner (Anacker et al. 2011c). Taken together, the results of the current study suggest that social influences on alcohol self-administration in prairie voles differ based on the sex of a social partner.
In the first experiment, we replicated previous findings that animals housed in same-sex social pairs have higher ethanol consumption and preference compared to animals in isolation (Figure 3). These animals show a robust pattern of coordinated drinking similar to those previously reported (Anacker et al. 2011b). We also found a significant relationship between drinking in isolated pairs, in contrast to the previous study. This may be due to a slightly different (and more robust) statistical approach used in the current study. Our present findings suggest that there are genetic and/or developmental factors that contribute to shared drinking patterns between siblings, which may influence drinking behavior regardless of the social environment. However, the strength of this relationship was significantly lower than in mesh-housed pairs, suggesting that social housing promotes social coordination beyond similarity due to shared family background. These results indicate that social influences in same-sex dyads are robust and replicable.
We also found that animals in social housing have lower BECs following an injection of ethanol than isolated animals, and this has not been previously reported. One potential explanation for this finding is differential experience with alcohol. Long-term alcohol use can reduce alcohol metabolism (Julien 2005; Kater et al. 1969; Misra et al. 1971). Therefore greater ethanol consumption in the socially housed animals could lead to slower ethanol metabolism compared to isolated animals. An intriguing additional possibility is that the social environment may influence ethanol metabolism independent of ethanol experience. This is more likely an effect of isolation, which is a significant stressor for this species, than mesh-housing, which is a relatively small modification to the physical environment that allows contact with a social partner. A decrease in ethanol metabolism due to isolation could promote lower ethanol intake and preference in these subjects (Figure 3; Anacker et al. 2011b). Investigating the sources of social influences on ethanol metabolism may be a particularly interesting direction for future research.
Importantly, we found no effects of gonadectomy on any alcohol drinking measures (Figure 2). Although the majority of studies in rats have found no effect of gonadectomy on ethanol intake (Almeida et al. 1998; Begg and Weisinger 2008; Cailhol and Mormede 2001; Vetter-O’Hagen and Spear 2011), others have found either an increase (Vetter-O’Hagen and Spear 2011), or even a decrease in alcohol self-administration (Begg and Weisinger 2008; Ford et al. 2002, 2004). These latter studies have methodological differences relative to the present studies, including the use of limited access paradigms and alcohol experienced subjects for gonadectomies. Our data suggest that in prairie voles, adult gonadectomy does not directly affect alcohol self-administration. It is still possible that gonadectomy may have altered social behavior and thus indirectly affected the relationship between social environment and ethanol drinking in Experiment 2. However, prairie voles display normal social behavior toward, and form partner preferences with, a gonadectomized partner (DeVries and Carter 1999) and will form and maintain pair bonds in the absence of mating (Cho et al. 1999; Resendez et al. 2012; Winslow et al. 1993). Moreover, we have observed a similar lack of social facilitation on gonadally intact males with female partners, although these males were unavoidably exposed to their own pups (Anacker and Ryabinin, unpublished data). For these reasons we believe it is unlikely that castration is a significant confound to our findings in Experiment 2.
In Experiment 2 prairie voles isolated from an opposite-sex partner did not differ in either alcohol consumption or preference when compared to voles that remained housed with their partner (Figure 4). This contrasts with previous findings in same-sex pairs, in which isolated subjects had lower alcohol consumption and preference than their socially housed counterparts (Figure 3; Anacker et al. 2011b). Additionally, in Experiment 3, pairing high-drinking voles with an opposite sex low-drinking partner did not alter their alcohol consumption or preference (Figure 5). This lack of directional social influence also contrasts with previous studies in the same-sex pairs (Anacker et al. 2011c). Although we did observe changes in alcohol preference across different social housing periods, the direction and timing of effects differed in each high and low drinkers, and was not dependent on the partner’s drinking level (Figure 5b). This suggests that pairing with or isolation from an opposite-sex partner leads to moderate changes in drinking behavior, but these changes are independent of their partner’s drinking. Although these effects were not reported in the previous study with same-sex pairs, that study was parsed into separate experiments and may have lacked significant power to find such moderate effects compared to the larger design employed here. Overall, these experiments suggest that social influences on alcohol self-administration in prairie voles differ based on the sex of a social partner: changes in drinking depend on the partner in same-sex pairs, whereas in male-female pairs they depend on the individual’s own drinking status.
A particularly interesting result was that in contrast to the lack of housing effects on ethanol drinking, quinine preference was significantly lower in socially housed voles (Figure 4b). This is consistent with previous data in same-sex pairs (Anacker et al. 2011b), and suggests that drinking behavior of some fluids, such as quinine, may be responsive to the general social environment, whereas ethanol drinking may be sensitive to a specific partner. We speculate that close affiliations between individuals in prairie vole pairs helps them better avoid aversive drinking solutions.
No drinking measures were significantly correlated within mesh-housed pairs or between voles that had been separated from each other in Experiment 2. In contrast, same sex pairs show strong positive correlations with their social partners at high concentrations of ethanol (10%: Experiment 1; Anacker et al. 2011b). Therefore, it appears that alcohol drinking is much less coordinated in male-female pairs, which is consistent with our lack of social housing effects on alcohol drinking in these animals.
We also examined the relationship between anxiety-like behavior and alcohol drinking in Experiment 2. In humans, a bidirectional relationship between drinking and anxiety has been described, such that anxiety may promote drinking behavior via acute anxiolytic effects, and prolonged ethanol consumption may increase anxiety (Allan 1995; Kushner et al. 2000; Robinson et al. 2009). The relationship between anxiety and drinking is less clear in rodents, as studies have found a positive (Colombo et al. 1995; Izidio and Ramos 2007; Spanagel et al. 1995; Stewart et al. 1993), negative (Fernandez-Teruel et al. 2002; Henniger et al. 2002; Langen and Fink 2004; Viglinskaya et al. 1995), or no relationship (Da Silva et al. 2004; Tuominen et al. 1990; Viglinskaya et al. 1995) between measures of these behaviors. Given that even brief isolation from a social partner can lead to changes in anxiety-like behavior in prairie voles (Bosch et al. 2009), we hypothesized that the relationship between anxiety and ethanol drinking in the present study would differ based on the social environment. Across all alcohol concentrations, single-housed males showed negative correlations between the number of arm entries (which may be a measure of locomotor activity or risk-taking) and ethanol consumption (Table 1). These same males had positive correlations between time spent autogrooming and ethanol intake. It is interesting to note that both autogrooming and alcohol drinking may function as self-directed anxiolytic behaviors (Kalueff and Tuohimaa 2005), but we found no significant relationship with open arm proportion, the traditional measure of anxiety-like behavior. These associations were not found in females, or generally in males that were mesh-housed. This suggests that perhaps there is a more significant relationship between EPM behaviors and drinking in males versus females, and that social or individual housing can moderate this relationship. Another interesting finding is that there are fewer significant correlations between alcohol preference and EPM behaviors (Table 2).
Although we did not find a strong relationship between anxiety-like behavior and ethanol consumption in prairie voles, this should be more rigorously examined in future studies. For example, it is unclear whether our findings were influenced by dosage effects, relative experience with alcohol, length of time since isolation, or multiple comparisons. Additionally, the lack of an influence of pre-existing anxiety-like behavior and locomotor activity in mesh-housed males suggests that social environment may still influence alcohol drinking in a way not previously assessed. It is possible that social housing with an opposite sex partner itself alters anxiety-like behavior in the mesh-housed subjects; however, Bosch and colleagues (2009) found no differences in EPM behaviors in prairie voles housed with same-sex siblings versus a novel opposite-sex partner. Further studies on the interactions between anxiety, social environment, and alcohol drinking are recommended.
A limitation for interpretation is that we did not test for the presence of pair bonds in these animals. The current study was designed to assess the role of the sex of the partner on alcohol intake, not to specifically test whether social influences in drinking are altered by pair bonding. Therefore, the experimental design was not optimal for partner preference testing. Additionally, a lack of partner preference in these animals would be difficult to interpret as alcohol exposure can itself affect pair bond formation (Anacker et al. 2011a). The data from these studies should not be interpreted as necessarily reflecting alcohol drinking in pair bonded animals. Future studies investigating the interaction of alcohol and pair bonding are critical.
The present study is broadly consistent with studies in humans indicating that there are unique contributions of peers and a married partner to drinking behavior in humans (Bachman et al. 2002; Homish and Leonard 2008; Leonard and Rothbard 1999; Merline et al. 2008). Marriage is frequently associated with lower alcohol use and abuse (Bachman et al. 2002; Gotham et al. 1997; Leonard and Rothbard 1999; Miller-Tutzauer et al. 1991; Robbins 1991) and may have a protective effect against the development of problematic alcohol use (Chilcoat and Breslau 1996). In contrast, separation and divorce are often associated with increased alcohol drinking and alcohol use disorder (Bachman et al. 1997; Chilcoat and Breslau 1996; Leonard and Rothbard 1999). Although we do not equate male-female cohabitation with marriage, nor isolation with divorce, there are interesting parallels in Experiment 3. We found that high drinkers reduced their ethanol preference during pairing, and low increased ethanol preference but only after isolation from their partner. Wilsnack and colleagues (1991) found that women who had no history of problem drinking were at increased risk for alcohol problems following separation or divorce. However, among women that were initially characterized as problem-drinkers, there was a reduced risk of problem drinking following separation or divorce. Therefore, individual differences in drinking history are differentially influenced by the social environment in both humans and prairie voles.
Taken with previous studies, this research suggests that the sex of the social partner influences patterns of drinking in this species. Specifically, direct social influences on drinking behavior may be primarily restricted to same-sex peers and not opposite-sex pairs of prairie voles. This has implications for humans, as different social partners, such as a peer or spouse, can positively or negatively influence alcohol drinking.
Acknowledgments
We gratefully acknowledge Davelle Cocking, Lindsay Swanson, and the Veterinary Medical Unit (VMU) animal care staff the Portland VA Medical Center for assistance on this project. This research was funded by NIH 5T32AA007468-24 (to CMH) and NIH AA016886 (to AER). This material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center, Portland, Oregon.
References
- Allan CA. Alcohol problems and anxiety disorders--a critical review. Alcohol Alcohol. 1995;30:145–151. [PubMed] [Google Scholar]
- Almeida SA, Anselmo-Franci JA, Rosa e Silva AA, Carvalho TL. Chronic intermittent immobilization of male rats throughout sexual development: a stress protocol. Exp Physiol. 1998;83:701–704. doi: 10.1113/expphysiol.1998.sp004151. [DOI] [PubMed] [Google Scholar]
- Anacker AM, Ahern TH, Young LJ, Ryabinin AE. Program No. 469.06. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011a. Alcohol self-administration inhibits the expression of partner preference in a sex-specific manner in prairie voles. 2011. Online. [Google Scholar]
- Anacker AMJ, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially-facilitated excessive drinking. Addict Biol. 2011b;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011c;35:1884–1890. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7:473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman JG, O’Malley PM, Schulenberg JE, Johnston LD, Bryant AL, Merline AC. The Decline of Substance Use in Young Adulthood: Changes in Social Activities, Roles, and Beliefs. Lawrence Erlbaum Associates, Inc; Mahwah, NJ: 2002. [Google Scholar]
- Bachman JG, Wadsworth KN, O’Mally PM, Johnston LD, Schulenberg JE. Smoking, Drinking, and Drug Use in Young Adulthood: The Impacts of New Freedoms and New Responsibilities. Lawrence Erlbaum Associates, Inc; Mahwah, NJ: 1997. [Google Scholar]
- Begg DP, Weisinger RS. The role of adrenal or testicular hormones in voluntary ethanol and NaCl intake of crowded and individually housed rats. Physiol Behav. 2008;93:408–413. doi: 10.1016/j.physbeh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiol Behav. 2002;76:559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Cooper HM. Effects of alcohol on human aggression: an integrative research review. Psychol Bull. 1990;107:341–354. doi: 10.1037/0033-2909.107.3.341. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Cavanaugh BL, Lonstein JS. Social novelty increases tyrosine hydroxylase immunoreactivity in the extended olfactory amygdala of female prairie voles. Physiol Behav. 2010;100:381–386. doi: 10.1016/j.physbeh.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Alcohol disorders in young adulthood: effects of transitions into adult roles. J Health Soc Behav. 1996;37:339–349. [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Da Silva GE, Ramos A, Takahashi RN. Comparison of voluntary ethanol intake by two pairs of rat lines used as genetic models of anxiety. Braz J Med Biol Res. 2004;37:1511–1517. doi: 10.1590/s0100-879x2004001000010. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Carter CS. Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster) Can J Zool. 1999;77:885–889. [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. The modulation of pair bonding by corticosterone in female prairie voles (Microtus ochrogaster) Proc Natl Acad Sci USA. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Carter CS. Social modulation of corticosteroid responses in male prairie voles. Ann N Y Acad Sci. 1997;807:494–497. doi: 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Driscoll P, Gil L, Aguilar R, Tobena A, Escorihuela RM. Enduring effects of environmental enrichment on novelty seeking, saccharin and ethanol intake in two rat lines (RHA/Verh and RLA/Verh) differing in incentive-seeking behavior. Pharmacol Biochem Behav. 2002;73:225–231. doi: 10.1016/s0091-3057(02)00784-0. [DOI] [PubMed] [Google Scholar]
- Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002;26:103–113. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Gotham HJ, Sher KJ, Wood PK. Predicting stability and change in frequency of intoxication from the college years to beyond: individual-difference and role transition variables. J Abnorm Psychol. 1997;106:619–629. doi: 10.1037//0021-843x.106.4.619. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Holter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26:729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Homish GG, Leonard KE. The social network and alcohol use. J Stud Alcohol Drugs. 2008;69:906–914. doi: 10.15288/jsad.2008.69.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Bales KL. DeltaFosB is increased in the nucleus accumbens by amphetamine but not social housing or isolation in the prairie vole. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Kowalczyk AS, Griffin LL, Bales KL. CART peptide following social novelty in the prairie vole (Microtus ochrogaster) Brain Res. 2011;1414:32–40. doi: 10.1016/j.brainres.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izidio GS, Ramos A. Positive association between ethanol consumption and anxiety-related behaviors in two selected rat lines. Alcohol. 2007;41:517–524. doi: 10.1016/j.alcohol.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Julien RM. A Primer of Drug Action. 10. Worth Publishers; New York, NY: 2005. [Google Scholar]
- Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods. 2005;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kater RM, Carulli N, Iber FL. Differences in the rate of ethanol metabolism in recently drinking alcoholic and nondrinking subjects. Am J Clin Nutr. 1969;22(12):1608–17. doi: 10.1093/ajcn/22.12.1608. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Langen B, Fink H. Anxiety as a predictor of alcohol preference in rats? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:961–968. doi: 10.1016/j.pnpbp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Eiden RD. Marital and family processes in the context of alcohol use and alcohol disorders. Annu Rev Clin Psychol. 2007;3:285–310. doi: 10.1146/annurev.clinpsy.3.022806.091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE, Rothbard JC. Alcohol and the marriage effect. J Stud Alcohol Suppl. 1999;13:139–146. doi: 10.15288/jsas.1999.s13.139. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci USA. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J Neurosci. 2011;31:7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Zucker RA, Molina BS, Ammon L, Ames GM, Longabaugh R. Social environmental influences on the development and resolution of alcohol problems. Alcohol Clin Exp Res. 2006;30:688–699. doi: 10.1111/j.1530-0277.2006.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline AC, Schulenberg JE, O’Malley PM, Bachman JG, Johnston LD. Substance use in marital dyads: premarital assortment and change over time. J Stud Alcohol Drugs. 2008;69:352–361. doi: 10.15288/jsad.2008.69.352. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, DeBold JF. Alcohol, benzodiazepine-GABAA receptor complex and aggression: ethological analysis of individual differences in rodents and primates. J Stud Alcohol Suppl. 1993;11:170–179. doi: 10.15288/jsas.1993.s11.170. [DOI] [PubMed] [Google Scholar]
- Misra PS, Lefévre A, Ishii H, Rubin E, Lieber CS. Increase of ethanol, meprobamate and pentobarbital metabolism after chronic ethanol administration in man and in rats. Am J Med. 1971;51(3):346–51. doi: 10.1016/0002-9343(71)90270-1. [DOI] [PubMed] [Google Scholar]
- Miller-Tutzauer C, Leonard KE, Windle M. Marriage and alcohol use: a longitudinal study of “maturing out”. J Stud Alcohol. 1991;52:434–440. doi: 10.15288/jsa.1991.52.434. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Disruption of maternal behavior by alcohol intoxication in the lactating rat: a behavioral and metabolic analysis. Alcohol Clin Exp Res. 2002;26:1205–1214. doi: 10.1097/01.ALC.0000025884.74272.BC. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Pinel JPJ. Alcohol inhibits and disinhibits sexual behavior in the male rat. Psychobiology. 1989;17:195–201. [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012 doi: 10.1523/jneurosci.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CA. Social roles and alcohol abuse among older men and women. Fam Community Health. 1991;13:37–48. [Google Scholar]
- Robinson JA, Sareen J, Cox BJ, Bolton JM. Correlates of self-medication for anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Nerv Ment Dis. 2009;197:873–878. doi: 10.1097/NMD.0b013e3181c299c2. [DOI] [PubMed] [Google Scholar]
- Russell DW, Booth B, Reed D, Laughlin PR. Personality, social networks, and perceived social support among alcoholics: a structural equation analysis. J Pers. 1997;65:649–692. doi: 10.1111/j.1467-6494.1997.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Steele CM, Southwick L. Alcohol and social behavior I: The psychology of drunken excess. J Pers Soc Psychol. 1985;48:18–34. doi: 10.1037//0022-3514.48.1.18. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and non-preferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Tuominen K, Hilakivi LA, Paivarinta P, Korpi ER. Behavior of alcohol-preferring AA and alcohol-avoiding ANA rat lines in tests of anxiety and aggression. Alcohol. 1990;7:349–353. doi: 10.1016/0741-8329(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague Dawley rats. Alcohol Clin Exp Res. 2011;35:2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglinskaya IV, Overstreet DH, Kashevskaya OP, Badishtov BA, Kampov-Polevoy AB, Seredenin SB, Halikas JA. To drink or not to drink: tests of anxiety and immobility in alcohol-preferring and alcohol-nonpreferring rat strains. Physiol Behav. 1995;57:937–941. doi: 10.1016/0031-9384(94)00368-f. [DOI] [PubMed] [Google Scholar]
- Wilsnack SC, Klassen AD, Shur BE, Wilsnack RW. Predicting onset and chronicity of women’s problem drinking: A five year longitudinal analysis. Amer J Publ Hlth. 1991;81:305–318. doi: 10.2105/ajph.81.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011a;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Dietz DM, Wang H, Kabbaj M, Wang Z. Amphetamine alters behavior and mesocorticolimbic dopamine receptor expression in the monogamous female prairie vole. Brain Res. 2011b;1367:213–222. doi: 10.1016/j.brainres.2010.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]