Abstract
Background
Guidelines differ on screening recommendations for latent tuberculosis infection (LTBI) prior to immunosuppressive therapy. We aimed to determine the most cost-effective LTBI screening strategy before long-term steroid therapy in a child with new onset idiopathic nephrotic syndrome.
Study Design
Markov state-transition model.
Setting and Population
Five year old male with new onset idiopathic nephrotic syndrome.
Model, Perspective, and Timeframe
The Markov model took a societal perspective over a lifetime horizon.
Intervention
Three strategies were compared: universal tuberculin skin testing (TST), targeted screening using a risk-factor questionnaire, and no screening. A secondary model included the newer interferon-γ release assays (IGRA), requiring only one visit and having greater specificity than TST.
Outcomes
Marginal cost-effectiveness ratios (2010 United States $) with effectiveness measured as quality-adjusted life years (QALYs).
Results
At a LTBI prevalence of 1.1% (the average United States childhood prevalence in our base-case), a no-screening strategy dominated ($2,201, 29.3356 QALYs) targeted screening ($2,218, 29.3356 QALYs) and universal TST ($2,481, 29.3347 QALYs). At a prevalence >10.3%, targeted screening with a risk-factor questionnaire was the most cost-effective option. Above a prevalence of 58.5%, universal TST was preferred. In the secondary model, targeted screening with a questionnaire followed by IGRA testing was cost-effective compared to no screening in the base case when the LTBI prevalence was >4.9%.
Limitations
There is no established gold standard for the diagnosis of LTBI. Results of any modeling task are limited by the accuracy of available data.
Conclusions
Prior to starting steroids, only patients in areas with a high prevalence of LTBI will benefit from universal TST. As more evidence becomes available on the use of IGRA testing in children, the assay may become a component of cost-effective screening protocols in populations with a higher burden of LTBI.
Index words: cost-effectiveness, nephrotic syndrome, tuberculosis, children
The Centers for Disease Control and Prevention and the World Health Organization have identified tuberculosis (TB) as an important infectious disease target.1,2 Treating patients for latent tuberculosis infection (LTBI) is critical to eliminating TB.3, 4 Current recommendations in the United States support screening for LTBI with tuberculin skin testing (TST) in high-risk populations, such as patients with HIV and certain immigrants.1, 5 Conversely, consensus guidelines do not support universal TST screening in healthy children without risk factors.6, 7
Recommendations for LTBI screening before starting immunosuppression are conflicting. While the American Academy of Pediatrics advocates universal TST screening before immunosuppressive therapy,6 the American Thoracic Society suggests targeted screening with a questionnaire to decrease false positive TST results in patients without risk factors.1 A high risk of LTBI reactivation has been reported in patients who receive anti-TNF-α (tumor necrosis factor α) agents.8–11 Adults taking prednisone >15 mg/day (or its equivalent) for two–four weeks are also likely at increased risk for LTBI reactivation.5, 6, 11–14
Idiopathic nephrotic syndrome is a common illness treated by pediatric nephrologists and its incidence varies around the world.15–17 At presentation and during relapses, children receive courses of high-dose steroids.17 Current literature on children with nephrotic syndrome supports TST testing prior to such steroid therapy.15, 16 Multidisciplinary guidelines encourage developing cost-effective strategies for the targeted testing and treatment of persons with LTBI, calling for studies to determine whether or not to test at presentation.1, 13, 18 Therefore, we developed comprehensive decision analytic models to examine the cost-effectiveness of strategies including universal TST testing, targeted testing following a positive screening questionnaire, and the newer interferon-γ release assays (IGRAs), to determine the optimal LTBI screening strategy prior to steroid therapy in children with idiopathic nephrotic syndrome.
METHODS
Population at risk
To assess the impact of immunosuppression on the period of highest risk, the base-case was a five-year old male with newly diagnosed steroid-sensitive idiopathic nephrotic syndrome who will receive high-dose prednisone for six weeks, with a tapering course over the next six months.16, 17, 19 This base-case was chosen because children with idiopathic nephrotic syndrome usually presents by five years of age,16, 17 children <five years of age are at the highest risk of acquiring severe TB disease,5, 20 and although IGRA testing is not recommended in children, evidence supports its use in children >five years of age.5, 21 Patients were assumed to be adherent to medication.
Model overview
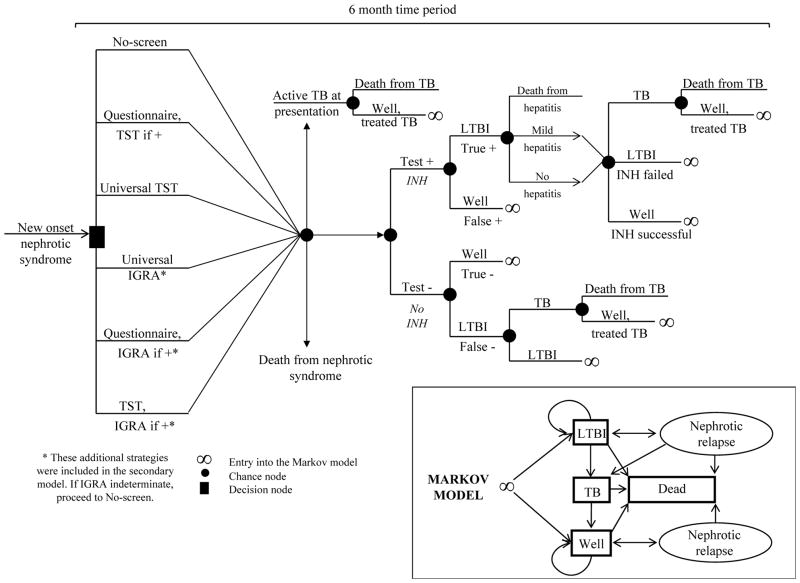
Events over the first six months following induction steroid therapy are modeled before entry into a Markov state-transition model (Fig 1). Longer term events are simulated within the Markov model22 (three month cycle length) using a lifetime horizon.
Figure 1. Model structure.
Patients enter the first six months of the model with idiopathic nephrotic syndrome. Those surviving the first six months of the model enter the four-state Markov state-transition model either well or with LTBI and remain in it until death. TST, tuberculin skin test; IGRA, interferon-γ release-assay; +, positive; TB, tuberculosis; LTBI, latent tuberculosis infection; INH, isoniazid; −, negative.
Base-case values for screening test characteristics, prevalence, mortality, and therapy (Table 1) were from the published literature. A TST ≥10 mm was considered positive. Given patients had active nephrotic syndrome, we assumed all patients returned to have their TST interpreted. The baseline annual risk of TB reactivation was derived from Horsburgh,23 using his assumption that the initial risk decreases by 10% per decade and we updated these rates to account for a current LTBI reactivation rate that is about half of the past risk (Table 2).24 Finally, the baseline rates were increased by a factor of 7.7 to account for the increased risk of TB while taking steroids.25 Patients receiving steroids at presentation or nephrotic syndrome relapse had this relative increased risk of TB reactivation for six or three months, respectively.
Table 1.
Model inputs: probabilities, rates, quality of life, and costs
| Variable | Base-Case Value* | Base-Case References | Distribution Type | Other references |
|---|---|---|---|---|
|
| ||||
|
Probabilities and Rates
| ||||
| Sensitivity TST | 0.90 (0.88–1.00) | 5, 58 | logit | 6, 31, 46, 47, 51, |
|
| ||||
| Specificity TST | 0.68 (0.52–0.84) | 5, 46 | logit | 55, 58–62 |
|
| ||||
| Sensitivity questionnaire | 0.46 | 34 | - | 63 |
|
| ||||
| Specificity questionnaire | 0.94 | |||
|
| ||||
| Sensitivity IGRA | 0.90 (0.77–0.99) | 3, 5, 31, 47, 53, 58 | logit | 46, 55, 59, 64 |
|
| ||||
| Specificity IGRA | 0.97 (0.90–1.00) | |||
|
| ||||
| IGRA indeterminate | 0.03 (0.02–0.05) | 31 | logit | 3, 21, 53 |
|
| ||||
| Prevalence of LTBI | 0.011 (0.005–0.024) | 42 | logit | 52, 65–67 |
|
| ||||
| RR of LTBI reactivation while on steroids | 7.7 (2.8–21.4) | 25 | log normal | 8–10, 68 |
|
| ||||
| Probability of death from TB on steroids | 0.01 | estimated | - | 11, 31, 68 |
|
| ||||
| INH effectiveness | 75% | 13 | - | 6, 7, 13, 31, 47, 50, 52, 69–71 |
|
| ||||
| INH hepatitis | 0.004 (0.001–0.010) | 6 | beta | 31 |
|
| ||||
| Death from INH hepatitis | 0.00002 | 6, 27 | ||
|
| ||||
| Probability of death from active NS | 0.007 | 72 | - | 16, 17, 73 |
|
| ||||
| Probability of active TB on initial presentation | 0.00001 | 74 | ||
|
| ||||
| Discount rate (per y) | 3% | |||
|
| ||||
|
Utilities
| ||||
| Well | 1.0 | - | ||
|
| ||||
| Latent TB | 0.997 | 31, 75, 76 | - | 30 |
|
| ||||
| Active TB | 0.68 (0.65–0.72) | 26, 30 | logit | 31 |
|
| ||||
| NS | 0.90 | 77 | - | |
|
| ||||
| INH hepatitis x 1 mo | 0.85 | 31 | - | 52 |
|
| ||||
| Death | 0.0 | - | ||
|
| ||||
|
Costs (2010 United States $)
| ||||
| TST | 39.64 | 36 | - | 31, 47, 51, 66, 78–80 |
| Test | 6.88 | |||
| Nurse Interpretation | 3.50 | |||
| Travel time/lost work | 29.26 | |||
|
| ||||
| Questionnaire | 1.62 | 51 | - | |
|
| ||||
| IGRA | 84.56 | 36 | 45 | |
|
| ||||
| LTBI | 772.29 | 6, 36, 37 | - | 13, 31, 47, 51, 52, 66, 78, 80 |
| Level 4 visit | 98.70 | |||
| Chest x-ray (2 views) | 30.45 | |||
| Level 3 visit x 8 | 312.64 | |||
| 9 mo of INH | 67.16 | |||
| Parent lost time x 9 | 263.34 | |||
|
| ||||
| Active TB | 60,000.00 | 31 | - | 18, 31, 35, 47, 50–52, 66, 78, 80, 81 |
|
| ||||
| INH hepatitis x 1 mo | 539.74 | 31, 36, 37 | - | |
| Level 4 visit x 2 | 197.40 | |||
| Liver function tests x 2 | 17.78 | |||
| Parent lost time x 2 | 58.52 | |||
| 6 mo of rifampin | 266.04 | |||
|
| ||||
| Death (TB, hepatic failure, or NS) | 100,000.00 | estimated31 | - | |
| NS onset | 420.48 | 36, 78, 82 | - | |
| Level 3 visit x 3 | 117.24 | |||
| Steroids x 6 mo | 215.46 | |||
| Parent lost time x 3 | 87.78 | |||
|
| ||||
| NS relapse | 140.16 | 36, 78, 82 | - | |
| Level 3 visit x 1 | 39.08 | |||
| Steroids x 1 mo | 71.82 | |||
| Parent lost time x 1 | 29.26 | |||
Values in parentheses are 95% confidence interval or clinically plausible range.
Abbreviations: TB, tuberculosis; LTBI, latent tuberculosis infection; IGRA, interferon-γ release assay; NS, nephrotic syndrome; INH, isoniazid; TST, tuberculin skin testing; RR, relative risk;
Table 2.
Markov model inputs that change over time
| Patient Age (y) | Baseline annual TB reactivation rate (no steroid therapy)23, 24 | NS annual relapse rate16, 17, 19, 33 |
|---|---|---|
| 5 | 0.00095 | 0.080 |
| 15 | 0.00085 | 0.080 |
| 25 | 0.00075 | 0.006 |
| 35 | 0.0007 | 0.006 |
| 45 | 0.0006 | 0.006 |
| 55 | 0.00055 | 0 |
| 65 | 0.0005 | 0 |
| 75 | 0.00045 | 0 |
| 85 | 0.0004 | 0 |
| 95 | 0.00035 | 0 |
| 105 | 0.00035 | 0 |
| 150 | 0 | 0 |
Abbreviations: TB, tuberculosis; NS, nephrotic syndrome
After presentation, patients would not be screened for LTBI again and isoniazid (INH), if effective, provided lifelong protection.26 Children developing INH-related mild hepatitis were switched to rifampin. Although the risk of severe complications of INH hepatitis are extremely rare in children,6 we included death from hepatic failure.6, 27
Patients could either present with active TB or later develop active TB if they had LTBI at presentation. Children could have active TB only once and did not develop multi-drug resistant disease.7, 28 Recent evidence supports that many patients surviving acute infection have decreased lung function.29 We accounted for this by assuming that children surviving active TB would have lower utility estimates30 than previously published.27, 31
Other model inputs included the baseline TB mortality rate,32 the TB case fatality rate while receiving steroids, and the decreasing risk of nephrotic syndrome relapse over time (Table 2).16, 17, 33
As shown in Fig 1, we developed two models. The primary model compared universal TST screening prior to starting steroids with targeted screening using a nine-item questionnaire34 (Table S1, available as online supplementary material) and only performing TST testing if the screen was positive. To minimize false-positive TST results at a base-case LTBI prevalence of 1.1%, patients were assumed to have a positive screen if at least three out of nine risk factors were present.34 The secondary model assessed combinations of screening strategies that included IGRA testing. Both models included a no-screen strategy for comparison.
Costs were estimated from the published literature (Table 1), the Healthcare Cost and Utilization Project,35 the Centers for Medicare and Medicaid Services website (Medicare non-facility fees),36 and the Red Book.37 Costs were adjusted to 2010 United States $ using the Department of Labor consumer price index inflation calculator.38 The model used a societal perspective. We assumed the economic cost of death was $100,000, adapted from the highest cost estimate in a recent economic model of TST screening in health care workers.31 Effectiveness was measured as quality-adjusted life years (QALYs) using utility estimates for health states reported in patients with TB, or when not available, in other conditions requiring similar types of treatment (Table 1). Results were reported as marginal cost-effectiveness ratios (mCERs), applying a standard discount rate of 3% per year for both costs and QALYs. Non-discounted results were also computed (Table 3). Cost-effective strategies were defined as those costing <$100,000/QALY. Analyses were performed with commercially available software (Decision Maker; Tufts Medical Center [www.tuftsmedicalcenter.org]).
Table 3.
Base-case results
| Screening strategy | Cost, $ | Effectiveness, QALYs | Marginal cost, $ | Marginal effectiveness QALYs | Marginal cost-effectiveness ratio, $/QALY |
|---|---|---|---|---|---|
| Primary Model: Discounted (3% per year) | |||||
| No screen | 2,200.96 | 29.33559 | - | - | - |
| Questionnaire/TST if + | 2,218.20 | 29.33555 | 17.24 | −0.00004 | dominated |
| Universal TST | 2,480.97 | 29.33471 | 280.01 | −0.00088 | dominated |
| Primary Model: Non-discounted | |||||
| No screen | 2,216.16 | 71.75844 | - | - | |
| Questionnaire/TST if + | 2,228.69 | 71.75839 | 12.53 | −0.00005 | dominated |
| Universal TST | 2,485.93 | 71.75731 | 269.76 | −0.00113 | dominated |
| Secondary Model: Discounted (3% per year) | |||||
| No screen | 2,200.96 | 29.33559 | |||
| Questionnaire/IGRA if + | 2,208.01 | 29.33560 | 7.05 | 0.00001 | 705,000 |
| Questionnaire/TST if + | 2,218.20 | 29.33555 | 10.02 | −0.00005 | dominated |
| TST/IGRA if + | 2,272.43 | 29.33558 | 64.42 | −0.00002 | dominated |
| Universal IGRA | 2,304.89 | 29.33553 | 96.88 | −0.00007 | dominated |
| Universal TST | 2,480.97 | 29.33471 | 272.97 | −0.00089 | dominated |
| Secondary Model: Non-discounted | |||||
| No screen | 2,216.16 | 71.75844 | - | - | - |
| Questionnaire/IGRA if + | 2,218.68 | 71.75845 | 2.52 | 0.00001 | 252,000 |
| Questionnaire/TST if + | 2,228.69 | 71.75839 | 10.01 | −0.00006 | dominated |
| TST/IGRA if + | 2,278.77 | 71.75844 | 60.09 | −0.00001 | dominated |
| Universal IGRA | 2,310.25 | 71.75836 | 91.57 | −0.00009 | dominated |
| Universal TST | 2,485.93 | 71.75731 | 267.24 | −0.00114 | dominated |
Dominated indicates the strategy is both more costly and less/equally effective compared to the next best strategy
Abbreviation: QALY, quality-adjusted life-year; ; IGRA, interferon-γ release assay; TST, tuberculin skin testing
Primary model: universal TST, targeted screening, and no screen
Following testing, patients could have true positive, false positive, true negative, or false negative results. We used Bayes’ Rule and sequential Bayesian revisions when screening tests were combined in series39 and assumed the second test was conditionally independent of the first test. This assumption was tested with one-way sensitivity analyses.
Patients with LTBI face a risk of TB reactivation, a risk reduced by the administration of INH in patients with true positive results. Patients with TB reactivation while receiving steroids are at higher risk of death during the next six months. Patients surviving TB, those with continued LTBI (either through no screening, false negative screening, or failed INH), and those without LTBI enter the Markov model.
Within the Markov model patients move between one of four health states (Fig 1), depending upon the occurrence of probabilistic events. Patients face a continued risk of nephrotic syndrome relapse, and relapsing patients with LTBI have a higher rate of reactivating and dying from TB due to reimplementation of steroid therapy. LTBI patients without nephrotic relapse have a lower risk of reactivation and death from TB. Finally, all patients may die from non-explicitly modeled mortality forces based on standard life tables.40
Secondary model: addition of IGRA testing strategies
Otherwise identical to the primary model, the secondary model included additional IGRA testing strategies (Fig 1), i.e. universal IGRA, universal TST followed by IGRA among those with positive TSTs, and targeted IGRA for those with a positive questionnaire. For IGRA testing, the secondary model did not differentiate between the two commonly used assays, the T-SPOT.TB test and the QuantiFERON-TB Gold InTube test. This model was considered secondary because IGRA testing is currently not approved in children or during immunosuppression.5
Sensitivity analyses
One-way and two-way deterministic sensitivity analyses assessed the impact of parameter uncertainty on model results. Probabilistic sensitivity analyses were performed on the primary and secondary models using second-order Monte Carlo simulations with 10,000 iterations.22 Variables were parameterized with mathematical distributions (Table 1), including beta (event versus no event: mild INH hepatitis6), logit (probabilities and utilities41), and log normal (relative risks) distributions (Table 1). For each model, three separate Monte Carlo simulations assessed decision-making for populations, varying the LTBI prevalence: 1) Low-risk population (base-case) with an LTBI prevalence of 1.1%,42 2) Moderate-risk population with an LTBI prevalence of 9.0%, representing the rate of infection in foreign born high-risk children living in the United States,43 and 3) High-risk population with an LTBI prevalence of 18.1%, representing the rate of infection in HIV-negative South African children living in high-prevalence regions of South Africa.44
RESULTS
Base-case
For the primary and secondary models, our base-case (United States LTBI prevalence of 1.1%) demonstrated that no screen was the dominant strategy, being both less costly and more effective than either universal TST or targeted screening (Table 3). Similarly, in the secondary model, no-screen dominated the other strategies.
Deterministic sensitivity analyses
In the primary model, the results were most sensitive to the prevalence of LTBI and the specificity of the risk factor questionnaire. Targeted screening cost <$100,000/QALY compared to the no-screen option when the prevalence of LTBI was between 10.3% and 58.5%. Universal TST did not become cost-effective compared to targeted screening until the prevalence of LTBI exceeded 58.5%. Even if the TST had 100% specificity for detecting LTBI, targeted screening (mCER = $264,530) and universal TST (mCER = $2,893,341) remained inferior to the no-screen strategy. Furthermore, a no-screen strategy dominated even if INH provided 100% protection against developing active TB.
In terms of the specificity of the questionnaire, we assumed that a positive questionnaire was defined as at least 3/9 risk factors present (Table S1). Targeted screening became cost-effective when the questionnaire specificity was >99.8%. This was equivalent to at least 6/9 questionnaire risk factors, resulting in a specificity of 99.98% and a sensitivity of 0.6% to detect a TST of ≥10 mm.34 Running the model with these inputs, targeted screening had a mCER of $102,138 compared to the no-screen strategy.
The inclusion of non-medical costs (travel time and lost work) is not universally used in cost-effectiveness models. After removing these non-medical costs from the primary model, the no-screen strategy continued to dominate targeted screening and universal TST. In a sensitivity analysis to assess the conditional independence of sequential screening tests in the primary model, the results remained the same if the TST was assumed 100% dependent on the questionnaire result. Specifically, no-screen remained dominant in the base-case. Similarly, the secondary model results were not sensitive to the assumption of conditional independence.
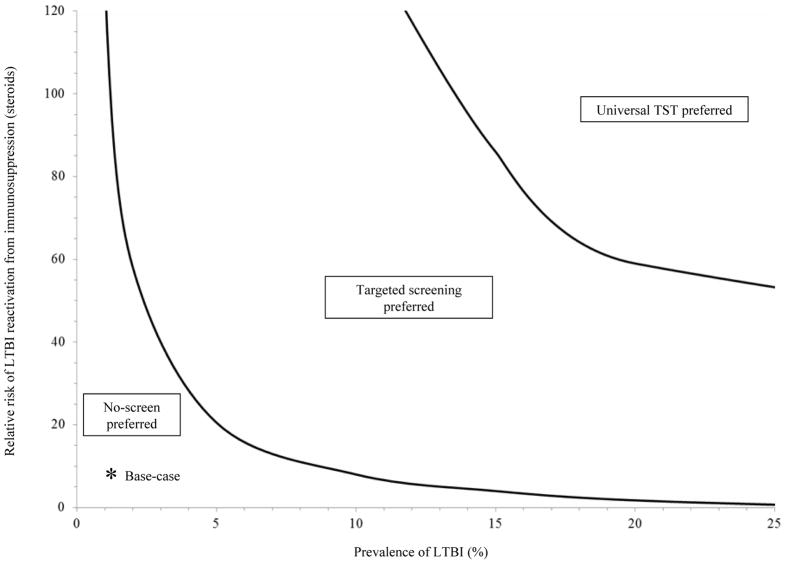
A two-way sensitivity analysis of the primary model compared the prevalence of LTBI versus the risk of LTBI reactivation while taking steroids (Fig 2). For example, universal TST was cost-effective compared to targeted screening if the LTBI prevalence was 20% and the relative risk of TB reactivation while receiving steroids was >60 (for comparison, the relative hazard in our base-case was 7.7 with a LTBI prevalence of 1.1%).
Figure 2. Two-way sensitivity analysis examining the risk of LTBI reactivation from immunosuppression and the LTBI prevalence in the primary model.
Solid lines represent the willingness to pay thresholds of $100,000/QALY demarcating the tested strategies. As the LTBI prevalence and relative risk of reactivation from immunosuppression increase from the base-case (relative hazard of 7.7 with a LTBI prevalence of 1.1%), targeted screening becomes preferred over no-screen. At a higher LTBI prevalence and relative risk of LTBI reactivation from immunosuppression, universal TST is preferred over targeted screening. TST, tuberculin skin test; LTBI, latent tuberculosis infection; QALY, quality-adjusted life years.
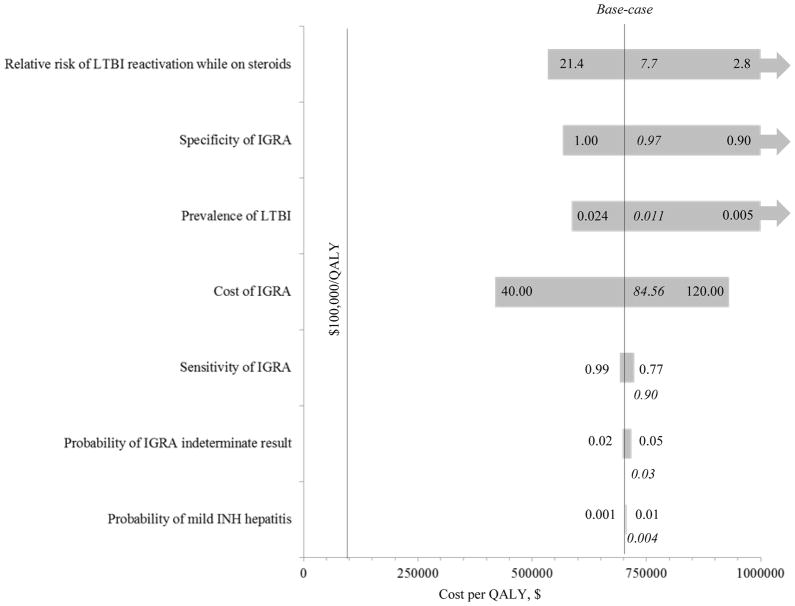
The secondary model was also sensitive to the prevalence of LTBI (Fig 3). For targeted screening with a questionnaire followed by IGRA testing if positive to be cost-effective, the prevalence of LTBI had to be >4.9%. Even with perfect IGRA sensitivity or specificity, targeted IGRA testing was not cost-effective at the base-case LTBI prevalence of 1.1%. At an IGRA indeterminate rate of 0%, targeted IGRA testing had a mCER of >$1,400,000/QALY. Assessing a LTBI prevalence of 9.0%,43 targeted IGRA testing was cost-effective as long as the IGRA specificity was >84%, its sensitivity was >54%, its cost was <$192, and the indeterminate rate was <12.6%. Finally, at a LTBI prevalence of 18.1%44, targeted IGRA testing was cost-effective as long as its cost was <$123 and the indeterminate rate was <6.7%.
Figure 3. Tornado plot (one-way sensitivity analyses, secondary model) examining targeted IGRA versus no-screen.
Parameters are varied across 95% confidence intervals or range of plausible inputs (i.e. the cost of IGRA testing was varied by ±50% of the base-case value of $84.56) with the model most sensitive to inputs (upper and lower values; base-case in italics) at the top of the figure with the widest bars. Arrows show mCERs >$1,000,000/QALY or where the no-screen strategy dominates.
Probabilistic sensitivity analyses
For the primary model, using a second-order Monte Carlo simulation at a LTBI prevalence of 1.1% (base-case), universal TST was always more costly and less effective than targeted screening and no-screening. At a LTBI prevalence of 9.0%,43 targeted screening was preferred over no-screening in 36.9% of the simulations, and universal TST never had a mCER of <$100,000/QALY. Furthermore, at a LTBI prevalence of 18.1%,44 universal TST had a mCER of <$100,000/QALY when compared to targeted screening in only 1 of the 10,000 iterations.
For the secondary model at a LTBI prevalence of 1.1%, compared to no-screening, targeted IGRA was preferred in 0.49% of the 10,000 simulations. At a LTBI prevalence of 9.0%, targeted IGRA testing was cost-effective in 99.9% of the 10,000 simulations compared to either universal IGRA or sequential TST and IGRA testing.
At a LTBI prevalence of 18.1%, targeted IGRA testing was cost-effective in 96.1% and 94.4% of the 10,000 iterations compared to universal IGRA and sequential TST and IGRA testing, respectively. Targeted TST testing was preferred to targeted IGRA testing in only 4.4% of the simulations.
DISCUSSION
Several guidelines recommend TST to screen for LTBI before immunosuppressive therapy, including before initiating steroid therapy for idiopathic nephrotic syndrome in children.6, 9, 15 Others have endorsed targeted screening prior to starting immunosuppression by first assessing a patient’s specific TB risk factors.1 At a base-case LTBI prevalence of 1.1%,42 we draw three main conclusions from our analysis.
First, universal TST is not cost-effective until the prevalence of LTBI becomes very high (>58.5%). Second, although targeted screening with a risk factor questionnaire also was not cost-effective in the base-case, this finding was sensitive to the prevalence of LTBI and the specificity of the questionnaire. While TST and questionnaire screening are relatively inexpensive procedures, screening may be harmful, resulting in higher costs and lower utility compared to a no-screen strategy. Potential harm results from false positive tests leading to unnecessary treatment with INH. Third, as more research becomes available, IGRA testing may become a cost-effective screening tool in this population, offering improved specificity and the need for only a single patient visit.
The underlying prevalence of LTBI and the specificity of screening tests are important variables in the model. Between a LTBI prevalence of 10.3% and 58.5%, screening only those who are positive on a risk-factor questionnaire is either cost-effective or dominates the other strategies. Above a LTBI prevalence of 58.5%, universal TST screening is cost-effective. To prevent excessive false positive TST results in areas with relatively lower LTBI prevalence (the United States),34 we used a more specific questionnaire cutoff, requiring at least 3/9 risk factors be present before performing a TST. If the cut point was changed to require the presence of at least 6/9 questionnaire risk factors,34 targeted screening had a mCER of $102,138 compared to no-screening. Conversely, in higher prevalence areas, we speculate it may be prudent to maximize sensitivity, requiring fewer risk factors to be present before skin testing.
Unfortunately, LTBI is not reportable in countries such as the United States, so lack of data regarding the underlying prevalence of LTBI in various populations may prevent targeted implementation of cost-effective screening approaches.34 Finally, if approved for use in children, IGRA testing of patients with a positive screening questionnaire may be a cost-effective option in higher prevalence settings, offering greater test specificity and requiring only one patient visit as compared to TST screening.5, 31
Results of any modeling task are limited by the accuracy of available data and model assumptions. In studying the risk of LTBI reactivation, estimating screening test sensitivity and specificity is particularly challenging because until recently the TST has served as the only accepted screening test and the gold-standard.6, 21, 31 Even in healthy patients, the TST is not a perfect test, in part due to exposure to non-tuberculous mycobacterium or prior BCG vaccination, which may lead to false-positive results.13, 14, 28, 45 Furthermore, because anergy can decrease TST sensitivity, potential T-cell dysregulation in nephrotic syndrome16 or other conditions could increase the risk of false-negative TST results. We tried to account for these effects by utilizing data on the validity of the TST from a recent study using advanced statistical modeling.46 Finally, our assumption that children are not screened again for LTBI may be an oversimplification, especially in a population exposed to immunosuppression with frequent nephrotic syndrome relapses. Future studies are needed to assess the cost-effectiveness of continued screening for children on prolonged or intermittent courses of these medications.
The clinical significance of small differences between the tested strategies must be interpreted with caution as over a modeled patient’s lifetime, many strategies differed by only a few dollars of expected costs and less than an hour of life expectancy (Table 3). While the societal impact of these differences may be great, the impact on a particular patient is likely much less, unless they develop a preventable case of TB. As modeling can only calculate expected outcomes, it cannot replace clinical judgment in the care of individual patients. To this point, we have shown that targeted screening may be cost-effective if patients have multiple (>6) risk factors present. These risk factors can be assessed by the screening questionnaire we used in our model. Although this questionnaire was validated in >30,000 children, the population may not represent the demographics of the United States at large.34 Furthermore, we understand that elements of the screening questionnaire (Table S1) may be asked during a routine history. We stress that an appropriate history should be taken before starting immunosuppression in any patient. Despite these caveats, we were unable to demonstrate the cost-effectiveness of universal TST testing unless the prevalence of LTBI was very high.
While prolonged steroid therapy is known to increases the risk for TB reactivation, the exact degree of this increase is uncertain.13 We assumed a 7.7 fold risk elevation,25 which is consistent with the known eight to 30-fold increased risk of LTBI reactivation in adults taking anti-TNF-α agents.8, 9 Along these lines, our two-way sensitivity analysis (Fig 2) allows decision makers to determine cost-effective screening approaches based on the population LTBI prevalence and the reactivation risk of a specific immunosuppressive agent.
To the best of our knowledge, no study has evaluated the cost-effectiveness of screening for LTBI prior to immunosuppression. Linas et al.27 reported that universal TST screening of adults taking immunosuppression (mean age 55 years with an LTBI prevalence of 5.3%) had a mCER of $129,000 compared to no-screening. Others have assessed the costs of screening other populations, such as adult health care workers,31 close contacts of TB patients,47–49 and healthy children in kindergarten.50–52 Similar to our findings, these additional studies were unable to demonstrate the cost-effectiveness of universal TST screening. However, many of these studies50–52 only examined the cost per case of TB averted, and there are no established standards for what is cost-effective relative to this metric. In contrast, our analysis used an effectiveness metric of QALYs, for which there are accepted thresholds for societal willingness-to-pay, giving decision makers a standardized means to compare competing interventions.
The strengths of our analysis include a lifetime horizon, adding non-medical costs, accounting for nephrotic syndrome relapses, and comprehensive deterministic and probabilistic sensitivity analyses. We chose base-case parameter values that would bias results towards universal TST testing such as a low QALY decrement from INH therapy, assuming all patients returned for TST interpretation, and severe consequences from developing active TB such as high treatment costs and a high case-fatality rate.
IGRA testing may offer benefits over the TST due to improved specificity and the need for only a single visit.7, 14, 28, 45 However, as with the TST, the validity of IGRA testing in patients with a concern for LTBI is difficult to measure due to the lack of gold standard.53, 54 Recent reports have highlighted a lower sensitivity of some IGRA assays, especially when used to detect TB disease in children.55, 56 Further limitations include the possibility of indeterminate results and the need for venipuncture.3 Indeterminate results leading to false negative interpretations may be higher in younger children and the immunosuppressed.57,53 Nevertheless, more experts are recommending the use of IGRA testing in children, especially in those with prior BCG vaccination, with a low risk of disease, or when there is concern they will not return to have their TST read.3, 5, 21 Our results support the cost-effectiveness of targeted IGRA testing only in populations with a higher prevalence of LTBI (>4.9%).
In conclusion, at a LTBI prevalence of 1.1%, both universal and targeted TST testing are not cost-effective prior to starting immunosuppression in five year-olds with newly diagnosed idiopathic nephrotic syndrome. Only patients who live in areas with very high rates of LTBI (>58%) will likely benefit from universal TST testing. As more research becomes available describing the international and sociodemographic variations in LTBI prevalence and the specific risk of LTBI reactivation from individual immunosuppressive agents, clinicians will be better able to tailor screening guidelines to their individual patients. While our results specifically address young children with new-onset nephrotic syndrome, we speculate that they are applicable to those treated with similar immunosuppressive drugs for other conditions. Finally, research on IGRA testing may lead to this test becoming a component of the most cost-effective screening options in areas with a higher prevalence of LTBI.
Supplementary Material
Acknowledgments
Support: Dr. Eckman is supported by the Foundation for Informed Medical Decision Making, Merck/Schering-Plough, Gilead Sciences, Inc., the Office of the National Coordinator for Health Information Technology (90BC0016/01), the National Center for Research Resources (1UL1RR026314) and (1U54 RR 025216), the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases; K23 DK075599), and the National Library of Medicine R01 (LM009533). Dr. Schauer is supported by the NIDDK (K23 DK075599). These funding sources played no part in the study design, collection, analysis, and interpretation of data, writing the report, or decision to submit the report for publication.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Footnotes
Table S1: Questionnaire to determine the risk of LTBI reactivation with a targeted screening approach.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Controlling Tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172(9):1169–1226. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [Accessed December 12, 2011.];Global Tuberculosis Control. 2011 http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf.
- 3.Cruz AT, Geltemeyer AM, Starke JR, Flores JA, Graviss EA, Smith KC. Comparing the tuberculin skin test and T-SPOT.TB blood test in children. Pediatrics. 2011;127(1):e31–38. doi: 10.1542/peds.2010-1725. [DOI] [PubMed] [Google Scholar]
- 4.Horsburgh CR, Jr, Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137(2):401–409. doi: 10.1378/chest.09-0394. [DOI] [PubMed] [Google Scholar]
- 5.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 6.Targeted Tuberculin Skin Testing and Treatment of Latent Tuberculosis Infection in Children and Adolescents. Pediatrics. 2004;114(4 pt 3):1175–1201. doi: 10.1542/peds.2004-1525. [DOI] [PubMed] [Google Scholar]
- 7.Cruz AT, Starke JR. Pediatric tuberculosis. Pediatr Rev. 2010;31(1):13–25. doi: 10.1542/pir.31-1-13. [DOI] [PubMed] [Google Scholar]
- 8.Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60(7):1884–1894. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50(2):372–379. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 10.Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69(3):522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 12.Cisneros JR, Murray KM. Corticosteroids in tuberculosis. Ann Pharmacother. 1996;30(11):1298–1303. doi: 10.1177/106002809603001115. [DOI] [PubMed] [Google Scholar]
- 13.Targeted tuberculin testing and treatment of latent tuberculosis infection. Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC) Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 14.Mack U, Migliori GB, Sester M, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33(5):956–973. doi: 10.1183/09031936.00120908. [DOI] [PubMed] [Google Scholar]
- 15.Gipson DS, Massengill SF, Yao L, et al. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124(2):747–757. doi: 10.1542/peds.2008-1559. [DOI] [PubMed] [Google Scholar]
- 16.Gbadegesin R, Smoyer WE. Nephrotic syndrome. In: Geary DF, Schaefer F, editors. Comprehensive Pediatric Nephrology. Philadelphia: Mosby Elsevier; 2008. pp. 205–218. [Google Scholar]
- 17.Hodson EM, Alexander SI, Graf N. Steroid-sensitive nephrotic syndrome. In: Geary DF, Schaefer F, editors. Comprehensive Pediatric Nephrology. Philadelphia: Mosby Elsevier; 2008. pp. 239–256. [Google Scholar]
- 18.Taylor Z, Marks SM, Rios Burrows NM, Weis SE, Stricof RL, Miller B. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis. 2000;4(10):931–939. [PMC free article] [PubMed] [Google Scholar]
- 19.Hodson EM, Craig JC, Willis NS. Evidence-based management of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2005;20(11):1523–1530. doi: 10.1007/s00467-005-1968-8. [DOI] [PubMed] [Google Scholar]
- 20.Lockman S, Tappero JW, Kenyon TA, Rumisha D, Huebner RE, Binkin NJ. Tuberculin reactivity in a pediatric population with high BCG vaccination coverage. Int J Tuberc Lung Dis. 1999;3(1):23–30. [PubMed] [Google Scholar]
- 21.Lewinsohn DA, Lobato MN, Jereb JA. Interferon-gamma release assays: new diagnostic tests for Mycobacterium tuberculosis infection, and their use in children. Curr Opin Pediatr. 2010;22(1):71–76. doi: 10.1097/MOP.0b013e3283350301. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 23.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004;350(20):2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 24.Horsburgh CR, Jr, O’Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med. 2010;182(3):420–425. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55(1):19–26. doi: 10.1002/art.21705. [DOI] [PubMed] [Google Scholar]
- 26.Tsevat J, Taylor WC, Wong JB, Pauker SG. Isoniazid for the tuberculin reactor: take it or leave it. Am Rev Respir Dis. 1988;137(1):215–220. doi: 10.1164/ajrccm/137.1.215. [DOI] [PubMed] [Google Scholar]
- 27.Linas BP, Wong AY, Freedberg KA, Horsburgh CR., Jr Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184(5):590–601. doi: 10.1164/rccm.201101-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LoBue PA, Enarson DA, Thoen TC. Tuberculosis in humans and its epidemiology, diagnosis and treatment in the United States. Int J Tuberc Lung Dis. 2010;14(10):1226–1232. [PubMed] [Google Scholar]
- 29.de Valliere S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8(6):767–771. [PubMed] [Google Scholar]
- 30.Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitzgerald JM. Health state utilities in latent and active tuberculosis. Value Health. 2008;11(7):1154–1161. doi: 10.1111/j.1524-4733.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 31.de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med. 2009;169(2):179–187. doi: 10.1001/archinternmed.2008.524. [DOI] [PubMed] [Google Scholar]
- 32.Jung RS, Bennion JR, Sorvillo F, Bellomy A. Trends in tuberculosis mortality in the United States, 1990–2006: a population-based case-control study. Public Health Rep. 2010;125(3):389–397. doi: 10.1177/003335491012500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fakhouri F, Bocquet N, Taupin P, et al. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis. 2003;41(3):550–557. doi: 10.1053/ajkd.2003.50116. [DOI] [PubMed] [Google Scholar]
- 34.Froehlich H, Ackerson LM, Morozumi PA. Targeted testing of children for tuberculosis: validation of a risk assessment questionnaire. Pediatrics. 2001;107(4):E54. doi: 10.1542/peds.107.4.e54. [DOI] [PubMed] [Google Scholar]
- 35.United States Department of Health and Human Services Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) [Accessed March 6, 2010.]; http://hcupnet.ahrq.gov/
- 36.Centers for Medicare and Medicaid Services (CMS) website. [Accessed June 3, 2011.]; http://www.cms.gov/pfslookup/02_PFSsearch.asp?agree=yes&next=Accept.
- 37.Red Book 2009: Pharmacy’s Fundamental Reference. Thomson Reuters. (113) 2009 [Google Scholar]
- 38. [Accessed March 8, 2010.];United States Department of Labor Consumer Price Index inflation calculator. http://www.bls.gov/data/inflation_calculator.htm.
- 39.McNeil BJ, Keller E, Adelstein SJ. Primer on certain elements of medical decision making. N Engl J Med. 1975;293(5):211–215. doi: 10.1056/NEJM197507312930501. [DOI] [PubMed] [Google Scholar]
- 40.Arias E. United States Life Tables, 2001. Natl Vital Stat Rep. 2004;52(14):1–38. [PubMed] [Google Scholar]
- 41.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5(2):157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 42.Bennett DE, Courval JM, Onorato I, et al. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999–2000. Am J Respir Crit Care Med. 2008;177(3):348–355. doi: 10.1164/rccm.200701-057OC. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Stable EJ, Levin R, Pineda A, Slutkin G. Tuberculin skin test reactivity and conversions in United States- and foreign-born Latino children. Pediatr Infect Dis. 1985;4(5):476–479. doi: 10.1097/00006454-198509000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14(4):406–412. [PMC free article] [PubMed] [Google Scholar]
- 45.Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis. 2006;6:47. doi: 10.1186/1471-2334-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadatsafavi M, Shahidi N, Marra F, et al. A statistical method was used for the meta-analysis of tests for latent TB in the absence of a gold standard, combining random-effect and latent-class methods to estimate test accuracy. J Clin Epidemiol. 2010;63(3):257–269. doi: 10.1016/j.jclinepi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Pooran A, Booth H, Miller RF, et al. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med. 2010;10:7. doi: 10.1186/1471-2466-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diel R, Nienhaus A, Loddenkemper R. Cost-effectiveness of interferon-gamma release assay screening for latent tuberculosis infection treatment in Germany. Chest. 2007;131(5):1424–1434. doi: 10.1378/chest.06-2728. [DOI] [PubMed] [Google Scholar]
- 49.Diel R, Wrighton-Smith P, Zellweger JP. Cost-effectiveness of interferon-gamma release assay testing for the treatment of latent tuberculosis. Eur Respir J. 2007;30(2):321–332. doi: 10.1183/09031936.00145906. [DOI] [PubMed] [Google Scholar]
- 50.Lowin A, Slater J, Hall J, Alperstein G. Cost effectiveness analysis of school based Mantoux screening for TB infection. Aust N Z J Public Health. 2000;24(3):247–253. doi: 10.1111/j.1467-842x.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 51.Flaherman VJ, Porco TC, Marseille E, Royce SE. Cost-effectiveness of alternative strategies for tuberculosis screening before kindergarten entry. Pediatrics. 2007;120(1):90–99. doi: 10.1542/peds.2006-2168. [DOI] [PubMed] [Google Scholar]
- 52.Mohle-Boetani JC, Miller B, Halpern M, et al. School-based screening for tuberculous infection. A cost-benefit analysis. JAMA. 1995;274(8):613–619. [PubMed] [Google Scholar]
- 53.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest. 2010;137(4):952–968. doi: 10.1378/chest.09-2350. [DOI] [PubMed] [Google Scholar]
- 54.Sester M, Sotgiu G, Lange C, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37(1):100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 55.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149(3):177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J. 2011;30(8):694–700. doi: 10.1097/INF.0b013e318214b915. [DOI] [PubMed] [Google Scholar]
- 57.Bergamini BM, Losi M, Vaienti F, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics. 2009;123(3):e419–424. doi: 10.1542/peds.2008-1722. [DOI] [PubMed] [Google Scholar]
- 58.Detjen AK, Keil T, Roll S, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45(3):322–328. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- 59.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146(5):340–354. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 60.Berkel GM, Cobelens FG, de Vries G, Draayer-Jansen IW, Borgdorff MW. Tuberculin skin test: estimation of positive and negative predictive values from routine data. Int J Tuberc Lung Dis. 2005;9(3):310–316. [PubMed] [Google Scholar]
- 61.Rose DN, Schechter CB, Adler JJ. Interpretation of the tuberculin skin test. J Gen Intern Med. 1995;10(11):635–642. doi: 10.1007/BF02602749. [DOI] [PubMed] [Google Scholar]
- 62.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17(6):968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 63.Ozuah PO, Ozuah TP, Stein RE, Burton W, Mulvihill M. Evaluation of a risk assessment questionnaire used to target tuberculin skin testing in children. JAMA. 2001;285(4):451–453. doi: 10.1001/jama.285.4.451. [DOI] [PubMed] [Google Scholar]
- 64.Mancuso J, Niebuhr D, Krauss MR, Dabbs C, Anderson K. Cost-effectiveness of tuberculosis screening in health care workers is not robust. Arch Intern Med. 2009;169(14):1336. doi: 10.1001/archinternmed.2009.211. author reply 1336–1337. [DOI] [PubMed] [Google Scholar]
- 65.Driver CR, Valway SE, Cantwell MF, Onorato IM. Tuberculin skin test screening in schoolchildren in the United States. Pediatrics. 1996;98(1):97–102. [PubMed] [Google Scholar]
- 66.Gounder CR, Driver CR, Scholten JN, Shen H, Munsiff SS. Tuberculin testing and risk of tuberculosis infection among New York City schoolchildren. Pediatrics. 2003;111(4 Pt 1):e309–315. doi: 10.1542/peds.111.4.e309. [DOI] [PubMed] [Google Scholar]
- 67.Lobato MN, Hopewell PC. Mycobacterium tuberculosis infection after travel to or contact with visitors from countries with a high prevalence of tuberculosis. Am J Respir Crit Care Med. 1998;158(6):1871–1875. doi: 10.1164/ajrccm.158.6.9804106. [DOI] [PubMed] [Google Scholar]
- 68.Kim HA, Yoo CD, Baek HJ, et al. Mycobacterium tuberculosis infection in a corticosteroid-treated rheumatic disease patient population. Clin Exp Rheumatol. 1998;16(1):9–13. [PubMed] [Google Scholar]
- 69.Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173(8):927–931. doi: 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 70.Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982;60(4):555–564. [PMC free article] [PubMed] [Google Scholar]
- 71.Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis. 2005;40(5):670–676. doi: 10.1086/427802. [DOI] [PubMed] [Google Scholar]
- 72.Ruth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid-sensitive nephrotic syndrome come of age: long-term outcome. J Pediatr. 2005;147(2):202–207. doi: 10.1016/j.jpeds.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 73.Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8(5):769–776. doi: 10.1681/ASN.V85769. [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention. Division of Tuberculosis Elimination. [Accessed April 20, 2012.];Reported Tuberculosis in the United States. http://www.cdc.gov/tb/statistics/reports/2010/pdf/report2010.pdf.
- 75.Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150(2):73–83. doi: 10.7326/0003-4819-150-2-200901200-00005. [DOI] [PubMed] [Google Scholar]
- 76.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–1836. [PubMed] [Google Scholar]
- 77.Piccoli A, Pillon L, Passerini P, Ponticelli C. Therapy for idiopathic membranous nephropathy: tailoring the choice by decision analysis. Kidney Int. 1994;45(4):1193–1202. doi: 10.1038/ki.1994.158. [DOI] [PubMed] [Google Scholar]
- 78.Finnell SM, Christenson JC, Downs SM. Latent tuberculosis infection in children: a call for revised treatment guidelines. Pediatrics. 2009;123(3):816–822. doi: 10.1542/peds.2008-0433. [DOI] [PubMed] [Google Scholar]
- 79.Christy C, Pulcino ML, Lanphear BP, McConnochie KM. Screening for tuberculosis infection in urban children. Arch Pediatr Adolesc Med. 1996;150(7):722–726. doi: 10.1001/archpedi.1996.02170320068011. [DOI] [PubMed] [Google Scholar]
- 80.Salpeter SR, Salpeter EE. Screening and treatment of latent tuberculosis among healthcare workers at low, moderate, and high risk for tuberculosis exposure: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2004;25(12):1056–1061. doi: 10.1086/502343. [DOI] [PubMed] [Google Scholar]
- 81.Salpeter SR, Sanders GD, Salpeter EE, Owens DK. Monitored isoniazid prophylaxis for low-risk tuberculin reactors older than 35 years of age: a risk-benefit and cost-effectiveness analysis. Ann Intern Med. 1997;127(12):1051–1061. doi: 10.7326/0003-4819-127-12-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 82.Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS. The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review. Health Technol Assess. 2007;11(21):iii–iv. ix–xi, 1–93. doi: 10.3310/hta11210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.