Abstract
The brain remains dynamic even in older age and can benefit from mental exercise. Thus, it is important to understand the concepts of positive neuroplasticity and negative neuroplasticity and how these mechanisms either support or detract from cognitive reserve. This article provides a brief review of these key concepts using four exemplary studies that clearly demonstrate the effects these neurological mechanisms exert on cognitive reserve and cognitive functioning. From this review, a working knowledge of how neuroplasticity and cognitive reserve are expressed in patients will be provided along with how this information can be incorporated into nursing practice and research.
Keywords: Cognition, Speed of Processing, Executive Functioning, Memory, Cognitive Remediation, Neuroplasticity, Environmental Press
According to Baltes and Baltes (1990), successful aging requires eight essential components: length of life, biological health, mental health, cognitive efficiency, social competence, productivity, personal control, and life satisfaction. Moreover, these components are dynamic and interactive with one another so that if one component is lacking, this may negatively affect the other components. Cognitive efficiency is clearly one component that influences the others. For example, experiencing normal age-related cognitive declines can adversely affect length of life and biological health (e.g., forgetting to take medications), mental health (e.g., less cognitive proficiency in emotional problem solving), social competence (e.g., not being able to remember someone’s name), productivity (e.g., taking longer to grocery shop), personal control (e.g., fear of developing Alzheimer’s disease), and life satisfaction (e.g., cognitive complaints are bothersome).
Studies show that such normal age-related cognitive declines, even in the absence of dementia or Mild Cognitive Impairment, can impair the accuracy and speed in which instrumental activities of daily living are performed (Edwards et al., 2005; McGuire, Ford, & Ajani, 2006); even subtle declines can reduce independence, autonomy, and life satisfaction (Ballard, 2010). Given that there will be 54 million adults 65 years and older in the United States by 2020 (United States Census Bureau, Population Division, 2005), it is important to understand how to facilitate successful cognitive aging in this growing population in order to facilitate successful aging in general.
The purpose of this article is to provide nurses and nurse practitioners with updated information about the importance of positive and negative neuroplasticity on cognitive reserve in order to help educate and facilitate successful cognitive aging in their older patients. To accomplish this, the concepts of positive and negative neuroplasticity will be explained through four well-known studies in the neuropsychology literature. Following this, the role of neuroplasticity on cognitive reserve will be reviewed with particular attention focusing on the putative biomechanisms that may reduce cognitive reserve and thus, cognitive functioning. From this, implications for nursing practice (i.e., cognitive exercise) and research (i.e., investigating cognitive remediation therapy) will be provided.
Positive and Negative Neuroplasticity
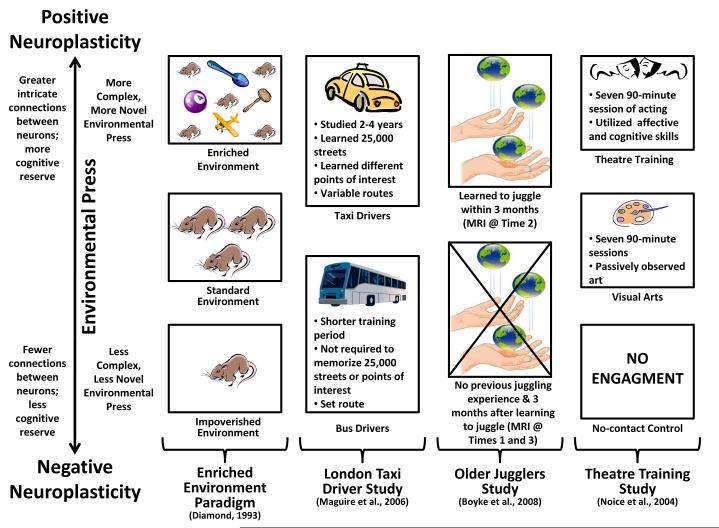
Both positive neuroplasticity and negative neuroplasticity refer to the actual morphological and neurochemical changes that occur in the nervous system and brain between neurons in response to adapting to environmental demands or press (Figure 1; Vance & Crowe, 2006; Vance, Roberson, McGuiness, & Fazeli, 2010). The guiding principles behind positive and negative neuroplasticity come from two sources: 1) knowledge about how glial cells, which account for 85% of the cells in the brain, assist neurons to maintain or rewire and form new connections with other neurons (Brown, 2009; Fields, 2009), and 2) the “Use It or Lose It” theory of aging which posits that systems in the body that are underutilized eventually atrophy and the energy needed to maintain them is stored or diverted to areas where there is need (Coyle, 2003; Vance et al., 2010). Based upon these principles, when animals and humans are exposed to a more complex, more novel environmental press, they adapt by learning the environment in order to negotiate it better (Figure 1). Such adaptation includes developing stronger and more intricate connections between neurons in order to have better communication between them, and as a result, gain better cognition in order to further explore and negotiate within that environment. This process increases cognitive reserve and is referred to as positive neuroplasticity. Likewise, when animals and humans are exposed to a less complex, less novel environmental press, there is little need to adapt since they already have the cognitive skills necessary to negotiate the environment. In fact, if their skills far surpass the environmental press, the energy needed to maintain such neuronal connections may be diverted and fewer connections may be needed; therefore, these neuronal connections may atrophy. This process decreases cognitive reserve and is referred to as negative neuroplasticity.
Figure 1.
The interaction of environmental press on positive and negative neuroplasticity.
Both positive and negative neuroplasticity function on a continuum of environmental press. The more challenging and novel the environmental press, the more positive neuroplasticity will be involved in adapting to this press by forming more neuronal connections and increasing cognitive reserve. Similarly, negative neuroplasticity functions in an opposite manner by not building and/or breaking down the neuronal connections that are needed to adapt to environmental press. These concepts are shown in Figure 1 which includes four exemplary studies from the neuropsychology literature that clearly demonstrate the effects these neurological mechanisms exert on cognitive reserve and thus cognitive functioning.
Enriched Environmental Paradigm
Diamond and other neuroscientists (Diamond, 1993; Kobayashi, Ohashi, & Ando, 2002) used various permutations (e.g., ages of rats, time exposures) of the enriched environmental paradigm to demonstrate the influence that the environment exerts on brain morphology, specifically positive and negative neuroplasticity. In this experimental paradigm, rats from the same colony (i.e., genetically similar) are randomly assigned to live in one of three environmental conditions: Enriched, standard, and impoverished. In the enriched environmental condition, rats are placed with several other rats in a large cage and are also given toys and objects to explore; these toys and objects are exchanged with others such items periodically. In this condition, rats have an enriched social and physical environment to explore. In the standard environmental condition, rats are placed three to a cage but are not given toys and objects to explore. In this condition, rats have a less enriched social and physical environment to explore. In the impoverished environmental condition, rats are placed in a cage by themselves and are not given toys and objects to explore. In this condition, rats have no social interaction and a minimal physical environment to explore.
After equal exposure to the environments, researchers found that rats in the enriched environmental condition developed larger brains, had more dendritic connections between neurons, and had larger amounts of neurotrophic growth factor compared to rats placed in the other two environmental conditions. Likewise, a similar pattern emerged for those rats in the standard environmental condition compared to those rats placed in the impoverished environmental condition. These findings support the role that environmental press exerts on brain morphology.
It has been shown in subsequent studies that these morphological changes translate into better cognitive functioning (i.e., memory and problem solving) as measured by time to complete mazes which is typical in studies of this nature. In other words, rats exposed to the enriched environmental condition and benefitted from neurological growth, performed faster and better in completing maze tasks compared to rats exposed to the other environmental conditions. These findings were found regardless of the amount of exposure to these environments (a few weeks to several months) or the various ages of the rats (Diamond, 1993; Kobayashi et al., 2002; Paban, Jaffard, Chamben, Malafosse, & Alescio-Lautier, 2005; Van Praag, Kempermann, & Gag, 2000; Vance & Crowe, 2006; Vance et al., 2010). Thus, the more complex and enriched the environment (a.k.a., environmental press), the more positive neuroplasticity was observed; similarly, the less complex and less enriched the environment, the more negative neuroplasticty was observed.
London Taxi Driver Study
In the London Taxi Driver Study (Figure 1), Maguire and colleagues (Maguire et al., 2000; Maguire, Woolett, & Spiers, 2006) examined the brains of London taxi drivers and London bus drivers using magnetic resonance imaging (MRI). In doing so, researchers capitalized upon a naturalistic phenomenon whereby to gain their license, taxi drivers had to undergo a 2 to 4 year training period to learn London’s many points of interest, familiarize and recall London’s 25,000 streets, and figure out how to drive and connect through them. This intense training period and driving in such a complex setting represents an enriched environmental (i.e., more complex/more novel environmental press) paradigm. In contrast, bus drivers did not have to receive such an intense training period and were assigned to bus routes that varied little. For the bus drivers, being in such a routine setting represents the standard environment (i.e., less complex and less novel environmental press). MRI scans were used to compare the taxi drivers and bus drivers; this revealed that in comparison to the bus drivers, the taxi drivers had larger mid-posterior hippocampi (i.e., the brain structures needed for memory formation and consolidation). Furthermore, the larger size of the hippocampi was significantly related to more years of taxi driving. As expected, bus drivers who were considered to be in a more standard routine environment did not have larger hippocampi associated with years of driving.
Older Jugglers Study
Such principles of positive and negative neuroplasticity are also observed in older adults. In a sample of 69 community-dwelling older adults (Mage = 60; range = 50 – 67), Boyke, Briemeyer, Gaser, Büchel, and May (2008) instructed participants how to juggle three balls at a time. In addition, researchers conducted MRIs with these participants at baseline (Time 1 – before juggling instruction), at posttest (Time 2 – after 3 months when participants were able to juggle for a minimum of 1 minute), and 3 months later (Time 3 – after participants were no longer juggling). Only 25 participants were able to learn to juggle. Comparing the MRI scans of these 25 participants, researchers found that the hippocampi and nucleus accumbens grew from baseline to immediate posttest when participants were learning to juggle; juggling represents a more complex and more novel environmental press. Albeit, these brain structures diminished in size from posttest to 3 months later when participants were no longer juggling; not juggling represents less complex and less novel environmental press.
Theatre Training Study
In a sample of 124 community-dwelling older adults (Mage = 73.7; range = 60 – 86), Noice, Noice, and Staines (2004) assigned groups of older adults to one of three groups of varying environmental press: theatre training, visual arts, and no-contact control. In the more complex and more novel environmental press condition, theatre training, participants received nine 90-minute group sessions over a 4-week period. During these sessions, participants engaged in acting exercises that required them to integrate cognitive, physiological, and affective abilities. In the medium environmental press condition, visual arts, participants received nine 90-minute group sessions where art was examined. In the less complex and less novel environmental press condition, no-contact control, participants did not receive any attention. Those participants in the theatre training group performed better on some of the cognitive measures of verbal memory and reasoning compared to those in the conditions with less complex and less novel environmental press. Other permutations of this study have resulted in similar findings (Noice & Noice, 2006, 2009; Noice, Noice, Perrig-Chiello, & Perrig, 1999).
Cognitive Reserve
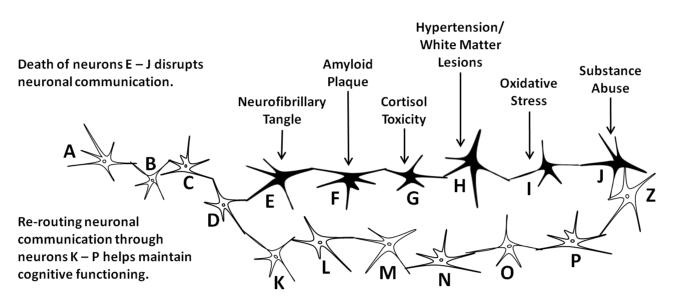
Cognitive reserve refers to the amount, sophistication, and strength of connections between neurons; it is from these neuronal connections and the information communicated between them from which cognition is expressed. The greater one’s cognitive reserve, the more likely one is to withstand neuronal insults and still maintain cognitive functioning (Roe et al., 2008; Vance et al., 2010). For example, as seen in Figure 2, the flow of communication between neurons conveyed by the action potentials and the release of neurotransmitters between the synaptic cleft processes in sequential manner from neurons A to B, B to C, and so forth until it arrives at neuron Z. Unfortunately with increasing age, some neurons may be compromised due to a variety of events (e.g., cortisol toxicity, oxidative stress) which may render such neurons either ineffective or dead. In either case, communication between the neurons is blocked, thereby reducing cognitive efficiency. But if there is sufficient cognitive reserve (i.e., other neuronal connections), adjacent functional neurons (i.e., neurons L – Q) may be able to re-route communication from the damaged neurons (i.e., neurons E – K), thereby maintaining cognitive efficiency. Such functioning is observed in other bodily systems. This is similar to collateral circulation that develops in occlusive coronary heart disease whereby coronary collateral arteries actually provide an alternate route for blood flow in an attempt to decrease myocardial ischemia from occurring (Berry et al., 2007).
Figure 2.
Cognitive reserve and cognitive functioning.
Similarly, positive and negative neuroplasticity are the mechanisms by which cognitive reserve is considered to be increased, maintained, or decreased. As elucidated in the previous section, being exposed to an enriching environment that provides the motivation for the person (or animal) to adapt promotes positive neuroplasticity, resulting in more dendritic connections, larger brains, and thus more cognitive reserve. Likewise, the opposite is observed for negative neuroplasticity. From this, studies exemplify how encouraging positive neuroplasticity promotes cognitive reserve and protects against cognitive impairment. For instance, in a sample of adults with (n = 37) and without Alzheimer’s disease (n = 161), Roe and colleagues (2008) examined whether educational attainment, as a form of environmental press and positive neuroplasticity that builds cognitive reserve, delays the onset of Alzheimer’s disease. Alzheimer’s disease severity in this study was measured by the amount of fibrillar beta-amyloid in the brain using radiotracers that highlighted this protein in positron emission tomography. Roe and colleagues found that higher education level was predictive of better cognitive functioning, even when fibrillar beta-amyloid was present in higher levels. As in other studies (Brayne et al., 2010), this finding suggests that educational level, as a measure of cognitive reserve, helps delay the symptoms of developing Alzheimer’s disease.
Neuofibrillary Tangles and Amyloid Plaques
Unfortunately, there are several other biomechanisms that can compromise neuronal health and reduce cognitive reserve. As just mentioned, neurofibrillary tangles and beta-amyloid plaques, the hallmark of Alzheimer’s disease, can lead to the death of neurons. Specifically, neurofibrillary tangles occur within the neurons and compromise cellular functioning which eventually kills them. Likewise, beta-amyloid plaques amass around neurons, severe connections with other neurons, and entangle neurons, eventually causing cell death (Gauthier & Ballard, 2009). Other biomechanisms also contribute to poorer functioning or the death of neurons; for this brief review, only a few are included such as cortisol toxicity, hypertension/white matter lesions, oxidative stress, and substance use.
Cortisol Toxicity
Research has suggested that repeated lifelong exposure to psychosocial hazards such as anxiety, depression, and stress may be harmful to neuronal health and thus cognitive functioning and reserve. Studies have hypothesized that the primary mechanism for this relationship is the response of the hypothalamic-pituitary-adrenal axis to these psychosocial hazards, thus triggering it to secrete glucocorticoids (most relevant is cortisol), which are hypothesized to be detrimental to hippocampal neurons (Chrousos & Gold, 1992; Lupien et al., 2005). One longitudinal study examined the predictive value of urinary excretion of cortisol on cognitive impairment (n = 538; age range 70-79 years) (Karlamangla, Singer, Chodosh, McEwen, & Seeman, 2005). Results indicated that those with higher urinary cortisol levels at baseline had a higher risk for developing cognitive impairment over a 7-year period. This relationship was not affected by age, gender, education, ethnicity, smoking, cardiovascular disease, or blood pressure. Another cross-sectional study examined salivary cortisol levels among older adults (N = 967; age range 50-70 years) and found higher cortisol levels to be predictive of poorer cognitive performance across multiple domains (i.e., language, processing speed, hand-eye-coordination, executive functioning, verbal memory and learning, and visual memory) (Lee et al., 2007). These associations did not differ by age, sex, ethnicity/race, or time day. Thus, dysregulation of cortisol by the hypothalamic-pituitary-adrenal axis which may be caused by lifelong stress, anxiety, and depression may cause reduced cognitive reserve and cognitive function among older adults.
Hypertension
Hypertension can also impair neuronal health and contribute to reduced cognitive reserve and thus cognitive impairment. Hypertension causes small-vessel cerebrovascular disease that eventually form cerebrovascular white matter lesions, which is basically small localized areas of damaged neuronal tissue (de Leeuw et al., 2002; Liao et al., 1996). Using MRI scans in a sample of 1,920 older adults (Mage = 64; range = 55 – 72 years), Liao and colleagues (1996) found that those with uncontrolled hypertension had statistically greater odds of having white matter lesions compared to those with controlled hypertension. Likewise, in a sample of 1,077 older adults (Mage = 51; range = 60 – 90 years), de Leeuw and colleagues (2002) found that the amount of both subcortical and periventricular white matter lesions were significantly correlated with the duration of hypertension. Furthermore, those with uncontrolled hypertension were significantly more likely to have such white matter lesions in both brain regions.
Oxidative Stress
Oxidative stress refers to a vast array of aberrant cellular and molecular changes that produce inflammation in tissues as a result of their contact with reactive oxygen species, sometimes known as free radicals (Dröge & Schipper, 2007). Although the body needs oxygen for survival, oxygen itself is corrosive and toxic to tissues; fortunately, the body possesses a complex array of antioxidant defenses that minimize the putative effects that these free radicals of oxygen exert on plasma cysteine homeostasis, brain calcium and iron homeostasis, and mitochondrial DNA. Unfortunately, these antioxidant defenses become less efficient with aging; this results in a progressive accumulation of these reactive oxygen species detrimentally interacting with tissues in the body, including the brain, thus creating oxidative stress (Asha Devi, 2009). In fact, it is widely held that brain aging, and aging in general, is due largely to oxidative stress. Succinctly, free radicals of oxygen interact with and damage DNA; over time, the damage accumulates resulting in a progressively degraded genetic code that is used to replicate the tissues of the body. As the degraded genetic code is used to produce these tissues, these tissues are degraded as a result – hence, aging occurs (Dröge & Schipper, 2007).
Oxidative stress in the brain has been studied extensively in rats and humans. Singh, Kanwar, Sood, and Nehru (2011) examined the role of folic acid, an antioxidant, on rats. In their study, rats in the experimental condition received 5 mg/kg body weight/day of folic acid for 8 weeks; these rats were compared to same age rats in the control condition that did not receive this antioxidant. Singh and colleagues found rats that received the folic acid exhibited better performance on cognitive indices as measured by maze tests, passive avoidance, and active avoidance. An examination of the rats’ brains revealed lipid peroxidant, an index of aging, was lower in those supplemented with folic acid. Similar studies with rats involving other antioxidants (e.g., blueberry, spinach, and strawberry extracts) found similar benefits (Joseph, Shukitt-Hale, Denisova, Bielinski et al., 1999; Joseph, Shukitt-Hale, Denisova, Prior et al., 1998). Such studies show that relieving oxidative stress in the brain promotes better cognitive reserve and subsequent cognitive functioning (Asha Devi, 2009).
The relationship between selenium, another antioxidant, and cognition was examined in 1,389 older adults (60 – 70 years). In this study, Akbarlay and colleagues (2007) conducted 6 waves of cognitive and biomarker data collection over 9 years. Controlling for a number of potential confounders using mixed logistic and linear models, they found that a decline in selenium corresponded with a decline in cognitive functioning. They concluded that since selenium levels decrease with age, perhaps this increases oxidative stress in the brain which impairs cognitive reserve and cognitive functioning. Given these findings, antioxidant supplementation is being vigorously examined to prevent cognitive aging and treat age-related neurodegenerative diseases (Mandel, Amit, Weinreb, & Youdim, 2011; Pocernich, Bader Lange, Sultana, & Butterfield, 2011).
Substance Use
In general, the prevalence of substance use declines with age (Moos, Brennan, Schutte, & Moos, 2005). However, past and current substance use, particularly abuse, has well documented effects on neurological functioning and cognition (Anstey, 2008; Kumar, & Kinsella, 2010). Substance use also interferes with the role of glial cells in providing support to neurons; this means that neurons are more vulnerable to other insults such as cortisol toxicity and oxidative stress (Brown, 2009; Fields, 2009). In adolescence, alcohol and substance abuse have been shown to negatively affect hippocampi volume and cognitive functioning; this can place such adolescents at-risk in later life because they will have less cognitive reserve (Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007).
Implications for Nursing Practice and Research
For many older adults, subtle cognitive declines are a normal part of aging; however, they do not necessarily have to interfere with everyday functioning and quality of life. In fact, as observed in this brief literature review, cognition is somewhat malleable and can be augmented through the process of neuroplasticity. This has implications for nursing practice and research.
In practice, nurses and nurse practitioners can incorporate this information on neuroplasticity and cognitive reserve in two ways: 1) addressing patients’ concerns and complaints about cognitive aging, and 2) understanding how the symptoms of dementia emerge. Many patients express concerns about their mental abilities as they grow older; likewise, some may voice cognitive complaints in certain cognitive domains such as memory, attention, and/or reasoning. Such concerns and complaints should be documented given that these may be benign such as vitamin B12 (cobalamin) deficiency (Ballard, 2010; Tangney, Tang, Evans, & Morris, 2009) which can be easily corrected or be an indication of something more serious such as dementia. Obviously, monitoring cognition and possible progression of cognitive decline should be documented in case a referral is needed (Vance, Farr, & Struzick, 2008). Albeit, nurses and nurse practitioners can recommend ways to promote positive neuroplasticity, reduce negative neuroplasticity, and thus facilitate cognitive reserve and cognitive functioning. In doing so, patients can be empowered to modify certain lifestyle factors known to benefit cognition.
Vance, Eagerton, Harnish, McKie-Bell, and Fazeli (2011) proposed an evidence-based format whereby nurses can provide a behavioral approach to modify such lifestyle factors with the goal of maintaining or improving cognition. In this cognitive prescription approach, several areas known to influence cognition are targeted: nutrition, physical exercise, intellectual exercise, social interaction, sleep hygiene, and positive mood (Vance & Crowe, 2006; Vance et al., 2010). In this holistic approach, motivational interviewing techniques are used to determine what goals patients want to set for themselves in each of these areas; then, patients are prescribed specific, observable goals in each area. For example, one patient’s physical exercise goal may be to go for a 30-min walk three times a week, while her intellectual goal may be to read one book a week. Using such cognitive prescriptions represents a concrete way to help patients address concerns about their cognitive concern and complaints while empowering them with a framework in which they may be able to actually improve cognitive functioning, as well as other areas of their lives.
Understanding how the symptoms of dementia emerge within the conceptualization of cognitive reserve will also help nurses and nurse practitioners monitor patients’ progression to dementia and help them explain it to patients, caregivers, and family members. Specifically, as alluded to in the Roe and colleagues (2008) study (Section – Cognitive Reserve), with greater cognitive reserve, the initial symptoms of Alzheimer’s disease are delayed, even when amyloid plaques and neurofibrillary tangles are present because alternative routes of neuronal communication can still occur which supports continued cognitive functioning (Figure 1). Unfortunately, once such cognitive reserve reaches a compromised threshold, as indicated by fewer or non-existent routes, neuronal communication becomes inconsistent or ceases which results in poorer cognitive functioning and eventually leads to intellectual failure (Stern, 2009).
In research, there are several areas in which nurses in gerontology can investigate but three areas of particular interest are: 1) cognitive remediation therapy, 2) the extent to which cognition can be improved, and 3) the use of antioxidants such as hydrogen water. Cognitive remediation therapy represents an exciting and emerging area of practice and research in which either global cognition or specific cognitive domains such as reasoning or speed of processing are targeted for improvement. Using the principles of neuroplasticity, older adults are given a set of mental exercises to improve cognition (Vance & Crowe, 2006; Vance et al., 2010).
In a randomized, multisite, single-blind, control trial, Ball, Edwards, Ross, and McGwin (2010) randomized 908 older drivers (65+ years) to one of four conditions: 1) memory training, 2) reasoning training, 3) speed of processing training, and 4) no-contact control. Those in the memory (n = 175) and reasoning (n = 145) conditions received ten hours of practicing either memory or reasoning strategies and using these strategies in group and one-on-one exercises. Those in the speed of processing (n = 179) condition received ten hours of computerized visual attention and speed of processing exercises designed to help them to process visual stimuli faster. State records of motor vehicle crashes were examined for the participants six years after enrollment. Controlling for several confounds (e.g, age, race, mental status, education, vision, health, depressive symptomatology, and testing site), participants in the reasoning and speed of processing conditions experienced approximately 50% reduction of at-fault motor vehicle crashes (per person-mile driven) compared to the control group. Although memory training did not produce a detectable change in motor vehicle crashes, this study did demonstrate that reasoning and speed of processing training improves driving performance long-term, which has dramatic public health consequences (Ball et al., 2010). Given the efficacy of this training in normal, community-dwelling older adults, it may be warranted to test such cognitive remediation therapy in cognitively at-risk adults such as those with mild cognitive impairment (i.e., a preclinical stage of dementia), those with early stage Alzheimer’s disease, or older adults with HIV suffering from neurological sequelae (Vance et al., 2010).
Fortunately, many of cognitive remediation therapies have been translated to computer programs making them more accessible and affordable (Vance, McNees, & Meneses, 2009). Despite the robust findings in the literature on the benefits of cognitive remediation therapy (e.g., Ball, Edwards, & Ross, 2007), it is unclear to what extent such cognitive remediation therapy can improve cognitive. For example, Kwok and colleagues (2011) found in a small pilot (N = 24) study with older adults (range = 67 – 88) using memory training and cognitive stimulation that some of these participants appeared to plateau in their cognitive performance. Clearly, a certain amount of training will most likely improve cognitive performance. Ostensibly, a certain amount of training will most likely improve cognitive functioning up to a point; what this ceiling for improvement is and what are its determinants has yet to be examined. There are several factors that may limit the gains derived such cognitive remediation including innate intelligence, genetics, the amount of insults to the brain, and personality factors such as motivation; yet, this has yet to be studied in detail.
Finally, in a recent advance, Gu and colleagues (2010) proposed that since the nervous system consumes a great amount of oxygen and is vulnerable to oxidative stress, coupled with an aging and impaired antioxidant defense system, this may be one of the driving putative biomechanisms leading to reduced cognitive functioning in older adults. Therefore, reducing oxidative stress is essential. In their study, Gu and colleagues gave some senescence-accelerated prone mice hydrogen water; hydrogen water protects the nervous system from such oxidative stress. Compared to the senescence-accelerated prone mice given normal water, researchers found that the senescence-accelerated prone mice provided the hydrogen water to drink for 30 days had increased serum antioxidant activity and elevated brain serotonin levels; in addition, mice in the hydrogen water condition exhibited better cognitive functioning, as exhibited by a water maze task, compared to the rats in the control condition. Furthermore, after 18 weeks the mice provided with the hydrogen water had better intact hippocampi compared to the mice given normal water. Since hydrogen water is inexpensive to make, well-tolerated, and safe, this represents a novel approach to protecting and ameliorating cognitive reserve in older adults. Obviously, more research must be conducted to examine this exciting research vector.
Conclusion
As the number of older adults increases, healthcare providers will need to be aware of the impact that even small declines in cognition can exert a negative impact on everyday functioning and quality of life. Fortunately, evidence demonstrates that older adults can experience cognitive benefits from intellectual exercise, health promoting behaviors, and cognitive remediation therapies. Although such evidence suggests that cognitive reserve can be increased in later life, it is equally important to focus on increasing cognitive reserve across the entire lifespan in order to better increase the chances of successful cognitive aging.
Acknowledgments
Dr. Vance was also supported by the UAB Edward R. Roybal Center for Translational Research on Aging and Mobility, NIA 2 P30 AG022838.
Contributor Information
David E. Vance, School of Nursing, University of Alabama at Birmingham (UAB), Birmingham, AL.
Jaspreet Kaur, Department of Psychology & Edward R. Roybal Center for Translational Research in Aging and Mobility, University of Alabama at Birmingham (UAB), Birmingham, AL.
Pariya L. Fazeli, Department of Psychology & Edward R. Roybal Center for Translational Research in Aging and Mobility, University of Alabama at Birmingham (UAB), Birmingham, AL.
Michele H. Talley, School of Nursing, University of Alabama at Birmingham (UAB), Birmingham, AL.
Hon K. Yuen, School of Health Professions, University of Alabama at Birmingham, Birmingham, AL.
Beth Kitchin, Nutrition Sciences, University of Alabama at Birmingham (UAB), Birmingham, AL.
Feng Lin, School of Nursing, University of Rochester, Rochester, NY.
References
- Akbaraly TN, Hininger-Favier I, Carrière I, Arnaud J, Gourlet V, Roussel AM, Berr C. Plasma selenium over time and cognitive decline in the elderly. Epidemiology. 2007;18(1):52–58. doi: 10.1097/01.ede.0000248202.83695.4e. doi: 10.1097/01.ede.0000248202.83695.4e. [DOI] [PubMed] [Google Scholar]
- Anstey KJ. Alcohol exposure and cognitive development: An example of why we need a contextualized, dynamic life course approach to cognitive aging – a mini-review. Gerontology. 2008;54(5):283–291. doi: 10.1159/000161735. doi: 10.1159/000161735. [DOI] [PubMed] [Google Scholar]
- Asha Devi S. Aging brain: Prevention of oxidative stress by vitamin E and exercise. Scientific World Journal. 2009;9:366–372. doi: 10.1100/tsw.2009.46. doi: 10.1100/tsw.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(Spec No 1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. doi: 62/suppl_Special_Issue_1/19. [DOI] [PubMed] [Google Scholar]
- Ball K, Edwards JE, Ross LA, McGwin G., Jr. Cognitive training decreases motor vehicle collision involvement in older drivers. Journal of the American Geriatics Society. 2010;58:2107–2113. doi: 10.1111/j.1532-5415.2010.03138.x. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard J. Forgetfulness and older adults: Concept analysis. Journal of Advanced Nursing. 2010;66(6):1409–1419. doi: 10.1111/j.1365-2648.2010.05279.x. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives on successful aging: The model of selective optimization with compensation. In: Baltes PB, Baltes MM, editors. Successful aging: Perspectives from the behavioral sciences. Cambridge University Press; Cambridge, England: 1990. pp. 1–34. [Google Scholar]
- Berry C, Balachandran KP, L’Allier PL, Lesperance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heasrt disease. European Heart Journal. 2007;28:278–291. doi: 10.1093/eurheartj/ehl446. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. Journal of Neuroscience. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Povikoski T, Sulkava R. Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133(Pt. 8):2210–2216. doi: 10.1093/brain/awq185. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- Brown D. Role of microglia in age-related changes to the nervous system. The Scientific World Journal. 2009;9:1061–1071. doi: 10.1100/tsw.2009.111. doi: 10.1100/tsw.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Coyle JT. Use it or lose it – Do effortful mental activities protect against dementia. New England Journal of Medicine. 2003;348:2489–2490. doi: 10.1056/NEJMp030051. [DOI] [PubMed] [Google Scholar]
- De Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hoffman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a propsective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- Diamond MC. An optimistic view of the aging brain. Generations. 1993;17(1):31–33. [Google Scholar]
- Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–379. doi: 10.1111/j.1474-9726.2007.00294.x. doi:10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging and Mental Health. 2005;9(3):262–271. doi: 10.1080/13607860412331336788. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Fields RD. The other brain: From dementia to schizophrenia, how new discoveries about the brain are revolutionizing medicine and science. Simon & Schuster; New York, NY: 2009. [Google Scholar]
- Gauthier S, Ballard C. Management of dementia: Second edition. Informa Healthcare USA, Inc; New York, NY: 2009. [Google Scholar]
- Gu Y, Huang CS, Inoue T, Yamashita T, Ishida T, Kang KM, Nakao A. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. Journal of Clinical and Biochemical Nutrition. 2010;46:269–276. doi: 10.3164/jcbn.10-19. doi: 10.3164/jcbn.10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G, Fisher D. Oxidative stress and inflammation in brain aging: Nutritional considerations. Neurochemical Research. 2005;30(6/7):927–935. doi: 10.1007/s11064-005-6967-4. doi:10.1007/s11064-005-6967-4. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reverals of age-related declines in neuronal signal transduction, cognitive and motor behavioral deficits with blueberry, spinach or strawberry dietary supplementation. Journal of Neuroscience. 1999;19(18):8,114–8,121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive beahavioral deficits. Journal of Neuroscience. 1998;18(19):8,047–8,055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiology of Aging. 2005;26S:S80–S84. doi: 10.1016/j.neurobiolaging.2005.09.037. doi:10.1016/j.neurobiolaging.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ohashi Y, Ando S. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. Journal of Neuroscience Research. 2002;70(3):340–346. doi: 10.1002/jnr.10442. doi: 10.1002/jnr.10442. [DOI] [PubMed] [Google Scholar]
- Kumar V, Kinsella LJ. Health brain aging: Effect of head injury, alcohol and environmental toxins. Clinics in Geriatric Medicine. 2010;26(1):29–44. doi: 10.1016/j.cger.2009.12.006. doi: 10.1016/j.cger.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Kwok TCY, Chau WW, Yuen KSL, Wong AYM, Li JCY, Shiu RYY, Ho FKY. Who would benefit from memory training? A pilot study examing the ceiling effect of concurrent cognitive stimulation. Clinical Interventions in Aging. 2011;6:83–88. doi: 10.2147/CIA.S16802. doi: 10.2147/CIA.S16802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Brandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore Maryland Study. Archives of General Psychiatry. 2007;64(7):810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler H. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: The ARIC Study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the USA. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–1101. doi: 10.1002/hipo.20233. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Mandel SA, Amit T, Weinreb O, Youdim MB. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. Journal of Alzheimer’s Disease. 2011 doi: 10.3233/JAD-2011-101803. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- McGuire LC, Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life. American Journal of Geriatric Psychiatry. 2006;14(1):36–42. doi: 10.1097/01.JGP.0000192502.10692.d6. doi: 10.1097/01.JGP.0000192502.10692.d6. [DOI] [PubMed] [Google Scholar]
- Medina K, Schweinsburg L, Cohen-Zion AD, Nagel M, B. J., Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratrology. 2007;29(1):141–152. doi: 10.1016/j.ntt.2006.10.010. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Brennan PL, Schutte KK, Moos BS. Older adults’ health and changes in late-life drinking patterns. Aging and Mental Health. 2005;9(1):49–59. doi: 10.1080/13607860412331323818. doi: 10.1080/13607860412331323818. [DOI] [PubMed] [Google Scholar]
- Noice H, Noice T. Theatrical intervention to improve cognition in intact residents of long term care facilities. Clinical Gerontologist. 2006;3:59–76. doi: 10.1300/J018v29n03.05. [Google Scholar]
- Noice H, Noice T. An arts intervention for older adults living subsidized retirement homes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009;16(1):56–79. doi: 10.1080/13825580802233400. doi: 10.1080/13825580802233400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noice H, Noice T, Perrig-Chiello P, Perrig W. Improving memory in older adults by instructing them in professional actors’ learning strategies. Applied Cognitive Psychology. 1999;13(4):315–328. [Google Scholar]
- Noice H, Noice T, Staines G. A short-term intervention to enhance cognitive and affective functioning in older adults. Journal of Aging and Health. 2004;16(4):562–585. doi: 10.1177/0898264304265819. doi: 10.1177/0898264304265819. [DOI] [PubMed] [Google Scholar]
- Paban V, Jaffard M, Chambon C, Malafosse M, Alescio-Lautier B. Time course of behavioral changes following basal forebrain cholinergic damage in rats: Environmental enrichment as a therapeutic intervention. Neuroscience. 2005;132(1):13–32. doi: 10.1016/j.neuroscience.2004.11.024. doi: 10.1016/j.neuroscience.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Bader Lange ML, Sultana R, Butterfield DA. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Current Alzheimer’s Research. 2011 doi: 10.2174/156720511796391908. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of Neurology. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kanwar SS, Sood PK, Nehru B. Beneficial effects of folic acid on enhancement of memory and antioxidant status in aged rat brain. Cellular and Molecular Neurobiology. 2011;31(1):83–91. doi: 10.1007/s10571-010-9557-1. doi: 10.1007/s10571-010-9557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologica. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. doi:10.1016;j.neuropsychologica.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72(4):361–367. doi: 10.1212/01.wnl.0000341272.48617.b0. doi: 10.1212/01.wnl.0000341272.48617.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau, Population Division [Retrieved November 20, 2010];U. S. Population Projections: State Interim Population Projections by Age and Sex: 2004 – 2030. 2005 from http://www.census.gov/population/www/projections/projectionsagesex.html.
- Van Praag H, Kempermann G, Gag FH. Neural consequences of environmental enrichment. Nature Reviews Neuroscience. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vance DE, Crowe M. A proposed model of neuroplasticity and cognitive reserve in older adults. Activities, Adaptation and Aging. 2006;30(3):61–79. doi: 10.1300/J016v30n03_04. [Google Scholar]
- Vance DE, Eagerton G, Harnish B, McKie-Bell P, Fazeli P. Cognitive prescription across the lifespan: A nursing approach to increasing cognitive reserve. Journal of Gerontological Nursing. 2011;37(4):22–29. doi: 10.3928/00989134-20101202-03. doi: 10.3928/00989134-20101202-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Farr KF, Struzick T. Assessing the clinical value of cognitive appraisal in adults aging with HIV. Journal of Gerontological Nursing. 2008;34(1):36–41. doi: 10.3928/00989134-20080101-11. doi: 10.3928/00989134-20080101-11. [DOI] [PubMed] [Google Scholar]
- Vance DE, Wright MA. Positive and negative neuroplasticity: Implications for promotion of cognitive health in aging. Journal of Gerontological Nursing. 2009;35(6):11–17. doi: 10.3928/00989134-20090428-02. doi: 10.3928100989134-20090428-02. [DOI] [PubMed] [Google Scholar]
- Vance DE, Roberson AJ, McGuinness TM, Fazeli PL. How neuroplasticity and cognitive reserve protect cognitive functioning. Journal of Psychosocial Nursing & Mental Health Services. 2010:1–8. doi: 10.3928/02793695-20100302-01. doi: 10.3928/02793695-20100302-01. [DOI] [PubMed] [Google Scholar]