Abstract
Objectives:
Haller cells are anterior ethmoid air cells located in the medial orbital floor immediately lateral to the maxillary infundibulum. The purpose of this study was to demonstrate the prevalence and relationship between the existence and size of these cells with ipsilateral maxillary sinusitis and orbital floor dehiscence as visualized on cone beam CT (CBCT) images.
Methods:
CBCT image volumes of 50 patients were retrieved and analysed. All CBCT images were acquired with a 9-inch field of view scan. χ2 and Cochran–Mantel–Haenszel tests were used for statistical analysis of the obtained data, and p-values of <0.05 were considered to be statistically significant.
Results:
There was no statistically significant association between the existence and size of Haller cells and maxillary sinusitis. There was a significant association between Haller cells and orbital floor dehiscence.
Conclusions:
The explanation of maxillary sinusitis on the basis of mechanical obstruction is unlikely. This study provides evidence for the usefulness of CBCT scan in delineation of the sinonasal anatomy.
Keywords: cone beam CT, Haller cells, orbital floor dehiscence, maxillary sinusitis
Introduction
Haller cells were first identified by Albrecht von Haller (1708–1777) in 1765 and were subsequently named after him.1 However, the terminology for these air cells has been changed, reflecting a trend away from naming structures after the anatomist who had first described them as the need grows for international standardization and descriptive nomenclature of anatomical terms. Therefore, Haller cells are alternatively called infraorbital ethmoid cells, as they arise from anterior ethmoid cells and are located in the medial orbital floor.
With the increasing popularity of endoscopic sinus surgery and recent advances in CT technology, there has arisen interest in the complex radiological anatomy of the paranasal sinuses and ostiomeatal system. It is well documented that some of the anatomical variations of the paranasal sinuses can predispose to sinus pathology or can even complicate sinus surgery, and Haller cells are no exception. These cells are frequently seen as incidental findings in CT examination of paranasal sinuses. The position of Haller cells in the medial portion of the orbital floor, lateral to the maxillary infundibulum, places them in a key position to disturb the normal pattern of mucociliary flow and predispose to recurrent maxillary sinusitis.2–4 Several radiographic studies have shown a significant relationship between Haller cells' size (greater than 3 mm) and maxillary sinusitis.5–7
To date, there have been few studies comparing image quality in cone beam CT (CBCT) scans with that in multislice CT.8 Cadaver and clinical studies have provided the principle evidence for the application of CBCT imaging to endoscopic sinus surgery, concluding that both spatial and soft-tissue contrast were sufficient to aid surgical navigation in the sinonasal cavity.9–11 Preliminary evidence suggests that CBCT may be suited to specific imaging tasks in the context of bony structural evaluations enabling low-dose assessment of sinonasal anatomy.
To the best of our knowledge, this is the first study that uses CBCT in the evaluation of Haller cells in human subjects. This study was primarily aimed at the following purposes:
to demonstrate prevalence of Haller cells as visualized in CBCT images
-
to evaluate the relationship between
presence of Haller cells and ipsilateral maxillary sinus disease
size of Haller cells and ipsilateral maxillary sinus disease
size of maxillary sinus ostia and ipsilateral maxillary sinus disease
presence of Haller cells and ipsilateral dehiscence of orbital floor.
Materials and methods
CBCT image volumes of 50 patients were retrieved from the digital imaging and communications in medicine archive folder. All CBCT images were acquired with a 9-inch field of view by CB MercuRay (Hitachi Medical Corporation, Tokyo, Japan) in the Department of Oral and Maxillofacial Radiology at the University of Connecticut School of Dental Medicine. Images were requested for various dentomaxillofacial indications, including dental implants, jaw lesions, orthodontic and temporomandibular joint evaluation. Patients with a history of sinus tumour or surgery, sinonasal polyposis or midfacial trauma, and patients younger than 16 years were excluded, as according to Gray's anatomy, sinonasal cavity does not reach its full development until adolescence.12 All digital imaging and communications in medicine files were viewed by CBWorks 3.0™ software (v. 3.0; CyberMed, Seoul, Republic of Korea). The study was approved by the institutional review board.
Criteria of recognition
We used meticulous criteria for defining Haller cells as air cells, of any size, located along the medial portion of the orbital floor and/or the lamina papyracea inferior to the bulla ethmoidalis, and continuous with the ethmoid capsule (Figure 1). The continuity with the ethmoid capsule distinguishes Haller cells from the infraoribital recess of the maxillary sinus13 (Figure 2).
Figure 1.
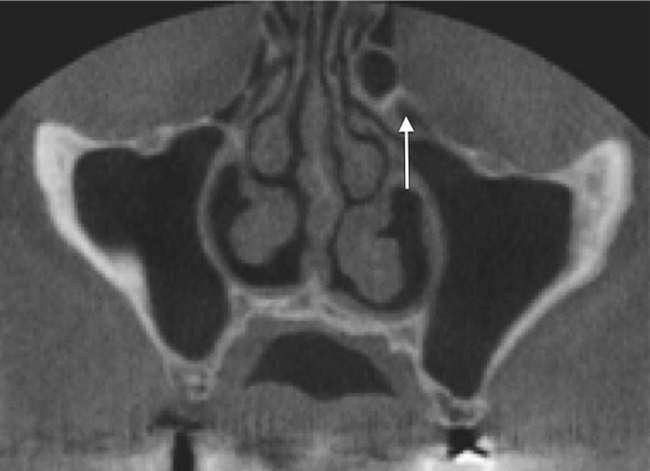
Coronal cone beam CT image shows Haller cells (arrow); note the continuity with the ethmoid capsule
Figure 2.
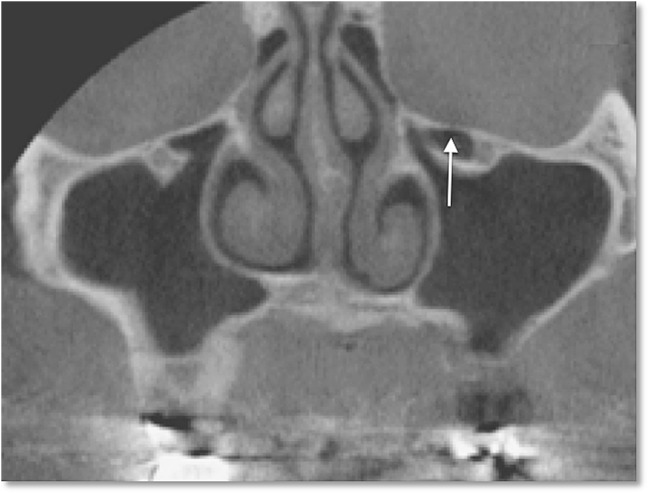
Coronal cone beam CT image shows infraorbital recess of maxillary sinus (arrow); note the discontinuity with the ethmoid capsule
Maxillary sinusitis was defined as radiographic evidence of thickening of sinus mucosa and/or fluid accumulation at any level (Figure 3). The finding of mucous retention phenomenon (cyst) was not considered as a sinus disease. Dehiscence of the adjacent orbital floor was recognized as loss of bone density at any level. Whenever a clear decision between “very thin bony wall” and “total dehiscence” was not feasible, the results were accepted as dehiscence (Figure 3). The size of the Haller cell was measured at the maximum mediolateral dimension. The maxillary sinus ostium was quantified by measuring the distance between the Haller cell at its most medial portion and the uncinate process. Both Haller cells and maxillary sinuses ostia were arbitrarily categorized based on the size into small (less than 2 mm), medium (2–4 mm) and large (greater than 4 mm) (Figure 4).
Figure 3.
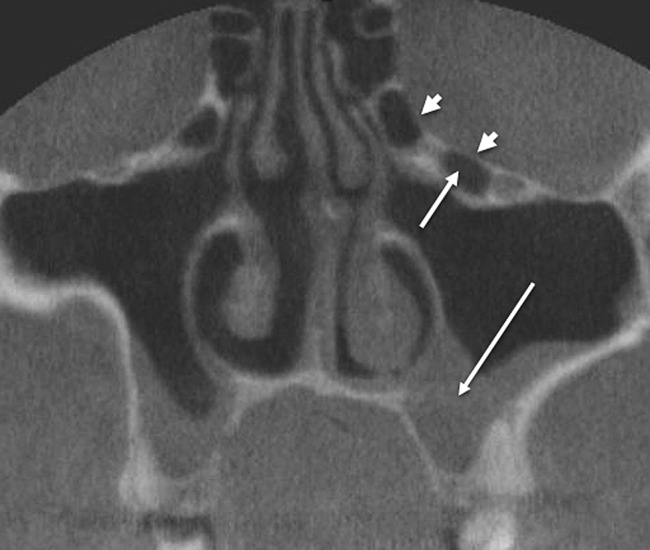
Coronal cone beam CT shows maxillary sinusitis (long arrow), multiple Haller cells (short arrow) and orbital dehiscence (arrowheads)
Figure 4.
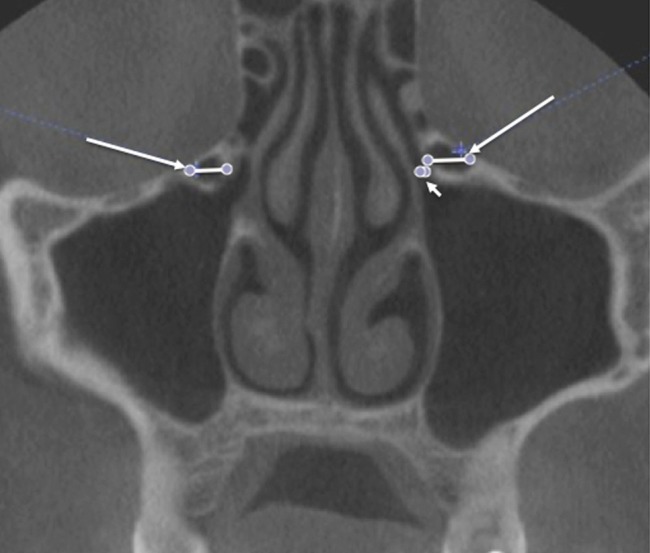
Coronal cone beam CT image shows measurement of Haller cells (long arrows) and maxillary sinus ostium (short arrow)
For statistical analysis, the χ2 test was used to evaluate the association of Haller cells with maxillary sinusitis and orbital dehiscence. The Cochran–Mantel–Haenszel test was used to evaluate the relationship between the size of the Haller cells and the maxillary sinuses ostia with maxillary sinusitis. p < 0.05 was considered to be statistically significant.
Results
Of the 50 patients included in the investigation, 28 were females and 22 were males, with ages ranging from 16 to 85 years (mean 37 years). Haller cells were recognized in 30 patients (60%); 17 (34%) were bilateral and 13 (26%) were unilateral; i.e. Haller cells were seen in 47 sides, in which 13 were small sized, 10 medium sized and 24 large sized. Small maxillary sinus ostia were encountered in 26, medium in 12 and large ostia in 9 out of 47 sides (Table 1). Haller cells concurring with ipsilateral maxillary sinusitis were encountered in 27 (54%) cases (14 on the right side, 13 on the left side).
Table 1.
Analysis of Haller cells and maxillary sinus ostia
| Prevalence of Haller cellsa | Unilateral | Bilateral | Total |
| 13 (26%) | 17 (34%) | 30 (60%) (47 sides) | |
| Size of Haller cellsb | Small | Medium | Large |
| 13 (28%) | 10 (21%) | 24 (51%) | |
| Size of maxillary sinus ostiab | Small | Medium | Large |
| 26 (55%) | 12 (26%) | 9 (19%) |
Out of 50 patients.
Out of 47 sides.
Concomitant presence of orbital floor dehiscence with Haller cells was encountered on the right and left sides in 19 and 20 patients, respectively. There was no statistically significant association between existence of Haller cells, size of Haller cells and size of maxillary sinus ostium with maxillary sinusitis (p > 0.05). However, there was a statistically significant association between Haller cells and orbital floor dehiscence (p = 0.0001).
Discussion
Prevalence of Haller cells in the English literature is remarkably variable, ranging from 2.7% to 45.1%.5,14 This enormous variability in the frequency of Haller cells is probably owing to the inconsistency in definition of Haller cells. Kennedy and Zinreich15 considered Haller cells as ethmoid cells projecting below the ethmoid bulla within the orbital floor in the region of the opening of the maxillary sinus. Bolger et al5 defined Haller cells as any cell located between the ethmoid bulla, the orbital lamina of the ethmoid bone and the orbital floor. Kainz et al6 recognized Haller cells as cells within the orbital floor. The variability in the prevalence of Haller cells could also be explained on the basis of the patients' age group and race, and on the CT techniques used.16
In the present study, we generated our own criteria of definition; the major criterion was based primarily on the distinction between Haller cells and infraorbital recess of maxillary sinus. The latter is sparsely described in the literature.13 According to Earwaker,13 the bony wall of the infraorbital recess of the maxillary sinus is discrete from the ethmoid capsule. We used this concept in articulating a solid definition of Haller cells. Prevalence of Haller cells in our study was relatively high (60%). This could be explained on the basis of the imaging tool used in the investigation, as CBCT is a volumetric imaging technique, so it captures any Haller cell when present, irrespective of size; by contrast, small-sized Haller cells could easily be missed in the interslice intervals in multislice CT scans. Many cells identified in this survey were less than 1 mm in size; such a cell will likely be missed in multislice CT scanning. The high percentage of Haller cells in our analysis may represent the greater sensitivity of CBCT scan in the detection of small delicate bony structures. This observation provides a piece of evidence of usefulness of CBCT technology in accurate imaging of sinonasal cavity at substantially lesser radiation dose.
Our study reviewed the prevalence of Haller cells with reference to their size. Bearing in mind the critical location of Haller cells immediately medial to the maxillary infundibulum, our side-specific analysis showed significant association between the presence or size of the Haller cell and ipsilateral maxillary sinus disease; hence, the overall rate of maxillary sinusitis was similar for the Haller and non-Haller cell populations (54% vs 46%, respectively).
Several authors are in agreement with this observation.17–19 However, others found Haller cells as important aetiological factor in maxillary sinusitis certainly when the cells are large enough (greater than 6 mm) to cause substantial narrowing of the maxillary infundibulum.20,21
A limitation of our study is that maxillary sinusitis could have been overrated because infectious sinusitis cannot be distinguished from allergic sinusitis on the basis of radiographic evaluation only.
The lack of association between the presence of Haller cells and the ipsilateral maxillary sinusitis could also be explained on the basis of the accessory maxillary sinus ostia in the lateral nasal wall; these ostia have previously been described in 14% of patients13 and would enhance maxillary sinus ventilation by functioning as an alternative route of drainage even in the case of mechanical obstruction of the maxillary infundibulum by a Haller cell.
A surprising additional finding is the lack of significant association between the size of maxillary size ostium and radiographic sinusitis; this could argue against the historical theory of mechanical obstruction. This observation suggests that maxillary sinusitis might rather be a primary condition than classically arising from narrowing or occlusion of the maxillary sinus ostium. We suggest that the role of Haller cells in sinus disease should be evaluated on an individual basis depending on the size of Haller cells and clinical evidence of sinus inflammation.
Our study showed a significant association between Haller cells and orbital floor dehiscence. We recognized dehiscence as loss of bone density with only mucoperiosteal covering separating the Haller cell from the orbit. Dehiscent orbital floor could make the orbit vulnerable either in the event of Haller cell disease or during surgical instrumentation of the ostiomeatal complex. Sebrechts et al21 presented three case reports of unilateral orbital cellulitis, resulting from isolated inflammation of Haller cells, and management required endoscopic incision and drainage of infected Haller cells. Accordingly, they considered the pathology of Haller cells to be of the potential cases of unilateral orbital cellulitis. Since there is no lymphatic drainage system in the orbit, they consequently assumed infection spreading through a dehiscence in the orbital floor, lamina papyracea or sutures in the medial orbital floor. Radiological experience shows that in the case of Haller cell inflammation, hypertrophic mucosa tends to obscure a coexistent orbital floor dehiscence; therefore, based on our findings, we postulate that in the case of inflamed Haller cells, a concurring orbital floor dehiscence should always be considered unless otherwise proven. The present study showed the usefulness of CBCT imaging in the delineation of the regional anatomy of ostiomeatal complex. This observation provides preliminary evidence suggesting that CBCT may be suited for pre-operative bony structural evaluation, enabling low-dose assessment of paranasal sinus anatomy.
In conclusion, our analysis showed that the prevalence of Haller cells was remarkably high; it also showed lack of association of existence or size of Haller cells with maxillary sinusitis. This finding could support doubts about the theory of obstruction of maxillary sinusitis. However, presence of Haller cells was strongly associated with ipsilateral orbital floor dehiscence. This study provides evidence for the usefulness of CBCT scan in delineation of the bony anatomy of sinonasal complex at substantially higher precision and lesser radiation. Further size-specific CBCT evaluation of patients with definite maxillary sinusitis is strongly recommended to investigate the association between Haller cells and maxillary or ethmoid sinusitis.
REFERENCES
- 1.Proetz AW. Essays on the applied physiology of the nose. 2nd edn St. Louis, MI: Annals Publishing Co; 1953 [Google Scholar]
- 2.Stammberger H, Wolf G. Headaches and sinus disease: the endoscopic approach. Ann Otol Rhinol Laryngol 1988; 97: 3–23 [DOI] [PubMed] [Google Scholar]
- 3.Stackpole SA, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol 1997; 11: 219–223 [DOI] [PubMed] [Google Scholar]
- 4.Kantarci M, Karasen RM, Alper F, Onbas O, Okur A, Karaman A. Remarkable anatomic variation in paranasal sinus region and their clinical importance. Eur J Radiol 2004; 50: 296–302 10.1016/j.ejrad.2003.08.012 [DOI] [PubMed] [Google Scholar]
- 5.Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope 1991; 101: 56–64 10.1288/00005537-199101000-00010 [DOI] [PubMed] [Google Scholar]
- 6.Kainz J, Braun H, Genser P. Haller's cells: morphologic evaluation and clinico-surgical relevance. Laryngorhinootologie 1993; 72: 599–604 [DOI] [PubMed] [Google Scholar]
- 7.Milczuk H. Nasal and paranasal sinus anomalies in children with chronic sinusitis. Laryngoscope 1993; 103: 247–52 10.1288/00005537-199303000-00002 [DOI] [PubMed] [Google Scholar]
- 8.Miracle AC, Mukherji SK. Cone beam CT of the head and neck, part 2: clinical applications. AJNR Am J Neuroradiol 2009; 30: 1285–1292 10.3174/ajnr.A1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafferty MA, Siewerdsen JH, Chan Y. Investigation of C-arm cone-beam CT-guided surgery of the frontal recess. Laryngoscope 2005; 115: 2138–2143 10.1097/01.mlg.0000180759.52082.45 [DOI] [PubMed] [Google Scholar]
- 10.Jackman AH, Palmer JN, Chiu AG. Use of intraoperative CT scanning in endoscopic sinus surgery: a preliminary report. Am J Rhinol 2008; 22: 170–174 10.2500/ajr.2008.22.3153 [DOI] [PubMed] [Google Scholar]
- 11.Batra PS, Kanowitz SJ, Citardi MJ. Clinical utility of intraoperative volume computed tomography scanner for endoscopic sinonasal and skull base procedures. Am J Rhinol 2008; 22: 511–515 [DOI] [PubMed] [Google Scholar]
- 12.Gray H. Gray’s anatomy. 37th edn Edinburgh, Scotland: Churchill Livingstone; 1989. pp 376–377 [Google Scholar]
- 13.Earwaker J. Anatomic variants in sinonasal CT. Radiographics 1993; 13: 381–415 [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Piñas I, Sabate J, Carmona A, Catalina-Herrera CJ, Jiménez-Castellanos J. Anatomical variations in the human paranasal sinus region studied by CT. J Anat 2000; 197: 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy DW, Zinreich SJ. The functional endoscopic approach to inflammatory sinus disease: current perspective and technique modifications. Am J Rhinol 1988; 2: 89–96 [Google Scholar]
- 16.Rysz M, Bakon L. Maxillary sinus anatomy variation and nasal cavity width: structural computed tomography imaging. Folia Morphol (Warsz) 2009; 68: 260–264 [PubMed] [Google Scholar]
- 17.Sivasli E, Sirikçi A, Bayazyt YA, Gümüsburun E, Erbagci H, Bayram M, et al. Anatomic variations of the paranasal sinus area in pediatric patients with chronic sinusitis. Surg Radiol Anat 2003; 24: 400–405 10.1007/s00276-002-0074-x [DOI] [PubMed] [Google Scholar]
- 18.Lerdlum S, Vachiranubhap B. Prevalence of anatomic variation demonstrated on screening sinus computed tomography and clinical correlation. J Med Assoc Thai 2005; 88: 110–115 [PubMed] [Google Scholar]
- 19.Kim HJ, Jung Cho M, Lee JW, Tae Kim Y, Kahng H, Sung Kim H, et al. The relationship between anatomic variations of paranasal sinuses and chronic sinusitis in children. Acta Otolaryngol 2006; 126: 1067–1072 10.1080/00016480600606681 [DOI] [PubMed] [Google Scholar]
- 20.Stackpole SA, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol 1997; 11: 219–223 [DOI] [PubMed] [Google Scholar]
- 21.Sebrechts H, Vlaminck S, Casselman J. Orbital edema resulting from Haller cell pathology: 3 case reports and review of literature. Acta Otorhinolaryngol Belg 2000; 54: 39–43 [PubMed] [Google Scholar]


