Abstract
Objectives:
As an uncommon neoplasm, parotid non-Hodgkin lymphoma (NHL) comprises mucosa-associated lymphoid tissue (MALT) and non-MALT lymphomas. Both types of lymphoma vary in prognosis and treatment. The aim of this study was to explore CT and MRI characteristics of these two types of lymphoma.
Methods:
61 cases of parotid NHL, 34 MALT and 27 non-MALT lymphomas with histopathological confirmation were examined with routine CT and MR scans prior to treatment, and retrospectively reviewed.
Results:
On CT and MRI, 34 MALT lymphomas presented with 11 solid and 23 solid-cystic forms, whereas 27 non-MALT lymphomas presented with 25 solid and 2 solid-cystic forms (p < 0.01).
Conclusions:
Parotid MALT lymphoma is characterized mainly by the solid-cystic form, whereas non-MALT lymphoma is characterized mainly by the solid form. The differences on CT and MRI can offer helpful information for differentiation of both types of parotid NHL.
Keywords: non-Hodgkin lymphoma, parotid gland, magnetic resonance imaging, computed tomography
Introduction
Primary non-Hodgkin lymphoma (NHL) involving the salivary glands is uncommon, comprising 5% of all primary extranodal NHL and 2% of all salivary gland neoplasms.1,2 In cases where NHL affects the salivary glands, 75% arise within the parotid gland.2,3 The most common subtypes of NHL present in the salivary glands are extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT) type, follicular B-cell lymphoma and diffuse large B-cell lymphoma.4,5 The different NHLs of the salivary gland vary in prognosis based on the histological subtypes and clinical stages.6 A study has shown that lymphomas with probable nodal origin have a worse prognosis than those of probable extranodal-parenchymal origin.7 The extranodal marginal zone B-cell lymphoma of the MALT type (MALT lymphoma) is believed to have a better prognosis than other histological subtype lymphomas (non-MALT lymphoma).6 Therefore, it is critical to differentiate MALT lymphoma from non-MALT lymphoma involving the parotid gland with regards to assessing prognosis and determining treatment procedures. In addition, recognizing the differences between parotid NHL and salivary epithelial tumours (benign or malignant) is important for clinicians because management of these tumours is different. The parotid NHL is usually treated with radiotherapy and chemotherapy, whereas parotidectomy is commonly performed for parotid epithelial tumours.
CT and MRI play a key role in clinical staging, assessment of prognosis and treatment planning of head and neck lymphomas.4,5 However, to our knowledge, there is no previous report comparing the CT and MRI characteristics of MALT vs non-MALT lymphomas in the parotid gland.
The aim of this study was to explore CT and MRI characteristics of both lymphomas and to observe what differences exist between MALT lymphoma and non-MALT lymphoma in the parotid gland.
Materials and methods
From January 1994 to October 2012, 298 subjects with head and neck NHL were examined by routine CT and MR scan in our institute. 61 subjects with both MALT and non-MALT lymphomas affecting the parotid gland were retrospectively reviewed, including 41 subjects with only CT scan (from 1994 to 2012), 17 subjects with only MR scan (from 2003 to 2012) and 3 subjects with both. The basic information for these 61 subjects is listed in Table 1.
Table 1.
The demographic information for and imaging examinations of 61 subjects with parotid NHL
| Types of parotid NHL | Number of subjects | Male/female | Age range (years) | Mean age (years) | Plain CT/enhanced CT | Plain MRI/enhanced MRI |
| MALT lymphoma | 34 | 8/26 | 29–78 | 52.1 | 21/21 | 15/12 |
| Non-MALT lymphoma | 27 | 13/14 | 36–93 | 64.5 | 23/21 | 5/4 |
| Total | 61 | 21/40 | 29–93 | 57.5 | 44/42 | 20/16 |
MALT, mucosa-associated lymphoid tissue; NHL, non-Hodgkin lymphoma.
CT scans were performed with either Siemens SOMATOM ART (Siemens Ltd, Medical Engineering Group; Erlangen, Germany) on 9 subjects and 16-section GE LightSpeed® (General Electric Medical Systems; Milwaukee, WI) on the remaining 35 subjects. CT scans were acquired at 130 kV/210 mAs (Somatom ART) and 120 kV/250 mAs (GE LightSpeed). Axial section of 5 mm thickness, reconstructed at 1.25 mm (in GE LightSpeed), was carried out using a soft-tissue algorithm. A bolus intravenous injection of 80 ml (injection rate of 1.5–2.0 ml s−1) of a non-ionic contrast medium (iodine 300 mg m–1) was administered to 42 of the 44 subjects.
MR scans were performed on a 1.5 T GE Signa TwinSpeed system (General Electric Medical Systems) and a neurovascular array coil was used. Axial plain MR images were obtained using a T1 weighted spin-echo sequence and a T2 weighted fast spin-echo sequence with or without fat suppression. Coronal MR images were acquired using a T2 weighted fast spin-echo sequence with fat suppression. The parameters of MRI are presented in Table 2. An intravenous injection of gadolinium contrast medium (0.1 mmol kg−1 of body weight) was administered to 16 of the 20 subjects.
Table 2.
The parameters of MRI examination in 20 subjects with parotid NHL
| Location of MRI | T1 weighted images [TR/TE (ms)] | Fast spin echo T2 weighted images [TR/TE (ms)] | Field of view (mm) | Acquisition | Matrix | Slice thickness (mm) | Spacing (mm) |
| Axial | 600/11 | 4700/85 | 24 | 3 | 256 × 192 | 5 | 1 |
| Coronal | — | 3200/100 | 20 | 3 | 320 × 160 | 5 | 1 |
NHL, non-Hodgkin lymphoma; TE, time of echo; TR, time of repetition.
Two experienced radiologists, who were blinded to the both types of parotid NHL, independently interpreted the CT and MR images. Final decisions regarding CT and MRI findings were reached by consensus. Fisher’s exact test was used to compare differences between both types of parotid NHL on CT and MR images. p < 0.05 was considered as a statistically significant difference. Sensitivity, specificity and accuracy were calculated based on the following formulas: sensitivity = true positive/true positive + false negative; specificity = true negative/true negative + false positive and accuracy = true positive + true negative/total.
Results
Parotid mucosa-associated lymphoid tissue lymphoma
Pathologically, 34 of 61 subjects (55.7%) was diagnosed as MALT lymphoma involving the parotid gland, and a background of lymphoepithelial lesions was presented in 31 of 34 (91.2%) subjects. CT and MRI appearances of MALT lymphoma were identified as four forms: solitary solid mass (Figure 1), solitary solid-cystic mass (Figure 2), diffusely mixed solid-cystic lesion (Figures 3–5) and multiple solid nodules or masses (Figure 6). The internal structure and marginal changes of 34 parotid MALT lymphomas shown on CT and MRI are listed in Table 3. Solid (Figures 1 and 6) and solid-cystic (Figures 2–5) changes of parotid MALT lymphoma were found in 11 of 34 subjects (32.3%) and in 23 of 34 subjects (67.6%), respectively. Of the 23 solid-cystic lesions, a large solid structure (solid volume of neoplasm is over 70%) was found in 14 of the lesions (60.9%) (Figure 3). Solitary masses, multiple masses and diffuse lesions were found in 11 of 34 (32.3%), 7 of 34 (17.6%) and 16 of 34 (47%) subjects, respectively.
Figure 1.
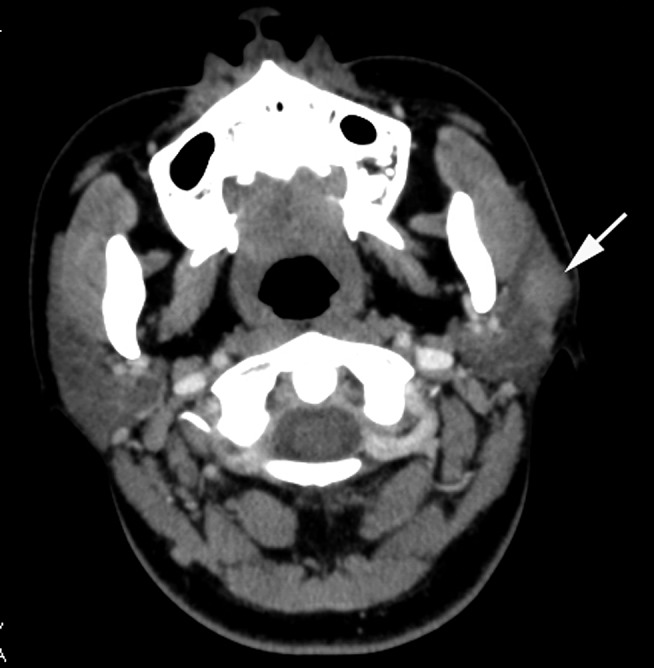
Mucosa-associated lymphoid tissue lymphoma of the left parotid gland (30 year old female). Contrast-enhanced axial CT image shows a solitary solid mass (white arrow) with ill-defined margins in the left parotid gland
Figure 2.
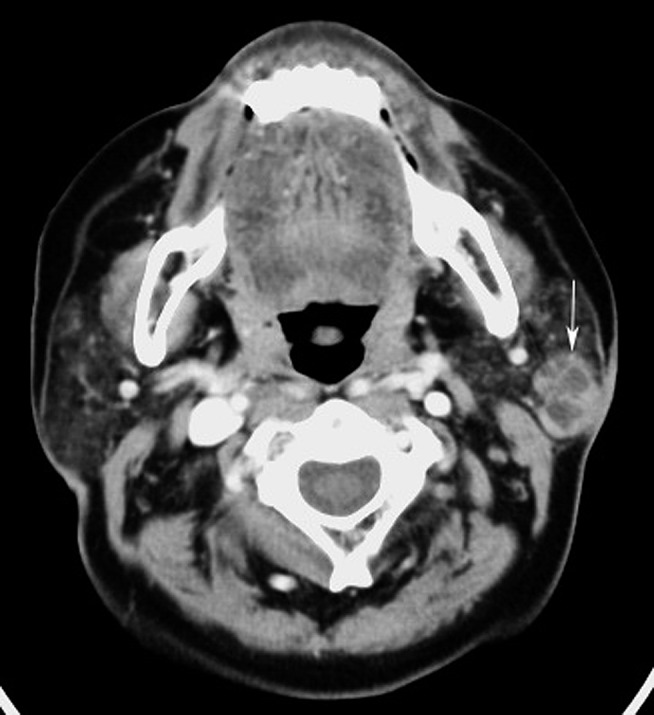
Mucosa-associated lymphoid tissue lymphoma of the left parotid gland (63 year old female). Contrast-enhanced axial CT image shows a solitary solid-cystic mass (white arrow) with a well-defined margin in the left parotid gland
Figure 3.
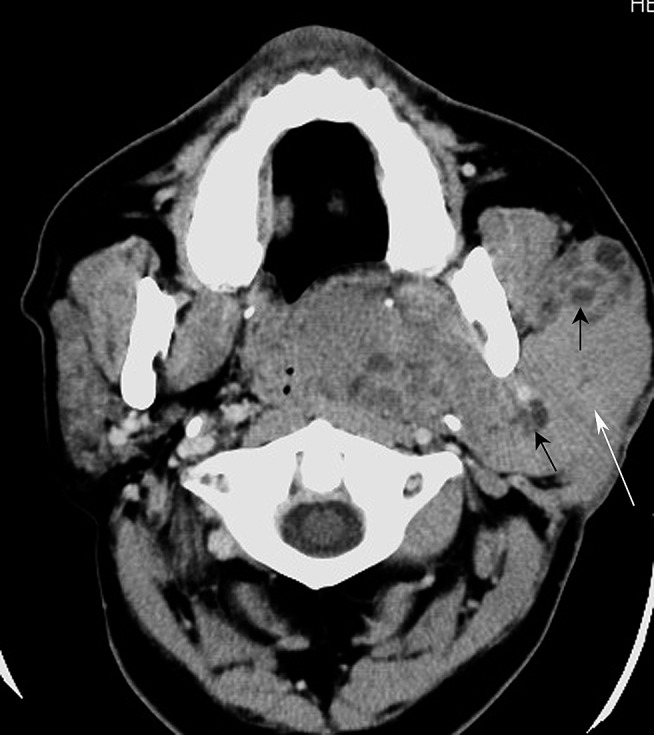
Mucosa-associated lymphoid tissue lymphoma and lymphoepithelial lesion of the left parotid gland (59 year old male). Axial-enhanced CT image shows a diffusely mixed solid-cystic lesion with ill-defined margins in the left parotid gland. The lesion, which has a large solid component (white arrow) and multicystic regions (black arrows), involves the superficial and deep lobes of the left parotid gland
Figure 5.
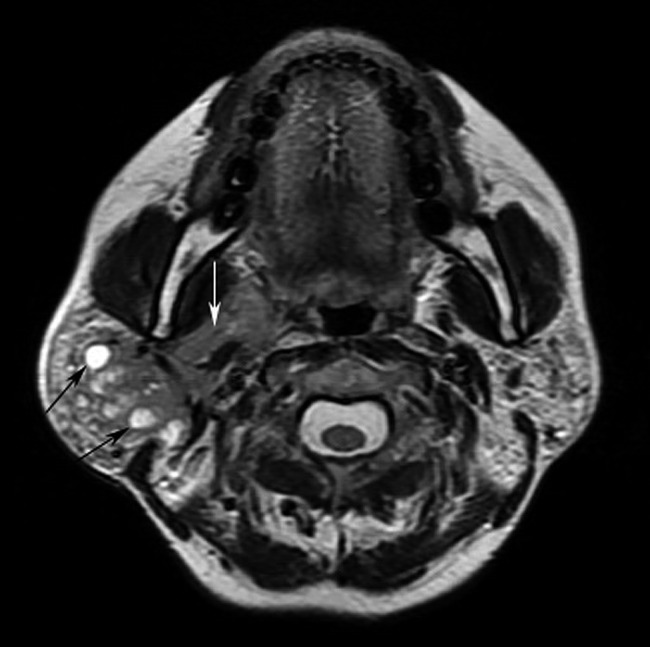
Mucosa-associated lymphoid tissue lymphoma and lymphoepithelial lesion of the right parotid gland (44 year old female). Axial T2 weighted imaging shows a diffuse mixed solid-cystic lesion containing small hyperintense multicystic (black arrows) and irregular solid (white arrow) components in the right parotid gland. The lesion has an ill-defined margin. The left parotid gland is also identified as abnormal with multiple solid nodules
Figure 6.
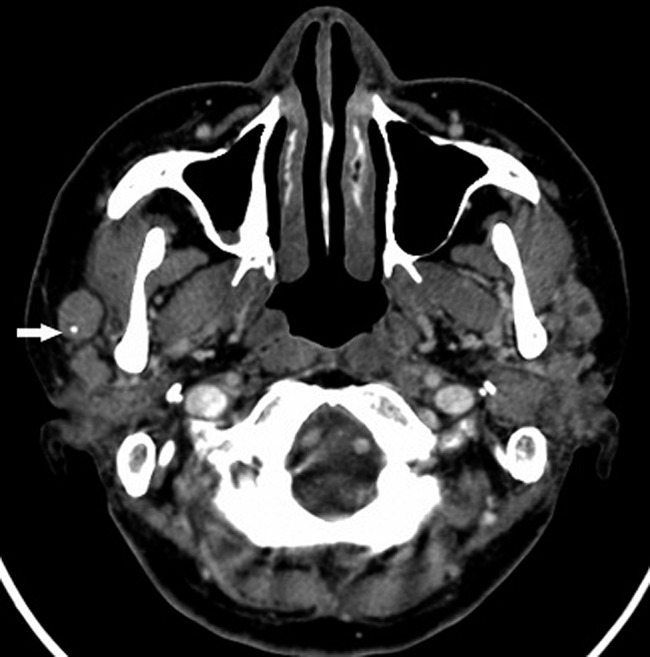
Mucosa-associated lymphoid tissue lymphoma and lymphoepithelial lesion of the parotid gland (50 year old woman). Axial contrast CT images show multiple solid nodules and masses with well-defined margins in the bilateral parotid glands (the lesion in the right parotid gland was pathologically confirmed). Punctuate calcification is shown in a soft tissue mass in the right parotid gland (white arrow)
Table 3.
The internal structure and marginal changes of 61 parotid NHLs shown on CT and MRI
| Types of parotid NHL | Smooth/irregular margin | Solitary solid mass | Solitary solid-cystic mass | Diffuse solid lesion | Diffuse solid-cystic lesion | Multiple solid masses | Calcification |
| MALT lymphoma | 15/19 | 4/34 | 7/34 | 0/34 | 16/34 | 7/34 | 8/34 |
| Non-MALT lymphoma | 15/12 | 9/27 | 0/27 | 5/27 | 2/27 | 11/27 | 2/27 |
| Total | 30/31 | 13/61 | 7/61 | 5/61 | 18/61 | 18/61 | 10/61 |
| p-value (two-tail) | 0.44 | 0.06 | 0.01 | 0.01 | 0.00 | 1.00 | 0.16 |
MALT, mucosa-associated lymphoid tissue; NHL, non-Hodgkin lymphoma.
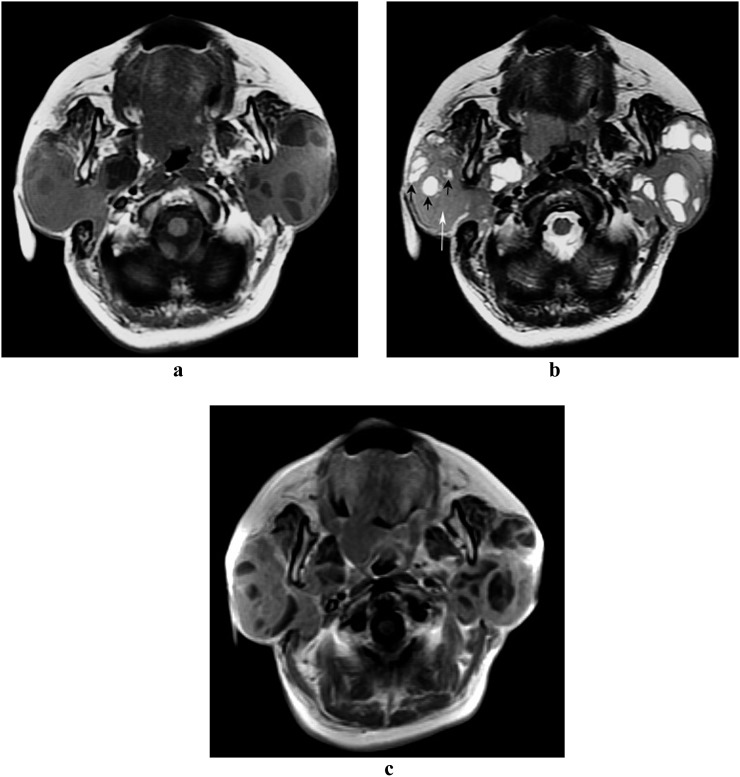
Figure 4.
Mucosa-associated lymphoid tissue lymphoma and lymphoepithelial lesion of the parotid gland (59 year old female). Axial MRI shows diffuse bilateral parotid disease in mixed solid-cystic form with an ill-defined margin (the lesion in the right parotid gland was pathologically confirmed). On pre-contrast T1 weighted image (a), multicystic and solid regions of the lesion are identified as low and intermediate signal intensities, respectively. On T2 weighted image (b), both multicystic (black arrows) and solid (white arrow) regions are revealed as heterogeneous high signal intensity. On post-contrast T1 weighted image (c), the signal of solid component is increased when compared with the pre-contrast T1 weighted image (a)
Of the 34 subjects, the contralateral parotid gland appeared as abnormal on CT and MRI (no pathological confirmation) in 24 subjects. These included multiple solid nodules or masses in 14 subjects, diffuse solid-cystic lesions in 6 subjects and diffuse honeycomb-like appearance in 4 subjects. Enlarged lymph nodes (no pathological confirmation) of the neck, excluding the parotid glands, were found in 10 subjects.
Parotid non-mucosa-associated lymphoid tissue lymphoma
Pathologically, 27 subjects with non-MALT-type lymphoma involving the parotid gland comprised both B-cell lymphoma (diffuse large B-cell lymphoma and follicular B-cell lymphoma) in 25 subjects and T-cell lymphoma in 2 subjects. No background of the lymphoepithelial lesions was detected in non-MALT lymphoma involving the parotid gland. CT and MRI appearances of non-MALT lymphoma were identified as four forms: solitary solid mass (Figure 7), diffuse solid lesion (Figure 8), diffusely mixed solid-cystic lesion (Figure 9) and multiple solid nodules or masses (Figure 10). The internal structure and marginal changes of 27 parotid non-MALT lymphomas shown on CT and MRI are listed in the Table 3. Solid (Figures 7, 8 and 10) and solid-cystic (Figure 9) appearances were seen in 25 of 27 subjects (92.6%) and 2 of 27 subjects (7.4%), respectively. Solitary masses, multiple masses and diffuse lesions were found in 9 of 27 (33.3%), 11 of 27 (40.7%) and 7 of 27 (25.9%) subjects, respectively.
Figure 7.
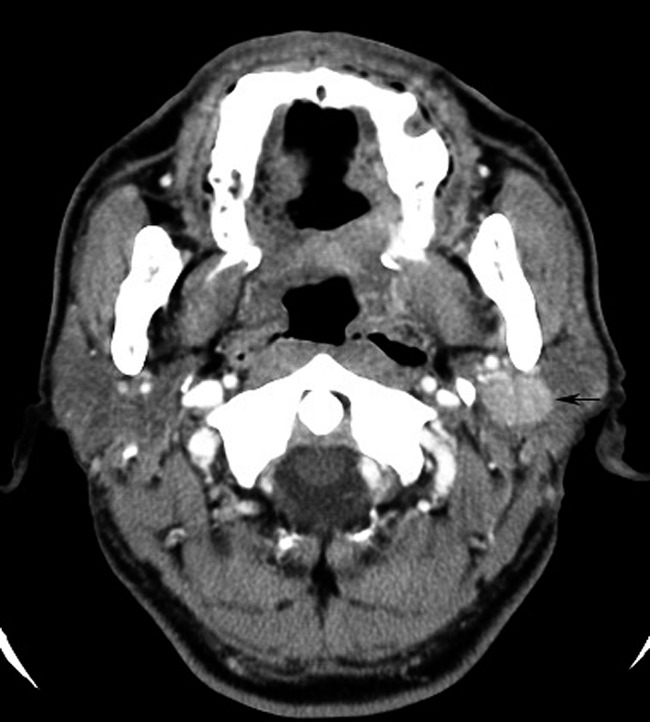
Non-mucosa-associated lymphoid tissue lymphoma (diffuse large B-cell lymphoma) of the left parotid gland (57 year old male). Contrast-enhanced axial CT image shows a solitary, oval and homogeneous mass (black arrow) with a well-defined margin in the deep lobe of the left parotid gland
Figure 8.
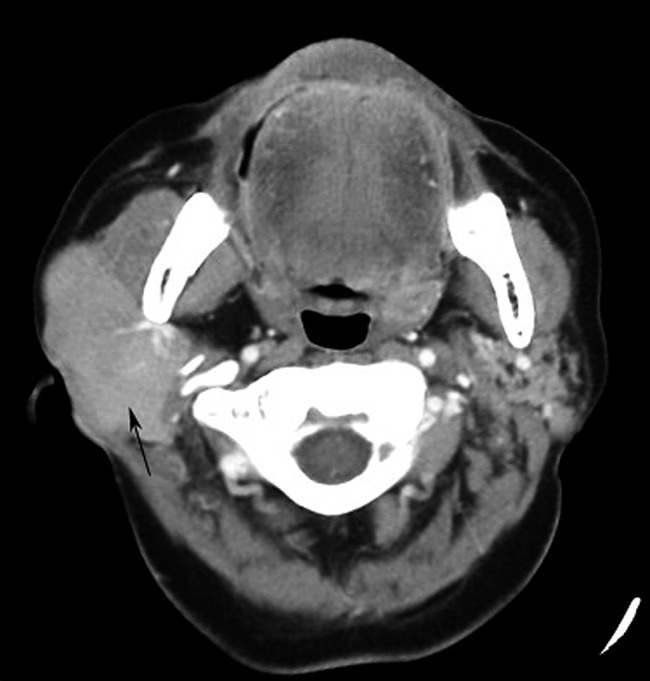
Non-mucosa-associated lymphoid tissue lymphoma (diffuse large B-cell lymphoma) of the right parotid gland (69 year old female). Contrast-enhanced axial CT image shows diffuse homogeneous neoplasm (black arrow) with an ill-defined margin occupied in the right parotid gland
Figure 9.
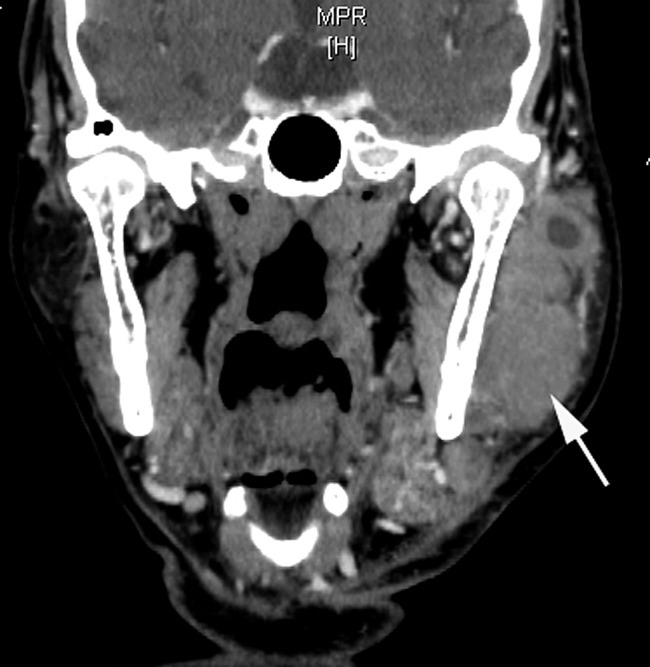
Non-mucosa-associated lymphoid tissue lymphoma (follicular B-cell lymphoma) of the left parotid gland (73 year old male). Coronal CT image reconstructed from axial contrast CT images shows diffuse mixed solid-cystic neoplasm (white arrow) with an ill-defined margin
Figure 10.
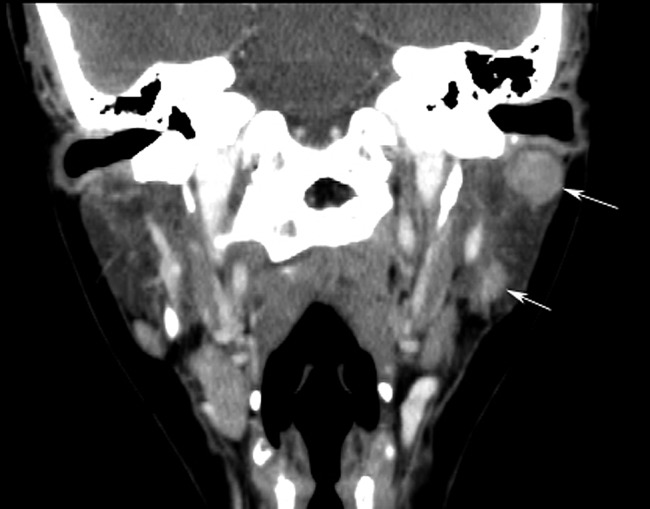
Non-mucosa-associated lymphoid tissue lymphoma (follicular B-cell lymphoma) of the left parotid gland (36 year old female). Coronal CT image reconstructed from axial contrast CT images shows the multiple solid masses (white arrows) with well-circumscribed margins in the left parotid
Abnormal CT and MR findings on the contralateral parotid gland (no pathological confirmation) were seen in 8 of 27 subjects. These included multiple solid nodules or masses in six subjects, diffuse honeycomb-like appearance in one subject and diffuse solid lesion in one subject. Enlarged lymph nodes (no pathological confirmation) of the neck, excluding lymph nodes in the parotid glands, were seen in nine subjects. The involvement of the muscles of mastication and masticator space adjacent to the neoplasm was found in six subjects. A solid mass of pharyngeal mucosal space and buccal space was seen in two subjects. The erosion of mandibular ramus was found in one subject.
Statistics confirm a significant difference with solid and solid-cystic changes between parotid MALT lymphoma and non-MALT lymphoma (p < 0.01). If solid-cystic changes of the neoplasm in the parotid gland are considered as signs of MALT lymphoma, true-positive detection of MALT lymphoma occurred in 23 of 34 and true-negative detection in 25 of 27 cases; and false-positive lesion detection occurred in 2 of 27 and false-negative detection in 11 of 34 cases. Therefore, CT and MRI have a sensitivity of 67.6%, specificity of 92.6% and accuracy of 78.7%. There is no statistically significant difference (p > 0.05) among solitary, multiple and diffuse patterns between parotid MALT-type and non-MALT-type lymphomas.
Discussion
CT and MRI findings of parotid mucosa-associated lymphoid tissue lymphoma
Salivary glands do not normally contain MALT but may acquire it as a result of an autoimmune inflammatory disorder, usually Sjögren's syndrome, which leads to the histological features of myoepithelial sialadenitis and lymphoepithelial lesions.8,9 The lymphocytic infiltrate of myoepithelial sialadenitis and lymphoepithelial lesions is thought to form a substrate for the possible development of MALT-derived salivary gland lymphoma.10 Lymphoma is reported to develop in up to 6% of subjects with Sjögren’s syndrome per year, and the risk of subjects in this group developing lymphoma has been calculated to be approximately 44 times greater than in a comparably healthy population.11 Histopathologically, MALT lymphoma involving the parotid gland occurs in association with lymphoepithelial lesions in almost all cases.6 The data of the current study demonstrate that approximately 90% of parotid MALT lymphomas are accompanied by background lymphoepithelial lesions, which suggests that there is a close relationship between parotid MALT lymphoma and lymphoepithelial lesions found in Sjögren’s syndrome.
CT and MRI findings of the parotid MALT lymphoma described in previous case reports5,9,12–17 included a significant swelling of the parotid parenchyma on unilateral or bilateral sides, solid nodules, multiple microcystic changes and multiple calculi in both the intracystic portion and the parenchymal gland. Corr et al17 reported that most solid nodules in MALT lymphoma correspond to hyperplastic lymphoid tissue or lymphoma, and cystic portions correspond to lymphoepithelial cysts arising from compression of terminal parotid ducts by contiguous hyperplastic or neoplastic lymphoid tissue. The data from this study demonstrate that CT and MR findings of parotid MALT lymphoma might vary in contour and internal structure, including solitary solid mass, solitary solid-cystic mass, diffusely solid-cystic lesion and multiple solid nodules or masses. Of these CT and MRI findings, solitary and diffuse solid-cystic changes are the most common form, accounting for 67.6% of all cases. The diffuse solid-cystic form of parotid MALT lymphoma is more common than the solitary solid-cystic form. The data of the current study indicate that the solid-cystic appearance is most suggestive of parotid MALT lymphoma, especially in subjects with a large solid component within the diffuse solid-cystic lesion.
Salivary lymphoepithelial lesions of Sjögren’s syndrome may be precursor lesions for MALT-type lymphoma.6,8–10 Based on the literature reports,9,18–22 CT and MRI findings of parotid lymphoepithelial lesions included bilateral enlargement with increased density, diffuse honeycomb-like appearance, or multiple solid-cystic changes of parenchyma, and dotted calcifications. Because the majority of parotid MALT lymphomas developed from lymphoepithelial lesions, the CT and MRI findings of both lesions may overlap each other. Therefore, it may be difficult to differentiate MALT lymphoma from lymphoepithelial lesions in terms of CT and MRI findings. However, the results of the current study indicate that the probability of parotid MALT lymphoma is increased once the dominant solid component within the solid-cystic lesion is revealed on CT and MRI.
CT and MRI findings of parotid non-mucosa-associated lymphoid tissue lymphoma
The main pathological subtypes of parotid non-MALT lymphomas in this study comprise both diffuse large B-cell lymphoma and follicular B-cell lymphoma. Shine et al23 described the CT findings of parotid lymphoma without the background of Sjögren's syndrome or any other autoimmune disorders. According to their observations, a unilateral single mass of relative soft-tissue homogeneity with a poorly defined margin was thought to be the most common sign of a parotid lymphoma. The CT findings of head and neck lymphomas described in previous literature also include an isodense mass with distinct margins, extranodal extension with less well-defined margins and areas of necrosis within the tumour matrix.4,23–26 To our knowledge, there are few reports that independently depict MR findings of non-MALT lymphoma in the parotid gland. The general description of MRI of head and neck lymphomas includes low signal intensity on T1 weighted images and low to high signal intensity on T2 weighted images, with variable, but usually low, enhancement following the introduction of gadolinium contrast material.25
Although CT and MRI manifestations of parotid non-MALT lymphoma were protean, the current study indicates that most of them presented as solitary and multiple solid forms. The solid-cystic form of parotid non-MALT lymphoma was infrequently seen. Based on previously published findings,4,23–26 the homogeneous solid change in head and neck lymphoma was more visible than necrosis and cystic changes, especially in nodal lymphoma. The results of this study support these viewpoints. In addition, we presume that the solid change of parotid non-MALT lymphoma corresponds to the neoplasm without the background of lymphoepithelial lesion.
Comparison of CT and MRI findings on parotid mucosa-associated lymphoid tissue and non-mucosa-associated lymphoid tissue lymphomas
There are few studies which compare CT and MRI findings of the parotid NHL in different pathological types. The current study demonstrates that differences on CT and MRI findings between MALT lymphoma and non-MALT lymphoma exist and are obvious. The CT and MRI characteristics of MALT lymphoma and MALT lymphoma in the parotid gland may be concluded as follows: (1) most parotid MALT lymphomas are characterized as solid-cystic lesions with a solitary or diffuse form, (2) diffuse solid lesions do not commonly occur in subjects with parotid MALT lymphoma and (3) solitary and diffuse solid-cystic lesions are infrequently seen in subjects with parotid non-MALT lymphoma.
Differential diagnosis of parotid NHL
The current study demonstrates that there are no differences in imaging findings between MALT lymphoma and non-MALT lymphoma among the solitary, multiple and diffuse forms. The differential diagnosis according to shape patterns between both types of parotid NHL may be difficult. Additionally, differential diagnosis between solid lymphomas and solid salivary epithelial tumours (such as plemorphic adenoma and Warthin tumour), and also between a metastatic necrotic lymph node and a solitary solid-cystic mass of MALT lymphoma in the parotid gland, remains difficult. However, in our experience, parotid pleomorphic adenoma, Warthin tumour and metastatic necrotic lymph nodes are usually without a history and signs of Sjögren’s syndrome in the clinic. Moreover, Warthin tumours occur predominantly in adult males and are associated with smoking. These differences may provide useful clinical information for the differential diagnosis.
Conclusions
This study has presented the different CT and MRI characteristics between parotid MALT lymphoma and non-MALT lymphoma. Although MALT lymphoma is characterized by solid-cystic changes of the neoplasm, non-MALT lymphoma shows a homogeneous solid change of the neoplasm.
In conclusion, the differences on CT and MRI findings between both types of parotid NHL can provide useful information for clinicians in predicting prognosis and selecting an appropriate treatment procedure.
REFERENCES
- 1.Freeman C, Berg FR, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972; 29: 252–260 [DOI] [PubMed] [Google Scholar]
- 2.Gleeson MJ, Bennett MH, Cawson RA. Lymphomas of salivary glands. Cancer 1986; 58: 699–704 [DOI] [PubMed] [Google Scholar]
- 3.Hyman GA, Wolff M. Malignant lymphomas of the salivary glands. Review of the literature and report of 33 new cases, including four cases with the lymphoepithelial lesion. Am J Clin Pathol 1976; 65: 421–438 [DOI] [PubMed] [Google Scholar]
- 4.Harnsberger HR, Bragg DG, Osborn AG, Smoker WRK, Dillon WP, Davis RK, et al. Non-Hodgkin’s lymphoma of the head and neck: CT evaluation of nodal and extranodal sites. AJR Am J Roentgenol 1987; 149: 785–791 10.2214/ajr.149.4.785 [DOI] [PubMed] [Google Scholar]
- 5.Aiken AH, Glastonbury C. Imaging Hodgkin and non-Hogkin lymphoma in the head and neck. Radiol Clin North Am 2008; 46: 363–378 10.1016/j.rcl.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Chan ACL, Chan JKC, Cheng MMC, Kapadia SB. Heamatolymphoid tumours. In: Barnes L, Eveson JW, Reichart P, Sidransky D. (eds). World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon, France: IARC Press; 2005. pp 277–80 [Google Scholar]
- 7.Jaehne M, Ussmuller J, Jakel KT, Zschaber R. The clinical presentation of non-Hodgkin lymphomas of the major salivary glands. Acta Otolaryngol 2001; 121: 647–651 [PubMed] [Google Scholar]
- 8.Balm AJ, Delaere P, Hilgers FJ, Somers R, Van Heerde P. Primary lymphoma of mucosa-associated lymphoid tissue (MALT) in the parotid gland. Clin Otolaryngol 1993; 18: 528–532 [DOI] [PubMed] [Google Scholar]
- 9.Stewar A, Blenkinsopp PT, Henry K. Bilateral parotid MALT lymphoma and Sjögren's syndrome. Br J Oral Maxillofac Surg 1994; 32: 318–322 [DOI] [PubMed] [Google Scholar]
- 10.Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer 1984; 53: 2515–2524 [DOI] [PubMed] [Google Scholar]
- 11.Moutsopoulos HM, Chused TM, Mann DL, Klippel JH, Fauci AS, Frank MM, et al. Sjörcn’s syndrome (Sicca Syndrome): current issues. Ann Intern Med l980; 92: 212–226 [DOI] [PubMed] [Google Scholar]
- 12.DePeña CA, Van Tassel P, Lee YY. Lymphoma of the head and neck. Radiol Clin North Am 1990; 28: 723–743 [PubMed] [Google Scholar]
- 13.Maksimovic O, Bethge WA, Pintoffl JP, Vogell M, Claussenl CD, Bares R, et al. Marginal zone B-cell non-Hodgkin’s lymphoma of mucosa-associated lymphoid tissue type: imaging findings. AJR Am J Roentgenol 2008; 191: 921–930 10.2214/AJR.07.2629 [DOI] [PubMed] [Google Scholar]
- 14.Tagnon BB, Theate I, Weynand B, Hamoir M, Coche EE. Long-standing mucosa-associated lymphoid tissue lymphoma of the parotid gland: CT and MR imaging findings. AJR Am J Roentgenol 2002; 178: 1563–1565 10.2214/ajr.178.6.1781563 [DOI] [PubMed] [Google Scholar]
- 15.Tonami H, Matoba M, Yokota H, Higashi K, Yamamoto I, Sugai S. Mucosa-associated lymphoid tissue lymphoma in Sjögren's syndrome: initial and follow-up imaging features. AJR Am J Roentgenol 2002; 179: 485–489 10.2214/ajr.179.2.1790485 [DOI] [PubMed] [Google Scholar]
- 16.Wickramasinghe A, Howarth A, Drage NA. Multiple bilateral parotid sialoliths in a patient with mucosa-associated lymphoid tissue lymphoma (MALT lymphoma) of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99: 496–498 10.1016/j.tripleo.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Corr P, Vaithilingum M, Thejpal R, Jeenn P. Parotid MALT lymphoma in HIV infected children. J Ultrasound Med 1997; 16: 615–617 [DOI] [PubMed] [Google Scholar]
- 18.Som PM, Brandwein MS. Salivary gland: anatomy and pathology. In: Som PM, Curtin HD. (eds). Head and neck imaging. St Louis, MO: Mosby; 2003. pp 2005–2133 [Google Scholar]
- 19.Vogl TJ, Dresel SH, Grevers G, Späth M, Bergman C, Balzer J, et al. Sjöegren's syndrome: MR imaging of the parotid gland. Eur Radiol 1996; 6: 46–51 [DOI] [PubMed] [Google Scholar]
- 20.Takashima S, Takeuchi N, Morimoto S, Tomiyama N, Ikezoe J, Shogen K, et al. MR imaging of Sjögren's syndrome: correlation with sialography and pathology. J Comput Assist Tomogr 1991; 15: 393–400 [DOI] [PubMed] [Google Scholar]
- 21.Tonami H, Ogawa Y, Matoba M, Kuginuki Y, Yokota H, Higashi K, et al. MR sialography in patients with Sjögren's syndrome. AJR Am J Neuroradiol 1998; 19: 1199–203 [PMC free article] [PubMed] [Google Scholar]
- 22.Izumi M, Eguchi K, Ohki M, Uetani M, Hayashi K, Kita M, et al. MR imaging of the parotid gland in Sjögren's syndrome: a proposal for new diagnostic criteria. AJR Am J Roentgenol 1996; 166: 1483–1487 10.2214/ajr.166.6.8633469 [DOI] [PubMed] [Google Scholar]
- 23.Shine NP, O'Leary G, Blake SP. Parotid lymphomas—clinical and computed tomographic imaging features. S Afr J Surg 2006; 44: 60, 62–64 [PubMed] [Google Scholar]
- 24.King AD, Lei KI, Ahuja AT. MRI of neck nodes in non-Hodgkin’s lymphoma of the head and neck. Br J Radiolo 2004; 77: 111–115 [DOI] [PubMed] [Google Scholar]
- 25.Weber AL, Rahemtullah A, Ferry JA. Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am 2003; 13: 371–92 [DOI] [PubMed] [Google Scholar]
- 26.Lee YY, Van Tassel P, Nauert C, North LB, Jing BS. Lymphomas of the head and neck: CT findings at initial presentation. AJR Am J Roentgenol 1987; 149: 575–581 10.2214/ajr.149.3.575 [DOI] [PubMed] [Google Scholar]